Abstract
Vasospasm of arterial conduits used for coronary artery surgery is an important cause of graft failure and is likely to result partly from raised levels of vasoconstrictor substances such as thromboxane A2 and endothelin-1. Our aim was to find pharmacological agents that could prevent agonist-induced vasospasm.
Isometric tension was recorded from discarded segments of human left internal mammary artery (LIMA). Submaximal contraction evoked by the thromboxane A2 mimetic U46619 (10 nM) was not inhibited by a blocker of store- and receptor-operated Ca2+ channels (30 μM SKF96365) in the presence of diltiazem. Furthermore, contractions to ⩽1 nM U46619 were preserved when extracellular Ca2+ was reduced from 2.5 mM to 60 nM. Thus, sustained U46619-evoked contraction occurred without Ca2+ influx.
We hypothesized that contraction might occur via Rho-kinase-mediated Ca2+-sensitization of myofilaments. Inhibitors of Rho-kinase (Y27632 and HA1077) were profound relaxants. If contraction was pre-evoked by 10 nM U46619, Y27632 and HA1077 caused full relaxation with EC50s of 1.67±0.22 μM and 3.58±0.35 μM respectively. Y27632 was also effective if applied before U46619, but was less potent.
Y27632 abolished contraction evoked by endothelin-1 and significantly reduced resting tone in the absence of a vasoconstrictor.
Rho-kinase-mediated Ca2+-sensitization appears to be a major mechanism of vasoconstriction in human LIMA. Rho-kinase inhibitors may have an important role in preventing vasospasm in arterial grafts used for coronary artery surgery.
Keywords: Arteries, calcium, calcium-sensitization, muscle, smooth, revascularization, rho-kinase, thromboxane, vasospasm
Introduction
Vasospasm of arterial conduits is an important cause of graft failure and morbidity in patients undergoing coronary artery surgery (He et al., 1989; 1995; Sarabu et al., 1987; Cable et al., 1998; He 1999; Rosenfeldt et al., 1999). With the current trend towards total arterial revascularization, it has become essential to identify both the causes of vasospasm and potential therapeutic targets.
The aetiology of graft vasospasm is likely to be multifactorial, including trauma at the time of surgery, endothelial disruption and the presence of vasoactive substances. Endothelin-1 (ET-1) and thromboxane A2 (TXA2) are two of the most potent endogenous vasoconstrictors (He et al., 1995), circulating levels of which have been shown to rise at the time of surgery (Watkins et al., 1982; Faymonville et al., 1986). ET-1 is released by the endothelium, whereas the prostanoid TXA2 is released from platelets. Other vasoconstrictor substances include noradrenaline, 5-hydroxytryptamine, acetylcholine, histamine and angiotensin II (He 1999), whilst exogenous vasoconstrictors such as phenylephrine, a drug used to support the circulation perioperatively, may also be implicated.
Clinically, a number of drugs have been used to prevent arterial graft spasm, including diltiazem, papaverine and nitrates. Other drugs have been studied in vitro as potential antispasmogens and include L-type Ca2+ channel blockers (e.g. diltiazem, verapamil, nifedipine) (He et al., 1989; Cable et al., 1998; He & Yang, 2000a; Sadaba et al., 2000), nitrates (He et al., 1989; Cable et al., 1998), phosphodiesterase inhibitors (e.g. papaverine, milrinone, enoximone, amrinone) (He et al., 1989; Salmenperä & Levy, 1996; He & Yang, 2000b), TXA2 antagonists (He & Yang, 1995) and nicorandil (Sadaba et al., 2000). The commonly administered Ca2+ antagonists may be completely ineffective against contractions evoked by some endogenous vasoconstrictors (Sadaba et al., 2000). Many other drugs have only modest relaxant effects or must be used at high concentrations in order to inhibit vasospasm effectively.
Until recently there has been poor understanding of the intracellular events leading to contraction in human vascular smooth muscle cells. Agents such as diltiazem, nifedipine and verapamil prevent entry of Ca2+ into the cell via voltage-operated Ca2+ channels (VOCCs) (Morel & Godfraind, 1993). Ca2+ may also enter the cell via receptor-operated Ca2+ channels (ROCCs) (Barritt, 1999). In addition, agonist occupancy of cell-surface receptors linked to phospholipase C generates inositol triphosphate, triggering the release of Ca2+ from the sarcoplasmic reticulum. Depletion of Ca2+ from intracellular stores is itself a trigger for the opening of store-operated Ca2+ entry channels (SOCCs) (Lewis, 1999). All these events lead to a rise in intracellular Ca2+ and increased activity of Ca2+-calmodulin-modulated myosin light chain kinase (MLCK) (Somlyo & Somlyo, 2000), an enzyme that phosphorylates myosin light chain (MLC) and consequently promotes contraction.
