Abstract
The anti-nociceptive effects of contralateral administration of κ-opioid agonist U-50,488H were investigated in rats.
Inflammation was induced by unilateral injection of 1% carrageenan into the right hindpaw. Prior to carrageenan injection, U-50,488H or saline was administered into the left hindpaw. Withdrawal responses to mechanical and heat stimulation and oedema levels were evaluated at 3, 6 and 24 h post-carrageenan injection.
The results showed that the inflammatory effect of 1% carrageenan peaked after 6 h with bilateral decreases in withdrawal latencies and ipsilateral oedema formation.
Contralateral treatment with 0.01, 0.05, 0.3 and 2 mg of U-50,488H attenuated nociceptive reflexes to mechanical stimulation on the inflamed side at 6 h. The anti-nociceptive effect of contralateral treatment was dose-dependent at 3 and 24 h. The hindpaw withdrawal latencies to heat stimulation were prolonged at 3 and 24 h after contralateral treatment with 0.3 mg U-50,488H. No effect on inflammatory oedema formation was observed, except for a decrease at 3 h after treatment with 2 mg of U-50,488H.
Sciatic nerve denervation on the contralateral side abolished the anti-nociceptive effects of U-50,488H (0.3 and 2 mg). In contrast, contralateral injection of 1 mg morphine prolonged paw latencies in denervated rats.
Both co-administration of the peripherally selective opioid antagonist naloxone methiodide with 0.3 mg U-50,488H, and alternatively, systemic administration of 0.3 mg U-50,488H reversed the anti-nociceptive effects induced by contralateral injection of U-50,488H.
Taken together, our findings indicate that the contralateral administration of U-50,488H attenuates nociceptive behaviour resulting from acute inflammation. The effect is mediated via peripheral neuronal κ-opioid receptors and, possibly, spinal cord mechanisms, suggesting a new treatment approach for acute inflammatory conditions.
Keywords: κ-opioid agonist; U-50,488H; opioid receptors; contralateral treatment; acute inflammation; nociception; oedema
Introduction
The nervous system responds to pro-inflammatory challenge with a local ‘triple response' which comprises wheal (oedema formation), redness (increased blood flow) and flare (spreading of increased blood flow) and is called ‘neurogenic inflammation'. This response is caused by the axon-reflex mediated by sensory nerves and the activation of Aδ- and C-fibres (Jancso et al., 1967) which results in the antidromic release of sensory vasodilating neuropeptides such as substance P (SP) (Gamse et al., 1980; Gamse & Saria, 1985) and calcitonin gene-related peptide (CGRP) (Maggi, 1995). Administration of opioids has been shown to reduce neurogenic inflammation mainly through the inhibition of SP release in the central and peripheral nervous systems (Lembeck & Donnerer, 1985; Lembeck et al., 1982; Smith & Buchan, 1984). Furthermore, it has been suggested that these effects are mediated by actions on specific opioid receptors located on primary afferent nerves (Bartho & Szolcsanyi, 1981; Laduron, 1984).
Activation of the nociceptive afferent fibres during inflammation also produces hyperalgesia (Hardy et al., 1950). This effect is mediated through spinal and supraspinal neuronal mechanisms (Melzack & Wall, 1965; Spiller & Martin, 1921) and enhanced by administration of pro-inflammatory neuropeptides into the inflamed tissue (Nakamura-Craig & Gill, 1991). Opioids exert analgesic effects through their actions within the central and peripheral nervous systems (Millan, 1986; Stein et al., 1993). Morphine has been widely used in clinics for pain relief (Jurna, 1997; Stein, 1993). The clinical utility of morphine is, however, severely limited by a plethora of undesirable side-effects including respiratory depression, constipation and physical dependence (Jurna, 1997). During the last decade, great interest has been focused on the peripheral neuronal mechanisms by which opioids exert their analgesic effects. Stein and colleagues reported that local administration of opioid agonists attenuated pain-related behaviour in experimental and clinical studies (Stein, 1993; Stein et al., 1996; 1998a). The involvement of endogenous opioids in peripheral pain mechanisms has also been described (Antonijevic et al., 1995; Stein et al., 1990; 1993).
