Abstract
We have used the whole-cell patch clamp technique to study the effect of the partial anti-oestrogens clomiphene and nafoxidine, the pure anti-oestrogens ICI 182,780 and RU 58,668 and the oestrogen β-estradiol, on the volume-regulated anion channel (VRAC) in cultured pulmonary artery endothelial (CPAE) cells.
In contrast to the pure anti-oestrogens and β-estradiol, clomiphene and nafoxidine potently inhibited the volume-sensitive chloride current, ICl,swell, activated by challenging CPAE cells with a 25% hypotonic solution. For clomiphene, the estimated IC50 and Hill coefficient were 1.03±0.14 μM and 1.40±0.21 respectively. In the case of nafoxidine, these values were 1.61±0.29 μM and 1.24±0.19.
The inhibition induced by the pure enantiomers of clomiphene, zuclomiphene and enclomiphene, was not different from that of the racemic mixture, indicating that the interaction between clomiphene and VRAC is not stereoselective.
Clomiphene and nafoxidine inhibited proliferation of CPAE cells. Half-maximal inhibition was found at 1.98±0.17 and 1.66±0.21 μM respectively, concentrations similar to those for half-maximal block of VRAC.
In conclusion, the nonsteroidal partial anti-oestrogens nafoxidine and clomiphene are potent inhibitors of volume-regulated anion channels. The inhibition by clomiphene is not stereoselective and occurs at concentrations close to therapeutically relevant concentrations. Finally, both drugs inhibit the proliferation of endothelial cells.
Keywords: Volume-regulated anion channels; anti-oestrogens; clomiphene; zuclomiphene; enclomiphene; nafoxidine; ICI 182,780; RU 58,668; β-estradiol; endothelial cells
Introduction
Tamoxifen (Nolvadex®), a non steroidal triphenylethylene anti-oestrogen, has been used to treat breast cancer for a quarter of a century. Anti-oestrogen treatment has revolutionized breast cancer therapy and is established as the endocrine therapy of choice for all stages of the disease. Extensive research has demonstrated additional physiological advantages and potential disadvantages of the drug (Gradishar et al., 1997). Most of the anti-oestrogenic effects of this group are mediated by binding to the oestrogen receptor. However, an increasing amount of evidence suggests that some of the actions assigned to anti-oestrogens do not involve an interaction with the oestrogen receptor.
Tamoxifen has been demonstrated to be a high-affinity blocker of volume-sensitive anion channels in several cell lines, including endothelial cells, fibroblasts, lung carcinoma cells and smooth muscle cells, by a mechanism independent of the interaction of tamoxifen with the oestrogen receptor (Greenwood & Large, 1998; Nilius et al., 1994b; Voets et al., 1995; Zhang et al., 1994). Volume-regulated anion channels (VRACs), expressed in most mammalian cells, are important regulators of various cell functions such as cell volume, pH control, transport of osmolytes and membrane potential (Kirk, 1997; Nilius et al., 1997a; Okada, 1997; Strange et al., 1996). It has also been suggested that VRACs may play a role in cell proliferation (Nilius et al., 1997a; Voets et al., 1995; 1997) and angiogenesis, since structurally different VRAC blockers were shown to be potent inhibitors of angiogenesis (Manolopoulos et al., 2000).
The molecular and functional characterization of VRACs is still hampered by the non-availability of highly sensitive and selective pharmacological modulators of these channels. Since tamoxifen has been shown to be a potent blocker of VRACs, it was the purpose of the present experiments to investigate the pharmacological modulation of these channels by anti-oestrogens in more detail. This pharmacological class of drugs can be divided into two groups (Figure 1): nonsteroidal compounds showing partial agonist activity such as tamoxifen, clomiphene (Clomid®) and nafoxidine; and steroid analogues devoid of partial agonist activity, including a series of 7α-derivatives of estradiol, such as ICI 182,780 (Faslodex®) and 11β-derivatives of estradiol such as RU 58,668 (Gradishar et al., 1997). The nonsteroidal anti-oestrogens, tamoxifen and clomiphene, are used for the treatment of breast cancer and female infertility, respectively. They belong to the triphenylethylene class of compounds derived from the same stilbene nucleus as diethylstilbestrol. We have investigated whether the triphenylethylene derivative clomiphene, the dihydronaphtylene derivative nafoxidine and the pure anti-oestrogens ICI 182,780 and RU 58,668 modulate VRAC in endothelial cells. In addition, we also examined the effect of a pure oestrogen, i.e. β-estradiol (Figure 1). Furthermore, given that clomiphene (Clomid®) is marketed as a racemic mixture of enclomiphene and zuclomiphene and that both isomers display distinct pharmacological activity, the pure enantiomers were tested on VRAC. Finally, since serum-induced proliferation of endothelial cells is arrested in the presence of VRAC blockers, among which tamoxifen (Voets et al., 1995) and a possible relation between VRAC and angiogenesis was put forward (Manolopoulos et al., 2000), we investigated whether clomiphene and nafoxidine displayed the same properties.