Smooth muscle contraction does not necessarily require an increase in intracellular Ca2+. A decade ago it was shown that U46619, a stable TXA2 mimetic, caused little or no rise in intracellular Ca2+ in rabbit pulmonary artery despite evoking contractions (Himpens et al., 1990). Sensitization of the contractile apparatus to Ca2+ is now an established phenomenon (Somlyo & Somlyo, 2000). Although protein kinase C and arachidonic acid may play a role in Ca2+-sensitization, the main mechanism appears to involve the monomeric G-protein RhoA (Somlyo & Somlyo, 2000; Uehata et al., 1997; Fu et al., 1998). Activation of cell-surface receptors activates RhoA which binds GTP and translocates to the cell membrane. Its effector is a serine-threonine kinase known as Rho-kinase (Fu et al., 1998). Rho-kinase phosphorylates myosin phosphatase (MLCP), which inhibits the activity of MLCP (Kimura et al., 1996). Inactive MLCP cannot dephosphorylate MLC, and so Rho-kinase activity removes inhibition of MLC phosphorylation, allowing MLCK, and contraction, to dominate. Thus, regulation of MLC phosphorylation depends on Ca2+-dependent (via MLCK) and Ca2+-independent (via Rho-kinase) mechanisms.
Our aim was to determine the dominant mechanisms responsible for agonist-induced contraction in human left internal mammary artery (LIMA) and thus reveal an important target for new anti-vasospastic drugs. We have previously shown that blockers of L-type Ca2+ channels have only weak effects (Sadaba et al., 2000) and so we focussed on other potential mechanisms including receptor- and store-operated Ca2+ entry and sensitization of the contractile apparatus to Ca2+.
Methods
Ethical approval was obtained from the Research Ethics Committee, Leeds Teaching Hospitals NHS Trust, Leeds, U.K. Samples were obtained anonymously from discarded segments of distal left internal mammary arteries from patients undergoing coronary artery bypass surgery. These samples were transported to the laboratory in Hanks solution at 4°C. Loose connective tissue was removed and 3 mm rings prepared to be used either on the day of surgery or the following day. They were stored in Hanks solution at 4°C or incubated overnight in Dulbecco's Modified Eagle Medium supplemented with 100 μg ml−1 penicillin G and 100 μg ml−1 streptomycin in a 5% CO2 incubator at 37°C. Hanks solution contained (in mM) NaCl 137, KCl 5.4, CaCl2 0.01, NaH2PO4 0.34, K2HPO4 0.44, glucose 8, and HEPES 5 (pH 7.40).
The rings were mounted on two stainless steel hooks, one of which was fixed and the other connected to an isometric tension transducer in a glass organ bath containing 15 ml of Krebs solution bubbled with 95% O2/5% CO2 at 37°C. Krebs solution contained (in mM) NaCl 118.3, KCl 4.6, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, CaCl2 2.5, and glucose 11. Low Ca2+ solution differed in that it contained 5 mM EGTA (calculated free Ca2+=60 nM; this and all other free Ca2+ values were calculated with Eqcal software) and was titrated to pH 7.40 with NaOH.
Arterial rings were routinely stretched to a resting tension of 1 g (Sadaba et al., 2000) and equilibrated for 90 min before being tested for three reproducible responses to phenylephrine.
All vessels were tested for the presence of functional endothelium by eliciting relaxation with carbachol (1 μM) in the presence of phenylephrine. Vessels exhibiting less than 10% relaxation were classed as intrinsically lacking functional endothelium. In experiments involving preincubation with Y27632 or GF 109203X, the arterial segments were contracted with Krebs solution containing 80 mM K+ to allow comparison between vessels. Thereafter, all experiments occurred in the presence of 10 μM diltiazem to abolish Ca2+ entry through voltage-gated Ca2+ channels.