Recently, we reported that contralateral injection of a local anaesthetic attenuated pain-related behaviour for 24 h and reduced oedema formation in rats with acute carrageenan-induced inflammation (Bileviciute-Ljungar & Lundeberg, 2000a). The aim of the present study was to investigate the effects of contralateral administration of the κ-opioid receptor agonist U-50,488H on nociceptive behaviour and oedema formation in rats with experimentally induced acute inflammation. The effects of contralateral treatment were also compared with those observed following systemic administration of the opioid compound. The contribution of neuronal mechanisms was investigated by denervation of the sciatic nerve on the contralateral side.
Methods
Experimental protocol for animal studies
Experiments were performed on freely moving male albino Sprague Dawley rats (B&K Universal AB, Sollentuna, Sweden) weighing 200 g, and were approved by the local Ethical Committee at the Karolinska Institutet, Stockholm.
Unilateral inflammation was induced by injection of 100 μl of 1% carrageenan into the right hindpaw. Prior to carrageenan injection, the left hindpaw was injected with 100 μl of either saline or 0.01, 0.05, 0.3 or 2 mg U-50,488H. Drugs were administered in a blind fashion whenever possible.
The contribution of the nervous system to the effects of contralateral treatment was studied by severing the left sciatic nerve before the administration of 0.3 mg of U-50,488H into the left and carrageenan into the right hindpaw. To investigate the possible involvement of systemic mechanisms in the effects of contralateral treatment, 100 μl of morphine (10 mg ml−1) was given contralateral to the carrageenan injection in the denervated rats. To compare the effects of systemic administration of U-50,488H versus contralateral injection, 100 μl of 0.3 mg of U-50,488H was injected subcutaneously (s.c.) into the back. Involvement of the peripheral opioid receptors in the effects of contralateral treatment with 0.3 mg of U-50,488H was investigated by its concomitant administration with a peripheral opioid antagonist, naloxone methiodide, in equimolar doses. Hindpaw withdrawal responses to mechanical and heat stimulation and the degree of oedema in both the right and left sides were determined at 3, 6 and 24 h following carrageenan or saline injection. All assessments were made at the same time of the day.
Surgical procedure for sciatic nerve denervation
Rats were anaesthetized with intraperitoneal chloralhydrate (350 mg kg−1) and the left sciatic nerve was cut. Ten days after denervation, 100 μl of either saline, morphine (1 mg) or U-50,488H (0.3 and 2 mg) was injected into the left hindpaw and 100 μl of 1% carrageenan was injected into the right hindpaw. Hindpaw withdrawal latencies (HWLs) and hindpaw volumes were measured only for the right hindpaw.
Experimental procedure for nociceptive behavioural tests
All rats were accustomed to the testing conditions six times daily for 3 days before the experiments were performed, using the hot-plate and Randall – Selitto tests and varying the testing 50/50 between the two. The volume of each hindpaw was measured following nociceptive tests. The order of measurements for the right and left hindpaw was continually alternated and the interval between measurements was approximately 5 – 7 min. Two measurements were carried out for each test and the average value was used to quantify the percentage changes from the start of the experiment (basal values) for statistical analysis.
The Randall – Selitto test (Ugo Basile, Type 7200, Italy) was used to assess withdrawal thresholds to mechanical stimulation. A wedge-shaped pusher with a loading rate of 48 g s−1 withdrawal latency was applied to the dorsal surface of the manually handled hindpaw and the time required to initiate the struggle response was measured in seconds. This is referred to as the HWL to mechanical stimulation. A cut-off time of 15 s was applied.
The withdrawal response to noxious heat was determined using the hot-plate test. The entire ventral surface of the rat's hindpaw was placed on a hot-plate which was maintained at a temperature of 50°C (49.7 – 50.5°C). The time to hindpaw withdrawal was measured in seconds and subsequently referred to as the HWL to thermal stimulation. The cut-off time was 20 s. The hindpaw volume was measured using a plethysmometer (UGO Basile, type 7150, Italy) and expressed in ml. Basal values for 64 rats were measured prior induction of inflammation and each rat served as its own control. The basal values to mechanical stimulation were 5.0±0.1 s and 5.0±0.1 s (right and left, respectively) and to thermal stimulation 5.6±0.3 s and 5.7±0.3 s (right and left, respectively); hindpaw volumes were 1.6±0.01 ml and 1.6±0.01 ml (right and left, respectively). The basal values on the right side of 33 sciatic nerve ligated rats to mechanical stimulation were 5.5±0.2 s and to thermal stimulation 7.9±0.8 s; right hindpaw volume was 1.8±0.02 ml. Changes in hindpaw withdrawal responses and oedema formation are presented as a percentage (%) change from the basal values. Percentage changes were obtained according to the formula:
where P is the parameter of inflammation, X is the time after carrageenan injection and 0 (zero) is the time before injection.
