Abstract
We have previously shown that tumour necrosis factor-α (TNF-α) activates p38 mitogen-activated protein (MAP) kinase to produce interleukin-8 (IL-8) by human pulmonary vascular endothelial cells. Reactive oxygen species (ROS) including H2O2 generated by TNF-α can act as signalling intermediates for cytokine induction; therefore, scavenging ROS by anti-oxidants is important for the regulation of cytokine production. However, the effect of N-acetylcysteine (NAC), which acts as a precursor of glutathione (GSH) synthesis, on TNF-α-induced activation of p38 MAP kinase pathway and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells has not been determined. To clarify these issues, we examined the effect of NAC on TNF-α-induced activation of p38 MAP kinase, MAP kinase kinase (MKK) 3 and MKK6 which are upstream regulators of p38 MAP kinase, and p38 MAP kinase-mediated IL-8 production.
Human pulmonary vascular endothelial cells that had been preincubated with NAC were stimulated with TNF-α and then the activation of p38 MAP kinase and MKK3/MKK6 in the cells and IL-8 concentrations in the culture supernatants were determined.
Intracellular GSH levels increased in NAC-treated cells.
NAC attenuated TNF-α-induced activation of p38 MAP kinase and MKK3/MKK6.
NAC attenuated p38 MAP kinase-mediated IL-8 production by TNF-α-stimulated cells.
These results indicate that the cellular reduction and oxidation (redox) regulated by intracellular GSH is critical for TNF-α-induced activation of p38 MAP kinase pathway and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells, and we emphasize that anti-oxidant therapy is an important strategy for the treatment of acute lung injury.
Keywords: NAC, p38 MAP kinase, IL-8, acute lung injury, pulmonary vascular endothelial cells
Introduction
Adult respiratory distress syndrome (ARDS), a form of acute lung injury, which is characterized by increased vascular permeability leading to pulmonary oedema and acute respiratory failure, is observed in severe insults such as bacteremia sepsis (Tate et al., 1983; Nogare, 1989; Matthy, 1990; Hashimoto et al., 1994). The pathogenesis of ARDS is complex and involves multiple inflammatory cells and mediators (Tate et al., 1983; Nogare, 1989; Matthy, 1990; Hashimoto et al., 1994). Neutrophils and their products including reactive oxygen species (ROS) have been suggested to play an important role in the production of acute lung injury (Chabot et al., 1998). The extravasation and accumulation of neutrophils at the sites of injury depends upon adhesion to and migration through endothelial linings (Hashimoto et al., 1994). Interleukin-8 (IL-8) which displays chemotactic activity for neutrophils participates in sequestration of neutrophils into the sites of injury (Huber et al., 1991). Vascular endothelial cells are well known to produce IL-8 (Mantovani et al., 1989), therefore, it is an important issue to clarify the mechanism in the production of IL-8 by pulmonary vascular endothelial cells.
Many extracellular stimuli elicit the specific biological responses through the activation of mitogen-activated protein (MAP) kinase cascades (Davis, 1994). The activation of p38 MAP kinase that belongs to MAP kinase superfamily elicits a variety of biological responses including cytokine expression (Han et al., 1994; Raingeaud et al., 1995; Moriguchi et al., 1996; Clerk et al., 1998; Gon et al., 1998; Matsumoto et al., 1998a; Hashimoto et al., 1999c). The mechanism of p38 MAP kinase activation has been extensively studied. p38 MAP kinase is activated by a variety of extracellular stresses. Among stresses, oxidative stresses can activate p38 MAP kinase cascade (Moriguchi et al., 1996; Clerk et al., 1998), and we have previously shown that (1) p38 MAP kinase regulates tumour necrosis factor-α (TNF-α)-induced IL-8 expression (Hashimoto et al., 1999b); (2) N-acetylcysteine (NAC) inhibits IL-8 production (Matsumoto et al., 1998b); and (3) a redox control protein, thioredoxin (TRX), negatively regulates p38 MAP kinase activation and p38 MAP kinase-mediated IL-6 expression (Hashimoto et al., 1999a). These observations indicate that there may be a link between the cellular reduction/oxidation (redox) state and p38 MAP kinase-mediated cytokine expression. However, a role of cellular redox regulated by intracellular glutathione (GSH) in TNF-α-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells have not been determined. In the present study, we examined the effect of NAC which acts as a precursor of GSH synthesis, thus scavenging ROS (Cotgreave et al., 1991) on TNF-α-induced threonine and tyrosine phosphorylation of p38 MAP kinase and p38 MAP kinase-mediated IL-8 production by pulmonary vascular endothelial cells in order to clarify these issues. We simultaneously examined the effect of NAC on TNF-α-induced serine phosphorylation of MAP kinase kinase (MKK) 3 and MKK6 which are upstream regulators of p38 MAP kinase.