All statistical comparisons were made using either paired or independent two-tailed Student's t-test and differences were taken to be statistically significant at P<0.05. Results are expressed as mean±s.e.mean, and the value n indicates the number of arterial segments. Data analysis and the mathematical fitting of functions to data using a least-squares method were performed by the program Origin (version 4.1; MicroCal Inc, Northampton, MA, U.S.A.). Concentration-effect data were fitted to the Hill equation:
where s is the slope and A is the maximum value of y. Normalized tension was calculated as the tension in grams divided by the wet weight of the arterial ring in grams.
Diltiazem, carbachol, EGTA, U46619 and HEPES were from Sigma (Poole, Dorset, U.K.). SKF96365, HA1077 (fasudil) and endothelin-1 were from Calbiochem (Beeston, Nottingham, U.K.). GF 109203X was from Research Biochemicals International (Natick, MA, U.S.A.). Y27632 was a gift from the Welfide Corporation (Saitama, Japan). General salts were from BDH (Merck Ltd.). Dulbecco's Modified Eagle Medium was from Gibco BRL (Paisley, Scotland).
Stock solutions of diltiazem, Y27632, HA1077 and SKF96365 were prepared in water, while GF 109203X was prepared in dimethylsulphoxide (DMSO), U46619 in ethanol and endothelin-1 in 5% acetic acid. The final concentration of DMSO, ethanol or acetic acid in the recording bath was always ⩽0.01%.
Results
Contraction without Ca2+ entry
Ca2+ entry pathways other than voltage-operated Ca2+ entry could be important for U46619-evoked contraction and thus we investigated the effect of SKF96365, a blocker of SOCCs and ROCCs in rat aorta smooth muscle cells (Merritt et al., 1990; Zhang et al., 1999). Concentration-response curves were created for U46619 alone and then (after a washout) for U46619 following a 30-min preincubation with 30 μM SKF96365. There was no significant change in the EC50 or maximum effect of U46619 in time-matched control experiments (not shown) or in the presence of SKF96365 (Figure 1A). Although these data indicate that Ca2+ entry pathways are not important for U46619-evoked contraction, they do not exclude a role for diltiazem- and SKF96365-resistant Ca2+-entry. To look more generally at Ca2+-entry we investigated the effect of lowering the external Ca2+ concentration from 2.5 mM to 60 nM, a value at or just below that expected in the cytoplasm. Importantly, contractions to U46619 were still observed (Figure 1B) and contractions to ⩽1 nM U46619 were unaffected (Figure 1C). The maximum contraction to U46619 was reduced by about half (P=0.038) and the EC50 decreased from 7.86±0.82 nM to 4.89±0.90 nM (P=0.021).
Figure 1.
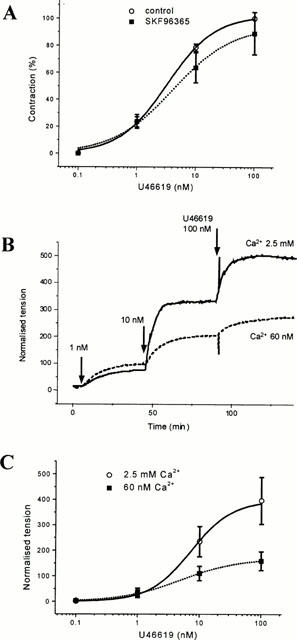
Role of Ca2+ entry in contraction evoked by U46619 in human LIMA. (A) Concentration-response curves for U46619 in control conditions and following preincubation with 30 μM SKF96365. EC50 values for U46619 were 3.30±0.41 nM (control, n=4) and 4.76±1.01 nM (SKF96365, n=4, P>0.05). (B) Typical original experimental traces showing contraction evoked by U46619 in one vessel segment bathed in Krebs solution (2.5 mM Ca2+) and another in low-Ca2+ Krebs solution (60 nM free Ca2+). (C) Summary of experiments as shown in (B). Data points are mean±s.e.mean normalized tension (tension (g)/wet weight of the vessel (g)) (n=6 for both groups). Experiments were performed in the presence of 10 μM diltiazem. The smooth curves in this and all other figures are fitted Hill equations.
Thus, at least at low concentrations of U46619, Ca2+ influx is not a prerequisite for contraction, and pharmacologically identified Ca2+ channels do not appear to play an important role even when U46619 concentrations are high. However, the possibility exists that contractions to high concentrations of U46619 may occur partly via Ca2+ release from the sarcoplasmic reticulum, and that these stores were depleted in 60 nM Ca2+ solution.
