Abstract
This study examines the relative contributions made by inhibition of mast cell degranulation, reduction of mast cell recruitment and maturation, and lowering the responsiveness of the vasculature to histamine, in the inhibition by glucocorticoids of the weal and flare in human skin.
One forearm of healthy human volunteers was treated for 24 h (n=6) or daily for 21 days (n=10) with 0.05% clobetasol propionate. The other arm served as control. Weal and flare responses were elicited by intradermal injection of 20 μl of 0.3 mM codeine. The areas of the responses were measured using scanning laser Doppler imaging. Microdialysis was used to assess histamine release. Mast cell numbers and tissue histamine content were assessed in 4-mm punch biopsies. Histamine (20 μl of 1 μM i.d.) was used to assess the status of the vasculature.
No significant effects were seen at 24 h. At 21 days, clobetasol reduced the areas of the codeine-induced weal and flare responses by 59 and 58% respectively (both P=0.006). Mast cell numbers were reduced by 47%, (P=0.014) and total tissue histamine content by 52% (P=0.006). Codeine-induced histamine release was reduced by 44% (P=0.022). The weal, but not the flare, induced by histamine was significantly inhibited (P=0.019). Echography revealed a 15% thinning of the skin by clobetasol.
These results demonstrate that reduction of the weal and flare responses to codeine following clobetasol treatment, results primarily from reduced mast cell numbers and tissue histamine content rather than inhibition by corticosteroids of mast cell degranulation.
Keywords: Glucocorticoids, clobetasol, mast cell, weal, flare, skin, histamine, human
Introduction
While a beneficial role for glucocorticoids in inhibiting the late phase of the allergic response and in reducing allergic inflammation is well established, their possible benefits in the mast cell-mediated early phase of the allergic response is less clear. In urticaria, for example, they are poorly effective when given acutely but may afford benefit when used for more prolonged periods. However, when considering the use of glucocorticoids for prolonged periods, clinicians have to balance the benefits of therapy against the potential long-term detrimental effects on the skin, which include thinning and a reduction of cutaneous immune defences.
There are three possible mechanisms by which glucocorticoids may lead to a suppression of the mast cell-dependent weal and flare response in the skin. The first is an inhibitory action on mast cell mediator release, the second an effect on mast cell recruitment and maturation, and the third a reduction of the responsiveness of the vasculature to mast cell mediators.
An inhibition of mediator release from mast cells is unlikely. In vitro studies have demonstrated that incubation for 24 h with the potent synthetic glucocorticoid, dexamethasone, does not inhibit mediator release from human mast cells derived from the lung, intestine or skin (Cohan et al., 1989; Schleimer et al., 1983). Also in vivo, administration of glucocorticoids for periods of 48 h or less fails to reduce the magnitude of early phase allergic responses, indicating that mast cell degranulation is not inhibited (Atkins et al., 1990; Booij Noord et al., 1971; Dunsky et al., 1979; Slott & Zweiman, 1974).
That administration of glucocorticoids for periods in excess of a week reduces the size of weal and flare responses provoked in the skin by allergen or codeine, led to the suggestion that the recruitment and maturation of mast cells within tissues is inhibited by steroids (Olson et al., 1990; Pipkorn et al., 1989). Direct evidence in support of this suggestion was then obtained from biopsy studies in which both mast cell numbers and tissue histamine levels were shown to decrease from week 3 onwards of treatment of the skin with glucocorticoids (Lavker & Schechter, 1985; Pipkorn et al., 1989). This was confirmed by a study using electron microscopy in which Lavker & Schechter (1985) described the mast cells in steroid treated skin as ‘degenerating' and ‘dying'. This observed effect is most likely to be due to a combination of a reduction of mast cell recruitment to the tissue and an increased rate of apoptosis of the mast cells already present.
Although the ability of glucocorticoids to induce vasoconstriction in the skin (the blanching effect) is well established, evidence that they modify the responsiveness of the vasculature to the effects of mast cell mediators is equivocal, some workers reporting an inhibition of histamine-induced effects (Andersson & Pipkorn, 1987; Olson et al., 1990; Perretti & Ahluwalia, 2000; Singh & Singh, 1986; Stahle & Hägermark, 1984) whilst others reported no effect (Goldsmith et al., 1996; Lopez-Campos et al., 1998; Slott & Zweiman, 1974).
In this study, we have compared the effects of clobetasol propionate applied to the skin for 24 h with application daily for three weeks to investigate the mechanisms by which glucocorticoids inhibit the mast cell-dependent weal and flare response in human skin in vivo. Scanning laser Doppler imaging was employed to measure weal and flare areas, microdialysis used to assess histamine release, biopsies taken to count mast cell numbers and assess total tissue histamine levels, and echography used to monitor the thickness of the skin.