Figure 1.
Chemical structures of anti-oestrogens and β-estradiol.
This is the first study dealing with modulation of volume-regulated anion channels by the widely used drug clomiphene (Clomid®) and nafoxidine. Our data reveal a potent block of VRAC in endothelial cells by the non-steroidal, partial anti-oestrogens clomiphene and nafoxidine, in contrast to the steroidal, pure anti-oestrogens ICI 182,780 and RU 58,668 who only weakly modulated this anion channel. In addition, we show that clomiphene and nafoxidine efficiently inhibit proliferation of endothelial cells.
Methods
Cell culture
Cultured pulmonary artery endothelial (CPAE) cells (American Tissue Type Culture Collection, CCL 209) were grown in Dulbecco's modified Eagle's medium containing 20% foetal calf serum, 2 mM L-glutamine, 2 u ml−1 penicillin and 2 mg ml−1 streptomycin. Cultures were maintained at 37°C in a fully humidified atmosphere of 10% CO2 in air. Cells were detached by exposure to 0.05% trypsin in a Ca2+- and Mg2+- free solution, reseeded on gelatine coated cover slips, and kept in culture for 2 – 4 days before use. For electrophysiological experiments, only non-confluent single endothelial cells were used.
To assess cell proliferation, cells were plated at a density of 20,000 viable cells per well of a 12-well plate. The culture medium (control and in the presence of the anti-oestrogens) was replaced every 24 h. Cells were counted at days 2, 4 and 6. For counting, the cells were trypsinized by incubation in 500 μl of an 0.05% trypsin/5 mM EDTA solution. Trypsinization was stopped by adding 1000 μl culture medium. An aliquot of 500 μl of this cell suspension was mixed with 70 μl trypan blue and the number of cells was counted using a BURKER chamber. Only viable cells (which excluded trypan blue) were counted. This enabled us to differentiate easily between a reduced cell proliferation rate and cell death.
Solutions and drugs
The standard extracellular solution contained (in mM): NaCl 150, KCl 6, MgCl2 1, CaCl2 1.5, glucose 10, N-(hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid) (HEPES) 10, titrated with NaOH to pH 7.4. The osmolarity, as measured with a vapour pressure osmometer (Wescor 5500, Schlag, Gladbach, Germany), was 320±5 mOsm. At the beginning of the patch-clamp recording, this solution was replaced by an isotonic-Cs+ solution (ISO, 320±5 mOsm) containing (in mM): NaCl 105, CsCl 6, CaCl2 1.5, MgCl2 1, D-mannitol 90, glucose 10, HEPES 10, adjusted to pH 7.4 with NaOH. Volume-sensitive Cl− currents, ICl,swell, were activated by exposing the cells to a 25% hypotonic extracellular solution (HTS, 240±5 mOsm), containing (in mM): NaCl 105, CsCl 6, CaCl2 1.5, MgCl2 1, glucose 10, HEPES 10, adjusted to pH 7.4 with NaOH. The standard pipette solution contained (in mM): CsCl 40, Cs+-aspartate 100, MgCl2 1, CaCl2 1.93, ethylene glycol-O,O′-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid (EGTA) 5, Na2ATP 4, HEPES 10, adjusted to pH 7.2 with CsOH (290±5 mOsm).
The substitution of K+ for Cs+ in extra- and intracellular solutions blocked the inwardly rectifying K+ currents, which are present in CPAE cells (Voets et al., 1996). To suppress the Ca2+-activated Cl− current, the free Ca2+ concentration in the pipette solution was buffered at 100 nM, which is below the threshold for activation of this current (Nilius et al., 1997c), but which is sufficient for full activation of ICl,swell during cell swelling in CPAE cells (Szücs et al., 1996).
Tamoxifen, clomiphene citrate and nafoxidine hydrochloride were purchased from Sigma-Aldrich. ICI 182,780 and RU 58,668 were kindly provided by Dr A. Gagliardi, University of Kentucky, Kentucky, U.S.A. β-Estradiol was kindly supplied by Dr H.J. Lang, Aventis, Germany.
The compounds were daily dissolved in the HTS and the final concentrations used were obtained with appropriate dilution of the stock solutions. The stock solutions were made in DMSO for clomiphene, zuclomiphene, enclomiphene, nafoxidine and tamoxifen and in ethanol for RU 58,668, ICI 182,780 and β-estradiol. The final concentrations of these solvents never exceeded 0.1 – 0.5%. These concentrations were tested in our laboratory and had no effect on membrane currents and cell proliferation. Rapid solution exchange and extracellular application of drugs occurred via a multi-barrelled pipette connected to solution reservoirs and controlled by a set of magnetic valves. The volume of the chamber was 0.5 ml and the perfusion rate 2.5 ml min−1.
