Abstract
The present study examines the effect of pregabalin (previously S-Isobutylgaba and CI-1008) in two distinct rat models of anxiety. Pregabalin binds with high affinity and selectivity to the α2δ subunit of voltage dependent calcium channels (VDCC). Its corresponding R-enantiomer (R-isobutylgaba) is approximately 10 fold weaker. Pregabalin dose-dependently induced anxiolytic-like effects in both the rat conflict test and elevated X-maze with respective minimum effective doses (MED) of 3 and 10 mg kg−1. In contrast, R-isobutylgaba only showed activity at the highest dose of 100 mg kg−1 in the conflict test. These data indicate that pregabalin may possess clinical utility as a novel anxiolytic agent and demonstrates the importance of the α2δ subunit of VDCC in the mediation of anxiety related behaviours.
Keywords: Anxiety, conflict test, elevated X-maze, rat, R-isobutylgaba, α2δ subunit of voltage-dependent calcium channels
Introduction
For the last four decades the pharmacological treatment of generalized anxiety has been dominated by benzodiazepines. However, the use of these compounds is limited due to their side-effects. They induce sedation and interact with other CNS depressants (Woods et al., 1992). Major problems with benzodiazepines are dependence and withdrawal symptoms which have hindered their long term use (Lader, 1999; Woods et al., 1992). Thus, a great deal of research has been dedicated towards the discovery of novel anxiolytic agents with an improved side-effect profile.
Pregabalin (previously S-isobutylgaba and CI-1008) is structurally related to the inhibitory neurotransmitter GABA. However, radioligand binding studies have shown that pregabalin has negligible affinity for GABA and many other receptors but binds with high affinity and selectivity to a novel binding site in the CNS, recently identified as the α2δ subunit of voltage-dependent calcium channels (VDCC) (Gee et al., 1996). The corresponding R-enantiomer (R-isobutylgaba) of pregabalin has a 10 fold lower affinity for the α2δ site (Taylor et al., 1993). The present study examines and compares the effects of pregabalin and R-isobutylgaba in animal models of anxiety.
Methods
Animals
Male Hooded Lister rats (200 – 250 g) were obtained from Interfauna Universal (Huntingdon, U.K.). Animals were housed in groups of six under a 12 h light/dark cycle (lights on at 0700 h) with food and water ad libitum, except rats used in the conflict procedure were maintained at 80% of their free feeding body weight.
X-maze
A standard elevated X-maze apparatus was used in these experiments (Singh et al., 1991). Animals were placed onto the centre of the X-maze facing one of the open arms. The time (TOA) and % time (%TOA) spent on open arms and time spent in the end half sections of the open arms (TEOA) together with total arm entries (TE) were measured in a 5 min test period.
Rat conflict test
These methods have been described before (Singh et al., 1991). Briefly, rats were trained to press levers for food reward in operant chambers. The schedule consisted of alternations of four 4-min unpunished periods on variable interval of 30 s signalled by chamber lights on and three 3-min punished periods on fixed ratio 5 (by foot shock concomitant to food delivery) signalled by chamber lights off. The degree of foot shock was adjusted for each rat to obtain approximately 80 – 90% suppression of responding in comparison with unpunished responding. Rats received saline vehicle on training days.
Drugs
Drugs used were pregabalin and R-isobutylgaba (Pfizer Global Research and Development, Ann Arbor Laboratories, MI, U.S.A.) and chlordiazepoxide (Sigma, Poole, U.K.). All compounds were dissolved in 0.9% w/v−1 NaCl (normal saline) and administered s.c. in a volume of 1 ml kg−1, except the top dose of 100 mg kg−1 pregabalin and R-isobutylgaba which were administered in a volume of 3 ml kg−1. The pretreatment times, route of administration and doses were taken from previous studies (Taylor et al., 1993; Singh et al., 1996).
