Abstract
Oxidative mechanisms have been implicated in neonatal cardiomyocyte hypertrophy. We and others have shown that a HMG-CoA reductase inhibitor preserves endogenous antioxidant enzyme activity and inhibits cardiac hypertrophy in vivo. We therefore have examined whether noradrenaline (NA) induces the generation of reactive oxygen species (ROS) during its induction of neonatal cardiomyocyte hypertrophy and whether simvastatin, a HMG-CoA reductase inhibitor, attenuates ROS production and thus NA-induced hypertrophy of cardiomyocytes.
NA increased the intracellular ROS levels in a concentration-dependent manner. This increase of ROS was significantly inhibited by simvastatin and catalase. Prazosin partially suppressed NA-induced increase of ROS and beating, while preincubation with both prazosin and propranolol completely abolished NA-evoked increase of ROS and beating. Simvastatin did not affect NA-induced increase of beating.
The NA-induced increase of protein content was partially suppressed by prazosin and completely abolished by preincubation with both prazosin and propranolol. Simvastatin inhibited the increase of NA-induced increase of RNA content and [3H]-leucine incorporation in a concentration-dependent manner. Mevalonic acid (MVA) reversed the inhibition of NA-induced RNA and protein increase by simvastatin. Catalase also inhibited the NA-induced increase of RNA and protein.
We conclude that the inhibitory effects of simvastatin on myocyte hypertrophy were associated with its antioxidant effects and inhibition of MVA-metabolism pathway in neonatal rat cardiomyocytes. NA-induced increases of intracellular ROS and cardiomyocyte hypertrophy requires both α and β adrenoceptors activation in neonatal rat cardiomyocytes. The increases of ROS induced by NA is required for hypertrophy.
Keywords: Antioxidants, cardiomyocyte hypertrophy, noradrenaline, reactive oxygen species, signal transduction, simvastatin
Introduction
Cardiac hypertrophy is an adaptive remodelling process of cardiomyocytes to haemodynamic overload from various causes. Unfortunately it is associated with an increased risk of arrhythmia (McLenachan et al., 1987) and development of congestive heart failure (Levy et al., 1990). Therefore, the elucidation of the mechanisms leading to cardiac hypertrophy is important for preventing these harmful consequences.
Recent studies have shown that angiotensin II, tumour necrosis factor-α and ouabain stimulate the production of intracellular reactive oxygen species (ROS) in cultured rat neonatal cardiomyocytes (Nakamura et al., 1998; Xie et al., 1999). The three stimuli induced ROS-dependent cardiomyocyte hypertrophy. Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase have antioxidant effects (Suzumura et al., 1999; Yamamoto et al., 1998) and can preserve endogenous antioxidant enzyme activity in the aorta of rabbits fed a high cholesterol diet (Chen et al., 1997). According to our previous results simvastatin, a HMG-CoA inhibitor, inhibits cardiac hypertrophy in rats with aortic stenosis (Luo et al., 1999a,1999b). Taken together these results suggest that ROS may play an important role in signal transduction underlying the cardiomyocyte hypertrophy. Thus, we hypothesized that abnormal ROS accumulation caused by the hypertrophic factors leads to cardiomyocyte hypertrophy. Therefore, we have examined whether noradrenaline, an important cardiac hypertrophy promoting substance, induces hypertrophic growth of the rat neonatal cardiomyocytes via an intracellular signalling pathway involving ROS. We have also examined effects of simvastatin on NA-induced ROS production and hypertrophy of the cultured neonatal cardiomyocytes.
Methods
Materials
Simvastatin was purchased from Merck, Sharp & Dohme Co (Hangzhou, China). Because cardiomyocytes lack lactonases to process simvastatin to its active form, simvastatin was chemically activated before use as previously described (Blum, 1994; Gerson et al., 1989). Transferrin, insulin, bromodeoxyuridine (BrdU), noradrenaline (NA), catalase, mevalonic acid, deoxyribonuclease (DNAase) I, prazosin, propranalol and [3H]-leucine were from Sigma Chemical Co. (St. Louis, MO, U.S.A.); 2′,7′-dichlorofluorescin diacetate (DCF-DA) and propidium iodide (PI) were from Molecular Probes Inc (Eugene, OR, U.S.A.). Medium 199 and foetal bovine serum (FBS) were from GIBCO BRL (Gaithersburg, MD, U.S.A.).