Methods
Reagents
NAC was obtained from Sigma Chemical Co. (St. Louis, MI, U.S.A.). The pyridinyl imidazole SB 203580, a specific inhibitor of p38 MAP kinase activity (Lee et al., 1994), was obtained from Calbiochem-Novabiochem Corporation (La Jolla, CA, U.S.A.) and was dissolved in dimethyl sulphoxide. The final concentration of dimethyl sulphoxide used in this experiment was 0.01%. This had no effect on the results. Human recombinant TNF-α was kindly provided by Dainippon Pharmaceutical Co. Ltd. (Osaka, Japan).
Cell culture
Human pulmonary arterial endothelial cells (HPAECs) derived from healthy normal subjects were used as pulmonary vascular endothelial cells, and were obtained from Clonetics (San Diego, CA, U.S.A.). The cells (1×104 cells ml−1) were placed in a tissue culture dish (Falcon 1007; Falcon Labware, Oxnard, CA, U.S.A.) for Western blot analysis. Cells were placed onto 24-well flat-bottom tissue culture plate (Corning, Corning, NY, U.S.A.) for determination of cytokine production using vascular endothelial growth medium (EGM-2; Clonetics) containing 0.2% foetal bovine serum (FBS), gentamycin-amphotericin B, epidermal growth factor (EGF), insulin-like growth factor (IGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), ascorbic acid, heparin and hydrocortisone. Cells were incubated in humidified 5% CO2 atmosphere at 37°C until subconfluence and the medium was replaced with EGM-2 medium without FBS, EGF, FGF, IGF, VEGF, ascorbic acid and hydrocortisone (growth factor free medium) for 16 h. To examine the effect of NAC on TNF-α-induced threonine and tyrosine phosphorylation of p38 MAP kinase, serine phosphorylation of MKK3/MKK6 and IL-8 production, growth factor-starved HPAECs that had been preincubated with or without NAC for 1 h were stimulated with TNF-α, and cultured for the desired times as indicated. In order to examine the effect of SB 203580 on IL-8 production, growth factor-starved HPAECs that had been preincubated with or without SB 203580 for 1 h were stimulated with TNF-α, and cultured for 24 h in humidified 5% CO2 atmosphere at 37°C. After 24 h of culture, the culture supernatants for determination of IL-8 protein were harvested, centrifuged and the supernatants retained, filtered through a Millipore filter (0.45 μm pore size; Millipore, Bedford, MA, U.S.A.) and stored at −80°C until assay.
Measurement of intracellular GSH and hydrogen peroxide
Intracellular GSH levels were measured using the methods described previously (Anderson, 1985). Briefly, the cells were washed with ice-cold PBS. After washing the cells, the cells were collected and suspended with ice-cold PBS, and then 10% trichloroloacetic acid was added to the cell suspension. The mixture was centrifuged at 4°C, thereafter, the supernatant was extracted with diethylether. The resulting extract was incubated with 200 μM of NADPH (Sigma, St. Louis, MO, U.S.A.) and 1 unit ml−1 of GSH reductase (Sigma) for 10 min at 37°C, and then 1 mM of 5,5-dithiobis(2-nitro-benzonic acid) (DTNB, Sigma) was added and the reaction was measured at 415 nm absorbance. Hydrogen peroxide (H2O2); intracellular H2O2 levels were measured using a fluorescent dye, 2′-7′-dichlorofluorescin diacetate (DCFH-DA) as described previously (Ohba et al., 1994). Briefly, the cells were stimulated with TNF-α for 5 min, and then medium was replaced with phosphate buffer saline (PBS) containing 5 μM of DCFH-DA. After 5 min of incubation with DCFH-DA, the fluorescence of intensity was measured by confocal laser microscopy (Olympus Kogyo Ltd., Tokyo, Japan). Relative fluorescence intensity was calculated using unstimulated control cells as standard.
Western blot analysis of p38 MAP kinase phosphorylation
Threonine and tyrosine phosphorylation of 38 MAP kinase was analysed by commercially available kits (PhosphoPlus p38 MAP kinase Antibody Kit, New England Biolabs, Inc., Beverly, MA, U.S.A.). The kit uses antiphospho-p38 MAP kinase that is specific for phosphorylated threonine and tyrosine kinase of p38 and does not cross-react with phosphorylated threonine and tyrosine of extracellular signal-regulated kinase (ERK) 1/2 or c-Jun-NH2-terminal kinase (JNK). Analysis of threonine and tyrosine phosphorylation of p38 MAP kinase was performed according to the manufacturer's instructions. Briefly, after separating proteins from the cell lysate by 15% SDS-polycarylamide gel electrophoresis (PAGE), the cell lysate containing 10 μg of protein was electrophoretically transferred to nitrocellulose membrane and the membrane was blotted with a specific antibody to phosphorylated threonine and tyrosine of p38 MAP kinase. In order to show the amounts of p38 MAP kinase immunoblotted, blots were stripped and reprobed using phosphorylation-state independent p38 MAP kinase-specific antibody to determine total p38 MAP kinase levels.
