Abstract
Application of ATP and α,β-methylene ATP (αβmeATP) to voltage-clamped guinea-pig pelvic neurons produced three types of inward currents. A fast-desensitizing response was present in 5% (25/660) of neurons, 70% gave slowly-desensitizing currents, and the remainder had biphasic responses.
Slowly-desensitizing responses were characterized pharmacologically. The response to αβmeATP 100 μM was 46±27% (range 0 – 100%) of that evoked by ATP 100 μM in the same cell. Cross-desensitization indicated the presence of αβmeATP-sensitive and -insensitive receptors.
The concentration-response curve for αβmeATP had an EC50 of 55 μM, and a Hill coefficient of 0.99, while at the αβmeATP-insensitive receptor, ATP had an EC50 of 73 μM, with a Hill coefficient of 1.78.
The response to αβmeATP was blocked by pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), suramin and Cibacron blue. However, the αβmeATP-insensitive receptor was inhibited by PPADS, but not by the other two antagonists.
2′- (or 3′-) O-trinitrophenyl-ATP was 10 times more potent in inhibiting responses to αβmeATP than to ATP (at the αβmeATP-insensitive receptor).
Lowering extracellular pH potentiated responses to αβmeATP and ATP, while raising pH attenuated them.
Co-application of Zn2+ (3 – 300 μM) inhibited the responses to αβmeATP and ATP, with IC50 values of 286 and 60 μM, respectively.
In conclusion, unlike rat and mouse pelvic ganglion neurons, which only express P2X2 homomers, at least three distinct P2X receptors are present in guinea-pig pelvic neurons, probably homomeric P2X2, P2X3 and heteromeric P2X2/3 receptors. However, some of the novel pharmacological properties observed suggest that the guinea-pig P2X receptor subtypes may differ from their rat orthologues.
Keywords: P2X receptor, pelvic ganglion, ATP
Introduction
ATP functions as a fast excitatory neurotransmitter by activating a class of ligand-gated cation channels, the P2X receptors (for review, see Ralevic & Burnstock, 1998). It also modulates fast synaptic transmission by acting presynaptically (Gu & MacDermott, 1997; Khakh & Henderson, 1998). Seven subunits, P2X1 – 7, have been cloned in this family. When expressed heterologously, most of them can form functional homomeric receptors with different, yet overlapping phenotypes (Brake et al., 1994; Valera et al., 1994; Bo et al., 1995; Chen et al., 1995; Collo et al., 1996; Surprenant et al., 1996). In addition, they can form heteromeric channels with novel characteristics (Lewis et al., 1995; Lê et al., 1998; Torres et al., 1998; King et al., 2000). For example, P2X3 receptors are activated by α,β-methylene ATP (αβmeATP) and desensitize rapidly, whereas P2X2 receptors do not respond to this ligand and desensitize slowly (Brake et al., 1994; Chen et al., 1995). In contrast, P2X2/3 heteromeric receptors respond to αβmeATP (a property of P2X3 receptors), but desensitize slowly (a property of P2X2 receptors) (Lewis et al., 1995).
A number of subtype-selective molecules have emerged in recent years, mainly from the studies on recombinant rat P2X (rP2X) receptors. While P2X2 receptors are antagonized by suramin, Cibacron blue and pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), P2X4 receptors were relatively insensitive to them (Brake et al., 1994; Bo et al., 1995). 2′- (or 3′-) O-trinitrophenyl-ATP (TNP-ATP) is a potent antagonist on P2X1, P2X3 and P2X2/3 receptors, with 1000-fold less potency on P2X2, P2X4 and P2X7 receptors (Virginio et al., 1998). Diinosine pentaphosphate (Ip5I) selectively antagonizes P2X1 and P2X3 receptors, is inactive on P2X2 and P2X2/3 receptors, and potentiates P2X4 receptors (King et al., 1999; Dunn et al., 2000). P2X receptors can also be influenced by allosteric modulators, thus extracellular acidification potentiates P2X2 and P2X2/3 receptors, while inhibiting P2X1, P2X3 and P2X4 receptors (Stoop et al., 1997). Zn2+ potentiates P2X2 and P2X2/3 receptors (Li et al., 1993; Wildman et al., 1998), inhibits P2X1 receptors (Wildman et al., 1999a), while producing a bell-shaped dose-response curve on P2X3 and P2X4 receptors (Wildman et al., 1999a,1999b). Ivermectin is a specific positive modulator of P2X4 and P2X4/6 receptors, while being inactive on P2X2, P2X3, P2X2/3 and P2X7 receptors (Khakh et al., 1999). These molecules may prove to be important pharmacological tools in characterizing endogenously expressed P2X receptors in native tissues.
In our previous studies, we have shown that the P2X receptors on rat pelvic ganglia neurons are of the P2X2 subtype (Zhong et al., 1998), similar to those on rat superior cervical ganglia (SCG) (Nakazawa, 1994), and coeliac ganglia (Zhong et al., 2000a). In contrast, two distinct P2X receptors coexist on the same guinea-pig SCG neurons (Zhong et al., 2000b). While both desensitize slowly, only one is sensitive to αβmeATP. The proportions of these two receptors vary considerably from cell to cell. Guinea-pig coeliac neurons also demonstrated αβmeATP-sensitivity (Khakh et al., 1995). Therefore, in the present study, we sought to examine the P2X receptors on guinea-pig pelvic ganglia neurons, to find out whether they resemble those on rat pelvic, or those on guinea-pig SCG neurons. In addition, some experiments have been conducted on guinea-pig nodose neurons for comparison. Preliminary accounts of part of the work have appeared in the form of abstracts (Zhong et al., 1999, 2000c, 2000d).
Methods
Cell culture
Single neurons from the pelvic and nodose ganglia of male guinea-pigs (150 – 250 g) were enzymatically isolated as described previously (Zhong et al., 1998). Briefly, guinea-pigs were killed by inhalation of a rising concentration of CO2 and death was confirmed by cardiac haemorrhage. The pelvic and nodose ganglia were rapidly dissected out, and placed in Leibovitz's L-15 medium (Life Technologies, Paisley, U.K.). The ganglia were then desheathed, cut and incubated in 4 ml Ca2+ and Mg2+ free Hanks' balanced salt solution with 10 mM HEPES buffer (pH 7.4) (HBSS; Life Technologies) containing 1.5 mg ml−1 collagenase (Class-II, Worthington Biochemical Corporation, Reading, U.K.) and 6 mg ml−1 bovine serum albumin (Sigma, Poole, U.K.) at 37°C for 40 min. The ganglia were then incubated in 4 ml HBSS containing 1 mg ml−1 trypsin (Sigma) at 37°C for 20 min. The solution was replaced with 3 ml growth medium comprising of L-15 medium supplemented with 10% bovine serum, 50 ng ml−1 nerve growth factor, 2 mg ml−1 NaHCO3, 5.5 mg ml−1 glucose, 200 IU ml−1 penicillin and 200 μg ml−1 streptomycin. The ganglia were dissociated into single neurons by gentle trituration. The cells were then centrifuged at 160 g for 5 min, resuspended in 1 ml growth medium and plated onto 35-mm Petri dishes coated with 10 μg ml−1 laminin (Sigma). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2, and used on the following day.
Electrophysiology
Whole cell voltage-clamp recording was carried out at room temperature using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, U.S.A.). Membrane potential was held at −60 mV. External solution contained (mM): NaCl 154, KCl 4.7, MgCl2 1.2, CaCl2 2.5, HEPES 10, Glucose 5.6; the pH was adjusted to 7.4 using NaOH. Recording electrodes (resistance 2 – 4 MΩ) were filled with internal solution which contained (mM): citric acid 56, MgCl2 3, CsCl 10, NaCl 10, HEPES 40, EGTA 0.1, tetraethylammonium chloride 10; the pH was adjusted to 7.2 using CsOH (total Cs+ concentration 170 mM). Series resistance compensation of 70 – 75% was used in all recordings. Data were acquired using pCLAMP software (Axon Instruments). Signals were filtered at 2 kHz (−3 dB frequency, Bessel filter, 80 dB per decade).
Drugs were applied rapidly through a 7-barrel manifold comprising fused glass capillaries inserted into a common outlet tube (tip diameter of ∼200 μm) which was placed about 200 μm from the cell (Dunn et al., 1996). Solutions were delivered by gravity flow from independent reservoirs. One barrel was used to apply drug free solution to enable rapid termination of drug application. Solution exchange measured by changes in open tip current was complete in 200 ms; however, complete exchange of solution around an intact cell was considerably slower (⩽1 s).