Cell culture
Primary cultures of cardiomyocytes were prepared from the ventricles of neonatal Sprague-Dawley rats by the method of Simpson (1985). After dissociation of the cardiac tissue with trypsin, cells were preplated for 2 h into 100-mm culture dishes in medium 199 with 10% FBS and maintained at 37°C in humidified air with 5% CO2 to reduce the number of non-myocyte cells. Cells that were not attached to the preplated dishes were plated onto 6-well culture plates (Corning) at a density of 1×103 cells mm−2. Non-myocytes on the cultures were limited to 10% of the total cell number by inclusion of BrdU (0.1 mmol l−1) in the medium (Simpson, 1985). The culture medium was replaced after 24 h with serum-free medium consisting of medium 199, transferring (10 μg ml−1), insulin (10 μg ml−1), vitamin B12 1.5 μmol l−1, and BrdU (0.1 mmol l−1). On culture day 4, the myocytes were treated with NA, simvastatin, catalase, prazosin, propranalol or their diluent, as indicated.
Assessment of beating frequencies
Beating frequencies were visually recorded in each culture according to the method of Clark et al. (1991). A random field was selected near the centre of each culture and a typical rhythmically contracting cell was identified. The elapsed time for 20 beats was determined with a stopwatch and converted to beats per minute. Beating was recorded on a daily basis in only a single field of each culture. Multiple readings from the same culture could have produced biased estimates of beating frequencies due to the high degree of cell contact, and essentially identical rates were recorded from multiple cells from the same culture. If no contractions were observed in the randomly selected field over a 30-s observation period, the culture was recorded as negative for beating.
Analysis of dichlorofluorescein fluorescence
The fluorescent probe, 2′,7′-dichlorofluorescin diacetate (DCF-DA), was used for the assessment of intracellular ROS formation in cultured rat cardiomyocytes. This assay is a reliable method for the measurement of intracellular ROS such as hydrogen peroxide (H2O2), hydroxyl radical, and hydroperoxides (Cathcart et al., 1983; Zhu et al., 1994). DCF-DA was dissolved in absolute ethanol at a concentration of 5 mmol l−1. On culture day 4, cultured rat cardiomyocytes were washed with Hanks' solution, and then NA (0.2 to 20 μmol l−1), simvastatin (10 – 1000 nmol l−1)+ NA (20 μmol l−1), catalase (200 u ml−1) +NA (20 μmol l−1), prazosin (20 μmol l−1) + NA (20 μmol l−1), or diluent (control) was administered simultaneously with DCF-DA (5 μmol l−1) in Hanks' solution. After incubation at 37°C for 1 h, cardiomyocytes were washed with Hanks' solution. Fluorescence intensity and images were obtained with laser confocal microscopy (MERIDIAN, ACAS 570). Randomly selected 2 fields in each well were examined for each condition.
Analysis of propidium iodide (PI) fluorescence
PI is a fluorescent dye that can intercalate specifically into double-helical regions of both DNA and RNA. To apply this to RNA, we stained RNA with PI after DNA digestion with deoxyribonuclease (DNAase) I. PI does not stain total cellular RNA, but only that fraction of RNA that is in double-stranded conformation. This fraction can be defined as the RNA that binds intercalating dyes, and changes in the proportion of double-stranded RNA are related to the total RNA (Frankfurt, 1980).
The cultured neonatal rat cardiac myocytes were treated with NA (20 μmol l−1), simvastatin (1 – 1000 nmol l−1) +NA (20 μmol l−1), catalase (200 u ml−1) +NA (20 μmol l−1), or diluent (control) from culture day 4 to 7. On the culture day 7, the cells were washed with Hanks' solution and fixed with 75% ethanol for 10 min. Ethanol-fixed cells were rinsed in Hanks' solution and incubated in solution containing 1 mg ml−1 DNAase, 0.25 mol l−1 sucrose, 5 mmol l−1 MgCl2, 20 mmol l−1 Tris-HCl (pH 6.5) for 40 min at 36°C. After incubation with enzymes, 1 ml of Hanks' solution containing 0.05 mg ml−1 PI were added to each well and kept for 30 min before laser confocal microscopy analysis. Randomly selected 2 fields in each well were examined for each condition.