Effects of Rho-kinase inhibitors
The Rho-kinase inhibitor HA1077 (Asano et al., 1989) completely reversed submaximal contraction evoked by 10 nM U46619 with an EC50 of 3.58±0.35 μM (Figure 2). Y27632, a more potent and selective inhibitor of Rho-kinase (Uehata et al., 1997; Ishizaki et al., 2000) also completely reversed the contraction to 10 nM U46619 in endothelium-intact vessels with an EC50 of 1.67±0.22 μM (Figure 3A,B). Y27632 was equally effective in vessels lacking a functional endothelium (n=3, data not shown). It was apparent that Y27632 caused relaxation to below pre-U46619 levels. Therefore, Y27632 (1 and 10 μM) was tested under basal conditions without precontraction with U46619 and was seen to cause a significant relaxation (Figure 3C). The absolute amplitude of the Y27632-induced relaxation was substantially smaller than that in the presence of U46619 (Figure 3A,C), and thus Y27632 did relax U46619-evoked as well as basal tension. Unlike the Rho-kinase inhibitors, the protein kinase C inhibitor GF 109203X (1 μM) had no significant effect on baseline tension (Figure 3C) or U46619-evoked contraction (n=4, data not shown).
Figure 2.
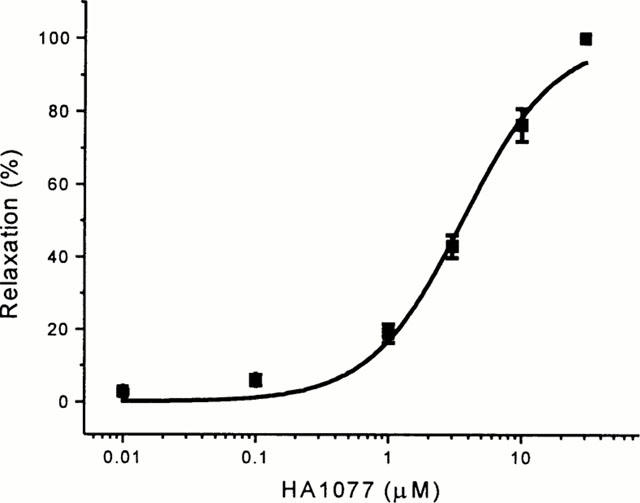
Concentration-response curve for HA1077-induced relaxation (EC50=3.58±0.35 μM, n=6) of LIMA pre-contracted with 10 nM U46619. Data points are mean±s.e.mean for tension as a percentage of that evoked by U46619. Experiments were performed in the presence of 10 μM diltiazem.
Figure 3.
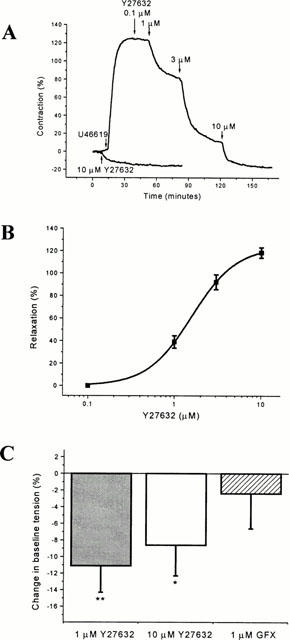
Effect of Y27632 on contraction pre-induced by U46619. (A) The upper (larger) experimental record is representative of contraction induced by 10 nM U46619 followed by relaxation in response to Y27632. The lower trace on the same plot is the effect of 10 μM Y27632 on another vessel segment in the absence of U46619. Contraction is expressed as a percentage of that evoked by 80 mM K+. (B) Mean±s.e.mean Y27632-induced relaxation of U46619-evoked tone in endothelium-intact vessels given as a percentage 80 mM K+-evoked contraction (EC50 1.67±0.22 μM, n=6). (C) Effect of Y27632 (1 and 10 μM) and GF 109203X on baseline tension in the absence of U46619 (n=4 in each group). Data points are mean±s.e.mean as a percentage of the maximum contractile responses to 80 mM K+ (*P=0.044, **P=0.0048). Experiments were performed in the presence of 10 μM diltiazem.