Data analysis
All responses were normalized to that evoked by 100 μM αβmeATP or ATP in the same cell, unless otherwise stated. Except where indicated to the contrary, all data are expressed as the means±s.e.mean. Statistical analysis (Student's t-test, F test) was performed using Prism v2, (Graphpad, San Diego, CA, U.S.A.).
Concentration-response data were fitted with the Hill equation: Y=A/[1+(K/X)nH], where: A is the maximum effect, K is the EC50, and nH is the Hill coefficient, using Prism v2, Graphpad. The combined data from the given number of cells were fitted, and the results are presented as values±s.e., determined by the fitting routine.
Traces were acquired using Fetchex (pCLAMP software) and plotted using Origin (Microcal, Northampton, MA, U.S.A.). The desensitization traces were fitted using Clampfit (pCLAMP software), to both the first and second order exponential decay. However, for the 2 min application of agonists, a significantly better fit was consistently found using the second order exponential decay (F test, P<0.0001).
Drugs
ATP, αβmeATP, Cibacron blue (Cibacron Blue 3GA, 65% pure) and ivermectin were obtained from Sigma Chemical Co. (Poole, U.K.). PPADS was obtained from Tocris Cookson (Bristol, U.K.). Suramin was a gift from Bayer plc (Newbury, U.K.). 2′- (or 3′-) O-trinitrophenyl-ATP was obtained from Molecular Probes (Leiden, Netherlands). Ip5I was prepared by enzymatic degradation of diadenosine pentaphosphate (Ap5A) (see King et al., 1999). Solutions (10 – 100 mM) of ATP and other drugs were prepared using deionized water and stored frozen, except for ivermectin, which was dissolved in dimethylsulphoxide to 1 mM. All drugs were then diluted in extracellular bathing solution to the final concentration.
Results
Three types of responses to αβmeATP and ATP
Rapid application of αβmeATP and ATP 100 μM on to isolated pelvic ganglia neurons (>600 cells) of guinea-pig, voltage clamped at −60 mV, induced three types of inward currents. About 5% (25/660) of neurons showed predominantly fast-desensitizing response (Figure 1A), whereas 70% (471/660) of neurons showed slowly-desensitizing response (Figure 2A,B). The remaining 25% (164/660) showed a biphasic response, with both fast-desensitizing and slowly-desensitizing components being present (Figure 1B – E). The shape of the response for a given cell was always same for both agonists.
Figure 1.
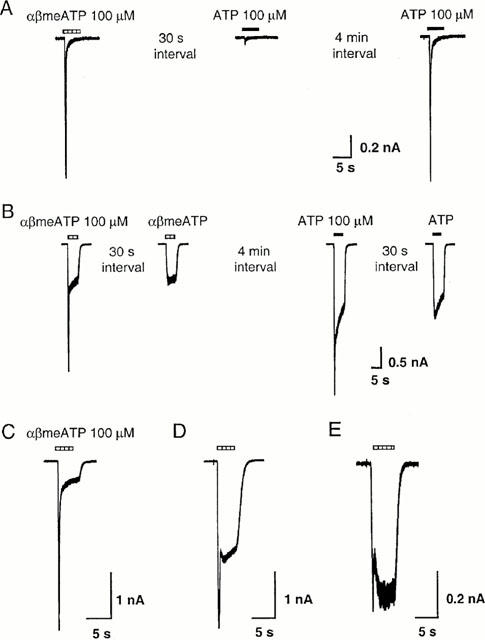
Heterogeneous responses to P2X agonists in isolated guinea-pig pelvic neurons. (A) Fast desensitizing inward current activated by αβmeATP and ATP (100 μM) in one neuron. Reapplying agonist 30 s after the first application evoked only a small response, but the response recovered fully after a 4 min interval. (B) In a different cell, application of αβmeATP and ATP evoked biphasic responses. Subsequent application of agonists 30 s later evoked only the slowly desensitizing response. (C – E) Examples of biphasic response to αβmeATP (100 μM) recorded from three guinea-pig pelvic neurons, illustrating the variation in the relative amplitude of the fast and slowly desensitizing components. All cells were voltage clamped at −60 mV. The horizontal bars above the traces indicate the duration of agonist application.
Figure 2.
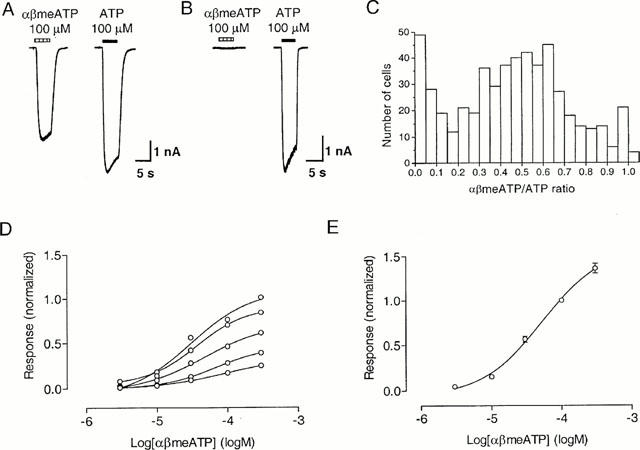
αβMeATP sensitivity of slowly-desensitizing responses in guinea-pig pelvic neurons. (A,B) Representative traces of slowly desensitizing responses evoked by αβmeATP (100 μM) and ATP (100 μM) from two neurons. Note the variation in the ratio of the slowly desensitizing responses to αβmeATP and ATP (αβmeATP/ATP ratio) in these two neurons. (C) The frequency distribution of the αβmeATP/ATP ratio for each of 531 cells. (D) Individual concentration-response curves for αβmeATP on six pelvic neurons, with responses normalized with respect to that obtained with 100 μM ATP on the same cell. (E) Concentration-response curves for αβmeATP, with responses normalized with respect to that obtained with 100 μM αβmeATP on the same cell. Points represent mean±s.e.mean for 12 cells. When not visible, error bars lie within the symbol. Agonists were applied for 5 s at 2 min intervals, which was sufficient for responses to be reproducible.
For cells demonstrating biphasic responses, the proportion of the fast-desensitizing and slowly-desensitizing components varied greatly from cell to cell (Figure 1C – E). Furthermore, in cells showing a fast-desensitizing inward current, reapplying agonist after an interval of 30 s evoked little response, but the current recovered fully after a 4 min interval (Figure 1A). In cells showing biphasic responses, subsequent application of agonists 30 s later evoked only the slowly-desensitizing component (Figure 1B).
The cells showing fast-desensitizing response had a capacitance of 17.5±6.4 pF (mean±s.d., n=25), which was significantly smaller than the capacitance of cells which demonstrated a slowly-desensitizing response (capacitance=33.4±17.6 pF, mean±s.d., n=471, P<0.001, Student's t-test) and those which demonstrated biphasic response (capacitance=26.6±13.4 pF, mean±s.d., n=164, P<0.001, Student's t-test). Because cells demonstrating a fast-desensitizing response comprised only 5% of the neurons, and it was difficult to isolate and study the transient component in cells showing biphasic responses, we have confined our pharmacological studies to the slowly-desensitizing responses.
Two receptors in the slowly-desensitizing response
As mentioned earlier, about 70% of guinea-pig pelvic ganglia neurons responded to both αβmeATP and ATP with slowly-desensitizing responses. However, the maximum response to αβmeATP was always smaller than that to ATP, and varied considerably from cell to cell (Figure 2A,B). In 531 cells where αβmeATP and ATP were tested on the same cells, the mean peak amplitude of the slowly-desensitizing current evoked by 100 μM αβmeATP was 2.2±2.2 nA (mean±s.d., n=531), while that evoked by 100 μM ATP was 5.2±4.1 nA (mean±s.d., n=531). The ratio of currents to αβmeATP and ATP at 100 μM from the same neuron (αβmeATP/ATP ratio) varied considerably from cell to cell. Thus the current elicited by 100 μM αβmeATP was 46±27% (mean±s.d., n=531) (range 0 – 100%) of that evoked by 100 μM ATP, although the EC50 values for these two agonists were similar (Figures 2 and 4). The frequency distribution of the αβmeATP/ATP ratio for each of 531 cells was clearly non-Gaussian (Figure 2C). While most neurons had a ratio about 0.5, there were substantial number of cells with a ratio of <0.1 or >0.9.