Methods
Study population
Ten male volunteers aged 19 – 35 years (mean 23.3±1.12 years) gave written informed consent and participated in the study. Ethical approval was granted from Southampton and South West Joint Research Ethical Committee (submission no.08/99). Exclusion criteria included subjects taking any drug with antihistaminic activity, non-steroidal anti-inflammatory drugs, corticosteroids or drugs that affect the cardiovascular system. Subjects who suffered from dermatological or cardiovascular conditions were also excluded.
Dermal provocation with histamine and codeine
Intradermal injections of 20 μl of 0.3 mM codeine (Martindale Pharmaceuticals, Romford, Essex, U.K.) in phosphate buffered saline (PBS) were used to cause mast cell degranulation. As a positive control, 20 μl of 1 μM histamine (Sigma, Poole, U.K.) was injected. Both injections were made 1 mm from and parallel to the microdialysis fibre using a 27 gauge needle and U-100 insulin syringe (Myjector, Terumo Europe NV, Leuven, Belgium).
Assessment of weal and flare responses
Changes in dermal blood flux were assessed using scanning laser Doppler imaging (SLDI, Moor LDI, Moor Instruments Ltd, Axminster, Devon, U.K.) as previously described (Clough et al., 1998). Laser Doppler images were made by scanning an area of skin 5 cm square, giving ∼16,000 data points for analysis. The areas of the weal and flare responses were calculated from the stored images using the manufacturer's software. Our experience has shown that the changes in weal and flare areas may be measured to an accuracy of ±0.05 cm2 and changes in perfusion to ±5% (Clough et al., 1998).
Assessment of histamine release in vivo
Two linear cutaneous microdialysis fibres of 2 kDa molecular mass cut off, 216 μm diameter, (Gambro model GFE 18, Gambro Dialysaten AG, Germany) were used in each subject. They were inserted 5 cm apart for a length of 20 mm at a depth of ∼0.6 – 0.8 mm into the volar surface of the forearm under topical local anaesthesia (EMLA cream; 2.5%, prilocaine, 2.5% lignocaine, Astra AB, Sweden) as previously described (Clough, 1999). After a 2 h period for recovery from local anaesthesia and trauma, the probes were perfused with sterile phosphate buffered 0.9% NaCl saline at a rate of 5 μl min−1 using a microinfusion pump (CMA/100, CMA/Microdialysis, Biotech, Luton, U.K.). Dialysate was collected every 2 min between 4 min before (baseline samples) and 20 min after injection and stored at −20°C prior to the spectrofluorimetric assay of histamine (Skov et al., 1985).
Determination of dermal mast cell numbers and histamine content
Following microdialysis, a single site in the treated area of each forearm, was infiltrated with 2% xylocaine/adrenaline and a 4 mm punch biopsy taken. Each specimen was cut in half, and each half treated as follows.
Determination of mast cell numbers
One half of the biopsy was fixed in 10% buffered formaldehyde and processed into paraffin from which 5 μm thick sections were cut. The sections were then stained immunocytochemically for human mast cell tryptase using antibody AA1 (Walls et al., 1989). Tryptase was visualised using aminoethylcarbazole (AEC) solution, counterstained with Mayers haematoxylin and the number of nucleated cells in the whole section which were tryptase-positive counted in a single blind fashion by a single observer. The area of intact dermis in each biopsy was measured using a computerized image analysis system (Colorvision 1.76 by Improvision, Coventry, U.K.) and the number of mast cells expressed per mm2.
Determination of tissue histamine
The other half of the 4-mm punch biopsy was put in a preweighed polypropylene tube with 200 μl distilled water, weighed again in order to determine the weight of the biopsy and frozen at −80°C. Just prior to histamine assay, the biopsies were thawed and frozen at −20°C six times to disrupt intact cells and release the histamine. Histamine was then measured by immunoassay (Immunotech, France). Histamine was then calculated as ng histamine mg−1 tissue.
Determination of skin thickness
The thickness of the combined epidermis and dermis was measured by echography (DermaScan C ver. 3, Cortex Technology, Denmark). Two separate scans of 2 cm2 in area were made within the treated areas of each forearm. From each scan, three measurements of skin thickness were made and the mean of the six measurements expressed as a single estimate of skin thickness in mm.