[3H]-leucine incorporation
To examine the inhibitory effects of simvastatin on myocyte protein synthesis induced by NA, the [3H]-leucine incorporation was measured as described previously (Thaik et al., 1995). Cultured myocytes were treated with NA (20 μmol l−1), simvastatin (1 – 1000 nmol l−1) +NA (20 μmol l−1), catalase (200 u ml−1) +NA (20 μmol l−1), or diluent (control) and coincubated with [3H]-leucine (1.5 Ci ml−1) from culture day 4 to 7. At the end of experiment, the cells were washed with Hanks' solution and scraped off the well, and then treated with 5% trichloroacetic acid at 4°C for 1 h to precipitate the protein. The precipitates were then dissolved in NaOH (0.1 mol l−1). Aliquots were counted with a scintillation counter.
Protein content
Cultured myocytes were treated with NA (20 μmol l−1), simvastatin (1 – 1000 nmol l−1) +NA (20 μmol l−1), catalase (200 u ml−1) +NA (20 μmol l−1), or diluent (control) from culture day 4 to 7. The cells were washed with Hanks' solution and scraped off the well and then treated with 5% trichloroacetic acid as described above. The precipitates were dissolved in NaOH (0.1 mol l−1). The protein content was measured by the Coomassie blue method (Khalid & Ashraf, 1993).
Statistical analysis
For the comparison of group means, one-way ANOVA and Student-Newman-Keuls tests were used as appropriate. A P-value <0.05 was considered statistically significant.
Results
Inhibitory effects of simvastatin on NA-induced increase of PI fluorescence
To determine whether simvastatin inhibited NA-induced RNA synthesis, cardiomyocytes treated with NA (20 μmol l−1) and simvastatin (1 – 1000 nmol l−1) for 3 days were incubated with PI. Simvastatin significantly inhibited the NA-induced increase of DNAas-treated PI fluorescence in a concentration-dependent manner compared with that in control cells (Figure 1A,B), indicating that simvastatin inhibited the RNA synthesis of NA-treated cardiomyocytes. The inhibitory effect of simvastatin (100 nmol l−1) on NA-induced RNA synthesis of cardiomyocytes was reversed by 100 μmol l−1 mevalonic acid (MVA). To examine whether the ROS mediates NA-induced RNA synthesis of cardiomyocytes, we tested the effect of catalase on RNA synthesis of cardiomyocyte. Catalase (200 u ml−1) significantly reduced NA (20 μmol l−1)-induced increase of PI fluorescence of cardiomyocytes. Simvastatin (1000 nmol l−1) alone exerted no effect on PI fluorescence intensity. These results indicate that ROS mediate NA-induced RNA synthesis of cardiomyocytes.
Figure 1.
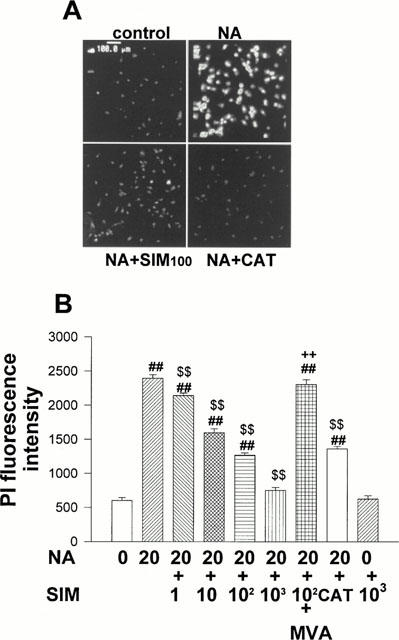
Inhibitory effects of simvastatin on PI fluorescence increase induced by NA. On culture day 4, cultured cardiomyocytes were treated with NA (20 μmol l−1), NA+SIM (1 – 1000 nmol l−1), NA+SIM+MVA (100 μmol l−1), NA+catalase (200 u ml−1) or diluent without NA (control). After 3 days of treatment, cardiomyocytes were incubated with PI and fluorescence intensity was measured by laser confocal microscopy. (A) Representative cardiomyocytes observed by laser confocal microscopy. (B) Histograms representing the mean±s.e.mean obtained from four separate experiments in which eight visual fields were quantified. ##P<0.01 vs control. $$P<0.01 vs NA. ++P<0.01 vs NA+SIM.