Figure 4.
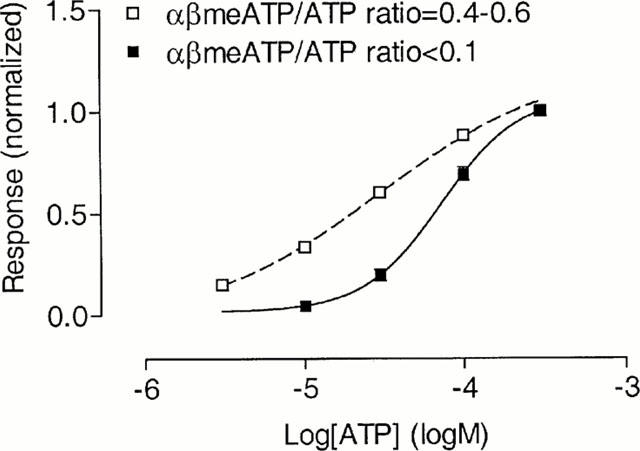
Concentration-response curves for the slowly-desensitizing responses to ATP on guinea-pig pelvic neurons. Concentration-response curves for ATP were constructed separately for guinea-pig pelvic neurons with an αβmeATP/ATP ratio <0.1, or in the range 0.4 – 0.6. For six cells possessing similar proportions of αβmeATP-sensitive and -insensitive receptors, (mean αβmeATP/ATP ratio=0.49), the concentration-response curve for ATP was fitted with a two-component curve, assuming equal proportions of high and low affinity binding sites, with an EC50 value of 73 μM for the αβmeATP-insensitive site as previously determined. Responses were normalized with respect to that obtained with 300 μM ATP on the same cell. When not visible, error bars lie within the symbol.
When the αβmeATP response was normalized with respect to that produced by 100 μM ATP from the same cell, the individual concentration-response curves yielded variable maximum responses (Figure 2D). However, when the response to αβmeATP was normalized with respect to that produced by 100 μM αβmeATP from the same cell, the data were very consistent (Figure 2E). Fitting the Hill equation to the concentration-response curve for αβmeATP yielded an EC50 value of 55 μM (log EC50=−4.26±0.08, n=12), and a Hill coefficient of 0.99.
The variable αβmeATP/ATP ratio found on these guinea-pig pelvic neurons was similar to our previous findings on guinea-pig SCG neurons, which possess both αβmeATP-sensitive and -insensitive P2X receptors on the same cell (Zhong et al., 2000b). To examine whether this situation also occurs in guinea-pig pelvic neurons, we studied the effect of cross-desensitization.
When αβmeATP 100 μM was applied for 2 min, the time course of the decline in the αβmeATP-induced current fitted well to the sum of two exponentials, with the time constants of decay (τ1 and τ2) being 7.0±1.0 s and 39.8±4.9 s, respectively (n=6). For six cells examined in this series of experiments, the peak response evoked by 100 μM αβmeATP was 41±6% of that produced by 100 μM ATP. After a 2-min application of αβmeATP, the response to αβmeATP declined to 15±2% of the peak (i.e. to 6% of the peak ATP response), while the response to 100 μM ATP was only reduced to 62±7% of control (Figure 3A). Therefore, the fractional reduction in the αβmeATP response was significantly greater than that of the ATP response (P<0.001, Student's t-test). On the other hand, when cells were desensitized by a prolonged exposure to 100 μM ATP, the responses to αβmeATP and ATP at the end of the 2-min desensitization were 15±6% and 15±3% of their respective controls (n=5, Figure 3B). In another series of experiments, we examined the desensitization rate of the ATP response in cells showing a negligible αβmeATP response, i.e., an αβmeATP/ATP ratio <0.1. In these neurons, the time course of the decline in the ATP-induced current was very similar to that of the αβmeATP response and fitted well to the sum of two exponential components, with time constants (τ1 and τ2) of 4.7±0.7 s and 47.5±9.6 s, respectively (n=6).
Figure 3.
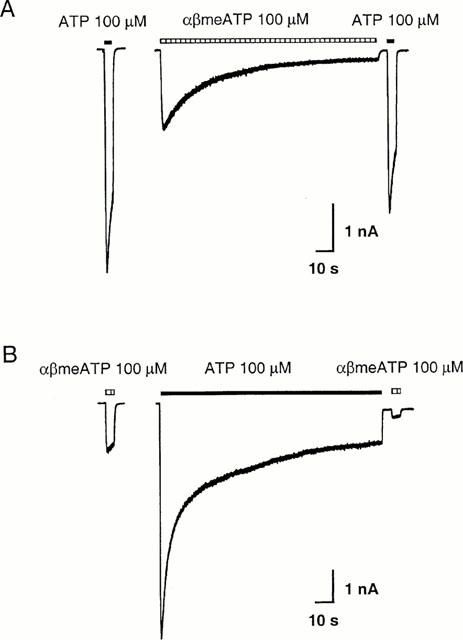
Cross-desensitization of P2X receptors on guinea-pig pelvic neurons by αβmeATP or ATP. (A) Traces of the membrane current recorded from one cell with a prolonged application of αβmeATP (100 μM). The control response evoked by 100 μM αβmeATP was 36% of that evoked by ATP (100 μM). A 2-min application of 100 μM αβmeATP produced a response which declined to 13% of the peak. In contrast, the response to 100 μM ATP was reduced to 73% of its own control. (B) Recordings from a different pelvic neuron with a prolonged application of 100 μM ATP. The control response evoked by 100 μM αβmeATP was 20% of that produced by 100 μM ATP. After 2 min desensitization by ATP 100 μM, the responses to αβmeATP and ATP were reduced to 18% and 14% of their respective controls. Cells were voltage clamped at −60 mV. The horizontal bars above the traces indicate the duration of agonist application.
These results suggested therefore, that in the 70% guinea-pig pelvic neurons that show slowly-desensitizing responses to agonists, two populations of P2X receptors are present. While both receptors are sensitive to ATP, only one can be activated by αβmeATP. To further characterize these two distinct P2X receptors pharmacologically, it was necessary to isolate each component of the response, and study them separately. To isolate the αβmeATP-sensitive slowly-desensitizing receptors, we used αβmeATP as a selective agonist. However, there is no selective agonist available for the αβmeATP-insensitive receptors. Thus, we selected neurons with an αβmeATP/ATP ratio <0.1, and used ATP as the agonist. This type of cell made up to only approximately 5% of total (see Figure 2C). Since this population of cells showed negligible response to αβmeATP, the ATP current could be regarded as due only to the activation of the αβmeATP-insensitive receptors. This strategy was used throughout the pharmacological characterization.
Further pharmacological characterization of the two slowly-desensitizing responses
The concentration-response relationship for ATP
The concentration-response relationship for ATP of nine cells having an αβmeATP/ATP ratio <0.1 (mean ratio=0.06) is shown in Figure 4. Fitting the Hill equation to the data yielded an EC50 of 73 μM (log EC50=−4.14±0.07, n=5 – 9 for each data point) and a Hill coefficient of 1.78. For six cells possessing similar proportions of αβmeATP-sensitive and -insensitive receptors (αβmeATP/ATP ratio of 0.4 – 0.6, mean=0.49), the concentration-response curve for ATP was fitted with a two-component curve, assuming equal proportions of high and low affinity binding sites, with an EC50 value of 73 μM for the αβmeATP-insensitive site as previously determined. This gave an EC50 value for the high affinity component of 13 μM.
Suramin
On rat pelvic neurons, suramin (100 μM) practically abolishes the response to ATP 100 μM (Zhong et al., 1998). On guinea-pig pelvic neurons, a 2 min preincubation with suramin (100 μM) reversibly inhibited the response evoked by αβmeATP (100 μM) to 5±1% (n=6) of control, with the response being 99±5% of control 2 min after washing out suramin (Figure 5A). In the presence of suramin 3 μM, the concentration-response curve of αβmeATP was shifted to the right, giving an EC50 of 104 μM (log EC50=−3.98±0.18, n=5) and a Hill coefficient of 1.00 (Figure 5C). From this increase in EC50, the pA2 value for suramin was estimated to be 5.45. Increasing suramin concentration to 10 μM shifted the concentration-response curve for αβmeATP further to the right, giving an estimated EC50 of 731 μM but with a Hill coefficient of only 0.83 (n=5). Thus, higher concentrations of suramin also appeared to have some non-competitive effects. In contrast, on cells showing an αβmeATP/ATP ratio <0.1 (mean ratio=0.01), after 2 min preincubation with suramin (100 μM), the response to ATP (100 μM) was 132±13% (n=4) of control (Figure 5B,D, P>0.05, Student's t-test).