Western blot analysis of MKK3/MKK6 phosphorylation
Serine phosphorylation of MKK3 and MKK6 kinase was analysed by commercially available kits (PhosphoPlus MKK3/MKK6 [Ser189/207] Antibody Kit, New England Biolabs). Phosphospecific MKK3/MKK6 antibody supplied in this kit selectively detects Ser189- and Ser207-phosphorylated MKK3 and MKK6, respectively. As the activation sites of both proteins are closely related, this antibody detects both phosphoproteins. Polyclonal antibodies were produced by immunizing rabbits with a synthetic phospho-Ser189 peptide (KLH coupled) corresponding with residues 185-194 of human MKK3. Analysis of serine phosphorylation of MKK3 and MKK6 was performed according to the manufacturer's instruction as described previously (Hashimoto et al., 2000). Briefly, after separating proteins from the cell lysate by 15% SDS-polyacrylamide gel electrophoresis (PAGE), the cell lysate containing 10 μg of protein was electophoretically transferred to nitrocellulose membrane and the membrane was blotted with a specific antibody to phosphorylated MKK3/MKK6. In order to show the amounts of MKK3 immunoblotted, blots were stripped and reprobed using phosphorylation-state independent MKK3-specific antibody to determine total MKK3 levels.
Measurement of IL-8
The concentrations of IL-8 in the culture supernatants from HPAECs were measured using commercially available enzyme-linked immunoabsorbent assay (ELISA) kits (Amersham International, Aylesbury, U.K.). ELISA was performed according to the manufacturer's instruction. All samples were assayed in duplicate.
Statistical analysis
Statistical significance was analysed with ANOVA. P values <0.05 were considered significant. When statistical significance was reached, post hoc tests (Fischer's Protected Least Significant Difference, Scheff's F) were performed.
Results
Intracellular GSH and hydrogen peroxide levels
We measured the intracellular GSH levels and the intracellular H2O2 levels in order to confirm the intracellular GSH levels and the intracellular H2O2 levels in this study. When HPAECs were incubated with various concentrations of NAC for 1 h, the intracellular GSH increased in a concentration-dependent manner (Figure 1a). We also measured the intracellular H2O2 as measured by the oxidation of DCF and the pretreatment with NAC attenuated TNF-α-induced increases in the intracellular H2O2 (Figure 1b).
Figure 1.
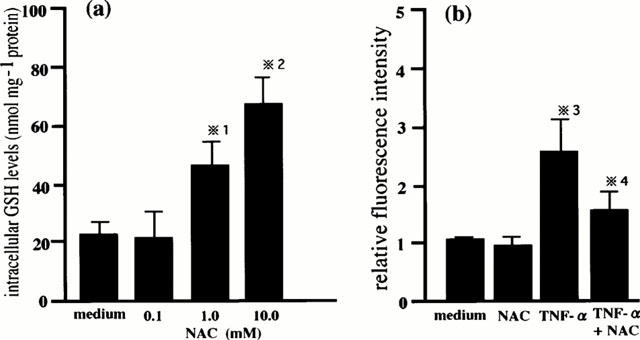
Cellular redox state. The intracellular GSH levels in HPAECs were measured at 1 h after incubation with various concentrations of NAC (a). The intracellular H2O2 levels in HPAECs that had been pretreated with or without 10 mM of NAC were measured at 5 min after TNF-α (10 ng ml−1) stimulation (b). Relative fluorescence intensity was calculated using unstimulated control cells as standard. The results are expressed as mean±s.d.mean in three different experiments. *1 P<0.05 compared with the intracellular GSH levels in the cells cultured with medium. *2 P<0.01 compared with the intracellular GSH levels in the cells cultured with medium. *3 P<0.01 compared with the intracellular H2O2 levels in the cells cultured with medium. *4 P<0.05 compared with the intracellular H2O2 levels in the cells cultured with TNF-α.
TNF-α activates p38 MAP kinase and NAC attenuates TNF-α-induced p38 MAP kinase activation
The increases in threonine- and tyrosine-phosphorylation of p38 MAP kinase reflect the activation state of p38 MAP kinase. Consequently, we examined the threonine- and tyrosine-phosphorylation of p38 MAP kinase. HPAECs were stimulated with TNF-α (10 ng ml−1) for 0 – 60 min and p38 MAP kinase was immunoblotted. Amounts of phosphorylated threonine and tyrosine of p38 MAP kinase were increased at 5 min, these levels being sustained between 10 and 30 min; thereafter, they returned to near-basal levels at 60 min, indicating that threonine- and tyrosine-phosphorylation of p38 MAP kinase was transient (Figure 2, upper panel). In order to examine the effect of NAC on TNF-α-induced p38 MAP kinase activation, the cell that had been preincubated with various concentrations of NAC for 1 h were stimulated with TNF-α, and amounts of phosphorylated threonine and tyrosine of p38 MAP kinase were analysed at 5 min after stimulation. NAC attenuated TNF-α-induced p38 MAP kinase activation in a concentration-dependent manner. Amounts of phosphorylated threonine and tyrosine of p38MAP kinase were lower in NAC-pretreated cells than those in NAC-untreated cells, indicating that the pretreatment with NAC attenuated TNF-α-induced p38 MAP kinase activation (Figure 3, upper panel). Lower panels of Figures 2 and 3 showed that equal amounts of p38 MAP kinase protein were immunoblotted using a phosphorylation state-independent p38 MAP kinase-specific antibody regardless of culture conditions. Ten mM of NAC also attenuated TNF-α-induced p38 MAP kinase activation at 15 and 30 min after stimulation (data not shown), indicating that the attenuation by NAC of TNF-α-induced p38 MAP kinase activation is not a delay.