Inhibitory effects of simvastatin on NA-induced [3H]-leucine incorporation
To investigate whether simvastatin inhibits NA-induced protein synthesis of cardiomyocytes, the incorporation of [3H]-leucine was assayed. NA (20 μmol l−1) significantly increased [3H]-leucine incorporation compared with the control group, and the increase was inhibited by simvastatin (1 – 1000 nmol l−1) in a concentration-dependent manner. Catalase (200 u ml−1) also reduced the NA-induced incorporation of [3H]-leucine in cardiomyocytes. MVA (100 μmol l−1) reversed the inhibitory effect of simvastatin (100 nmol l−1) on protein synthesis (P<0.01, Figure 2A). Simvastatin (1000 nmol l−1) alone exerted no effect on protein synthesis.
Figure 2.
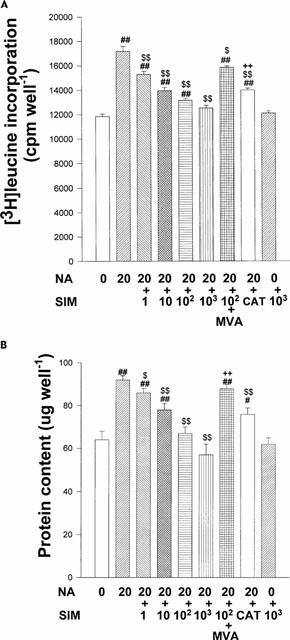
(A) Inhibitory effects of simvastatin on NA- induced increase in [3H]-leucine incorporation. On culture day 4, cultured cardiomyocytes were treated with NA (20 μmol l−1), NA+SIM (1 – 1000 nmol l−1), NA+SIM+MVA (100 μmol l−1), NA+catalase (200 u ml−1) or diluent without NA (control). After 3 days of treatment, incorporated [3H]-leucine was counted. Each point is mean±s.e.mean obtained from four separate experiments. ##P<0.01 vs control. $P<0.05, $$P<0.01 vs NA. ++P<0.01 vs NA+SIM. (B) Inhibitory effects of simvastatin on increase in protein content induced by NA. On culture day 4, cultured cardiomyocytes were treated with NA (20 μmol l−1), NA+SIM (1 – 1000 nmol l−1), NA+SIM+MVA (100 μmol l−1), NA+catalase (200 u ml−1) or diluent without NA (control). After 3 days of treatment, protein content was measured. Each point is mean±s.e.mean obtained from four separate experiments. #P<0.05, ##P<0.01 vs control. $P<0.05, $$P<0.01 vs NA. ++P<0.01 vs NA+SIM.
Inhibitory effects of simvastatin on NA-induced increase in protein content
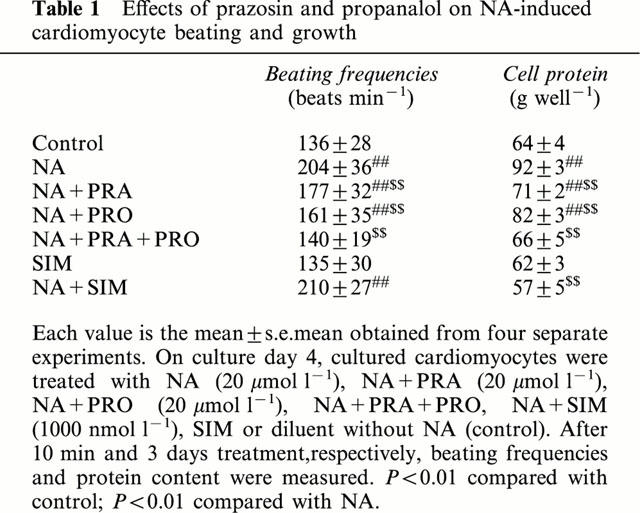
The protein content in NA (20 μmol l−1)-treated cardiomyocytes was significantly increased compared with control group (P<0.01). Prazosin (20 μmol l−1) and propranalol (20 μmol l−1) partially inhibited increase of protein content evoked by NA respectively and preincubation with both prazosin and propranalol completely abolished the NA-induced increase of protein content in the cultured neonatal cardiomyocytes (Table 1). These results suggested that NA induces cardiomyocyte hypertrophy through both α- and β-adrenergic stimulation. Simvastatin (1 – 1000 nmol l−1) and catalase (200 u ml−1) reduced the increase of protein content in NA-treated cardiomyocytes. MVA (100 μmol l−1) reversed the effect of simvastatin (100 nmol l−1) on protein content (Figure 2B). Simvastatin (1000 nmol l−1) alone exerted no effect on protein content.