Figure 5.
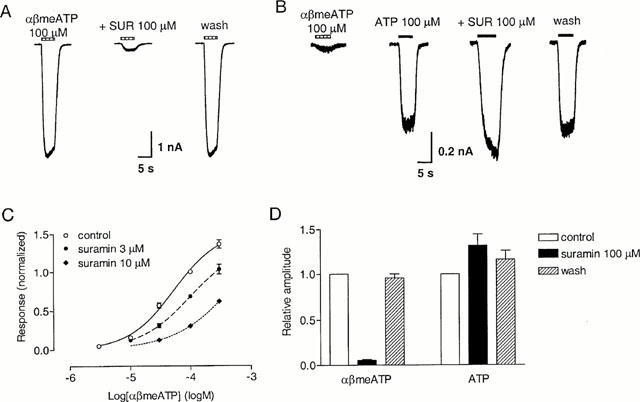
Effect of suramin on slowly-desensitizing responses evoked by αβmeATP and ATP in guinea-pig pelvic neurons. (A) Traces of three consecutive currents evoked from the same neuron by αβmeATP (100 μM) before, in the presence of suramin (100 μM) and 2 min after washing out the antagonist. Suramin was present for 2 min before and during the second application of αβmeATP. The horizontal bars above the traces indicate the duration of agonist application. (B) Traces from one cell (αβmeATP/ATP ratio <0.1) showing the response to ATP (100 μM) in the presence and absence of suramin (100 μM). Suramin was present for 2 min before and during the reapplication of ATP. (C) Concentration-response curve for αβmeATP in the absence and presence of suramin, 3 and 10 μM. (D) Averaged peak currents induced by αβmeATP 100 μM or ATP (100 μM, on cells with an αβmeATP/ATP ratio <0.1) in the presence and absence of suramin (100 μM). Bars represent responses in control condition, in the presence of suramin 100 μM and 2 min after washing out the antagonist. Responses were normalized with respect to that obtained with αβmeATP or ATP (100 μM) in the absence of suramin on the same cell. Each column represents the mean±s.e.mean of 4 – 6 cells.
Cibacron blue
On rat and mouse pelvic neurons, 2-min preincubation with 10 μM Cibacron blue abolished the response to 100 μM ATP (Zhong et al., 1998, 2000a). In contrast, on guinea-pig pelvic neurons, the response to αβmeATP (100 μM) was 106±7% (n=9) of control after a 2 min preincubation with Cibacron blue (10 μM). Increasing the concentration of Cibacron blue to 50 μM inhibited the αβmeATP response to 35±1% (n=4) of control (Figure 6A, C), and the response recovered to 86±6% of control 2 min after washing out the antagonist. On cells showing an αβmeATP/ATP ratio <0.1 (mean ratio=0.02), the response to ATP (100 μM) was 113±8% (n=6) and 122±8% (n=4, P>0.05, Student's t-test) of control, after 2 min preincubation with Cibacron blue 10 μM and 50 μM, respectively (Figure 6B,C). Two minutes after washing out Cibacron blue 50 μM, the response to ATP was 94±7% of control.
Figure 6.
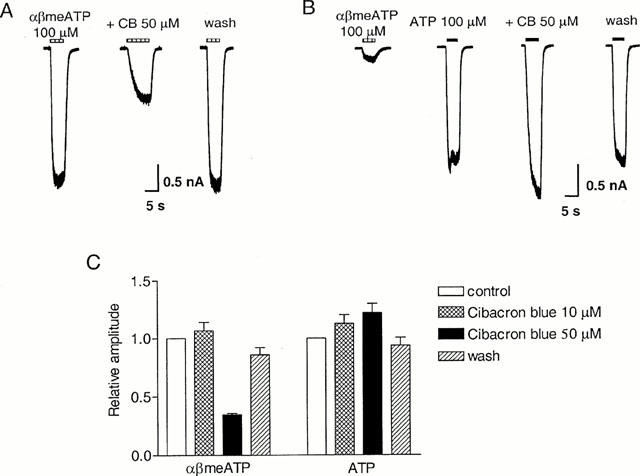
Effect of Cibacron blue on slowly-desensitizing responses evoked by αβmeATP and ATP on guinea-pig pelvic neurons. (A) Traces of three consecutive currents evoked from one cell by 100 μM αβmeATP before, in the presence of Cibacron blue (50 μM) and 2 min after washing out the antagonist. The horizontal bars above the traces indicate the duration of agonist application. (B) On another neuron with an αβmeATP/ATP ratio <0.1, four consecutive currents are shown to illustrate the control responses to αβmeATP (100 μM) and ATP (100 μM), and the responses to ATP in the presence of Cibacron blue (50 μM) and 2 min after washing out the antagonist. Cibacron blue was present for 2 min before and during the re-application of ATP. (C) Averaged peak currents induced by αβmeATP (100 μM) or ATP (100 μM, on cells with an αβmeATP/ATP ratio <0.1) in the presence and absence of Cibacron blue (10 and 50 μM). Bars represent responses in control condition, in the presence of 10 and 50 μM Cibacron blue and 2 min after washing out the antagonist. Responses were normalized with respect to that obtained with αβmeATP or ATP 100 μM in the absence of Cibacron blue on the same cell. Each column represents the mean±s.e.mean of 4 – 9 cells.
PPADS
The responses of guinea-pig pelvic neurons to both αβmeATP and ATP (on cells showing an αβmeATP/ATP ratio <0.1) were inhibited by PPADS (10 μM) with a slow time profile (Figure 7A). The decrease in response amplitude to αβmeATP (100 μM) could be described by a single exponential with a time constant of 1.7 min. The amplitude was 6±1% (n=9) of control after 10-min preincubation with PPADS 10 μM, and it only partially recovered to 20±3% of control 4 min after washing out the antagonist. Similarly, on cells showing an αβmeATP/ATP ratio <0.1 (mean ratio=0.03), the response to ATP (100 μM) was inhibited by PPADS (10 μM) with a time constant of 2.0 min. The ATP response was 3±1% (n=4) of control after 10-min preincubation with PPADS 10 μM, and recovered to 14±3% of control 4 min after washing out the antagonist.
Figure 7.
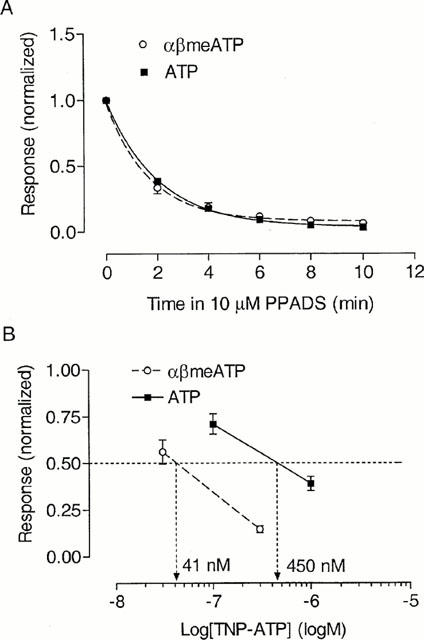
Inhibition of slowly-desensitizing responses to αβmeATP and ATP in guinea-pig pelvic neurons by PPADS and 2′- (or 3′-) O-trinitrophenyl-ATP (TNP-ATP). (A) Time profile of the inhibition by PPADS, using αβmeATP (100 μM) or ATP (100 μM) as the agonist. After determining the control responses to αβmeATP, or to ATP (on cells with an αβmeATP/ATP ratio <0.1), PPADS (10 μM) was applied to the cells and the response to agonists were redetermined every 2 min. Response was normalized with respect to that obtained with agonists in the absence of PPADS on the same neuron. Curves show single exponential fits to the data. Points represent mean±s.e.mean from 4 – 9 cells. (B) Inhibition by TNP-ATP of responses to αβmeATP (100 μM) or ATP (100 μM) (on cells with an αβmeATP/ATP ratio <0.1). Points represent mean±s.e.mean from four neurons. Responses were normalized with respect to that obtained with agonists in the absence of TNP-ATP on the same neuron. Each concentration of TNP-ATP was tested on a different sample of cells.