Chemicals
U-50,488H, (trans-(±)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benzeneacetamide methanesulphonate), and naloxone methiodide were purchased from Research Biochemicals (Sigma, MA, U.S.A.). U-50,488H was dissolved in sterile 0.9% saline and naloxone methiodide was dissolved in 0.1 M HCl and adjusted to pH 7 with 0.1 M NaOH. Morphine (10 mg ml−1) was obtained from Pharmacia & Upjohn (Sweden). Carrageenan (1%) solution (Carrageenan Lambda, Sigma Chemical Co, U.S.A.) was prepared in sterile water.
Statistical analysis
Statistical analysis was carried out using SPSS software (Statistical Product and Service Solutions, release 8). Each experimental group included 7 – 10 rats. The Student's t-test for independent samples was used to compare differences in HWL to mechanical and thermal stimulation and hindpaw volume expressed in percentage changes between the saline and carrageenan injected rats receiving saline injection on the contralateral side. Student's t-test was also used to compare differences between the contralateral and systemic routes of U-50,488H administration and between the contralateral treatment with U-50,488H alone versus treatment with U-50,488H and naloxone methiodide. The ANOVA test with Duncan's test for significance was used to compare the parameters of inflammation expressed in percentage changes between the contralateral treatment with either saline or U-50,488H in carrageenan injected intact rats. When a significant difference was detected, the Student's t-test was used to identify the differences between the groups treated with higher and lower doses of U-50,488H. The ANOVA test with Duncan's test for significance was also used to compare differences between sciatic nerve denervated rats treated with either contralateral saline, or U-50,488H or morphine. When a significant difference was detected, the Student's t-test was used to identify the differences between the groups treated with either morphine or U-50,488H.
Results
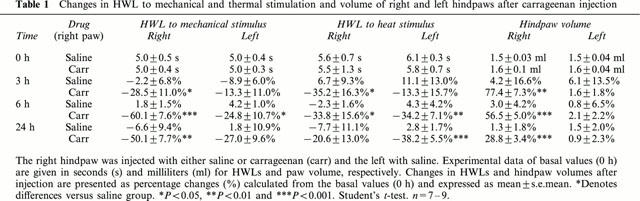
The effects of carrageenan on hindpaw withdrawal responses and oedema formation are summarised in Table 1. Injection of 100 μl of carrageenan caused a decrease in withdrawal latencies 3, 6 and 24 h. A bilateral decrease in HWLs was observed at 6 h, while the inflamed hindpaw volume remained significantly increased at 3, 6 and 24 h.
Table 1.
Changes in HWL to mechanical and thermal stimulation and volume of right and left hindpaws after carrageenan injection
Effects of contralateral treatment with increased doses of U-50,488H on the inflamed and non-inflamed paw in intact rats
HWL to mechanical stimulation
Contralateral injection of 0.3 and 2 mg of U-50,488H increased the inflamed (right) paw latency to mechanical stimulation for 3 – 24 h post-carrageenan injection (Table 2). Administration of either 0.01 or 0.05 mg of U-50,488H increased the latency at 6 h, and a dose of 0.05 mg also increased the latency at 24 h (Table 2). On the left side, HWL was increased at 3 and 6 h (2 mg) and at 24 h (0.01, 0.05, 0.3 and 2 mg) (Table 2). No difference was found between the effects of 0.3 and 2 mg of U-50,488H. Injection of either 0.3 or 2 mg induced a more pronounced increase in the right HWL compared with the lower doses of 0.01 and 0.05 at 3 and 6 h and 0.01 mg at 24 h (Figure 1).
Table 2.