Table 1.
Effects of prazosin and propanalol on NA-induced cardiomyocyte beating and growth
Concentration-dependent increase in DCF-DA fluorescence in NA-treated cardiomyocytes
To assay whether NA stimulates intracellular ROS production, cardiomyocytes treated with NA (0.2 – 20 μmol l−1, 1 h) were incubated with DCF-DA (5 mol l−1). NA significantly increased DCF-DA fluorescence in a concentration-dependent manner compared with that in controls (Figure 3A,B). NA-induced production of ROS was partially inhibited by prazosin (20 μmol l−1) and propranalol (20 μmol l−1) and completely abolished by preincubation with both prazosin and propranalol in the cultured neonatal cardiomyocytes. These data indicate that NA induces production of ROS in cardiac myocytes through both α- and β-adrenergic stimulation. Although DCF-DA is oxidized by both H2O2 and other peroxides, the significant inhibition of the fluorescence signal of NA-treated neonatal cardiomyocytes by addition of catalase (200 u ml−1) indicates that the fluorescence signal evoked by NA was predominantly derived from H2O2.
Figure 3.
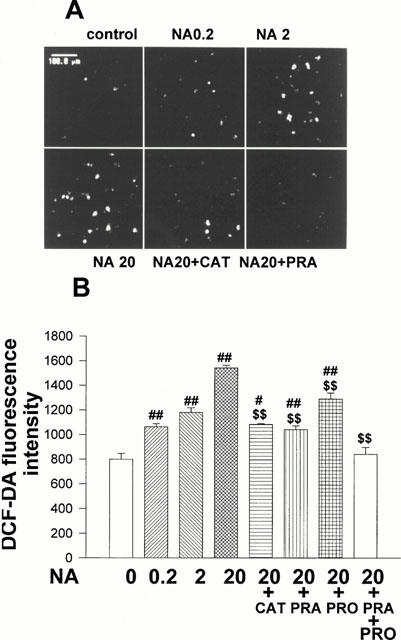
Concentration-dependent increase in DCF-DA fluorescence by NA. On culture day 4, cultured cardiomyocytes were treated with NA (0.2 – 20 μmol l−1), NA (20 μmol l−1)+catalase (200 u ml−1), NA+PRA (20 μmol l−1), NA+PRO (20 μmol l−1), NA+PRA+PRO or diluent without NA (control) and simultaneously with DCF-DA (5 μmol l−1). After 1 h of incubation, fluorescence intensity was measured by laser confocal microscopy. (A) Representative living cardiomyocytes observed by laser confocal microscopy. (B) Histograms representing the mean±s.e.mean obtained from four separate experiments in which eight visual fields were quantified. #P<0.05, ##P<0.01 vs control. $$P<0.01 vs NA (20 μmol l−1).
Inhibitory effects of simvastatin on NA-induced DCF-DA fluorescence
Since ROS are involved in NA-induced cardiomyocyte hypertrophy, we examined whether simvastatin reduces increase of NA induced DCF-DA fluorescence in cardiomyocytes. Cardiomyocytes treated with NA (20 μmol l−1) and simvastatin (10 – 1000 nmol l−1) for 1 h were incubated with DCF-DA. Simvastatin inhibited the NA-induced increase of DCF-DA fluorescence in cardiomyocytes in a concentration-dependent manner (Figure 4). Simvastatin (1000 nmol l−1) alone exerted no effect on DCF-DA fluorescence intensity in cardiomyocytes.