Because it has been reported that Y27632 is less effective if applied prior to the vasoconstrictor agonist (Fu et al., 1998; Iizuka et al., 1999) vessels were preincubated with either Y27632 or vehicle for 30 min before a concentration-response curve was constructed to U46619 (Figure 4A). Y27632 (10 μM) abolished contractions to low concentrations (1 – 3 nM) of U46619, but at 100 nM U46619 there was only partial inhibition (49.3±9.0% vs 125.9±17.7% contraction, P=0.0075). Y27632 (10 μM) significantly shifted the U46619 concentration-response curve to the right (EC50 of 18.69±5.39 nM compared to 5.04±0.83 nM in control conditions, P=0.046). Preincubation with 1 μM Y27632 did not significantly inhibit the contractions to 100 nM U46619 (119.3±20.6% vs 125.9±17.7%) (Figure 4A). There was, however, a trend towards attenuation of contractions to low U46619 concentrations and, using a more sensitive protocol, a statistically significant difference was detected (Figure 4B,C). Two concentration-response curves were constructed for U46619, the first with U46619 alone and the second (after a washout) with U46619 following a 30-min preincubation with 1 μM Y27632. There was no change in the time-matched control experiments (Figure 4B), but in the Y27632 group the contractile responses to low concentrations of U46619 (1 – 10 nM) were significantly attenuated (Figure 4C). A comparison of the pre- and post-incubation protocols (Figure 5) suggests that Y27632 was slightly less potent if applied before rather than after U46619 had evoked contraction.
Figure 4.

Effect of Y27632 applied before contraction with U46619. (A) Mean±s.e.mean contraction to U46619 as a percentage of contraction evoked by 80 mM K+. Data were collected in the absence of Y27632 and following preincubation with either 1 μM or 10 μM Y27632. EC50 values in control conditions and in the presence of 1 μM Y27632 were not significantly different (5.94±0.74 nM and 7.37±1.11 nM respectively, n=4 for each). Preincubation with 10 μM Y27632 changed the EC50 to 18.69±5.39 nM (P=0.046, n=4). (B,C) Use of a double concentration-response protocol to detect an effect of 1 μM Y27632. (B) Control experiments (n=5) showing no effect on maximum contraction to U46619 (100% vs 90.8±6.3%) or the EC50 (8.43±1.55 nM vs 9.03±2.00 nM) between the first and second concentration-response curves. (C) Preincubation with 1 μM Y27632 attenuated the contractile response to low concentrations of U46619 (1 – 10 nM) but not to higher concentrations. Y27632 increased the EC50 for U46619 from 4.81±0.56 nM to 8.93±0.69 nM (P=0.0017, n=5). Experiments were performed in the presence of 10 μM diltiazem. (*P<0.05, **P<0.01, #P<0.005, ##P<0.001, +P<0.0001).
Figure 5.
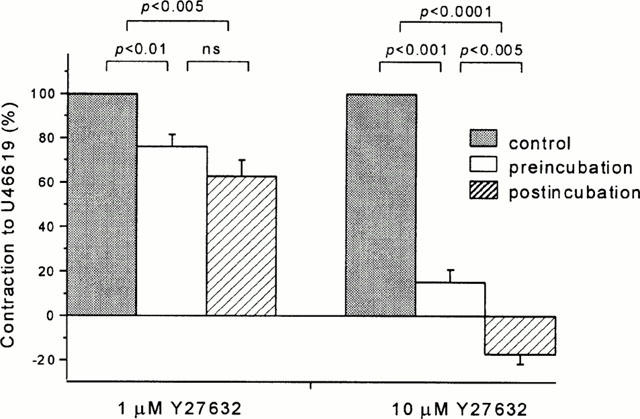
Comparison of the effectiveness of Y27632 applied before or after U46619. Data points are mean±s.e.mean responses as a percentage of the maximum response to 10 nM U46619 in the absence of Y27632.
Y27632 was also a relaxant of contraction evoked by a submaximally effective concentration of endothelin-1 (Figure 6), acting with an EC50 of 0.53±0.11 μM (n=5).
Figure 6.
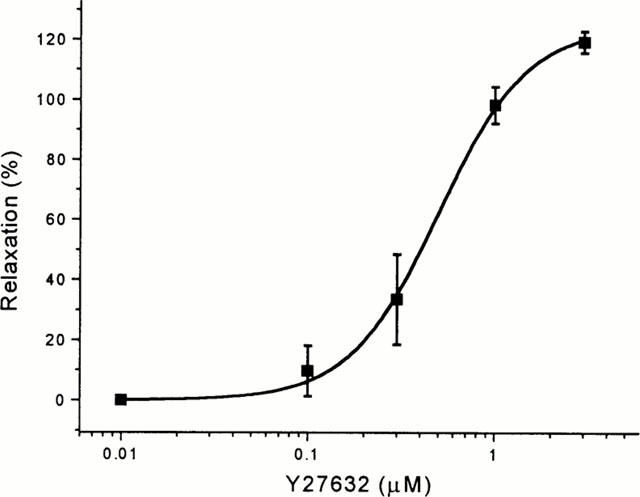
Concentration-response curve for Y27632-induced relaxation (EC50=0.53±0.11 μM, n=5) of LIMA pre-contracted with 10 nM endothelin-1. Data points are mean±s.e.mean for tension as a percentage of that evoked by endothelin-1. Experiments were performed in the presence of 10 μM diltiazem.