Current measurements and data analysis
Whole-cell membrane currents were measured in ruptured patches. All experiments were performed at room temperature (20 – 23°C). Currents were monitored with an EPC-7 patch clamp amplifier (List Electronic, Germany) and sampled at 2 ms intervals (1024 points per record, filtered at 200 Hz), unless otherwise mentioned. Patch electrodes had a resistance between 3 and 5 MΩ. An Ag-AgCl wire was used as reference electrode. Cell capacitance and series resistance were compensated. The mean value of the series resistance was around 6 MΩ. Approximately 50% of the series resistance was compensated.
In most experiments we applied a ‘ramp' protocol, which consisted of a step to −80 mV for 0.4 s, followed by a step to −150 mV for 0.1 s and a 1.3 s linear voltage ramp to +100 mV. This voltage protocol was repeated every 15 s from a holding potential of −20 mV. Current-voltage relations were constructed from the ramp current, and time courses were obtained by averaging the current in a small voltage window around +100 mV and −150 mV. In some experiments we used a ‘step' protocol consisting of 1 s voltage steps, applied every 5 s from a holding potential of −20 mV to test potentials from −100 to +100 mV with increments of 20 mV. Currents were sampled at 1 ms intervals.
Data were analysed in Winascd (G. Droogmans) and in Origin (MicroCal Software, Inc.). Pooled data are given as the mean±s.e.mean.
Results
VRAC inhibition by (anti-)oestrogens
Volume-activated chloride channels and the corresponding current, ICl,swell, were activated by replacing the isotonic solution (ISO) by the hypotonic solution (HTS), as described in detail elsewhere (Nilius et al., 1994a). Figure 2A – C show typical time course experiments in which 10 μM of the different anti-oestrogens were added to the external solution during a maintained superfusion with hypotonic solution. The time course in Figure 2D shows the effect of three different concentrations of β-estradiol added to the HTS solution from a stock solution in ethanol. The finding that higher concentrations (25 and 50 μM) induced the same percentage of inhibition as 10 μM is probably due to the fact that β-estradiol is almost insoluble in water.
Figure 2.
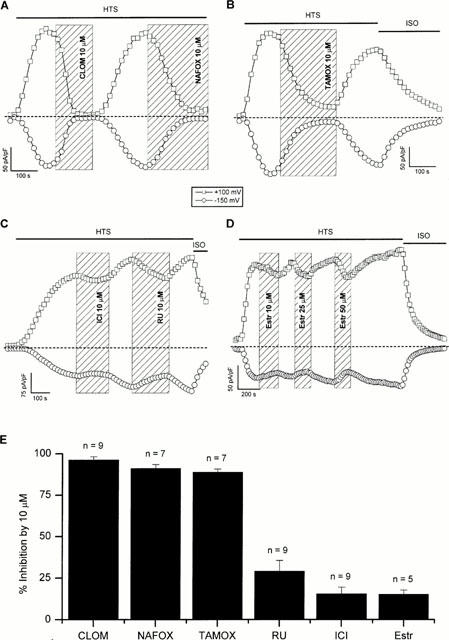
(A) Time course of activation of ICl,swell during superfusion with hypotonic solution (HTS) (following superfusion with isotonic-Cs+ solution) showing the inhibitory effect of 10 μM clomiphene (CLOM) and nafoxidine (NAFOX). The dashed line indicates zero current density. (B) Time course of activation of ICl,swell during superfusion with hypotonic solution (HTS) showing the inhibitory effect of 10 μM tamoxifen (TAMOX) and deactivation of the current after returning to isotonic solution (ISO). (C) Time course of ICl,swell demonstrating the effect of 10 μM ICI 182,780 (ICI) and RU 58,668 (RU). (D) Time course of ICl,swell showing the effect of 10, 25 and 50 μM β-estradiol (Estr). (E) Column graph summarizing the inhibitory effect of 10 μM clomiphene, nafoxidine, tamoxifen, RU 58,668, ICI 182,780 and β-estradiol on ICl,swell. The inhibition is expressed as the percentage reduction of the background corrected ICl,swell at +100 mV.
The results of several experiments (n=5 – 9) are summarized in Figure 2E, showing the inhibitory effect of 10 μM of clomiphene, nafoxidine, tamoxifen, RU 58,668, ICI 182,780 and β-estradiol on VRAC. The inhibition is expressed as the percentage reduction of the background (i.e. current under isotonic conditions) corrected ICl,swell at +100 mV. These compounds affected the background current only in cells that showed a clear outwardly rectifying current component in isotonic solution, which indicates that in these cells ICl,swell was already partially activated under isotonic conditions.