Results
Rat elevated X-maze
The s.c. administration of pregabalin (3 – 30 mg kg−1) 40 min before test, showed an anxiolytic-like action in the elevated X-maze as indicated by an increase in time and per cent time spent on the open arms and time spent in the end half sections of the open arms with a MED of 10 mg kg−1 (Figure 1a – c). The administration of chlordiazepoxide (3 mg kg−1) produced a similar anxiolytic action on the X-maze to that of pregabalin (Figure 1a – c). Neither compound had any adverse effect on the total number of arm entries indicating a lack of sedative action at the doses used (Figure 1d).
Figure 1.
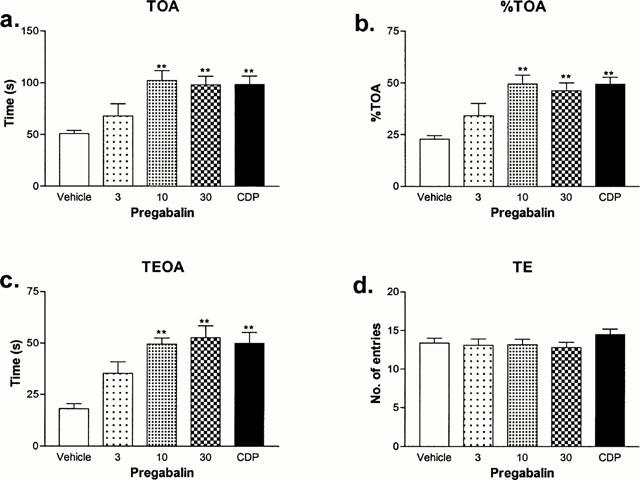
Effect of pregabalin in the rat elevated X-maze. Pregabalin (3.30 mg kg−2) or CDP (chlordiazepoxide, 3 mg kg−1 s.c.) was administered s.c. 40 min before test. The time (a: TOA) and per cent time spent (b: % TOA) on open arms and time spent in the end half sections of the open arms (c: TEOA) together with total arm entries (d: TE) were measured. The duration of each test was 5 min. Results are shown as the mean (vertical bars represent ±s.e.mean) of 7 – 10 animals per group. **Significantly different from vehicle treated controls P<0.01 (ANOVA followed by Dunnett's t-test).
Rat conflict test
The s.c. administration of pregabalin 40 min before test, dose-dependently (1 – 100 mg kg−1) increased lever pressing in the punished period with a MED of 3 mg kg−1 which produced a 48.1% increase (Figure 2a). A maximal effect (304.9% increase) was observed with 30 mg kg−1 (Figure 2a). Unpunished responding was only significantly reduced at the highest dose of 100 mg kg−1 (Figure 2a). R-isobutylgaba failed to affect punished responding except at the highest dose of 100 mg kg−1 which produced a 76.4% increase (Figure 2b).
Figure 2.
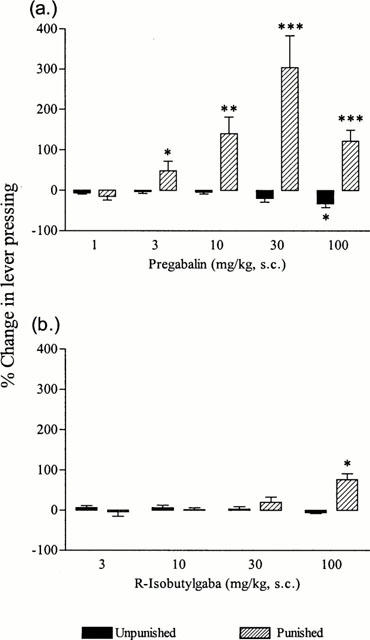
Effect of (a) pregabalin and (b) R-isobutylgaba in the rat conflict test. Pregabalin or R-isobutylgaba was administered s.c. 40 min before test. The results are expressed as mean per cent increase or decrease of lever pressing of at least six animals per group on test day compared with mean performances obtained the two previous days following vehicle administration. Significantly different from previous control days *P<0.05, **P<0.01, ***P<0.001 (paired student t-test).