Figure 2.
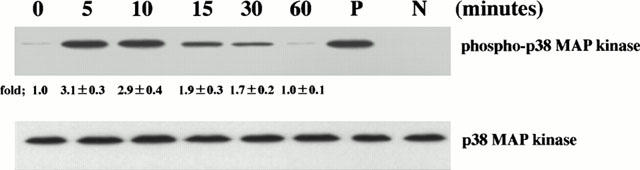
TNF-α activates p38 MAP kinase. HPAECs were stimulated with TNF-α (10 ng ml−1) for the desired times as indicated. The HPAEC lysates were separated by a 15% SDS – PAGE, transferred to membranes, and probed with a specific antibody directed against the phosphorylated threonine and tyrosine of p38 MAP kinase (phospho-p38 MAP kinase; upper panel). These blots were then stripped and reprobed using a phosphorylation state-independent p38 MAP kinase-specific antibody to determine the amounts of p38 MAP kinase blotted (p38MAP kinase; lower panel). P: positive control, protein prepared from C-6 glioma cells stimulated with anisomycin to phosphorylate the threonine and tyrosine of p38 MAP kinase; N: negative control, protein prepared from C-6 glioma cells not stimulated with anisomycin. Blots are representative of three identical experiments independently performed. The amounts of phosphorylated p38 MAP kinase were quantitated by National Institutes of Health (NIH) image analyzer (National Institute of Health, Bethesda, MD, USA) and are presented as the amounts of phosphorylated p38 MAP kinase relative to control cells treated without agonist (1.0). Fold increase in amounts of phosphorylated p38 MAP kinase proteins as indicated as below are expressed as mean±s.d.mean in three different experiments.
Figure 3.
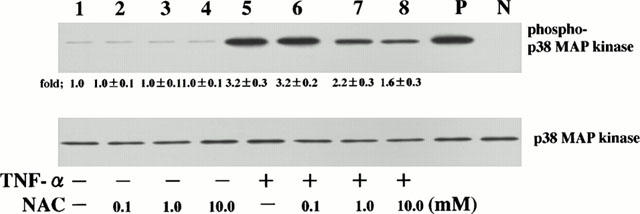
NAC attenuates TNF-α-induced p38 MAP kinase activation. HPAECs that had been pretreated either with medium or NAC (10 mM) for 1 h were stimulated with TNF-α (10 ng ml−1) for 5 min. The HPAEC lysates were separated by a 15% SDS – PAGE, transferred to membranes, and probed with a specific antibody directed against the phosphorylated threonine and tyrosine of p38 MAP kinase (phospho-p38 MAP kinase; upper panel). These blots were then stripped and reprobed using a phosphorylation state-independent p38 MAP kinase-specific antibody to show the amounts of p38 MAP kinase blotted (p38MAP kinase; lower panels). The cells were cultured with medium (lane 1), NAC 0.1 mM (lane 2), NAC 1.0 mM (lane 3), NAC 10 mM (lane 4), TNF-α (lane 5), TNF-α+NAC 0.1 mM (lane 6), TNF-α+NAC 1.0 mM (lane 7), and TNF-α+NAC 10 mM (lane 8). Lane P, lane N and fold were described as Figure 2 legend. Blots are representative of three identical experiments independently performed. The amounts of phosphorylated p38 MAP kinase were quantitated by National Institutes of Health (NIH) image analyzer (National Institute of Health, Bethesda, MD, U.S.A.) and are presented as the amounts of phosphorylated p38 MAP kinase relative to control cells treated without agonist (1.0). Fold increase in amounts of phosphorylated p38 MAP kinase proteins as indicated as below are expressed as mean±s.d.mean in three different experiments. Amounts of phosphorylated threonine and tyrosine of p38MAP kinase were significantly lower in NAC-pretreated cells than those in NAC-untreated cells (P<0.01).
TNF-α activates MKK3/MKK6 and NAC attenuates TNF-α-induced MKK3/MKK6 activation
In order to examine the effect of NAC on TNF-α-induced MKK3/MKK6 phosphorylation, the cells that had been preincubated with 10 mM of NAC for 1 h were stimulated with TNF-α, and amounts of serine phosphorylation of MKK3/MKK6 were analysed at 5 min after stimulation. Amounts of phosphorylated serine of MKK3/MKK6 were lower in NAC-pretreated cells than those in NAC-untreated cells, indicating that the pretreatment with NAC attenuated TNF-α-induced MKK3/MKK6 phosphorylation (Figure 4, upper panel). Lower panels of Figure 4 showed that equal amounts of MKK3 protein were immunoblotted using a phosphorylation state-independent MKK3-specific antibody regardless of culture conditions.