Figure 4.
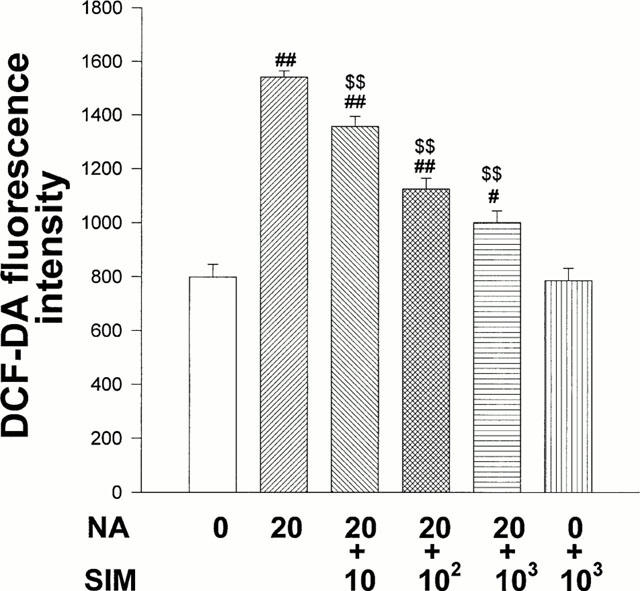
Inhibitory effects of simvastatin on DCF-DA fluorescence increase induced by NA. On culture day 4, cultured cardiac myocytes were treated with NA (20 μmol l−1), NA+SIM (10 – 1000 nmol l−1) or diluent without NA (control) and simultaneously with DCF-DA (5 μmol l−1). After 1 h of incubation, fluorescence intensity was measured by laser confocal microscopy. Each point is mean±s.e.mean obtained from four separate experiments in which eight visual fields were quantified. #P<0.05, ##P<0.01 vs control. $$P<0.01 vs NA.
Effects of prazosin and propranolol on NA-induced beating
It has been shown that an increase in the beating rate of neonatal cardiomyocytes requires both α- and β-adrenoceptors activation. Recently, it has been reported that NA synergistically induces cardiomyocyte hypertrophy through both α- and β-adrenergic stimulation (Yamazaki et al., 1997). To evaluate the role of beating in NA-induced neonatal rat cardiomyocyte hypertrophy, we preincubated cardiomyocytes with prazosin (20 μmol l−1), and/or propranalol (20 μmol l−1) for 10 min and then exposed cardiomyocytes to NA (20 μmol l−1) for 3 min. Prazosin and propranolol each partially suppressed NA-induced increase of beating, and preincubation with both prazosin and propranolol completely abolished the NA-evoked increase of beating rate. There were no effects of simvastatin (1000 nmol l−1) on increase of beating induced by NA (Table 1).
Discussion
The present study for the first time showed that simvastatin can inhibit the NA-induced ROS production and hypertrophic reaction in the neonatal rat cardiomyocytes. MVA can reverse the antihypertrophic effects of simvastatin. These results suggest that simvastatin may inhibit neonatal rat cardiomyocytes hypertrophy through blocking the biosynthesis of MVA. MVA, an intracellular product of HMG-CoA reductase, is necessary for cell growth (Goldstein & Brown, 1990). MVA metabolism yields a series of isoprenoid derivatives that are vital for diverse cellular functions, ranging from cholesterol synthesis to growth control (Goldstein & Brown, 1990; Raiteri et al., 1997).
Another new finding of our work is that the NA-induced increase of ROS and hypertrophy requires α- and β-adrenoceptor activation. In cardiomyocytes, NA activates at least two types of receptors, α- and β-adrenoceptors. Simpson et al. (1983) reported that NA stimulates hypertrophy of cultured neonatal rat cardiomyocytes through α1- but not through β- adrenoceptors. They also showed that an increase in beating rate requires both α- and β-adrenoceptor activation. It has also been reported that β-adrenergic activation produces adult feline cardiomyocyte hypertrophy by activating the beating of cardiomyocytes (Clark et al., 1991). Recently, Yamazaki et al. (1997) indicated that NA activates the raf-1 kinase/mitogen activated protein (MAP) kinase cascade through both α- and β-adrenergic stimulation, and signalling pathways from the two receptors synergistically induce cardiomyocyte hypertrophy. Our results presented here clearly show that blockade of α- and β-adrenoceptors partially inhibits the NA-induced increase of ROS, beating and protein content, whereas only combined inhibition yields complete inhibition. These results indicate that NA induces increase of ROS, beating and hypertrophy through both α- and β-adrenergic stimulation. Neither α- nor β-adrenoceptor stimulation alone can fully mediate the effects of NA.