TNP-ATP
TNP-ATP is a selective antagonist on rP2X1, rP2X3 and rP2X2/3 receptors relative to rP2X2 receptors (Virginio et al., 1998). In the present study, we investigated the antagonism by TNP-ATP on guinea-pig pelvic neurons. As shown in Figure 7B, against the response to αβmeATP 100 μM, TNP-ATP inhibited the response with an estimated IC50 of 41 nM (n=4 for each concentration). While on cells with an αβmeATP/ATP ratio <0.1 (mean ratio=0.05), the response to ATP 100 μM was inhibited with an estimated IC50 of 450 nM (n=4 for each concentration). This is similar to the 8-fold difference in the potency of TNP-ATP in antagonizing the αβmeATP-sensitive and -insensitive receptors on guinea-pig SCG neurons (Zhong et al., 2000b).
pH
Lowering extracellular pH from 7.4 to 6.8 increased the response to αβmeATP 30 μM to 120±2% of control (n=4), whereas raising pH to 8.0 attenuated the response to 82±5% of control (n=4) (Figure 8A,C). Acidification of the extracellular solution to pH 6.8 caused a leftward shift in the concentration-response curve of αβmeATP by 0.21±0.03 log units (n=4, P<0.01, Student's t-test).
Figure 8.
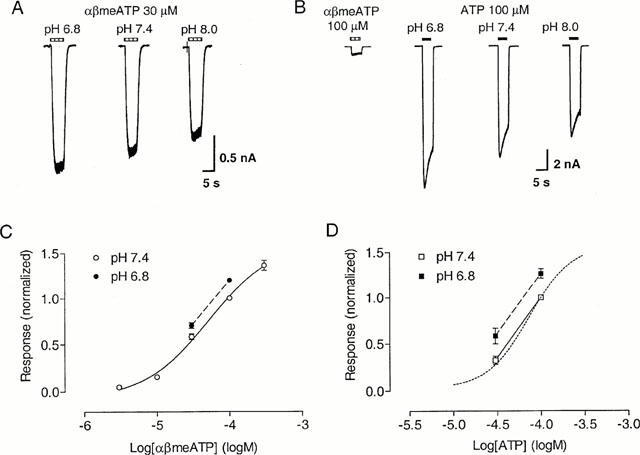
Effect of pH on slowly-desensitizing responses to αβmeATP and ATP on guinea-pig pelvic neurons. (A) Traces showing responses to αβmeATP (30 μM) of one neuron at pH 6.8, 7.4 and 8.0. (B) Traces showing responses to ATP (100 μM) of a neuron with an αβmeATP/ATP ratio <0.1 at pH 6.8, 7.4 and 8.0. The horizontal bars above the traces indicate the duration of agonist application. (C) Lowering extracellular pH from 7.4 to 6.8 displaced the αβmeATP concentration-response relationship to the left (n=4). (D) Changing extracellular pH from 7.4 to 6.8 shifted the ATP concentration-response relationship (for cells with αβmeATP/ATP ratio <0.1) to the left (n=4). The control concentration-response curve for ATP (pH 7.4) taken from Figure 4 has been superimposed (dotted curve) for comparison. Responses were normalized with respect to that evoked by αβmeATP or ATP (100 μM) at pH 7.4 on the same cell.
At the αβmeATP-insensitive receptor, lowering pH from 7.4 to 6.8 enhanced the response to ATP 100 μM to 126±5% of control (n=4), while raising pH to 8.0 attenuated the response to 79±5% of control (n=4) (Figure 8B,D). Change in pH from 7.4 to 6.8 shifted the concentration-response curve of ATP to the left by 0.15±0.02 log units (n=4, P<0.01).
Zn2+
Co-application of Zn2+ (3 – 300 μM) produced a reversible concentration-dependent inhibition of the response evoked by αβmeATP on guinea-pig pelvic neurons (Figure 9). Inclusion of a 2-min preincubation of Zn2+ did not produce any additional inhibition (data not shown). The response to αβmeATP 100 μM was inhibited by Zn2+ with an IC50 of 286 μM (log IC50=−3.54±0.10, n=8), and a Hill coefficient of 0.59. In the presence of 300 μM Zn2+, the EC50 for αβmeATP was estimated as 120 μM (log EC50=−3.92±0.47, n=4), with a Hill coefficient of 0.9 and an Emax of 0.85, compared with an EC50 of 55 μM, a Hill coefficient of 0.99 and an Emax of 1.62 in the control condition. Thus, the potency as well as the maximum response to αβmeATP appeared to have been reduced, indicating that the inhibition by Zn2+ (300 μM) is not competitive.
Figure 9.
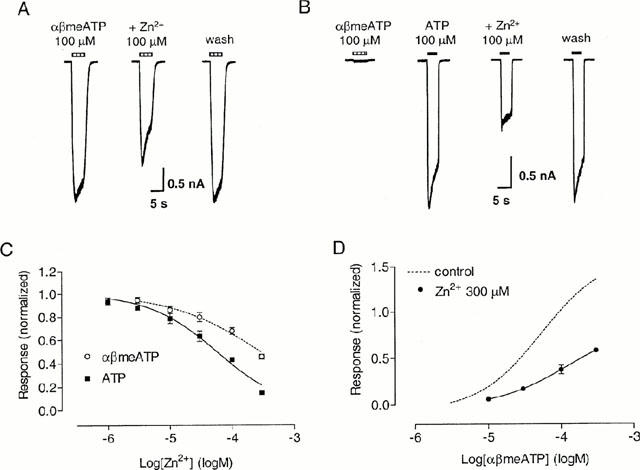
Effect of Zn2+ on slowly-desensitizing responses to αβmeATP and ATP on guinea-pig pelvic neurons. (A) Traces of three consecutive currents evoked from one neuron by 100 μM αβmeATP before, in the presence of Zn2+ 100 μM and 2 min after washing out Zn2+. Zn2+ was co-applied with αβmeATP. (B) Traces from one cell (αβmeATP/ATP ratio <0.1) showing the response to 100 μM ATP alone, when co-applied in the presence of 100 μM Zn2+ and 2 min after washing out Zn2+. The horizontal bars above the traces indicate the duration of agonist application. (C) Inhibition curve for Zn2+ when αβmeATP (100 μM) was used as the agonist, or on cells where the αβmeATP/ATP ratio was <0.1 with ATP (100 μM) as the agonist. Points represent mean±s.e.mean from 4 – 8 neurons. (D) Concentration-response curve for αβmeATP in the presence of Zn2+ (300 μM). The control concentration-response curve taken from Figure 2E has been superimposed for comparison. Responses were normalized with respect to that obtained with αβmeATP or ATP 100 μM in the absence of Zn2+ on the same cell.
At the αβmeATP-insensitive receptor, co-application of Zn2+ inhibited the response to ATP 100 μM with an IC50 of 60 μM (log IC50=−4.23±0.04, n=4), and a Hill coefficient of 0.82. Thus, the potency of Zn2+ on these receptors was significantly higher than that on the αβmeATP-sensitive receptors (P<0.001).
Ip5I and ivermectin
The rat P2X4 receptor is resistant to the antagonists suramin and Cibacron blue (Bo et al., 1995; Buell et al., 1996b). We therefore investigated the possible involvement of the P2X4 subunit in the αβmeATP-insensitive receptor. Ip5I is a P2X-subtype selective antagonist, which potently inhibits rP2X1 and rP2X3 receptors, but is inactive on rP2X2 receptors at concentrations up to 30 μM, and enhances the action of ATP at rP2X4 receptors (King et al., 1999). In four cells with a mean αβmeATP/ATP ratio of 0.03, after 2-min preincubation with Ip5I 0.1 μM or 10 μM, the response to 100 μM ATP was 92±2% (P<0.05) and 80±1% (P<0.001) of control, respectively (Figure 10A,B).
Figure 10.