Study protocols
Subjects attended the laboratory on two occasions. At the first visit, the skin thickness of both forearms was measured. Subjects were then supplied with 0.05% clobetasol propionate ointment (Dermovate, Glaxo, Middlesex, U.K.) and base control (white soft paraffin, Adams Healthcare, Leeds, U.K.) and asked to apply a 1 cm length of ointment to an area of approximately 10×5 cm on each forearm either once 24 h before returning to the laboratory or daily for 21 days.
At the second visit of both studies, two microdialysis fibres were inserted into each forearm and codeine and histamine injected intradermally. Dialysate was collected every 2 min for 4 min before and 20 min after injection. Laser Doppler images were taken before and at 5, 10, 15 and 20 min after injection to estimate the weal and flare areas. As estimates of weal and flare areas were qualitatively similar at all time points, only the data for 10 min are cited in the results section. In the 21-day study only, a biopsy was taken from each forearm on a healthy area of skin treated by the ointments. Skin thickness measurements were repeated.
The tubes of ointment were also re-weighed from which it was calculated that the mean amounts of clobetasol propionate and white soft paraffin applied per day were 307±20 and 432±44 mg respectively.
Statistics
As the data obtained in this study were not normally distributed, results are expressed as medians (95% confidence limits). Differences between clobetasol treated and base control sites were analysed using the Wilcoxon Signed Rank Test. A significance level of P<0.05 was taken as statistically significant.
Results
Weal responses
In the study in which clobetasol was applied for 24 h only, there were no statistically significant inhibitions of weal responses. The weal areas in the clobetasol and control sites following codeine injection were 0.60 (0.33 – 0.84) cm2 (median with 95% confidence limits) and 0.89 (0.52 – 1.40) cm2 (P=0.059, n=6) respectively. The corresponding areas of the weals in response to histamine were 0.53 (0.36 – 0.76) cm2 and 0.47 (0.39 – 0.76) cm2 (P=1.00, n=6).
In the 3-week study, comparison of responses in the control and clobetasol propionate treated sites showed marked differences in the weal responses to codeine and smaller differences in those to histamine. As the data obtained at 5, 10 and 20 min were qualitatively similar, only the results obtained at 10 min are illustrated (Figure 1). At this time after codeine injection, the median weal area in the clobetasol treated arm was 0.26 (0.20 – 0.36) cm2 compared with 0.63 (0.50 – 0.95) cm2 in the control arm, a reduction of 59% (P=0.006, n=10). The corresponding values following histamine injection were 0.30 (0.24 – 0.45) cm2 and 0.41 (0.38 – 1.01) cm2 respectively, a reduction of 27% (P=0.019, n=10).
Figure 1.
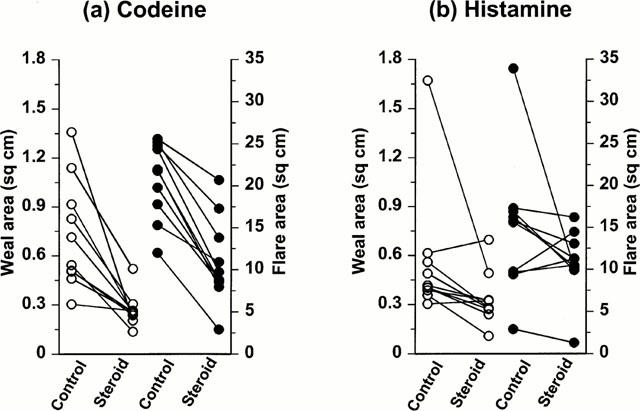
The effect of clobetasol on weal and flare responses induced by (a) codeine and (b) histamine. Areas of the volar forearm skin were treated for 21 days with either white soft paraffin (control) or 0.05% clobetasol propionate (steroid) before provocation of weal and flare responses by the intradermal injection of codeine (20 μl of 0.3 mM) or histamine (20 μl of 1 μM). Weal (open symbols) and flare (closed symbols) responses were assessed 10 min after provocation.
Flare responses
In the 24-h study, there were no statistically significant inhibitions of flare responses. The flare areas in the clobetasol and control sites following codeine injection were 20.8 (16.2 – 4.8) cm2 and 24.2 (18.0 – 29.0) cm2 (P=0.402, n=6) respectively. The corresponding areas of the flares in response to histamine were 16.7 (11.7 – 25.7) cm2 and 23.9 (13.8 – 30.6) cm2 (P=0.295, n=6).