Percentage changes in HWL to mechanical and thermal stimulation and volume of right and left hindpaws after contralateral treatment with either saline or various doses of U-50,488H in carrageenan-injected rats
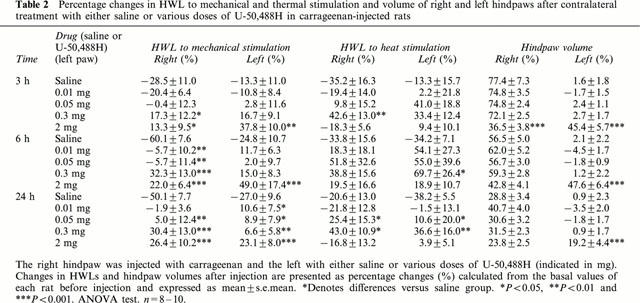
Figure 1.
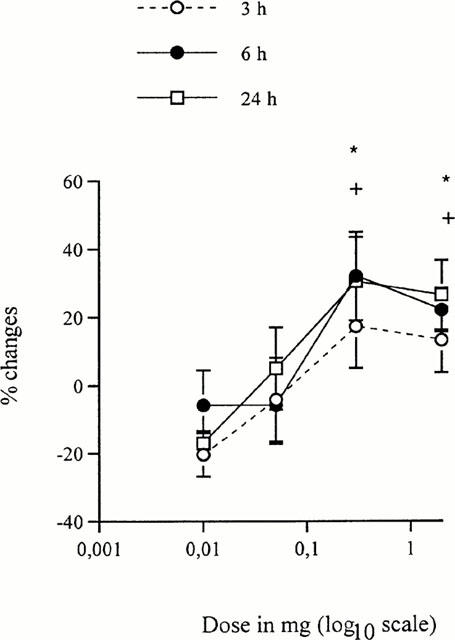
Dose effect of contralateral treatment with U-50,488H at 3, 6 and 24 h after carrageenan injection. Changes in HWL to mechanical stimulation of the right hindpaw injected with carrageenan are shown. The left hindpaw was treated with increasing doses of U-50,488H. Data are presented as percentage (%) changes and expressed in mean±s.e.mean (vertical axis). Horizontal axis indicates U-50,488H doses presented in logarithmic scale. Significant differences are denoted as (*) 0.3 and 2 mg of U-50,488H versus 0.01 and 0.05 mg at 3 and 6 h and (+) 0.3 and 2 mg of U-50,488H versus 0.01 mg at 24 h. Student's t-test. n=8−10. * and+P<0.05.
HWL to heat stimulation
Contralateral injection of 0.3 mg of U-50,488H increased HWL to heat stimulation on the inflamed side at 3 and 24 h (Table 2). A tendency to significant increase was also observed at 6 h (P<0.071). On the left side, HWL was increased at 6 and 24 h after injection of 0.3 mg U-50,488H (Table 2). An increase in HWL bilaterally at 24 h was observed for 0.05 mg of U-50,488H as compared with saline (Table 2). A dose of 0.3 mg of U-50,488H was found to induce a more pronounced anti-nociceptive effect on the right HWL than 0.01, 0.05 and 2 mg at 3 h, and 0.01 mg and 2 mg at 24 h (t-test, P<0.05).
Hindpaw volume
Contralateral injection of U-50,488H in doses of 0.01, 0.05 and 0.3 mg did not affect carrageenan-induced oedema formation (Table 2). Administration of 2 mg of U-50,488H significantly decreased the inflamed hindpaw volume at 3 h, while a tendency was observed at 6 h as compared to saline injection (P<0.082). An increase in the left hindpaw volume was found at 3, 6 and 24 h following 2 mg U-50,488H injection (Table 2). Comparison between the different doses of U-50,488 showed that contralateral injection of 2 mg significantly decreased carrageenan-induced oedema formation for the right hindpaw at 3 h and increased left hidpaw volume at 3 – 24 h (t-test, P<0.05−0.001) as compared with lower doses of U-50,488H (0.01, 0.05 and 0.3 mg).
Effects of contralateral treatment with U-50,488H and morphine on the inflamed paw in rats with contralateral sciatic nerve denervation
HWL to mechanical stimulation
No difference was found between the effects of saline and U-50,488H (0.3 or 2 mg) treatments on inflamed paw latencies of denervated rats (Figure 2). However, a strong tendency (P<0.06) to an increased HWL to mechanical stimulation was found at 3 h in rats treated with 2 mg of U-50,488H as compared with saline. Contralateral injection of 1 mg of morphine increased the inflamed paw latency to mechanical stimulation at 6 and 24 h compared with saline, and a tendency to such increase was observed at 3 h (P<0.07) (Figure 2).