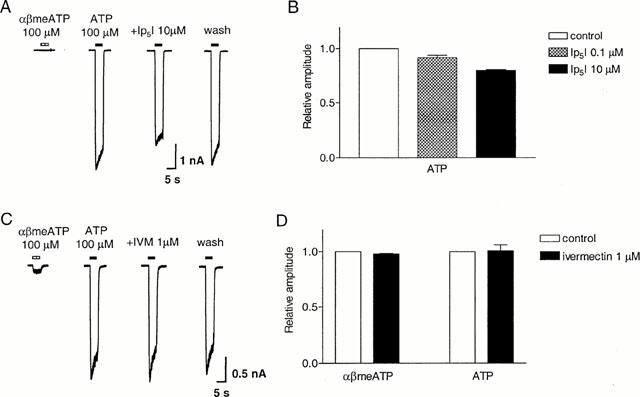
Effect of diinosine pentaphosphate (Ip5I) and ivermectin on slowly-desensitizing responses to αβmeATP and ATP on guinea-pig pelvic neurons. (A) Traces of responses to ATP (100 μM) in the absence and presence of Ip5I (10 μM) in a cell with an αβmeATP/ATP ratio <0.1. Ip5I was present 2 min before and during reapplication of ATP. The horizontal bars above the traces indicate the duration of agonist application. (B) Averaged peak amplitudes of the current induced by ATP (100 μM) in the absence and presence of Ip5I, 0.1 and 10 μM. (C) Traces of responses to ATP (100 μM) in the absence and presence of ivermectin (1 μM) in a cell with an αβmeATP/ATP ratio <0.1. Ivermectin was present 2 min before and during reapplication of ATP. (D) Averaged peak responses to αβmeATP or ATP (100 μM). (αβmeATP/ATP ratio <0.1), in the absence and presence of ivermectin (1 μM). Responses were normalized to that in the absence of Ip5I or ivermectin from the same cell. Each column represents the mean±s.e.mean of four cells.
Another subtype selective modulator of P2X receptors, ivermectin, selectively increases the potency and efficacy of ATP on recombinant rP2X4 receptors (Khakh et al., 1999), but has no effect on rP2X2, rP2X2/3 and rP2X3 receptors. Therefore, we sought to test its effect on the slowly-desensitizing responses on guinea-pig pelvic neurons. After 2-min preincubation with 1 μM ivermectin, the response to 100 μM αβmeATP was 98±1% of control (n=4) (Figure 10D). In four cells with a mean αβmeATP/ATP ratio of 0.06, the response to 100 μM ATP after 2 min preincubation with ivermectin 1 μM was 101±5% of control (n=4, P>0.05, Figure 10C,D).
Effect of Zn2+ on guinea-pig nodose neurons
Slowly-desensitizing αβmeATP sensitive P2X receptors are present on rat nodose neurons, where responses are potentiated by Zn2+ (Li et al., 1993). However, we observed inhibition by Zn2+ of the response to αβmeATP on guinea-pig pelvic neurons. To test whether this might be due to a species difference between rat and guinea-pig P2X receptors, we examined the effect of Zn2+ on αβmeATP response on guinea-pig nodose neurons.
On guinea-pig nodose neurons, αβmeATP evoked a slowly desensitizing response. Co-application of Zn2+ produced concentration-dependent inhibition of the αβmeATP response, similar to that seen on guinea-pig pelvic neurons (Figure 11). In the presence of 300 μM Zn2+, the response to 100 μM αβmeATP was 39±3% of control (n=6).
Figure 11.
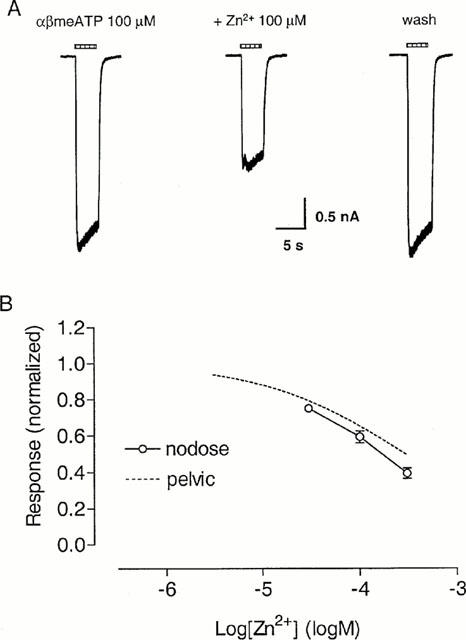
Effect of Zn2+ on slowly-desensitizing responses to αβmeATP on guinea-pig nodose neurons. (A) Traces showing responses to αβmeATP (100 μM) in the absence and presence of Zn2+ (100 μM) on one guinea-pig nodose neuron. Zn2+ was co-applied with αβmeATP. The horizontal bars above the traces indicate the duration of agonist application. (B) Concentration-response relationship for the inhibition by Zn2+ of responses to αβmeATP (100 μM) on guinea-pig nodose neurons. Zn2+ was co-applied with the agonist. Responses were normalized with respect to that obtained with αβmeATP (100 μM) in the absence of Zn2+ on the same cell. The inhibition curve for Zn2+ against αβmeATP on guinea-pig pelvic neurons (taken from Figure 9C) has been superimposed for comparison.
Discussion
Two major findings have come out of this study. Firstly, we have demonstrated the presence of at least three distinct populations of P2X receptors on guinea-pig pelvic neurons. This is in contrast to the situations in rat and mouse pelvic neurons, where only a single population of P2X2 homomeric receptors is present. Secondly, using currently available pharmacological tools, we have shown that these guinea-pig P2X receptors display novel pharmacological properties, which may reflect a species difference in the characteristics of P2X receptors, or may indicate the presence of novel P2X receptors.
Co-existence of three distinct P2X receptors
We have previously demonstrated that in guinea-pig SCG neurons two populations of P2X receptors are present, both desensitize slowly, but only one is activated by αβmeATP. The relative proportions of these receptors vary from cell to cell (Zhong et al., 2000b). In the present study, we have observed two similar populations of receptors in neurons of guinea-pig pelvic ganglia. In addition, however, we also observed in some neurons, the presence of the third receptor type, which was activated by both ATP and αβmeATP, desensitized very rapidly, and required >3 min between agonist application to achieve reproducible responses. The presence of two populations of slowly desensitizing receptors was indicated by the variability in the relative maximum responses to αβmeATP and ATP, confirmed by cross-desensitization experiments, and by the pharmacological characterization of the receptors (see below and Table 1). Therefore, three distinct populations of P2X receptors can co-exist on the same guinea-pig pelvic neuron, one is activated by αβmeATP and rapidly-desensitizing, one is αβmeATP-insensitive and desensitizes slowly and the third is slowly-desensitizing but αβmeATP-sensitive.
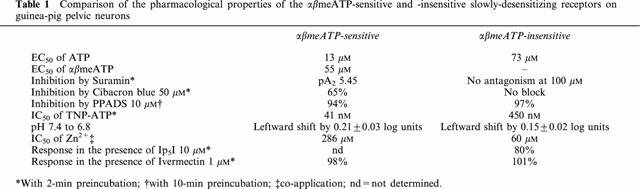
Table 1.
Comparison of the pharmacological properties of the αβmeATP-sensitive and -insensitive slowly-desensitizing receptors on guinea-pig pelvic neurons
The presence of both rapidly- and slowly-desensitizing responses in the same neuron gave rise to biphasic currents, which were encountered in about 25% of guinea-pig pelvic neurons. Biphasic P2X-mediated currents have previously been described in a sub-population of rat trigeminal and dorsal root ganglia (DRG) neurons, and have been attributed to the presence of homomeric P2X3 receptors and heteromeric P2X2/3 receptors (Cook et al., 1997; Burgard et al., 1999; Grubb & Evans 1999). Fast- and slowly-desensitizing ATP currents were previously observed in guinea-pig myenteric neurons, although no biphasic current was reported in these cells (Zhou & Galligan, 1996).
Possible identity of P2X receptors
Of the recombinant homomeric P2X receptors, only P2X1 and P2X3 respond significantly to αβmeATP. These receptors, however, desensitize very rapidly. In contrast, heteromeric P2X2/3 receptors respond to αβmeATP with a slowly-desensitizing profile. We have previously demonstrated the presence of P2X2 and P2X3 immunoreactivity in neurons of the guinea-pig pelvic ganglia (Zhong et al., 2000b). Thus, the simplest explanation of our results is that these neurons express varying proportions of P2X2 and P2X3 subunits which assemble to produce three different receptors, namely homomeric P2X2 and P2X3, and heteromeric P2X2/3.
This explanation would account for the temporal profile of the responses, and the presence of αβmeATP-sensitive and -insensitive components. Due to the infrequency of encountering cells showing transient response, we have been unable to characterize the pharmacological properties of the rapidly desensitizing receptors. Another possible explanation for a rapidly desensitizing αβmeATP sensitive receptor is the involvement of P2X1. However, since we were unable to detect the P2X1 immunoreactivity (Zhong et al., 2000b), this hypothesis is unlikely.