In the 3-week study, codeine-induced flare responses were reduced by clobetasol (Figure 1), the median flare area at 10 min in the clobetasol treated arm being 9.3 (8.2 – 4.7) cm2 compared with 21.9 (17.6 – 24.6) cm2 in the control arm, a reduction of 58% (P<0.006, n=10). The corresponding values following histamine injection were 10.8 (7.9 – 13.3) cm2 compared with 15.7 (9.5 – 21.7) cm2 respectively. The apparent reduction of 31% was not statistically significant (P=0.126).
Codeine-induced histamine release
Histamine released into the dermis following codeine injection was recovered using microdialysis. There were no differences in the time course of histamine release between the control and clobetasol propionate treated sites in both the 24-h and 3-week studies, peak releases occurring at 2 – 4 min.
In the 24-h study, clobetasol treatment did not significantly inhibit codeine-induced histamine release, the median concentrations for steroid treated and control sites being 1.74 (0.72 – 7.14) μM and 1.41 (0.70 – 3.65) μM (P=0.059). In the 3-week study, peak histamine levels (Figure 2) were reduced by clobetasol treatment to a median concentration of 0.67 (0.34 – 1.43) μM from control levels of 1.20 (0.95 – 2.56) μM (44% reduction, P=0.022, n=7).
Figure 2.
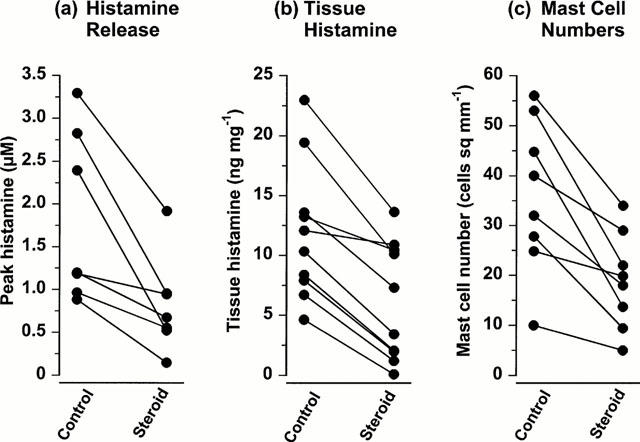
The effect of clobetasol on codeine-induced histamine release, tissue histamine levels and dermal mast cell numbers. Areas of the volar forearm skin were treated for 21 days with either white soft paraffin (control) or 0.05% clobetasol propionate (steroid). (a) The peak histamine concentration recovered by microdialysis following intradermal injection of codeine (20 μl of 0.3 mM). (b) Tissue histamine levels and (c) mast cell numbers in biopsies taken at 21 days.
Dermal mast cell numbers and histamine content
The mast cells present in the dermis, the majority of which appeared to be clustered around the blood vessels, were fewer at the site treated for 3 weeks with clobetasol, 18.9 (11.5 – 6.9) cells mm−2 compared with 36.0 (24.8 – 48.9) cells mm−2 in the control arm (Figure 2). The reduction in mast cell numbers of 47% was statistically significant (P<0.014, n=8). In the same biopsies, the median tissue histamine content was 5.37 (1.98 – 10.44) ng histamine mg−1 of tissue at the clobetasol treated site while at the control site it was 11.20 (7.90 – 16.33) ng histamine mg−1 of tissue, a reduction of 52% (P=0.006, n=10) (Figure 2).
Skin thickness
The combined dermal and epidermal thickness was reduced by 15% by 3 weeks treatment with clobetasol (Figure 3) from an original median thickness of 1.49 (1.22 – 1.89) mm to 1.27 (1.08 – 1.70) mm (P=0.014, n=8). There was no significant change in the thickness of the skin in the arm treated with base, the values at the beginning and end of treatment being 1.49 (1.24 – 1.85) mm and 1.51 (1.34 – 1.96) mm respectively (P=0.272, n=8).
Figure 3.
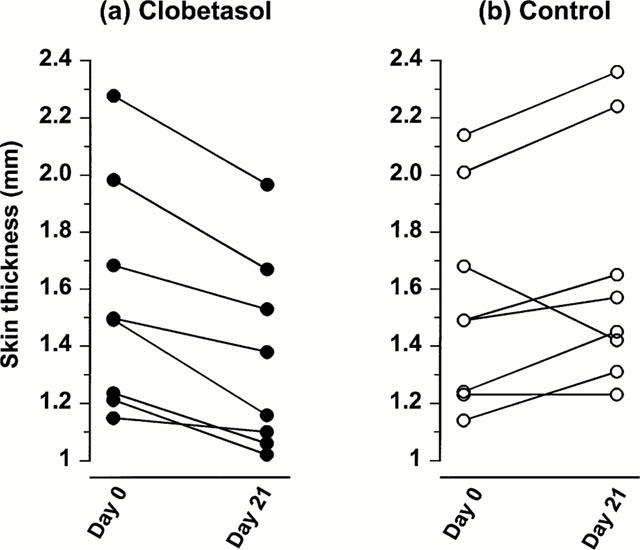
The effect of clobetasol on skin thickness. Areas of the volar forearm skin were treated for 21 days with either white soft paraffin (control) or 0.05% clobetasol propionate. Measurements of skin thickness were taken in both arms before (day 0) and at the 21st day of treatment.