Figure 2.
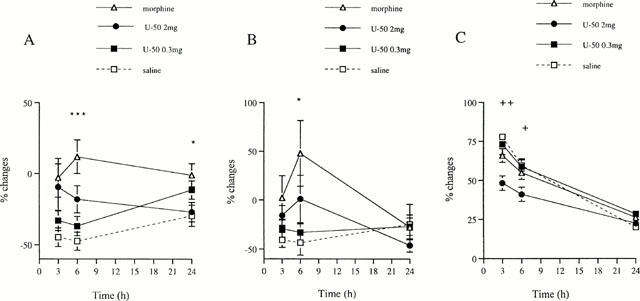
Effect of the left sciatic nerve denervation on the contralateral treatment with U-50,488H. Changes in HWLs to mechanical (A) and thermal (B) stimulation and volume (C) of the right hindpaw injected with carrageenan are shown. The left hindpaw was given either saline, or 0.3 or 2 mg of U-50,488H (U-50) or 1 mg of morphine. Data are presented as percentage (%) changes and expressed in mean±s.e.mean (vertical axis). Horizontal axis indicates hours (h) following injection. Significant differences are denoted as (*) morphine versus saline and (+) 2 mg of U-50,488H versus saline, 0.3 mg of U-50,488H and morphine. ANOVA and Student's t-test. n=8 – 10. * and+P<0.05; ++P<0.01; ***P<0.001.
HWL to heat stimulation
No difference was found between latencies of denervated rats after saline and U-50,488H (0.3 or 2 mg) treatments. Contralateral injection of 1 mg morphine increased HWL to heat stimulation at 6 h compared with saline (Figure 2).
Hindpaw volume
No difference was found in hindpaw volumes of denervated rats after treatment with either saline, morphine or 0.3 mg U-50,488H. However, in comparison with these, contralateral injection of 2 mg of U-50,488H decreased the right hindpaw volume at 3 and 6 h (Figure 2).
Effects of concomitant contralateral administration of U-50,488H (0.3 mg) and naloxone methiodide and of systemic administration of U-50,488H (0.3 mg) on the inflamed paw in intact rats
HWLs to mechanical and heat stimulation
Contralateral treatment with equimolar dose of U-50,488H (0.3 mg) and naloxone methiodide did not affect HWLs to either mechanical or heat stimulation compared with saline treatment. Latencies were significantly decreased at 3, 6 and 24 h when compared with the contralateral administration of 0.3 mg U50,488 (Figure 3). In comparison to its contralateral injection, s.c. systemic administration of 0.3 mg U-50,488H resulted in significantly shorter HWLs at 3 – 24 h (Figure 3).
Figure 3.
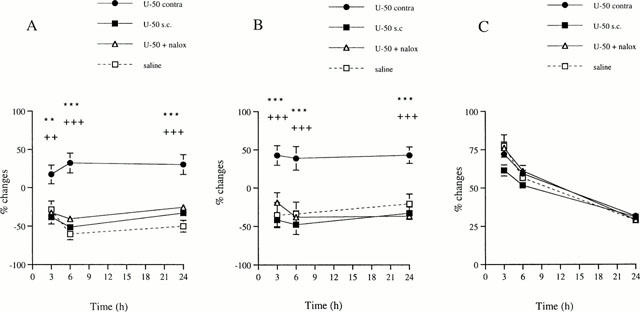
Changes in HWLs to mechanical (A) and thermal (B) stimulation and hindpaw volume (C) of the right hindpaw injected with carrageenan. The left hindpaw was injected with either saline, 0.3 mg of U-50,488H alone (U-50 contra) or equimolar concentrations of U-50,488H and naloxone methiodide (U-50+nalox). In systemic treatment, 0.3 mg of U-50,488H was injected subcutaneously (U-50 s.c.). Data are presented as percentage changes (%) and expressed in mean±s.e.mean (vertical axis). Horizontal axis indicates hours following injection. Significant differences are denoted as (*) contralateral U-50,488H versus systemic and (+) contralateral U-50,488H alone versus together with naloxone methiodide. Student's t-test. n=8 – 10. ** and ++P<0.01; *** and +++P<0.001.