The experimental results clearly show that the non steroidal compounds with partial agonist activity (clomiphene, nafoxidine and tamoxifen) are potent inhibitors of VRAC, whereas the steroid analogues devoid of partial agonist activity (ICI 182,780 and RU 58,668) and the oestrogen β-estradiol are modest or even weak inhibitors. The data obtained with tamoxifen are consistent with earlier experiments performed in our laboratory (Nilius et al., 1994b; Voets et al., 1995). We will focus in the following experiments on clomiphene and nafoxidine.
Inhibition of VRAC by clomiphene and nafoxidine
Figure 3A shows a typical time course experiment in which 2 μM clomiphene and 5 μM nafoxidine were added to the external solution during a maintained superfusion with hypotonic solution. clomiphene and nafoxidine induce a rather fast and concentration-dependent block of ICl,swell. This block is fully reversible and recovery of the current upon washout is fast. Current-voltage relationships (Figure 3B) are obtained from voltage ramps in control conditions (basal current and fully activated ICl,swell) and in the presence of the two anti-oestrogens. It is clear that these compounds inhibit inward as well as outward currents through VRAC. The voltage-independence of inhibition becomes evident by comparing the average per cent inhibitions in the voltage ranges [−100, −50 mV] and [+50, +100 mV]. The values for clomiphene were 87±14 and 78±21% respectively, those for nafoxidine 93±17 and 86±19%. ICl,swell reverses at potentials slightly more positive than the theoretical equilibrium potential for Cl− because of permeation of organic anions such as aspartate in the pipette (for a detailed discussion see (Nilius et al., 1997a)).
Figure 3.
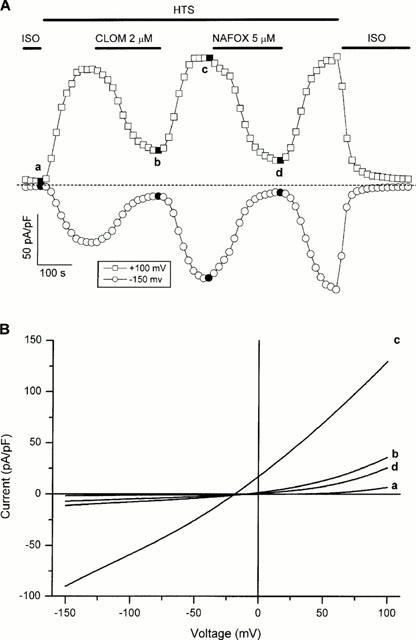
(A) Time course of activation of ICl,swell during superfusion with hypotonic solution (HTS) (following superfusion with standard extracellular solution and isotonic-Cs+ solution (ISO)), reversible inhibition of the current by 2 μM clomiphene (CLOM) and 5 μM nafoxidine (NAFOX) and deactivation of the current after returning to isotonic-Cs+ solution. The dashed line indicates zero current. Data were obtained from ramp protocols by averaging the current in a small voltage window around +100 mV and −150 mV. (B) Current-voltage relations obtained from voltage ramps at the times indicated in (A).
From the experimental protocols shown in Figure 3, we have evaluated the concentration dependence of the inhibition of ICl,swell for clomiphene and nafoxidine at +100 mV (Figure 4, filled squares for clomiphene, filled circles for nafoxidine). The inhibition is expressed as the percentage reduction of the background corrected ICl,swell at +100 mV. The dose-inhibition curve derived from responses at four different concentrations (each data point for clomiphene was obtained from 4 – 9 cells, and between 3 and 8 cells for nafoxidine) fitted to the Hill equation. The estimated IC50 and Hill coefficient for clomiphene were 1.03±0.14 μM and 1.40±0.21 respectively, the corresponding values for nafoxidine were 1.61±0.29 μM and 1.24±0.19.
Figure 4.
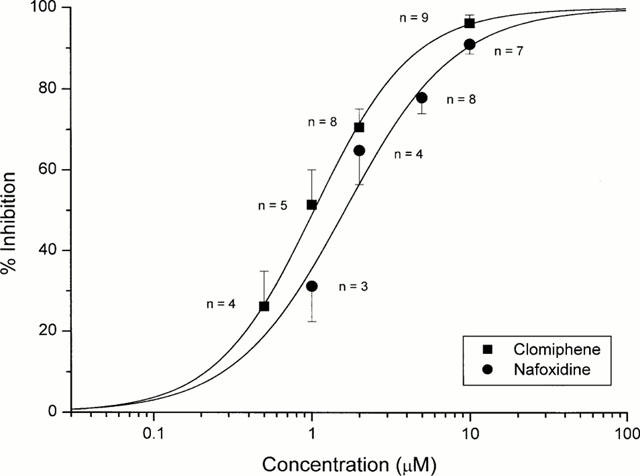
Dose-response curve for inhibition of ICl,swell by clomiphene and nafoxidine. Each data point represents the mean±s.e.mean of n cells as indicated. The filled lines represent the best fit of the data to the Hill equation.