Figure 4.
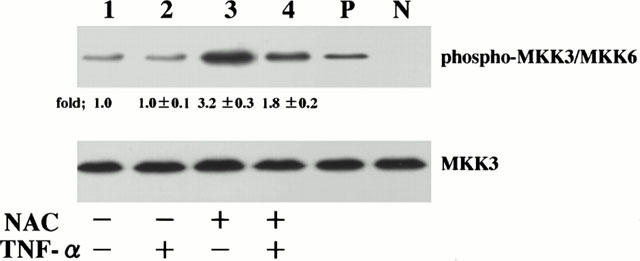
NAC attenuates TNF-α-induced MKK3 and MKK6 activation. HPAECs that had been pretreated either with medium or NAC (10 mM) for 1 h were stimulated with TNF-α (10 ng ml−1) for 5 min. The HPAEC lysates were separated by a 15% SDS – PAGE, transferred to membranes, and probed with a specific antibody directed against the phosphorylated serine of MKK3 and MKK6 (phospho-MKK3/MKK6; upper panel). These blots were then stripped and reprobed using a phosphorylation-state independent MKK3-specific antibody to determine total MKK3 levels (MKK3; lower panels). The cells were cultured with medium (lane 1), NAC 10 mM (lane 2), TNF-α (lane 3), TNF-α+NAC 10 mM (lane 4). Lane P: positive protein prepared from NIH3T3 cells stimulated with UV treatment for phosphorylated serine of MKK3 and MKK6; Lane N: negative protein prepared from NIH3T3 cells without UV treatment. Blots are representative of three identical experiments independently performed. The amounts of phosphorylated MKK3/MKK6 were quantitated by National Institutes of Health (NIH) image analyzer (National Institute of Health, Bethesda, MD, U.S.A.) and are presented as the amounts of phosphorylated MKK3/MKK6 relative to control cells treated without agonist (1.0). Fold increase in amounts of phosphorylated MKK3/MKK6 proteins as indicated as below are expressed as mean±s.d.mean in three different experiments. Amounts of phosphorylated threonine and tyrosine of p38MAP kinase were significantly lower in NAC-pretreated cells than those in NAC-untreated cells (P<0.01).
NAC attenuates TNF-α-induced IL-8 production
Finally, we examined the effect of NAC on TNF-α-induced IL-8 production. Simultaneously, we examined the effect of SB 203580 on TNF-α-induced IL-8 production to confirm the role of p38 MAP kinase in IL-8 production. As shown in Figure 5, the pretreatment of the cells with NAC attenuated TNF-α-induced IL-8 production in a concentration-dependent manner. SB 203580 attenuated TNF-α-induced IL-8 production, indicating and confirming that p38 MAP kinase plays an important role in TNF-α-activated signalling pathway which regulates IL-8 production by HPAECs. These results indicated that cellular GSH increasing agent, NAC, negatively regulated TNF-α-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production. The total number of cells and cell viability determined by means of the trypan blue exclusion dye assay, which was performed after 24 h of culture for the determination of IL-8 concentrations, did not differ with culture conditions (data not shown), suggesting that TNF-α-induced IL-8 production, its attenuation by NAC and SB 203580 did not result from cell cytotoxicity.
Figure 5.
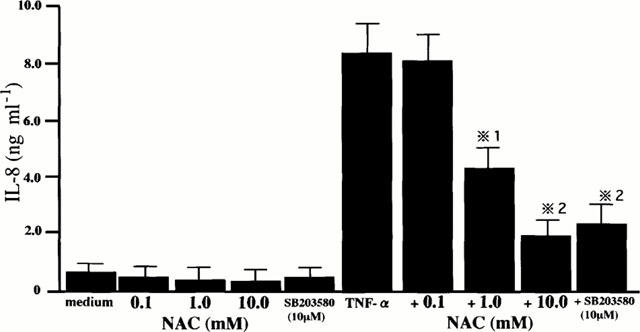
NAC attenuates TNF-α-induced IL-8 production. HPAECs that had been pretreated either with medium or various concentrations of NAC for 1 h were cultured with medium or TNF-α (10 ng ml−1). Simultaneously, the cells that had been pretreated with SB 203580 (10 μM) were stimulated with TNF-α (10 ng ml−1) to examine the effect of SB 203580 on TNF-α-induced IL-8 production. The concentrations of IL-8 in the culture supernatants were determined after 24 h of culture. The results are expressed as mean±s.d. in five different experiments. *1 P<0.05 compared with IL-8 concentrations in the cells cultured with TNF-α only. *2 P<0.01 compared with IL-8 concentrations in the cells cultured with TNF-α only.