Hindpaw volume
No differences in paw volume were found following contralateral treatment with 0.3 mg U-50,488H alone or together with naloxone methiodide when comparing with saline injection (Figure 3). Systemic administration of 0.3 mg U-50,488H did not affect the inflammatory oedema formation as compared to its contralateral injection (Figure 3).
Discussion
The results of the present study show that contralateral administration of the κ-opioid receptor agonist U-50,488H attenuated the bilateral decrease in withdrawal latencies in rats with carrageenan-induced inflammation. Recently, a similar anti-nociceptive effect was observed following contralateral administration of the local anaesthetic bupivacaine (Bileviciute-Ljungar & Lundeberg, 2000a). In both cases, the effect was abolished by sciatic nerve denervation, while systemic administration of the drug did not induce the same response, thus excluding the possible involvement of systemic mechanisms.
It is known that opioids produce anti-nociceptive effects in inflamed, but not in non-inflamed paw (Stein et al., 1988a,1988b), indicating that inflammatory process increases the expression of opioid receptors on peripheral nerve terminals (Hassan et al., 1993). Electrophysiological findings demonstrate that κ-opioid receptor agonist U-50,488H exert more pronounced inhibitory effect on the peripheral nerve activity when administered into the inflamed cat knee as compared to other opioid agonists (μ- or σ-opioid receptor agonists) (Russell et al., 1987). The results of the present study also indicate that contralateral administration of U-50,488H has a more pronounced effect compared to morphine on pain-related behaviour. Moreover, we suggest that the effect of contralateral treatment with morphine on withdrawal responses were due to systemic mechanisms since they were not abolished by sciatic nerve denervation.
Recently, it was described that a leakage of perineurial barrier following tissue damage is an important factor for peripheral opioid analgesia (Antonijevic et al., 1995). In the present study, no inflammatory tissue reaction in terms of oedema formation was found at the contralateral site injected with U-50,488H. However, the hindpaw withdrawal latencies were decreased bilaterally, indicating that primary afferent nerves were sensitized on the contralateral side. This observation suggests that contralateral administration of U-50,488H inhibits the increased nerve activity on the contralateral side. The inhibition from the contralateral side might be due to the same mechanisms as the ipsilateral, i.e. by presynaptic inhibition of calcium-dependent component of the action potential (Werz & Macdonald, 1982). Furthermore, a bilateral increase of β-endorphin receptors in sciatic nerve following acute unilateral inflammation was reported (Hassan et al., 1993). It could be hypothesized that contralateral administration of U-50,488H modulates the expression and/or affinity of κ-opioid receptors at the injected site, thereby affecting the transmission of nociceptive information to the central nervous system and resulting in reduced nociception.
Recently, Stein and colleagues demonstrated that an intraplantar injection of 0.05 mg of U-50,488H into the inflamed rat hindpaw reduced nociceptive behaviour, measured as paw pressure threshold, for 15 – 30 min (Stein et al., 1988a). In the present study, the same dose of U-50,488H given contralaterally, produced anti-nociceptive effects lasting for 24 h. The more pronounced effect of the contralateral treatment might be partly due to the reduced metabolic activity and minor dilution of the compound in the non-inflamed hindpaw. Another possible mechanism for the long-lasting effect could be related to changes in nociceptive transmission taking place at the level of spinal cord. The contribution of the central nervous system in the long-lasting anti-nociceptive effects following contralateral treatment is also evidenced by the fact that xylocaine administered contralaterally into mononeuropathic rats was found to produce effects that lasted for several days (Bileviciute-Ljungar & Lundeberg, 2000b). Moreover, an intrathecal injection of saline has been demonstrated to interrupt the bilateral responses (Bileviciute et al., 1998) and the anti-nociceptive effects of contralateral treatment with local anaesthetics (Bileviciute-Ljungar & Lundeberg, 2000a).
The highest dose of U-50,488H (2 mg) used in this study did not produce more analgesia than that of 0.3 mg. Indeed, 2 mg of U-50,488H showed a decrease in the HWL to heat stimulation compared with 0.3 mg. These paradoxical findings might be explained by pharmacological or systemic effects of 2 mg U-50,488H. Indeed, a leakage of the higher dose of U-50,488H into the systemic circulation is supported by the observation of a decrease in paw volume on the inflamed side and an increase on the non-inflamed side. The anti-nociceptive effects of contralateral U-50,488H were blocked by concomitant administration with equimolar doses of naloxone methiodide, a peripherally restricted opioid antagonist, indicating that anti-nociceptive effects are due to activation of peripheral but not central κ-opioid receptors.