In contrast with nafoxidine, clomiphene consists of a racemic mixture of two enantiomers, i.e. zuclomiphene and enclomiphene. In order to investigate whether clomiphene modulated VRAC in a stereoselective manner, we compared the effect of 10 μM enclomiphene with zuclomiphene and with the racemic mixture. The inhibitory effect of 10 μM, expressed as the percentage reduction of the background corrected ICl,swell at +100 mV, was 94.15±2.26% (n=4) and 94.31±2.78% (n=3) for enclomiphene and zuclomiphene, respectively (data not shown). These values are in the same range as the inhibition induced by the racemic mixture, where we found a value of 96.27±2.02 (n=9) (Figure 2).
Effects of clomiphene and nafoxidine on endothelial cell proliferation
Figure 5A,C show the effect of clomiphene and nafoxidine on the growth of CPAE cells. Cells, initially seeded at a density of 20,000 cells, were counted at days 2, 4 and 6 after plating (6 wells per day and per condition). Both anti-oestrogens induced a sensitive inhibition of the proliferation of CPAE cells. The presence of clomiphene or nafoxidine in the culture medium significantly inhibited growth of CPAE cells at a concentration of 1 μM, whereas 10 μM reduced the number of cells by more than 90% (average of days 2, 4 and 6). The cells did not survive if exposed to a concentration of 100 μM clomiphene or 30 μM nafoxidine. The concentration dependence of the growth inhibition (Figure 5B,D) was calculated from the fit of the pooled data points, normalised to the number of cells under control conditions, to the Hill equation. For clomiphene (Figure 5B), the estimated IC50 value was 1.98±0.17 μM, the Hill coefficient was 1.45±0.14. In the case of nafoxidine (Figure 5D), we found an IC50 value of 1.66±0.21 μM and a Hill coefficient of 1.49±0.27. These values are in the same range as the IC50 for block of ICl,swell.
Figure 5.
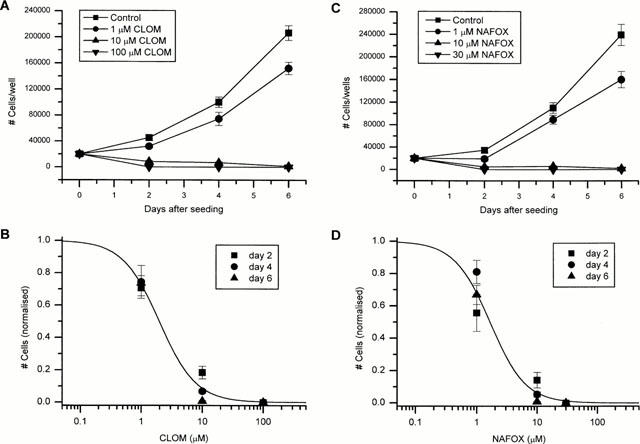
(A) Cell growth measured at various days after plating in the absence and presence of different concentrations of clomiphene (CLOM). Number of cells expressed per well. Each data point represents the mean±s.e.mean of six wells. (B) Number of cells normalised to the number of cells in the absence of clomiphene. For each day, cell numbers from all six wells were plotted as a function of the clomiphene concentration. The filled line represents the best fit of the data to the Hill equation. (C) Cell growth measured at various days after plating in the absence and presence of different concentrations of nafoxidine (NAFOX). Number of cells expressed per well. Each data point represents the mean±s.e.mean obtained from six wells. (D) Number of cells normalized to the number of cells in the absence of nafoxidine. For each day, cell numbers from all six wells were plotted as a function of the nafoxidine concentration. The filled line represents the best fit of the data to the Hill equation.
Discussion
The molecular and functional analysis of volume-regulated anion channels is still hampered by the non-availability of sufficiently sensitive and selective pharmacological tools as modulators of these channels. In previous studies it was shown that tamoxifen efficiently inhibited volume-regulated anion channels. This block was independent of the interaction of tamoxifen with the oestrogen receptor and therefore reflected an alternative cellular target (Voets et al., 1995; Zhang et al., 1994). The aim of this study was to further investigate the modulation of these channels by the pharmacological class of anti-oestrogens, which can be divided into two groups: nonsteroidal compounds showing partial agonist activity such as tamoxifen, clomiphene (Clomid®) and nafoxidine; and steroid analogues devoid of partial agonist activity, including a series of 7α-derivatives of estradiol such as ICI 182,780 (Faslodex®) and 11β-derivatives of estradiol such as RU 58,668 (Gradishar et al., 1997).