Of the slowly-desensitizing responses, the properties of the αβmeATP-sensitive receptors are most consistent with those of P2X2/3 receptors, e.g., the slowly desensitizing profile of the αβmeATP response, the sensitivity to antagonists suramin, Cibacron blue and PPADS and the potentiation by acidification (Lewis et al., 1995; Stoop et al., 1997; Liu & King, personal communication). The sensitivity to TNP-ATP of these receptors was close to that found previously on guinea-pig SCG neurons (Zhong et al., 2000b), and may suggest a slightly lower sensitivity to TNP-ATP of guinea-pig P2X2/3 receptors compared with those of the rat. The modulation by Zn2+ on guinea-pig neurons however, appeared to be different from that on rat. While the slowly-desensitizing αβmeATP-sensitive responses on rat nodose neurons were potentiated by Zn2+ (Li et al., 1993), those on guinea-pig pelvic neurons were inhibited. Although a novel P2X receptor might be involved here, it is also possible that guinea-pig P2X2/3 receptors differ from rP2X2/3 in their modulation by Zn2+. We found that on guinea-pig nodose neurons, which showed specific immunoreactivity for rP2X2 and rP2X3 antibodies in a pattern similar to that on rat nodose ganglia (Xiang et al., 1998; Zhong et al., 2000b), Zn2+ also inhibited the responses to αβmeATP. Similar inhibition by Zn2+ has been reported for the persistent response to ATP on bullfrog DRG neurons, which cannot be accounted for by the decrease in the concentration of one or more active forms of ATP (Li et al., 1997). This indicates that the difference in the modulation by Zn2+ seen here may reflect a species difference, i.e. the rP2X2/3 receptors are potentiated, while those of guinea-pig and bullfrog are inhibited.
We used αβmeATP as a selective agonist to isolate the αβmeATP-sensitive slowly-desensitizing receptors. To study the αβmeATP-insensitive receptors, we had to select neurons that express exclusively this type of receptor and use ATP as the agonist. Unfortunately, this type of cell made up only 5% of the total number (see Figure 2C), which added considerable difficulty to the study. While these receptors had many pharmacological properties observed for recombinant rP2X2 receptors, there were some clear differences. Thus, responses were potentiated by acidification and antagonized by PPADS – rP2X2-like (Zhong et al., 1998). However, these αβmeATP-insensitive responses were not blockable by Cibacron blue (50 μM) or suramin (100 μM). In view of the antagonist resistance observed, we considered the possible involvement of P2X4 subunits, although we have been unable to detect this protein using immunohistochemistry (Zhong et al., 2000b). Ip5I and ivermectin both potentiate P2X4 receptors (King et al., 1999; Khakh et al., 1999). However, responses in guinea-pig pelvic neurons were not potentiated by these modulators, which argues against the involvement of this subunit.
So far, P2X2 is the only subunit cloned from the guinea-pig, and three spliced variants have been identified (Parker et al., 1998). When expressed heterologously, homomeric guinea-pig P2X2-1 and P2X2-2 receptors gave rise to large currents which desensitized quite fast in a double exponential fashion, whereas homomeric P2X2-3 receptors gave rise to small, but sustained, currents (Chen et al., 2000). ATP was a potent full agonist on all of them and αβmeATP evoked little response at concentrations up to 100 μM. Interestingly however, co-application of ATP with suramin or PPADS produced variable degrees of inhibition on these recombinant guinea-pig receptors. Partial inhibition by suramin, Cibacron blue and PPADS at high concentrations was previously reported for guinea-pig chromaffin cells (Liu et al., 1999). In contrast, ATP responses on guinea-pig myenteric neurons were enhanced by suramin and Cibacron blue, but inhibited by PPADS (Barajas-López et al., 1996). Thus, the phenotype of αβmeATP-insensitive receptors on guinea-pig pelvic neurons was very similar to that on guinea-pig myenteric neurons. The discrepancy in the antagonist sensitivity seen here may result from the co-assembly of different spliced variants of guinea-pig P2X2 receptors in various native cells. Alternatively, this may be due to hetero-multimeric receptors containing P2X2 as well as other subunits. The third possibility is that novel P2X receptors are yet to be cloned from guinea-pig pelvic and myenteric neurons.
Inter-species variation
The findings in the present study support and extend our previous suggestion that the expression of P2X receptors is different in rat and guinea-pig. Thus, homomeric P2X2 receptors may be the dominant subtype in neurons of rat SCG (Nakazawa, 1994), coeliac (Zhong et al., 2000a) and pelvic ganglia (Zhong et al., 1998). In contrast, neurons in guinea-pig SCG express two distinct P2X receptors (Zhong et al., 2000b), and guinea-pig pelvic neurons express three P2X receptors, with different desensitizing kinetics and pharmacological properties (present study). Neurons in guinea-pig coeliac ganglia have also been reported to be αβmeATP-sensitive (Khakh et al., 1995). It remains to be seen whether in fact multiple P2X receptors are also present in guinea-pig coeliac neurons. In addition, while rat chromaffin cells lack P2X receptors, those of the guinea-pig are sensitive to ATP (Liu et al., 1999). Similarly, most outer hair cells of rat do not appear to possess P2X receptors, while those of guinea-pig do (Chen et al., 1997). The pharmacological and immunohistochemical evidence suggests that, in guinea-pig, P2X3 subunits may be present in autonomic as well as sensory neurons (Zhong et al., 2000b). However, in the rat, high levels of P2X3 subunit expression can only be detected in sensory neurons (Buell et al., 1996a; Xiang et al., 1998).
Inter-species difference is also evident from the differences in pharmacological properties between P2X receptor orthologues. For example, recombinant hP2X4 receptors displayed notably higher sensitivity to antagonists suramin and PPADS compared with the rat homologue. A single-point mutation on rP2X4 (Q78K) was sufficient to account for this increase in suramin sensitivity (Garcia-Guzman et al., 1997). Similarly, a lysine residue at position 249 on rP2X1 and rP2X2 seems to be crucial for the potency and kinetics of the antagonism by PPADS (Buell et al., 1996b). In the light of these findings, it is possible that the difference in the amino acids sequences between rat and guinea-pig P2X receptors at certain positions may confer distinct pharmacological phenotypes. The three spliced variants of guinea-pig P2X2 receptors share 80 – 83% homology with those of the rat (Brändle et al., 1997; Parker et al., 1998). Thus, the construction of chimeric rat/guinea-pig receptors should enable the amino acids involved in the Zn2+ binding site to be identified.
Neurons in the guinea-pig pelvic plexus provide motor innervation to urogenital organs and the lower digestive tracts. We found that neurons expressing only the fast-desensitizing P2X receptors are significantly smaller than the others. It remains to be seen whether there is a correlation between the property of the neuron, their target organ, and the type of P2X receptors expressed. On the other hand, it will be interesting to determine whether the proportions of multiple P2X receptors within a given cell remain constant, or change during development or under pathological conditions.
To conclude, we have identified three different P2X receptors on guinea-pig pelvic neurons. The proportion and combination of each type vary greatly from cell to cell. The novel pharmacological properties demonstrated by these neurons suggest that guinea-pig P2X receptors have different properties compared with those of the rat orthologues. Alternatively, a novel P2X receptor or heteromultimer with novel subunit combinations may be present in these neurons.
Acknowledgments
The authors are grateful to Mr E.W. Moules for excellent technical support in cell culture, to Mr R. Jordan for the editorial assistance with the manuscript and to Mr J. Gualix for preparation of Ip5I. Y. Zhong and P.M. Dunn were supported by Roche Bioscience (Palo Alto, California, U.S.A.).