Discussion
In the present study, we examined the effect of NAC on TNF-α-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by HPAECs. The results showed that (1) Intracellular GSH levels increased in NAC-treated cells; (2) NAC attenuated TNF-α-induced activation of p38 MAP kinase and MKK3/MKK6; and (3) NAC attenuated p38 MAP kinase-mediated IL-8 production by TNF-α-stimulated cells. These results indicate that cellular redox regulated by intracellular GSH is critical for TNF-α-induced activation of p38 MAP kinase pathway and p38 MAP kinase-mediated IL-8 production by HPAECs.
The cellular redox changes have been implicated in the activation of MAP kinase superfamily (Guyton et al., 1996; Moriguchi et al., 1996; Laderoute et al., 1997; Clerk et al., 1998) and the induction of cytokine expression (Shreck et al., 1991; 1992). ROS generated by TNF-α stimulation have been described to act as signalling intermediates for TNF-α-induced cytokine expression, since ROS are generated by TNF-α stimulation (Meier et al., 1989; Shreck et al., 1991; 1992) and antioxidants inhibit TNF-α-induced cytokine expression (Droge et al., 1992; Talley et al., 1995; Matsumoto et al., 1998b). In addition, ROS per se can activate p38 MAP kinase (Moriguchi et al., 1996; Clerk et al., 1998). We have previously shown that p38 MAP kinase regulates TNF-α-induced IL-8 expression in human pulmonary vascular endothelial cells (Hashimoto et al., 1999b). In addition, an increase in intracellular GSH levels has been shown to negatively regulate JNK activation (Wilhelm et al., 1997). However, the role of cellular redox regulated by intracellular GSH in TNF-α-induced activation of p38 MAP kinase pathway and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells have not been determined. To clarify these issues, we examined the effect of NAC on TNF-α-induced activation of p38 MAP kinase and IL-8 production. In addition, we examined the effect of NAC on MKK3 and MKK6 activation in order to clarify the effect of NAC on the upstream kinase of p38 MAP kinase. The results showed that NAC attenuated TNF-α-induced activation of p38 MAP kinase activation and MKK3/MKK6 as well as p38 MAP kinase-mediated IL-8 production. These results indicate that TNF-α-induced activation of p38 MAP kinase pathway and p38 MAP kinase-mediated IL-8 production are negatively regulated by increasing intracellular GSH levels.
There are several possible mechanisms by which cellular redox regulated by intracellular GSH modulates TNF-α-induced p38 MAP kinase activation. p38 MAP kinase activation is mediated by dual phosphorylation of threonine and tyrosine by MKK3 and MKK6, which are upstream regulators of p38 MAP kinase (Clerk et al., 1998). Recently, apoptosis signal-regulating kinase 1 (ASK1) was identified as a MAP kinase kinase kinase that activates p38 MAP kinase cascade (Ichijo et al., 1997; Saitoh et al., 1998). It has been shown that TNF-α-induced ASK1 activation is regulated by cellular redox state. TNF-α causes ASK1 activation through ROS-mediated dimerization of ASK1 and NAC reduces ASK1 activity (Gotoh et al., 1998). Alternatively, it has been shown that thioredoxin (TRX) bounds directly to the N-terminal portion of ASK1 and overexpression of TRX inhibits ASK1 activity (Saitoh et al., 1998). TRX-mediated inhibition of ASK1 activity depends on their interaction and a reduced form of TRX is critical for the direct inhibition of ASK1 activity (Saitoh et al., 1998). Conversely, when the cells are exposed to oxidative stress, the oxidation of TRX may disrupt their interaction and consequently activate ASK1. In the present study, we showed that TNF-α induced activation of p38 MAP kinase and MKK3/MKK6 in HPAECs, and that intracellular GSH modulated TNF-α-induced activation of p38 MAP kinase and MKK3/MKK6. Although we did not examine ASK1 activity, TNF-α-induced activation of p38 MAP kinase pathway might be mediated through ASK-1 activation by ROS-mediated ASK1 dimerization or dissociation of TRX from ASK1. The attenuation of TNF-α-induced activation of MKK3/MKK6 and p38 MAP kinase by NAC might result from the modulation of ASK1 activity by intracellular GSH. However, further study should be undertaken to clarify these issues.
The pathogenesis of acute lung injury is complex. The important role of the oxidant-antioxidant imbalance and inflammatory cytokines including TNF-α and IL-8 in the pathogenesis of acute lung injury has been described (Hyers et al., 1991; Miller et al., 1992). Oxidative stress-induced cellular damage participates in the production of acute lung injury (Chabot et al., 1998). The present results may provide an alternative explanation for a role of oxidative stress in the production of acute lung injury. According to these observations, we emphasize that anti-oxidant therapy is an important strategy for the treatment of acute lung injury.
From the data presented here, we conclude that cellular redox regulated by intracellular GSH is critical for TNF-α-induced activation of p38 MAP kinase pathway and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells, and that anti-oxidant therapy is an important strategy for the treatment of acute lung injury.
Acknowledgments
This work was financially supported, in part, by Grant-in-Aid for High-Tech Research Center from the Japanese Ministry of Education, Science, Sports and Culture to Nihon University.