Our experimental results show that the pure anti-oestrogens RU 58,668 and ICI 182,780 are only modest or even weak inhibitors of VRAC. The inhibition observed with 10 μM ICI 182,780 (15.6%) is slightly different from that observed by Zhang et al. (1995), who did not observe a significant effect of 30 μM on ICl,swell, in T84, HeLa and C1300 cells. The minor effects observed with β-estradiol are largely in agreement with the work of Greenwood & Large (1998), where it was shown that β-estradiol had no substantial effects on the swelling-activated chloride current in smooth muscle cells. In contrast to the pure anti-oestrogens, the partial anti-oestrogens tamoxifen, clomiphene and nafoxidine potently inhibit VRAC. The results obtained with 10 μM tamoxifen are consistent with the work of Voets et al. (1995), who reported an IC50 value of 3.8 μM for the inhibition of ICl,swell in CPAE cells. This is the first study dealing with modulation of volume-regulated anion channels by the widely used drug clomiphene (Clomid®) and nafoxidine. We show that both drugs are potent inhibitors of these channels, even more potent than tamoxifen. The estimated IC50 values are 1.61±0.29 and 1.03±0.14 μM for nafoxidine and clomiphene respectively. The modulation of VRAC by the latter does not seem to be stereoselective since the inhibitory effects of the enantiomers zuclomiphene and enclomiphene and the racemic mixture clomiphene were similar. Since potent inhibition of VRACs was also demonstrated for the nonsteroidal partial anti-oestrogen toremifene (Diaz, 1996), we conclude that VRAC block seems to be a general property of this group of anti-oestrogens. Maximal block of ICl,swell is reached within several minutes of exposure and subsequent wash out is rather fast, an effect that cannot be explained by an interaction of the partial anti-oestrogen with the oestrogen receptor. The fact that the pure anti-oestrogens and the oestrogen β-estradiol hardly modulate VRACs, strengthens the hypothesis that VRAC block by the nonsteroidal partial anti-oestrogens occurs independent from the oestrogen receptor. VRAC inhibition is therefore a non-genomic short term effect and this block probably occurs by a direct, non-stereoselective interaction of the anti-oestrogen with the channel protein.
The finding that non-steroidal partial anti-oestrogens are potent inhibitors of VRACs suggests a mechanism whereby these compounds might elicit oestrogen receptor-independent effects or specific side effects when administered therapeutically. It has been shown that VRAC is an ubiquitously expressed channel with similar properties in many cell types (Nilius et al., 1994c). The functional importance of this channel has been discussed in detail and comprises among others, effects on volume regulation, osmolyte transport and electrogenesis (Nilius et al., 1997a; Okada, 1997; Strange et al., 1996). Because of these multiple effects, it is not unlikely that modulation of VRAC contributes to the effects or side-effects of this class of drugs. This has been reported for tamoxifen (Nolvadex®), where block of VRAC has been put forward as the molecular mechanism by which this drug causes cataract formation, since chloride channels in the lens of the eye were shown to be essential for maintaining normal lens hydration and transmittance (Zhang et al., 1994). The pharmacological importance of clomiphene (Clomid®) rests on its ability to stimulate ovulation in women with ovulatory disturbances. Free serum concentrations during clomiphene therapy in female infertility range from 0.2 to 0.4 μM (Janicke et al., 1986). Since this concentration range approaches the IC50 for VRAC block, it is not improbable that this inhibition is responsible for some of the described side-effects of this widely used drug, especially in women who are taking a high and prolonged dose. One of the reported side-effects is indeed blurred vision (Goldfien, 1995; Williams et al., 1996) and various ocular side effects have been described in women taking the drug. They occur in up to 10% of the patients (Bishai et al., 1999) and could now be explained by the same mechanism as for tamoxifen. In addition, taking into account the fact that VRAC is critically involved in volume regulation and maintaining the osmotic composition of the fluid compartments in the central nervous system (Strange, 1992) our results could, at least partly, explain the central nervous symptoms that are experienced by 3.5% of women undergoing hormonal stimulation treatment with clomiphene (Siedentopf et al., 1997).
In previous studies it has been shown that clomiphene, tamoxifen and nafoxidine are effective inhibitors of angiogenesis, the formation of new blood vessels (De Lorenzo et al., 2000; Gagliardi et al., 1993; 1996; Manolopoulos et al., 2000). The finding that the angiostatic activity was not altered in the presence of excess oestrogen suggested that this activity is exerted via mechanisms other than their inhibition of oestrogen action. Several mechanisms have been put forward, among which a possible physiological role for endothelial volume-regulated anion channels (Manolopoulos et al., 2000). Previous studies (Nilius et al., 1997b; Voets et al., 1995) had already proposed a possible role for VRAC in cell proliferation and had shown that structurally different VRAC blockers inhibited endothelial cell proliferation at similar concentrations as those reported by Manolopoulos et al. (2000) to be effective in angiogenesis models. This may be one of the mechanisms responsible for the inhibitory effect on angiogenesis. We show here that clomiphene and nafoxidine induce a sensitive inhibition of the proliferation of CPAE cells. Half maximal block of endothelial cell proliferation was at 2.0 and 1.7 μM for clomiphene and nafoxidine respectively, which is in the same range as the IC50 for block of ICl,swell. These findings further support the view that VRAC might be involved in the control of proliferation of endothelial cells and angiogenesis. This knowledge could have possible therapeutic implication, since inhibition of angiogenesis is considered to be one of the most promising strategies that might lead to the development of novel antineoplastic therapies (Augustin, 1998). Blockers of VRAC might therefore be candidate drugs the therapy of angiogenesis-dependent tumour growth.