Discussion
The present studies demonstrate that a single application of clobetasol 24 h before test had no significant effect on weal and flare responses induced by either codeine or histamine. In contrast, treatment with clobetasol for 3 weeks caused a marked reduction in the responses to codeine and a smaller inhibition to those induced by histamine. The major mechanism underlying this reduction is a reduction in the number of dermal mast cells present within the skin with a consequential reduction in total histamine. A reduction of the responsiveness of the vasculature to histamine appears to be a minor contributory factor.
Twenty-four hours was chosen as the earliest time at which to test clobetasol as the majority of its actions are a consequence of steroid-induced modification of transcription, the effects of which take several hours to become manifest (Barnes, 1998). Also, a ‘skin blanching' effect, indicative of an effect on the vasculature, is seen at this time. While skin blanching may be observed by eye and by colorimetry, it was not detectable by scanning laser Doppler imaging using the configuration optimal for detecting increases in skin blood flow (Huang et al., 1996). However, clobetasol did not change the ability of the vasculature to respond to vasodilator stimuli at this time as evidenced by the unchanged responsiveness to histamine. Also, the observations that neither the weal and flare response nor histamine release was inhibited confirms that the steroid did not inhibit mast cell degranulation (Cohan et al., 1989; Schleimer et al., 1983).
The ability of the glucocorticoid, when applied topically for 3 weeks, to reduce the size of weal and flare response confirms previous reports (Olson et al., 1990; Pipkorn et al., 1989). To investigate the mast cell dependent mechanisms of this reduction, we used two approaches, microdialysis and biopsy. In the microdialysis study, the time course of histamine release, both in the presence and absence of glucocorticoid treatment, was similar to that reported previously (Petersen et al., 1997). However, the median peak histamine concentration was 44% lower in the clobetasol treated arm compared with control. In the biopsy study, the number of dermal mast cells was 47% lower, and the tissue histamine 52% lower in clobetasol treated skin compared with control. The corresponding reductions in median weal and flare areas were 59 and 59% respectively. Taken together, these data suggest that a reduction in the amount of histamine released in response to codeine is a major determinant in inhibiting the weal and flare response. Furthermore, the reduced histamine release is a consequence of reduced mast cell numbers rather than an inhibition of degranulation.
The failure of glucocorticoids to inhibit degranulation of human mast cells confirms previous observations (Cohan et al., 1989; Lavker & Schechter, 1985; Pipkorn et al., 1989; Schleimer et al., 1983). However, in rodent mast cells, where most mechanistic studies have been performed, a clear inhibition of mediator release by glucocorticoids is seen (Church et al., 1972; Daeron et al., 1982; Marquardt & Wasserman, 1983). The most likely explanation for this difference is that rats and mice are ‘cortisone sensitive' animals whilst humans, like other primates and the guinea-pig, are ‘cortisone resistant' (Shewell & Long, 1956).
The reduction of mast cell numbers and histamine levels in the skin confirms previous studies (Lavker & Schechter, 1985; Pipkorn et al., 1989). This reduction in mast cell numbers along with tissue histamine content could result from one of three possible mechanisms; a reduction in mast cell precursor recruitment, a reduction in factors present in the dermis required for mast cell maturation and histamine synthesis and an increase in apoptosis. Of these, a reduction of histamine synthesis by suppressing histidine decarboxylase activity (Telford & West, 1961) would seem unlikely as the calculated histamine content per mast cell was similar, 2.84 and 3.11 pg cell−1, in biopsies from steroid treated and control skin respectively. A more likely explanation is the reduction by glucocorticoids of the local production of mast cell growth factors. One possible factor is stem cell factor, a cytokine crucial for mast cell recruitment, development and survival (Castells et al., 1996; Costa et al., 1996; Demitsu et al., 1999; Finotto et al., 1997) whose NF-κB-stimulated transcription by dermal fibroblasts is inhibited by glucocorticoids of (Barnes & Larin, 1997). Another possibility is IL-4, depletion of which by glucocorticoids has been shown to increase apoptosis of cultured murine mast cells (Yoshikawa et al., 1999).