Abbreviations
- ARDS
adult respiratory distress syndrome
- EGF
epidermal growth factor
- ERK
extracellular signal-related kinase
- FBS
foetal bovine serum
- FGF
fibroblast growth factor
- GSH
glutathione
- HPAEC
human pulmonary artery endothelial cell
- IGF
insulin-like growth factor
- IL
interleukin
- IL-8
interleukin-8
- JNK
c-Jun-NH2-terminal kinase
- MAP
mitogen-activated protein kinase
- MKK
MAP kinase kinase
- NAC
N-acetylcysteine
- ROS
Reactive oxygen species
- TNF-α
tumour necrosis factor-α
- VEGF
vascular endothelial growth factor
References
- ANDERSON M.E. Determination of glutathione and glutathione disulfide in biological samples. Meth. Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- CHABOT F., MITCHELL J.A., CUTERIDGE J.M.C., EVANS T.W. Reactive oxygen species in acute lung injury. Eur. Respir. J. 1998;11:745–757. [PubMed] [Google Scholar]
- CLERK A., FULLER S.J., MICHAEL A., SUGDEN P.H. Stimulation of ‘stress-regulated' mitogen-activated protein kinases (stress-activated protein kinases/c-jun N-terminal kinases and p38-mitogen-activated protein kinase) in perfused rat hearts by oxidative and other stresses. J. Biol. Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- COTGREAVE I., MOLDEUS P., SCHUPPE I. The metabolism of N-acetylcysteine by human endothelial cells. Biochem. Pharmacol. 1991;42:13–16. doi: 10.1016/0006-2952(91)90674-t. [DOI] [PubMed] [Google Scholar]
- DAVIS R.J. MAPKs: new JNK expand the group. Trends Biochem. Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- DROGE W., ECK H.-P., MIHM S. HIV-induced cysteine deficiency and T-cell dysfunction – a rationale for treatment with N-acetylcysteine. Immunol. Today. 1992;13:211–214. doi: 10.1016/0167-5699(92)90156-2. [DOI] [PubMed] [Google Scholar]
- GON Y., HASHIMOTO S., MATSUMOTO K., NAKAYAMA T., TAKESHITA I., HORIE T. Cooling and rewarming-induced IL-8 expression in human bronchial epithelial cells through p38 MAP kinase-dependent pathway. Biochem. Biophy. Res. Commun. 1998;249:156–160. doi: 10.1006/bbrc.1998.9115. [DOI] [PubMed] [Google Scholar]
- GOTOH Y., COOPER J.A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-α signal transduction. J. Biol. Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- GUYTON K.Z., LIN Y., GOROSPE M., XU Q., HOLBROOK N.J. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J. Biol. Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- HAN J., LEE J.D., BUBBS L., ULEVITCH R.J. A Map kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO S., HORIE T.Adult respiratory distress syndrome and adhesion molecules Basic and clinical aspects of pulmonary fibrosis 1994CRS Press, Boca Bacon; 433–443.ed. Takishima T pp [Google Scholar]
- HASHIMOTO S., MATSUMOTO K., GON Y., FURUICHI S., MARUOKA S., TAKESHITA I., HIROTA K., YODOI J., HORIE T. Thioredoxin negatively regulates p38 MAP kinase activation and IL-6 production by tumor necrosis factor-α. Biochem. Biophy. Res. Commun. 1999a;258:443–447. doi: 10.1006/bbrc.1999.0658. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO S., MATSUMOTO K., GON Y., MARUOKA S., TAKESHITA I., HAYASIH S., KOURA K., KUJIME K., HORIE T. p38 mitogen-activated protein kinase regulate IL-8 expression in human pulmonary vascular endothelial cells. Eur. Respir. J. 1999b;13:1357–1364. [PubMed] [Google Scholar]
- HASHIMOTO S., MATSUMOTO K., GON Y., NAKAYAMA T., TAKESHITA I., HORIE T. Hyperosmolarity-induced IL-8 expression in human bronchial epithelial cells through p38 MAP kinase-dependent pathway. Am. J. Respir. Crit. Care Med. 1999c;159:634–640. doi: 10.1164/ajrccm.159.2.9712090. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO S., MATSUMOTO K., GON Y., MARUOKA S., KUJIME K., HAYASHI S., TAKESHITA I., HORIE T. p38 MAP kinase regulates TNF-α, IL-1 α- and PAF-induced RANTES and GM-CSF production by human bronchial epithelial cells. Clin. Exp. Allergy. 2000;30:48–55. doi: 10.1046/j.1365-2222.2000.00641.x. [DOI] [PubMed] [Google Scholar]
- HUBER A.R., KUNKEL S.L., TODD R.F., III, WEISS S.J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- HYERS T.M., TRICOMI S.M., DETTRNMEIER P.A., FOULER A. Tumor necrosis factor levels in serum and bronchoalveolar lavage fluid of patients with the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1991;144:268–271. doi: 10.1164/ajrccm/144.2.268. [DOI] [PubMed] [Google Scholar]