In conclusion, the nonsteroidal partial anti-oestrogens nafoxidine and clomiphene are potent inhibitors of volume-regulated anion channels. The inhibition by clomiphene is not stereoselective and occurs at concentrations close to therapeutically relevant concentrations. Finally, both drugs inhibited the proliferation of endothelial cells.
Acknowledgments
We thank Dr A. Gagliardi for providing the pure anti-oestrogens. The technical help of M. Schuermans, M. Crabbé, H. Van Weijenbergh, D. Hermans and J. Prenen is greatly acknowledged. C. Maertens is a Research Assistant of the Flemish Fund for Scientific Research (F.W.O.-Vlaanderen). This work was supported by the Belgian Federal Government, the Flemish Government and the Onderzoeksraad KU Leuven (GOA 99/07, F.W.O. G.0237.95, F.W.O. G.0214.99, F.W.O. G. 0136.00; Interuniversity Poles of Attraction Program, Prime Ministers Office IUAP Nr.3P4/23, and C.O.F./96/22-A069), by ‘Levenslijn' (7.0021.99) and a grant from the ‘Alphonse and Jean Forton – Koning Boudewijn Stichting' R7115 B0.
Abbreviations
- CPAE
cultured pulmonary artery endothelial
- EGTA
ethylene glycol-O,O′-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid
- HEPES
N-(hydroxyethyl)piperazine-N′-(2-ethanesulphonic acid)
- HTS
hypotonic solution
- ICl,swell
swelling activated chloride current
- ISO
isotonic solution
- VRAC
volume-regulated anion channel
References
- AUGUSTIN H.G. Antiangiogenic tumour therapy: will it work. Trends. Pharmacol. Sci. 1998;19:216–222. doi: 10.1016/s0165-6147(98)01211-5. [DOI] [PubMed] [Google Scholar]
- BISHAI R., ARBOUR L., LYONES C., KOREN G. Intrauterine exposure to clomiphene and neonatal persistent hyperplastic primary vitreous. Teratology. 1999;60:143–145. doi: 10.1002/(SICI)1096-9926(199909)60:3<143::AID-TERA9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- DE LORENZO M.S., ALONSO D.F., GOMEZ D.E. Nafoxidine modulates the expression of matrix-metalloproteinase-2 (MMP-2) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in endothelial cells. Anticancer Res. 2000;20:395–400. [PubMed] [Google Scholar]
- DIAZ M. Volume-activated chloride channels in neuroblastoma cells are blocked by the anti-oestrogen toremifene. Cell. Mol. Neurobiol. 1996;16:403–409. doi: 10.1007/BF02088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAGLIARDI A., COLLINS D.C. Inhibition of angiogenesis by anti-oestrogens. Cancer Res. 1993;53:533–535. [PubMed] [Google Scholar]
- GAGLIARDI A.R., HENNIG B., COLLINS D.C. Anti-oestrogens inhibit endothelial cell growth stimulated by angiogenic growth factors. Anticancer Res. 1996;16:1101–1106. [PubMed] [Google Scholar]
- GOLDFIEN A.The gonadal hormones and inhibitors Basic and Clinical Pharmacology 1995East Norwalk: Appleton & Lange; 608–636.ed. Katzung, G. pp [Google Scholar]
- GRADISHAR W.J., JORDAN V.C. Clinical potential of new anti-oestrogens. J. Clin. Oncol. 1997;15:840–852. doi: 10.1200/JCO.1997.15.2.840. [DOI] [PubMed] [Google Scholar]
- GREENWOOD I.A., LARGE W.A. Properties of a Cl− current activated by cell swelling in rabbit portal vein vascular smooth muscle cells. Am. J. Physiol. 1998;275:H1524–H1532. doi: 10.1152/ajpheart.1998.275.5.H1524. [DOI] [PubMed] [Google Scholar]
- JANICKE F., ESTOFAN D., BOOS H., BOTTGER I. Endocrine fertility-restricting factors in clomiphene therapy. Geburtsh. Frauenheilk. 1986;46:228–233. doi: 10.1055/s-2008-1035904. [DOI] [PubMed] [Google Scholar]
- KIRK K. Swelling-activated organic osmolyte channels. J. Membrane Biol. 1997;158:1–16. doi: 10.1007/s002329900239. [DOI] [PubMed] [Google Scholar]
- MANOLOPOULOS V., LIEKENS S., KOOLWIJK P., VOETS T., PETERS E., DROOGMANS G., LELKES P., DE CLERCQ E., NILIUS B. Inhibition of angiogenesis by blockers of volume-regulated anion channels. Gen. Pharmacol. 2000;34:107–116. doi: 10.1016/s0306-3623(00)00052-5. [DOI] [PubMed] [Google Scholar]