Contralateral administration of U-50,488H in doses lower than 2 mg induced a dose-related response to mechanical but not to heat stimulation. It is known that thin myelinated Aδ-fibres mediate the transmission of mechanical stimuli, while unmyelinated C-fibres are involved in heat sensation (Jessell & Kelly, 1991). The different effects of U-50,488H on HWLs suggests that κ-opioid receptors are predominantly expressed on Aδ- rather than C-fibres. Moreover, in the present study, the anti-nociceptive effects of 0.3 mg U-50,488H were not accompanied by reduction in oedema formation. This might be due to different mechanisms or nerve fibres involved in nociception and oedema formation. Interestingly, we have shown recently that contralateral treatment with bupivacaine reduced both nociception and oedema formation in rats with acute carrageenan-induced inflammation (Bileviciute-Ljungar & Lundeberg, 2000a). The results of the present study suggests that rather C- than Aδ-fibres play a major role in acute oedema formation.
In conclusion, the present results indicate that contralateral administration of the κ-opioid agonist U-50,488H attenuates nociceptive behaviour following acute inflammation. The effect is mediated via peripheral neuronal κ-opioid receptors and, possibly, spinal cord mechanisms, thus indicating that peripherally acting opioids might be used for pain management during acute inflammatory conditions.
Acknowledgments
This study was supported by grants from Lars Hiertas Memorial Foundation and Karolinska Institutet Foundation.
Abbreviations
- CGRP
calcitonin gene related peptide
- HWL
hindpaw withdrawal latency
- SP
substance P
- U-50,488H
trans-(±)-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]-benzeneacetamide methanesulphonate
References
- ANTONIJEVIC I., MOUSA S.A., SCHÄFER M., STEIN C. Perineural defect and peripheral opioid analgesia in inflammation. J. Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHO L., SZOLCSANYI J. Opiate agonists inhibit neurogenic plasma extravasation in the rat. Eur. J. Pharmacol. 1981;73:101–104. doi: 10.1016/0014-2999(81)90152-7. [DOI] [PubMed] [Google Scholar]
- BILEVICIUTE I., STENFORS C., THEODORSSON E., LUNDEBERG T. Unilateral injection of calcitonin-gene-related peptide (CGRP) induces bilateral oedema formation and release of CGRP-like immunoreactivity. Br. J. Pharmacol. 1998;125:1304–1312. doi: 10.1038/sj.bjp.0702192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILEVICIUTE-LJUNGAR I., LUNDEBERG T. Contralateral but not systemic administration of bupivacaine reduces acute inflammation in the rat hindpaw. Somatosens. Motor. Res. 2000a;17:285–293. doi: 10.1080/08990220050117637. [DOI] [PubMed] [Google Scholar]
- BILEVICIUTE-LJUNGAR I., LUNDEBERG T. Contralateral treatment with xylocaine reduces nociceptive behaviour in mononeuropathic rats. NeuroReport. 2000b;11:291–295. doi: 10.1097/00001756-200002070-00014. [DOI] [PubMed] [Google Scholar]
- GAMSE R., SARIA A. Potentiation of tachykinin-induced plasma protein extravasation by calcitonin gene-related peptide. Eur. J. Pharmacol. 1985;114:61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- GAMSE R., HOLZER P., LEMBECK F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br. J. Pharmacol. 1980;68:207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDY J.D., WOLFF H.G., GOODELL H. Experimental evidence on the nature of cutaneous hyperalgesia. J. Clin. Invest. 1950;29:115–140. doi: 10.1172/JCI102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSAN A.H.S., ABLETNER A., STEIN C., HERZ A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience. 1993;55:185–195. doi: 10.1016/0306-4522(93)90465-r. [DOI] [PubMed] [Google Scholar]