Abbreviations
- HBSS
Hanks' balanced salt solution with 10 mM HEPES buffer
- Ip5I
diinosine pentaphosphate
- αβmeATP
α,β-methylene ATP
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
- SCG
superior cervical ganglia
- TNP-ATP
2′-(or 3′-) O-trinitrophenyl-ATP
References
- BARAJAS-LÓPEZ C., HUIZINGA J.D., COLLINS S.M., GERZANICH V., ESPINOSA-LUNA R., PERES A. P2X-purinoceptors of myenteric neurons from the guinea-pig ileum and their unusual pharmacological properties. Br. J. Pharmacol. 1996;119:1541–1548. doi: 10.1111/j.1476-5381.1996.tb16070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BO X., ZHANG Y., NASSAR M., BURNSTOCK G., SCHOEPFER R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-q. [DOI] [PubMed] [Google Scholar]
- BRAKE A.J., WAGENBACH M.J., JULIUS D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. doi: 10.1038/371519a0. [DOI] [PubMed] [Google Scholar]
- BRÄNDLE U., SPIELMANNS P., OSTEROTH R., SIM J., SURPRENANT A., BUELL G., RUPPERSBERG J.P., PLINKERT P.K., ZENNER H.-P., GLOWATZKI E. Desensitization of the P2X2 receptor controlled by alternative splicing. FEBS Lett. 1997;404:294–298. doi: 10.1016/s0014-5793(97)00128-2. [DOI] [PubMed] [Google Scholar]
- BUELL G., COLLO G., RASSENDREN F. P2X receptors: an emerging channel family. Eur. J. Pharmacol. 1996a;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- BUELL G., LEWIS C., COLLO G., NORTH R.A., SURPRENANT A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996b;15:55–62. [PMC free article] [PubMed] [Google Scholar]
- BURGARD E.C., NIFORATOS W., BIESEN T.V., LYNCH K.J., TOUMA E., METZGER R.E., KOWALUK E.A., JARVIS M.F. P2X receptor-mediated ionic currents in dorsal root ganglia neurons. J. Neurophysiol. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- CHEN C.-C., AKOPIAN A.N., SIVILOTTI L., COLQUHOUN D., BURNSTOCK G., WOOD J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- CHEN C., LEBLANC C., BOBBIN R.P. Differences in the distribution of responses to ATP and acetylcholine between outer hair cells of rat and guinea-pig. Hear. Res. 1997;110:87–94. doi: 10.1016/s0378-5955(97)00069-5. [DOI] [PubMed] [Google Scholar]
- CHEN C., PARKER M.S., BARNES A.P., DEININGER P., BOBBIN R.P. Functional expression of three P2X2 receptor splice variants from guinea-pig cochlea. J. Neurophysiol. 2000;83:1502–1509. doi: 10.1152/jn.2000.83.3.1502. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK S.P., VULCHANOVA L., HARGREAVES K.M., ELDE R., McCLESKEY E.W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- DUNN P.M., BENTON D.C., CAMPOS-ROSA J., GANELLIN C.R., JENKINSON D.H. Discrimination between subtypes of apamin-sensitive Ca2+-activated K+ channels by gallamine and a novel bis-quaternary quinolinium cyclophane, UCL 1530. Br. J. Pharmacol. 1996;117:35–42. doi: 10.1111/j.1476-5381.1996.tb15151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN P.M., LIU M., ZHONG Y., KING B.F., BURNSTOCK G. Diinosine pentaphosphate: an antagonist which discriminates between recombinant P2X3 and P2X2/3 receptors and between two P2X receptors in rat sensory neurons. Br. J. Pharmacol. 2000;130:1378–1384. doi: 10.1038/sj.bjp.0703404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-GUZMAN M., SOTO F., GOMEZ-HERNANDEZ J.M., LUND P.-E., STÜHMER W. Characterisation of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol. Pharmacol. 1997;51:109–118. doi: 10.1124/mol.51.1.109. [DOI] [PubMed] [Google Scholar]
- GRUBB B.D., EVANS R.J. Characterization of cultured dorsal root ganglia neuron P2X receptors. Eur. J. Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- GU J.G., MACDERMOTT A.B. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., HENDERSON G. ATP receptor-mediated enhancement of fast excitatory neurotransmitter release in the brain. Mol. Pharmacol. 1998;54:372–378. doi: 10.1124/mol.54.2.372. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., HUMPHREY P.P.A., SURPRENANT A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurons. J. Physiol. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAKH B.S., PROCTOR W.R., DUNWIDDIE T.V., LABARCA C., LESTER H.A. Allosteric control of gating and kinetics at P2X4 receptor channels. J. Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., LIU M., PINTOR J., GUALIX J., MIRAS-PORTUGAL M.T., BURNSTOCK G. Diinosine pentaphosphate (Ip5I) is a potent antagonist at recombinant rat P2X1 receptors. Br. J. Pharmacol. 1999;128:981–988. doi: 10.1038/sj.bjp.0702876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., TOWNSEND-NICHOLSON A., WILDMAN S.S., THOMAS T., SPYER K.M., BURNSTOCK G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J. Neurosci. 2000;20:4871–4877. doi: 10.1523/JNEUROSCI.20-13-04871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LÊ K.-T., BABINSKI K., SÉGUÉLA P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J. Neurosci. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C., NEIDHART S., HOLY C., NORTH R.A., BUELL G., SURPRENANT A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., LI Z., WEIGHT F.F. Zn2+ potentiates excitatory action of ATP on mammalian neurons. Proc. Natl. Acad. Sci. USA. 1993;90:8264–9267. doi: 10.1073/pnas.90.17.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Inhibition of ATP-activated current by zinc in dorsal root ganglia neurons of bullfrog. J. Physiol. 1997;505:641–653. doi: 10.1111/j.1469-7793.1997.641ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU M., DUNN P.M., KING B.F., BURNSTOCK G. Rat chromaffin cells lack P2X receptors while those of the guinea-pig express a P2X receptor with novel pharmacology. Br. J. Pharmacol. 1999;128:61–68. doi: 10.1038/sj.bjp.0702790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAZAWA K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J. Neurosci. 1994;14:740–750. doi: 10.1523/JNEUROSCI.14-02-00740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER M.S., LARROQUE M.L., CAMPBELL J.M., BOBBIN R.P., DEININGER P.L. Novel variant of the P2X2 ATP receptor from the guinea-pig organ of Corti. Hear. Res. 1998;121:62–70. doi: 10.1016/s0378-5955(98)00065-3. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- STOOP R., SURPRENANT A., NORTH R.A. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J. Neurophysiol. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., RASSENDREN F., KAWASHIMA E., NORTH R.A., BUELL G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- TORRES G.E., HAINES W.R., EGAN T.M., VOIGT M.M. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol. Pharmacol. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- VALERA S., HUSSY N., EVANS R.J., ADAMI N., NORTH R.A., SURPRENANT A., BUELL G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- VIRGINIO C., ROBERTSON G, SURPRENANT A., NORTH R.A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3 and heteromeric P2X2/3 receptors. Mol. Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence of extracellular pH. Br. J. Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br. J. Pharmacol. 1999a;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Modulation of ATP-responses at recombinant rP2X4 receptors by extracellular pH and zinc. Br. J. Pharmacol. 1999b;126:762–768. doi: 10.1038/sj.bjp.0702325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIANG Z., BO X., BURNSTOCK G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci. Lett. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- ZHONG Y., DUNN P.M., BURNSTOCK G. P2X receptors on mouse and guinea-pig pelvic ganglion neurons exhibit different Zn2+ and pH sensitivities. Br. J. Pharmacol. 1999;126:23P. doi: 10.1038/sj.bjp.0703778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHONG Y., DUNN P.M., BURNSTOCK G. Pharmacological comparison of P2X receptors on rat coeliac, mouse coeliac and mouse pelvic ganglion neurons. Neuropharmacology. 2000a;39:172–180. doi: 10.1016/s0028-3908(99)00145-8. [DOI] [PubMed] [Google Scholar]
- ZHONG Y., DUNN P.M., BURNSTOCK G. Guinea-pig sympathetic neurons express varying proportions of two distinct P2X receptors. J. Physiol. 2000b;523:391–402. doi: 10.1111/j.1469-7793.2000.t01-1-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHONG Y., DUNN P.M., BURNSTOCK G. P2X receptors on guinea-pig pelvic ganglion neurons exhibit novel sensitivity to antagonists suramin, Cibacron blue and PPADS. Br. J. Pharmacol. 2000c;129:46P. doi: 10.1038/sj.bjp.0703778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHONG Y., DUNN P.M., BURNSTOCK G. Effects of allosteric modulators on P2X receptors of guinea pig pelvic ganglion neurons. Auton. Neurosci. 2000d;82:71. [Google Scholar]
- ZHONG Y., DUNN P.M., XIANG Z., BO X., BURNSTOCK G. Pharmacological and molecular characterization of P2X receptors in rat pelvic ganglia neurons. Br. J. Pharmacol. 1998;125:771–781. doi: 10.1038/sj.bjp.0702118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X., GALLIGAN J.J. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. J. Physiol. 1996;496:719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]