- NILIUS B., EGGERMONT J., VOETS T., BUYSE G., MANOLOPOULOS V., DROOGMANS G. Properties of volume-regulated anion channels in mammalian cells. Prog. Biophys. Mol. Biol. 1997a;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- NILIUS B., OIKE M., ZAHRADNIK I., DROOGMANS G. Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J. Gen. Physiol. 1994a;103:787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., KAMOUCHI M., VIANA F., VOETS T., DROOGMANS G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca2+- and volume-activated Cl− channels in macrovascular endothelial cells. Br. J. Pharmacol. 1997b;121:547–555. doi: 10.1038/sj.bjp.0701140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., SZÜCS G., WEI L., TANZI F., VOETS T., DROOGMANS G. Calcium-activated chloride channels in bovine pulmonary artery endothelial cells. J. Physiol. 1997c;497:95–107. doi: 10.1113/jphysiol.1997.sp021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., SEHRER J., DROOGMANS G. Permeation properties and modulation of volume-activated Cl−-currents in human endothelial cells. Br. J. Pharmacol. 1994b;112:1049–1056. doi: 10.1111/j.1476-5381.1994.tb13189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., SEHRER J., VIANA F., DE GREEF C., RAEYMAEKERS L., EGGERMONT J., DROOGMANS G. Volume-activated Cl− currents in different mammalian non-excitable cell types. Pflüg. Arch. Eur. J. Phy. 1994c;428:364–371. doi: 10.1007/BF00724520. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- SIEDENTOPF F., HORSTKAMP B., STIEF G., KENTENICH H. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum. Reprod. 1997;12:706–707. doi: 10.1093/humrep/12.4.706. [DOI] [PubMed] [Google Scholar]
- STRANGE K. Regulation of solute and water balance and cell volume in the central nervous system. J. Am. Soc. Nephrol. 1992;3:12–27. doi: 10.1681/ASN.V3112. [DOI] [PubMed] [Google Scholar]
- STRANGE K., EMMA F., JACKSON P.S. Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- SZÜCS G., HEINKE S., DROOGMANS G., NILIUS B. Activation of the volume-sensitive chloride current in vascular endothelial cells requires a permissive intracellular Ca2+ concentration. Pflüg. Arch. Eur. J. Phy. 1996;431:467–469. doi: 10.1007/BF02207289. [DOI] [PubMed] [Google Scholar]
- VOETS T., DROOGMANS G., NILIUS B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J. Physiol. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOETS T., SZÜCS G., DROOGMANS G., NILIUS B. Blockers of volume-activated Cl− currents inhibit endothelial cell proliferation. Pflüg. Arch. Eur. J. Phy. 1995;431:132–134. doi: 10.1007/BF00374387. [DOI] [PubMed] [Google Scholar]
- VOETS T., WEI L., DESMET P., VANDRIESSCHE W., EGGERMONT J., DROOGMANS G., NILIUS B. Downregulation of volume-activated Cl− currents during muscle differentiation. Am. J. Physiol. 1997;272:C667–C674. doi: 10.1152/ajpcell.1997.272.2.C667. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.L., STANCEL G.M.Oestrogens and progestins Goodman and Gilman's The Pharmacological basis of therapeutics 1996New York: McGraw-Hill; 1411–1440.ed. Hardman, J.G. & Limbird, L.E. pp [Google Scholar]
- ZHANG J.J., JACOB T.J., HARDY S.P., HIGGINS C.F., VALVERDE M.A. Lens opacification by antiooestrogens: tamoxifen vs ICI 182,780. Br. J. Pharmacol. 1995;115:1347–1348. doi: 10.1111/j.1476-5381.1995.tb16622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG J.J., JACOB T.J., VALVERDE M.A., HARDY S.P., MINTENIG G.M., SEPULVEDA F.V., GILL D.R., HYDE S.C., TREZISE A.E., HIGGINS C.F. Tamoxifen blocks chloride channels. A possible mechanism for cataract formation. J. Clin. Invest. 1994;94:1690–1697. doi: 10.1172/JCI117514. [DOI] [PMC free article] [PubMed] [Google Scholar]