- ICHIJO J., NISHIDA E., IRIE K., TEN DIJIKE P., SAITOH M., MORIGUCHI T., TAKAGI M., MATSUMOTO K., MIYAZONO K., GOTOH Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathway. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- LADEROUTE K.R., WEBSTER K.A. Hypoxia/reoxygenation stimulates Jun kinase activity through redox signaling in cardiac myocytes. Cir. Res. 1997;80:336–344. doi: 10.1161/01.res.80.3.336. [DOI] [PubMed] [Google Scholar]
- LEE J.C., LAYDON J.T., MCDONNELL P.C., GALLAGHER T.F., KUMMER S., GREEN D., MCNULTY D., BLUMENTHAL M.J., HEYS J.R., LANDVATTER S.W., STRICKLER J.E., MCLAIGHLIN M.M., SIEMENS I.R., FISHER S.M., LIVI G.P., WHITE J.R., ADAMS J.L., YOUNG P. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- MANTOVANI A., DEJANA E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol. Today. 1989;10:370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO K., HASHIMOTO S., GON Y., NAKAYAMA T., HORIE T. Proinflammatory cytokine- and chemical mediator-induced IL-8 expression in human bronchial epithelial cells through p38 MAP kinase-dependent pathway. J. Allergy & Clin. Immunol. 1998a;101:825–831. doi: 10.1016/S0091-6749(98)70311-2. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO K., HASHIMOTO S., GON Y., NAKAYAMA T., HORIE T. N-acetylcysteine inhibits IL-1α-induced IL-8 secretion by human bronchial epithelial cells. Respir. Med. 1998b;92:512–515. doi: 10.1016/s0954-6111(98)90300-6. [DOI] [PubMed] [Google Scholar]
- MATTHAY M.A. The adult respiratory distress syndrome, definition and prognosis. Clin. Chest. Med. 1990;11:575–580. [PubMed] [Google Scholar]
- MEIER B., RADEKE H.H., SELLE S., YOUNES M., SIES H., RESCH K., HABERMEHL G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 and tumor necrosis factor-α. Biochem. J. 1989;263:539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER E.J., COHEN A.B., NAGAO S., GRIFFITH D., MAUNDER R.J., MARTIN T.R., WEINER-KRONISH J.P., STICHERLING M., CHRISTOPHERS E., MATTHY M.A. Elevated levels of NAP-1/interleukin-8 are present in the airspace of patients with adult respiratory distress syndrome and are associated with increased mortality. Am. Rev. Respir. Dis. 1992;146:427–432. doi: 10.1164/ajrccm/146.2.427. [DOI] [PubMed] [Google Scholar]
- MORIUCHI T., TOYOSHIMA F., GOTOH Y., IWAMATSU K., IRIE E., MORI N., KUROYANAGI M., HAGIWARA K., MATSUMOTO K., NISHIDA E. Purification and identification of a major activator for p38 from osmotically shocked cells. Activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-α and H2O2. J. Biol. Chem. 1996;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- NOGARE N.D. Adult respiratory distress syndrome. Am. J. Med. Sciences. 1989;298:413–431. doi: 10.1097/00000441-198912000-00011. [DOI] [PubMed] [Google Scholar]
- OHBA M., SHIBAMURA M., KUROKI T., NOSE K. Production of hydrogen peroxide transforming growth factor-β1 and its involvement in induction of erg-1 in mouse osteoblastic cells. J. Cell Biol. 1994;126:1079–1088. doi: 10.1083/jcb.126.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAINGEAUD J., GUPTA S., ROGERS J.S., DICKENS M., HAN J., ULEVITSH R.J., DAVIS R. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- SAITOH M., NISHITOH H., FUJII M., TAKEDA K., TOBIUM K., SAWADA Y., KAWABATA M., MIYAZONO K., ICHIJO H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRECK R., ALBERMANN K., BAEURELE P.A. Nuclear factor κB: an oxidative stress-responsive transcriptional factor of eukaryotic cells (a review) Free Rad. Res. Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- SCHRECK R., BAEUERLE P.A. A role of oxygen radicals as second messengers. Trend Cell Biol. 1991;1:39–42. doi: 10.1016/0962-8924(91)90072-h. [DOI] [PubMed] [Google Scholar]
- TALLEY A.K., DEWHURST S., PERRY S.W. Tumor necrosis factor alpha-induced apoptosis in human neural cells: protection by the antioxidant N-acetylcysteine and the genes bcl-2 and crmA. Mol. Cell Biol. 1995;15:2359–2366. doi: 10.1128/mcb.15.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATE R.M., REPINE J.E. Neutrophils and the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1983;128:552–559. doi: 10.1164/arrd.1983.128.3.552. [DOI] [PubMed] [Google Scholar]
- WILHELM D., BENDER K., KNBEL A., ANGEL P. The levels of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol. Cell Biol. 1997;17:4792–4800. doi: 10.1128/mcb.17.8.4792. [DOI] [PMC free article] [PubMed] [Google Scholar]