When considering the effects of steroids on the vasculature, it is essential to distinguish between the direct and indirect effects of mast cell mediators. The direct effects of histamine at the site of its injection are an initial vasodilatation followed by the development of a weal. The latter results from a direct effect of histamine in causing endothelial cell contraction to allow exudation of plasma proteins into the extravascular space (Moy et al., 2000) which may be susceptible to inhibition by steroids (Hossmann et al., 1983; Perretti & Ahluwalia, 2000). However, there is also evidence that tolerance develops to these effects of steroids following 6 – 10 days continuous treatment (Singh & Singh, 1986; Singh et al., 1996). Our data showed a modest, but statistically significant inhibitory effect of clobetasol at 21 days.
In contrast to the weal, the flare is an indirect effect of histamine, the more widespread vasodilator response being mediated by neuropeptides released during axon reflexes stimulated by histamine (Petersen et al., 1997). The failure of clobetasol to cause a statistically significant inhibition of the flare response to histamine indicates that, in this study, glucocorticoids did not effect either activation of sensory nerves by histamine or the responsiveness of the vasculature to vasodilator neuropeptides.
Measurement of the combined dermal and epidermal thickness revealed a reduction of 15% with 21 days daily treatment with the potent topical glucocorticoid, clobetasol. Informal measurements made on the subjects 2 – 3 weeks later, showed that this acute thinning was reversed on cessation of treatment. These results confirm previous data that show a substantial thinning of the skin after even a relatively short period of corticosteroid treatment (Haapasaari et al., 1998; Schwartz et al., 1994) and illustrate the need for care to be used when using potent corticosteroids in dermatology.
These studies indicate that the primary mechanism by which prolonged treatment with glucocorticoids inhibit the weal and flare in humans is by reducing the number of mast cells in the skin although an inhibitory effect on the responsiveness of the vasculature is a contributory factor.
Acknowledgments
Z.A. Cole was supported by a British Pharmacological Society award for her Intercalated BSc. G.F. Clough was supported by The Wellcome Trust (057474/Z/99).
Abbreviations
- AEC
aminoethylcarbazole
- SLDI
scanning laser Doppler imaging
References
- ANDERSSON M., PIPKORN U. Inhibition of the dermal immediate allergic reaction through prolonged treatment with topical glucocorticosteroids. J. Allergy Clin. Immunol. 1987;79:345–349. doi: 10.1016/0091-6749(87)90153-9. [DOI] [PubMed] [Google Scholar]
- ATKINS P.C., SCHWARTZ L.B., ADKINSON N.F., VON ALLMEN C., VALENZANO M., ZWEIMAN B. In vivo antigen-induced cutaneous mediator release: simultaneous comparisons of histamine, tryptase, and prostaglandin D2 release and the effect of oral corticosteroid administration. J. Allergy Clin. Immunol. 1990;86:360–370. doi: 10.1016/s0091-6749(05)80099-5. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., LARIN M. Mechanisms of disease–Nuclear factor-kappa b – A pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BOOIJ NOORD H., ORIE N.G.M., VRIES K. Immediate and late bronchial obstructive reactions to inhalation of house dust and protective effects of disodium cromoglycate and prednisolone. J. Allergy Clin. Immunol. 1971;48:344–354. doi: 10.1016/0091-6749(71)90080-7. [DOI] [PubMed] [Google Scholar]
- CASTELLS M.C., FRIEND D.S., BUNNELL C.A., HU X.Z., KRAUS M., OSTEEN R.T., AUSTEN K.F. The presence of membrane-bound stem-cell factor on highly immature nonmetachromatic mast-cells in the peripheral-blood of a patient with aggressive systemic mastocytosis. J. Allergy Clin. Immunol. 1996;98:831–840. doi: 10.1016/s0091-6749(96)70133-1. [DOI] [PubMed] [Google Scholar]