- JANCSO N., JANCSO-GABOR A., SZOLCSANYI J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESSELL T.M., KELLY D.D.Pain and analgesia Principles of Neural Science 1991New York: Elsevier Science Publishing Co; 385–399.eds. Kandell, E.R., Schwartz, J.M. & Jessell, T.M. [Google Scholar]
- JURNA J. Opioid and nonopioid analgesic agents: some aspects of central (spinal) and peripheral actions. Pain Rev. 1997;41:205–209. [Google Scholar]
- LADURON P.M. Axonal transport of opiate receptors in capsaicin-sensitive neurones. Brain Res. 1984;294:157–160. doi: 10.1016/0006-8993(84)91322-2. [DOI] [PubMed] [Google Scholar]
- LEMBECK F., DONNERER J. Opioid control of the function of primary afferent substance P fibres. Eur. J. Pharmacol. 1985;114:241–246. doi: 10.1016/0014-2999(85)90365-6. [DOI] [PubMed] [Google Scholar]
- LEMBECK F., DONNERER J., BARTHO L. Inhibition of neurogenic vasodilatation and plasma extravasation by substance P antagonists, somatostatin and (D-met2,pro5)enkephalinamide. Eur. J. Pharmacol. 1982;85:171–176. doi: 10.1016/0014-2999(82)90462-9. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- MELZACK R., WALL P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J. Multiple opioid systems and pain. Pain. 1986;271:303–347. doi: 10.1016/0304-3959(86)90158-2. [DOI] [PubMed] [Google Scholar]
- NAKAMURA-CRAIG M., GILL B.K. Effect of neurokinin A, substance P and calcitonin gene-related peptide in peripheral hyperalgesia in the rat paw. Neurosci. Lett. 1991;124:49–51. doi: 10.1016/0304-3940(91)90819-f. [DOI] [PubMed] [Google Scholar]
- RUSSELL N.J.W., SCHAIBLE H.-G., SCHMIDT R.F. Opiates inhibit the discharges of the fine afferent units from inflamed knee joint of the cat. Neurosci. Lett. 1987;76:107–112. doi: 10.1016/0304-3940(87)90201-1. [DOI] [PubMed] [Google Scholar]
- SMITH T.W., BUCHAN P. Peripheral opioid receptors located on the rat saphenous nerve. Neuropeptides. 1984;5:217–220. doi: 10.1016/0143-4179(84)90066-0. [DOI] [PubMed] [Google Scholar]
- SPILLER W.G., MARTIN E. The treatment of persistent pain of organic origin in the lower part of the body by division of the anterolateral column of the spinal cord. J. Am. Med. Ass. 1921;58:1489–1490. [Google Scholar]
- STEIN C. Peripheral mechanisms of opioid analgesia. Anest. Analg. 1993;76:182–191. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- STEIN C., HASSAN A.H.S., LEHRBERGER K., GIEFING J., YASSOURIDIS A. Local analgesic effect of endogenous opioid peptides. Lancet. 1993;342:321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- STEIN C., HASSAN A.H.S., PRZEWLOCKI R., GRAMSCH C., PETER K., HERZ A. Opioids from immunocytes interact with receptors on sensory nerves to inhibit nociception in inflammation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5935–5939. doi: 10.1073/pnas.87.15.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN C., MILLAN M.J., SHIPPENBERG T.S., PETER K., HERTZ A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of Mu, Delta and Kappa receptors. J. Pharmacol. Exper. Ther. 1988a;248:1269–1275. [PubMed] [Google Scholar]
- STEIN C., MILLAN M.J., YASSOURIDIS A., HERTZ A. Antinociceptive effects of μ- and κ-agonists in inflammation are enhanced by a peripheral opioid receptor-specific mechanism. Eur. J. Pharmacol. 1988b;155:255–264. doi: 10.1016/0014-2999(88)90511-0. [DOI] [PubMed] [Google Scholar]
- STEIN C., PFLUGER M., YASSAOURIDIS A., HOELZL J., LEHRBERGER K., WELTE C. No tolerance to peripheral analgesia in presence of opioid expression in inflamed synovia. J. Clin. Invest. 1996;98:793–799. doi: 10.1172/JCI118852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERZ M.A., MACDONALD R.L. Heterogenous sensitivity of cultured dorsal root ganglion neurones to opioid peptides selective for μ- and δ-opioid receptors. Nature. 1982;299:730. doi: 10.1038/299730a0. [DOI] [PubMed] [Google Scholar]