Our results also showed that NA-induced increases of ROS are required for hypertrophy in neonatal rat cardiomyocyte. This conclusion was supported by the following results. Firstly, we have shown that NA generates ROS and induces hypertrophic growth in a concentration-dependent manner in the cultured neonatal cardiomyocytes. Second, prazosin+ propranolol completely inhibited the increase of ROS and protein content in the cultured neonatal cardiomyocytes induced by NA. Third, catalase, an enzyme that specifically decomposes H2O2 to water and molecular oxygen, inhibited the NA-induced increase of ROS, RNA content and [3H]-leucine incorporation in cultured neonatal cardiomyocytes.
In summary, we have shown that the inhibitory effects of simvastatin on cardiomyocyte hypertrophy were associated with its antioxidant effects and inhibition of the MVA-metabolism pathway in neonatal rat cardiomyocytes. NA-induced increases of intracellular ROS and cardiomyocyte hypertrophy requires both α- and β-adrenoceptor activation in neonatal rat cardiomyocytes. The increases of ROS induced by NA is required for hypertrophy.
Acknowledgments
This work was supported by the Education Committee of P.R. China, Guangzhou Education Committee and a PhD grant of Guangzhou Medical College.
Abbreviations
- BrdU
bromodeoxyuridine
- CAT
catalase
- DCF-DA
2′,7′-dichlorofluorescin diacetate
- DNAase
deoxyribonuclease
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- MAP
mitogen activated protein
- MVA
mevalonic acid
- NA
noradrenaline
- PI
propidium iodide
- PKC
protein kinase C
- PRA
prazosin
- PRO
propranolol
- ROS
reactive oxygen species
- SIM
simvastatin
References
- BLUM C.B. Comparison of properties of four inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Am. J. Cardiol. 1994;73:3D–11D. doi: 10.1016/0002-9149(94)90626-2. [DOI] [PubMed] [Google Scholar]
- CATHCART R., SCHWIERS E., AMES B.N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal. Biochem. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- CHEN L., HAUGHT W.H., YANG B., SALDEEN T.G., PARATHASARATHY S., MEHTA J.L. Preservation of endogenous antioxidant activity and inhibition of lipid peroxidation as common mechanisms of antiatherosclerotic effects of vitamin E, lovastatin and amlodipine. J. Am. Coll. Cardiol. 1997;30:569–575. doi: 10.1016/s0735-1097(97)00158-7. [DOI] [PubMed] [Google Scholar]
- CLARK W.A., STEVEM J.R., JOHN J.L., MICHAEL L., ROBERT S.D. Hypertrophy of isolated adult feline heart cells following α-adrenergic-induced beating. Am. J. Physiol. 1991;261:C530–C542. doi: 10.1152/ajpcell.1991.261.3.C530. [DOI] [PubMed] [Google Scholar]
- FRANKFURT O.S. Flow cytometric analysis of double-stranded RNA content distributions. J. Histochem. Cytochem. 1980;28:663–669. doi: 10.1177/28.7.6156201. [DOI] [PubMed] [Google Scholar]
- GERSON R.J., MACDONALOL J., ALBERTS A.W., KOMBRUST J., MAJKA J.A., STUBBS J., BOKELMAN D.L. Animal safety and toxicology of simvastatin and related hydroxymethyglutary-coenzyme A reductase inhibitors. Am. J. Med. 1989;87:28–38. doi: 10.1016/s0002-9343(89)80596-0. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN J.L., BROWN M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- KHALID M.A., ASHRAF M. Direct detection of endogenous hydroxyl radical production in cultured adult cardiomyocytes during anoxia and reoxygenation. Circ. Res. 1993;72:725–736. doi: 10.1161/01.res.72.4.725. [DOI] [PubMed] [Google Scholar]