- CHURCH M.K., COLLIER H.O.J., JAMES G.W.L. The inhibition by dexamethasone and disodium cromoglycate of anaphylactic bronchoconstriction in the rat. Br. J. Pharmacol. 1972;46:56–65. doi: 10.1111/j.1476-5381.1972.tb06848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOUGH G.F. Role of nitric oxide in the regulation of microvascular perfusion in human skin in vivo. J. Physiol. 1999;516:549–557. doi: 10.1111/j.1469-7793.1999.0549v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLOUGH G.F., BENNETT A.R., CHURCH M.K. Effects of H-1 antagonists on the cutaneous vascular response to histamine and bradykinin: a study using scanning laser Doppler imaging. Br. J. Dermatol. 1998;138:806–814. doi: 10.1046/j.1365-2133.1998.02217.x. [DOI] [PubMed] [Google Scholar]
- COHAN V.L., UNDEM B.J., FOX C.C., ADKINSON N.F., LICHTENSTEIN L.M., SCHLEIMER R.P. Dexamethasone does not inhibit the release of mediators from human mast cells residing in airway, intestine or skin. Am. Rev. Respir. Dis. 1989;140:951–954. doi: 10.1164/ajrccm/140.4.951. [DOI] [PubMed] [Google Scholar]
- COSTA J.J., DEMETRI G.D., HARRIST T.J., DVORAK A.M., HAYES D.F., MERICA E.A., MENCHACA D.M., GRINGERI A.J., SCHWARTZ L.B., GALLI S.J. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J. Exp. Med. 1996;183:2681–2686. doi: 10.1084/jem.183.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAERON M., STERK A.R., HIRATA F., ISHIZAKA T. Biochemical analysis of glucocorticoid-induced inhibition of IgE-mediated histamine release from mouse mast cells. J. Immunol. 1982;129:1212–1218. [PubMed] [Google Scholar]
- DEMITSU T., KIYOSAWA T., KAKURAI M., MURATA S., YAOITA H. Local injection of recombinant human stem cell factor promotes human skin mast cell survival and neurofibroma cell proliferation in the transplanted neurofibroma in nude mice. Arch. Dermatol. Res. 1999;291:318–324. doi: 10.1007/s004030050416. [DOI] [PubMed] [Google Scholar]
- DUNSKY E.H., ZWEIMAN B., FISCHLER E., LEVY D.A. Early effects of corticosteroids on basophils, leukocyte histamine, and tissue histamine. J. Allergy Clin. Immunol. 1979;63:426–432. doi: 10.1016/0091-6749(79)90217-3. [DOI] [PubMed] [Google Scholar]
- FINOTTO S., MEKORI Y.A., METCALFE D.D. Glucocorticoids decrease tissue mast cell number by reducing the production of the c-kit ligand, stem cell factor, by resident cells: in vitro and in vivo evidence in murine systems. J. Clin. Invest. 1997;99:1721–1728. doi: 10.1172/JCI119336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSMITH P., BUNKER C., LESLIE T., FOREMAN J., DOWD P.M. The effect of topical steroid on the actions of vasoconstrictor and vasodilator peptides in human skin. Skin Pharmacol. 1996;9:289–297. doi: 10.1159/000211427. [DOI] [PubMed] [Google Scholar]
- HAAPASAARI K., ROSSI O., RISTELI J., OIKARINEN A. Effects of long term inhaled corticosteroids on skin collagen synthesis and thickness in asthmatic patients. Eur. Respir. J. 1998;11:139–143. doi: 10.1183/09031936.98.11010139. [DOI] [PubMed] [Google Scholar]
- HOSSMANN K.A., HURTER T., OSCHLIES U. The effect of dexamethasone on serum protein extravasation and edema development in experimental brain tumors of cat. Acta Neuropathol. (Berlin) 1983;60:223–231. doi: 10.1007/BF00691870. [DOI] [PubMed] [Google Scholar]
- HUANG X., LU L., GUSH R.J., BOGGETT D.M.A new fast high resolution laser Doppler imager for clinical and research use Proceedings of the 6th World Congress for Microcirculation 1996Bologna: Monduzzi Editore; 115–119.ed. Messmer, K. & Kubler, W.M. pp [Google Scholar]
- LAVKER R.M., SCHECHTER N.M. Cutaneous mast cell depletion results from topical corticosteroid usage. J. Immunol. 1985;135:2368–2373. [PubMed] [Google Scholar]
- LOPEZ-CAMPOS C., RINCON-CASTANEDA C.B., CANO-RIOS P., MARTINEZ-ORDAZ V.A., VELASCO-RODRIGUEZ V.M. Is the histamine skin test inhibited by prednisone. Arch. Med. Res. 1998;29:63–65. [PubMed] [Google Scholar]
- MARQUARDT D.L., WASSERMAN S.I. Modulation of rat serosal mast-cell biochemistry by in vivo dexamethasone administration. J. Immunol. 1983;131:934–939. [PubMed] [Google Scholar]