Discussion
The results presented here show that pregabalin possesses anxiolytic-like activity in both ethological and conflict models of anxiety. Thus, it showed good anxiolytic-like effects in the rat elevated X-maze and the rat conflict test. The R-isomer of pregabalin, which possesses weaker affinity for the α2δ site only had a modest effect in the conflict model at the highest dose examined. These results indicate the importance of the α2δ subunit of VDCC in mediating the anxiolytic-like action of pregabalin. This is consistent with previous studies demonstrating activity for pregabalin and limited activity of R-isobutylgaba in both epilepsy and pain models (Field et al., 1997; 1999; Partridge et al., 1998; Taylor et al., 1993). It has been shown that α2δ binding sites are present throughout the CNS, including the limbic system (Hill et al., 1993). This area has high concentrations of benzodiazepine receptors and is known to play a major role in the control of emotional behaviours.
It is known that the α2δ subunit is common to all VDCC (Hofmann et al., 1994; Isom et al., 1994) and it is also clear that these channels play an important role in neurotransmission (e.g. control of neurotransmitter release). It seems unlikely that pregabalin interacts with all VDCC as an unselective blockade of Ca2+ channels would lead to severe side effects. Animal studies examining pregabalin in models of nociception indicate that it has a good side effect profile (Field et al., 1997). Recently, it has been reported there are at least three subtypes of α2δ subunits (Klugbauer et al., 1999) and pregabalin may interact specifically with one of these.
In the present study the magnitude of the anxiolytic effects of pregabalin was similar to those of the benzodiazepine CDP in both models (Singh et al., 1996). Most non-benzodiazepine ligands that induce potent anxiolytic-like effects in the rat elevated X-maze show much weaker activity in shock-induced conflict tests. For example both CCKB and 5-HT3 receptor antagonists have been shown to possess anxiolytic like action in the ethological models but are only weakly active or inactive in conflict tests (Costall et al., 1990; Singh et al., 1991). To date, it is unclear as to which preclinical models are more predictive of clinical utility. However, it is interesting to note that some non-benzodiazepine compounds with good activity in ethological models and poor activity in conflict models failed to make a significant impact on the anxiolytic market (Rodgers & Johnson, 1995). For example, CCK compounds have been unsuccessful to date in a number of clinical trials despite the positive ethological preclinical data (Griebel, 1999). This may suggest that activity in conflict models is most desirable in preclinical anxiolytic drug candidates. Further studies are required to determine the dependence liability of pregabalin. However, it should be noted that gabapentin, a related compound which also selectively binds to the α2δ subunit of VDCC, is anxiolytic in animals (Singh et al., 1996) and has been in clinical use as an antiepileptic agent for some years now with a very clean side effect profile. Thus, it has been shown to be safe and well tolerated with the main side effects being somnolence/dizziness and no reports of any abuse liability (Crawford, 1996; Walker & Patsalos, 1996). In conclusion, results of the present study suggest that pregabalin is a novel anxiolytic agent and demonstrate for the first time the importance of VDCC in anxiety related behaviours.