- LEVY D., GARRISON R.J., SAVAGE D.D., KANNEL W.B., CASTELLI W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- LUO J.D., ZHANG W.W., ZHANG G.P., LIU X.H., GUAN J.X. Effects of simvastatin on left ventricular hypertrophy and function in rats with aortic stenosis. Acta Pharmacol. Sinica. 1999a;20:345–348. [PubMed] [Google Scholar]
- LUO J.D., ZHANG W.W., ZHANG G.P., GUAN J.X., CHEN X. Simvastatin inhibits cardiac hypertrophy and angiotensin-converting enzyme activity in rats with aortic stenosis. Clin. Exp. Pharmacol. Physiol. 1999b;26:903–908. doi: 10.1046/j.1440-1681.1999.03165.x. [DOI] [PubMed] [Google Scholar]
- MCLENACHAN J.M., HENDERSON E., MORRIS K.I., DARGIE H.J. Ventricular arrhythmias in patients with hypertensive left ventricular hypertrophy. N. Engl. J. Med. 1987;317:787–792. doi: 10.1056/NEJM198709243171302. [DOI] [PubMed] [Google Scholar]
- NAKAMURA K., FUSHIMI K., KOUCHI H., MIHARA K., MIYAZAKI M., OHE T., NAMBA M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-α and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- RAITERI M., ARNABOLDI L., MCGEADY P., GELB M.H., VERRI D., TAGLIABUE C., QUARATO P., FERRABOSCHI P., SANTANIELLO E., PAOLETTI R., FUNAGALLI R., CORSONI A. Pharmacological control of the mevalonate pathway: effect on arterial smooth muscle cell proliferation. J. Pharmacol. Exp. Ther. 1997;281:1144–1153. [PubMed] [Google Scholar]
- SIMPSON P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an α-adrenergic receptor and induction of beating through an α- and β-adrenergic receptor interaction. Circ. Res. 1985;56:884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- SIMPSON P., MCGRATH A. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cell is an alpha, adrenergic response. J. Clin. Invest. 1983;72:732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUMURA K., YZSUHARA M., TANAKA K., SUZUKI T. Protective effect of fluvastatin sodium(XU-62-320), a 3-hydroxy-3-methylglutaryl coenzyme A(HMG-CoA) reductase inhibitor, on oxidative modification of human low-density lipoprotein in vitro. Biochem. Pharmacol. 1999;57:697–703. doi: 10.1016/s0006-2952(98)00341-4. [DOI] [PubMed] [Google Scholar]
- THAIK C.M., CALDERONE A., TAKAHASHI N., COLUCCI W.S. Interleukin-1 beta modulates the growth and phenotype of neonatal rat cardiac myocytes. J. Clin. Invest. 1995;96:1093–1099. doi: 10.1172/JCI118095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE Z., KOMETIANI P., LIU J., LI J., SHAPIRO J.I., ASKARI A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J. Biol. Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO A., HOSHI K., ICHIHARA K. Fluvastatin, an inhibitor of a 3-hydroxy-3-methylglutaryl coenzyme A reductase, scavenges free radicals and inhibits lipid peroxidation in rat liver microsomes. Eur. J. Pharmacol. 1998;361:143–149. doi: 10.1016/s0014-2999(98)00692-x. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI T., KOMURO I., ZOU Y., KUDOH S., SHIOJIMA I., HIROI Y., MIZUNO T., AIKAWA R., TAKANO H., YAZAKI Y. Norepinephrine induces the raf-1 kinase/mitogen-activated protein kinase cascade through both α- and β-adrenoceptors. Circulation. 1997;95:1260–1268. doi: 10.1161/01.cir.95.5.1260. [DOI] [PubMed] [Google Scholar]
- ZHU H., BANNENBERG G.L., MOLDEUS P., SCHERTZER H.G. Oxidation pathways for the intracellular probe 2′,7′-dichlorofluorescin. Arch. Toxicol. 1994;68:582–587. doi: 10.1007/s002040050118. [DOI] [PubMed] [Google Scholar]