- MOY A.B., WINTER M., KAMATH A., BLACKWELL K., REYES G., GIAEVER I., KEESE C., SHASBY D.M. Histamine alters endothelial barrier function at cell-cell and cell-matrix sites. Am. J. Physiol Lung Cell Mol. Physiol. 2000;278:L888–L898. doi: 10.1152/ajplung.2000.278.5.L888. [DOI] [PubMed] [Google Scholar]
- OLSON R., KARPINK M.H., SHELANSKI S., ATKINS P.C., ZWEIMAN B. Skin reactivity to codeine and histamine during prolonged corticosteroid therapy. J. Allergy Clin. Immunol. 1990;86:153–159. doi: 10.1016/s0091-6749(05)80060-0. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., AHLUWALIA A. The microcirculation and inflammation: site of action for glucocorticoids. Microcirculation. 2000;7:147–161. [PubMed] [Google Scholar]
- PETERSEN L.J., CHURCH M.K., SKOV P.S. Histamine is released in the weal but not the flare following challenge of human skin in vivo: A microdialysis study. Clin. Exp. Allergy. 1997;27:284–295. doi: 10.1046/j.1365-2222.1997.d01-502.x. [DOI] [PubMed] [Google Scholar]
- PIPKORN U., HAMMARLUND A., ENERBÄCH L. Prolonged treatment with topical glucocorticoids results in an inhibition of the allergen-induced weal-and-flare response and a reduction in skin mast cell numbers and histamine content. Clin. Exp. Allergy. 1989;19:19–25. doi: 10.1111/j.1365-2222.1989.tb02338.x. [DOI] [PubMed] [Google Scholar]
- SCHLEIMER R.P., SCHULMAN E.S., MACGLASHAN D.W., JR, PETERS S.P., HAYES E.C., ADAMS G.K., III, LICHTENSTEIN L.M., ADKINSON N.F., JR Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J. Clin. Invest. 1983;71:1830–1835. doi: 10.1172/JCI110938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ E., MEZICK J.A., GENDIMENICO G.J., KLIGMAN L.H. In Vivo prevention of corticosteroid-induced skin atrophy by tretinoin in the hairless mouse is accompanied by modulation of collagen, glycosaminoglycans, and fibronectin. J. Invest. Dermatol. 1994;102:241–246. doi: 10.1111/1523-1747.ep12371770. [DOI] [PubMed] [Google Scholar]
- SHEWELL J., LONG D.A. A species difference with regard to the effect of cortisone acetate on the body weight, γ-globulin and circulating antitoxin levels. J. Hyg., Camb. 1956;54:452–460. doi: 10.1017/s0022172400044739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH G., SINGH P.K. Tachyphylaxis to topical steroid measured by histamine-induced wheal suppression. Int. J. Dermatol. 1986;25:324–326. doi: 10.1111/j.1365-4362.1986.tb02258.x. [DOI] [PubMed] [Google Scholar]
- SINGH S., GUPTA A., PANDEY S.S., SINGH G. Tachyphylaxis to histamine-induced weal suppression by topical 0.05% clobetasol propionate in normal versus croton oil-induced dermatitic skin. Dermatology. 1996;193:121–123. doi: 10.1159/000246225. [DOI] [PubMed] [Google Scholar]
- SKOV P.S., MOSEBECH H., NORN S., WEEKE B. Sensitive glass microfibre based-histamine analysis for allergy testing in washed blood cells. Allergy. 1985;40:213–218. doi: 10.1111/j.1398-9995.1985.tb00219.x. [DOI] [PubMed] [Google Scholar]
- SLOTT R.I., ZWEIMAN B. A controlled study of the effect of corticosteroids on immediate skin test reactivity. J. Allergy Clin. Immunol. 1974;54:229–234. doi: 10.1016/0091-6749(74)90065-7. [DOI] [PubMed] [Google Scholar]
- STAHLE M., HÄGERMARK O. Effects of topically applied Clobetasol 17 propionate on histamine release in human skin. Acta Derm. Venereol. 1984;64:239–242. [PubMed] [Google Scholar]
- TELFORD J.M., WEST G.B. Some effects of corticosteroids on metabolism of histamine and 5-hydroxytryptamine in rat. Br. J. Pharmacol. 1961;16:360–368. doi: 10.1111/j.1476-5381.1961.tb01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLS A.F., BENNETT A.R., MCBRIDE H.M., GLENNIE M.J., HOLGATE S.T., CHURCH M.K. Human mast cell tryptase: a biochemical marker for mast cell activation. Biochem. Soc. Trans. 1989;17:728–729. [Google Scholar]
- YOSHIKAWA H., NAKAJIMA Y., TASAKA K. Glucocorticoid suppresses autocrine survival of mast cells by inhibiting IL-4 production and ICAM-1 expression. J. Immunol. 1999;162:6162–6170. [PubMed] [Google Scholar]