Abbreviations
- CCK
cholecystokinin
- CDP
chlordiazepoxide
- CNS
central nervous system
- MED
minimum effective dose
- TOA
time on open arms
- TEOA
time on ends of open arms
- TE
total entries
- VDCC
voltage-dependent calcium channels
References
- COSTALL B., NAYLOR R.J., TYERS M.B. The psychopharmacology of 5-HT3 receptors. Pharmacol. Ther. 1990;47:181–202. doi: 10.1016/0163-7258(90)90086-h. [DOI] [PubMed] [Google Scholar]
- CRAWFORD P.M. The clinical efficacy of gabapentin. Rev. Contemp. Pharmacother. 1996;7:215–225. [Google Scholar]
- FIELD M.J., OLES R.J., LEWIS A.S., MCCLEARY S., HUGHES J., SINGH L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br. J. Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD M.J., MCCLEARY S., HUGHES J., SINGH L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- GEE N.S., BROWN J.P., DISSANAYAKE V.U.K., OFFORD J., THURLOW R., WOODRUFF G.N. The novel anti-convulsant drug, Gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J. Biol. Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- GRIEBEL G. Is there a future for neuropeptide receptor ligands in the treatment of anxiety disorders. Pharmacol. Ther. 1999;82:1–61. doi: 10.1016/s0163-7258(98)00041-2. [DOI] [PubMed] [Google Scholar]
- HILL D.R., SUMAN-CHAUHAN N., WOODRUFF G.N. Localization of [3H]gabapentin to a novel site in rat brain: autoradiographic studies. Eur. J. Pharmacol. 1993;244:303–309. doi: 10.1016/0922-4106(93)90156-4. [DOI] [PubMed] [Google Scholar]
- HOFMANN F., BIEL M., FLOCKERZI V. Molecular basis for Ca2+ channel diversity. Annu. Rev. Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- ISOM L.L., DE-JONGH K.S., CATTERALL W.A. Auxiliary subunits of voltage-gated ion channels. Neuron. 1994;12:1183–1194. doi: 10.1016/0896-6273(94)90436-7. [DOI] [PubMed] [Google Scholar]
- KLUGBAUER N., LACINOVA L., MARAIS E., HOBOM M., HOFMANN F. Molecular diversity of the calcium channel alpha2delta subunit. J. Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LADER M.H. Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified. Eur. Neuropsychopharmacol. Suppl. 1999;6:S399–S405. doi: 10.1016/s0924-977x(99)00051-6. [DOI] [PubMed] [Google Scholar]
- PARTRIDGE B.J., CHAPLAN S.R., SAKAMOTO E., YAKSH T.L. Characterization of the effects of gabapentin and 3-isobutyl-γ-aminobutyric acid on substance P-induced thermal hyperalgesia. Anesthesiology. 1998;88:196–205. doi: 10.1097/00000542-199801000-00028. [DOI] [PubMed] [Google Scholar]
- RODGERS R.J., JOHNSON N.J. Cholecystokinin and anxiety: promises and pitfalls. Crit. Rev. Neurobiol. 1995;9:345–369. [PubMed] [Google Scholar]
- SINGH L., FIELD M.J., HUGHES J., MENZIES R., OLES R.J., VASS C.A., WOODRUFF G.N. The behavioural properties of CI-988, a selective cholecystokininB receptor antagonist. Br. J. Pharmacol. 1991;104:239–245. doi: 10.1111/j.1476-5381.1991.tb12413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH L., FIELD M.J., FERRIS P., HUNTER J.C., OLES R.J., WILLIAMS R.G., WOODRUFF G.N. The antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-Serine. Psychopharmacology. 1996;127:1–9. doi: 10.1007/BF02805968. [DOI] [PubMed] [Google Scholar]
- TAYLOR C.P., VARTANIAN M.G., YUEN P.W., BIGGE C., SUMAN-CHAUHAN N., HILL D.R. Potent and stereospecific anticonvulsant activity of 3-isobutyl GABA relates to in vitro binding at a novel site labelled by tritiated gabapentin. Epilepsy Res. 1993;14:11–15. doi: 10.1016/0920-1211(93)90070-n. [DOI] [PubMed] [Google Scholar]
- WALKER M.C., PATSALOS P.N. The tolerability and safety profile of gabapentin. Rev. Contemp. Pharmacother. 1996;7:249–257. [Google Scholar]
- WOODS J.H., KATZ J.L., WINGER G. Benzodiazepines: use, abuse, and consequences. Pharmacol. Rev. 1992;44:151–347. [PubMed] [Google Scholar]