Discussion
An important finding in this study is that contraction of human internal mammary artery induced by low concentrations of thromboxane A2 occurs independently of Ca2+ influx. Even contractions induced by high concentrations of agonist are resistant to established blockers of plasma membrane Ca2+ channels such as diltiazem and SKF96365. Strikingly, however, inhibitors of Rho-kinase abolished contractions. The Rho-kinase inhibitor Y27632 was also shown to be a potent inhibitor of endothelin-induced contraction and resting (agonist-independent) contraction.
The antivasospastic properties of Y27632 and HA1077 are likely to occur as a result of their ability to inhibit Rho-kinase. In biochemical assays, Y27632 and HA1077 inhibit Rho-kinase with Ki values of 0.14 – 0.30 μM (Uehata et al., 1997; Ishizaki et al., 2000) and 0.33 – 0.40 μM (Uehata et al., 1997; Nagumo et al., 2000). These values are lower than the IC50 values from our study (0.53/1.67 μM and 3.58 μM for Y27632 and HA1077 respectively) and lower than the ‘functional' IC50 values previously reported (Uehata et al., 1997; Nagumo et al., 2000; Yoshii et al., 1999). The approximate 10 fold difference between Ki and IC50 values may arise because of competition between ATP and drug on the Rho-kinase protein (Ishizaki et al., 2000). At the concentrations used in our study, Y27632 is not thought to have significant inhibitory effects on proteins other than Rho-kinase (Uehata et al., 1997; Ishizaki et al., 2000; Nagumo et al., 2000).
In a porcine model of coronary artery spasm, intravenous administration of either HA1077 (Shimokawa et al., 1999) or Y27632 (Kandabashi et al., 2000) strongly inhibits agonist-induced vasospasm. Indeed, expression of Rho-kinase mRNA is significantly increased at the spastic site, suggesting up-regulation of Rho-kinase is in part responsible for coronary artery spasm in ischaemic heart disease. So far, the only in vivo human studies have involved the intra-arterial infusion of HA1077 to successfully treat the cerebral vasospasm associated with subarachnoid haemorrhage (Tachibana et al., 1999). Ours is the first demonstration of the effectiveness of a selective Rho-kinase inhibitor in human artery.
It has previously been suggested that Y27632 is less effective when applied before rather than after the vasoconstrictor agonist (Fu et al., 1998; Iizuka et al., 1999). This effect, of unexplained mechanism, might represent a practical problem in the use of Rho-kinase inhibitors to prevent vasospasm if applied to arteries prior to grafting on to the heart, and thus prior to exposure to endogenous vasoconstrictors. Although a comparison of pre- and post-application of Y27632 did reveal a slightly stronger effect with a post-application protocol, this was only statistically significant at a high concentration of Y27632. Thus, the discrepancy between pre- and post-application protocols does not appear to be a major factor in human conduit artery.
It has recently been shown that sodium nitroprusside and raised levels of intracellular cyclic GMP (acting through G-kinase) inhibit RhoA in smooth muscle (Sauzeau et al., 2000). This suggests that nitrovasodilators and phosphodiesterase inhibitors act in part via inhibition of Rho-mediated Ca2+-sensitization. It is thus perhaps not surprising that the most effective vasodilators of human LIMA and radial artery previously described act either as nitric oxide donors or by increasing intracellular levels of cyclic GMP (He et al., 1989; Cable et al., 1998; Sadaba et al., 2000; Salmenperä & Levy, 1996; He & Yang, 2000b).
Intracellular Ca2+ acts concentration-dependently to regulate contraction. The EC50 of this concentration-response curve (or the position of the ‘pCa curve') is modulated as a consequence of Rho-kinase activation, the EC50 for Ca2+ becoming smaller (the pCa curve shifting to the left) as a consequence of Ca2+-sensitization. Because RhoA appears to be basally active (Fu et al., 1998; Gong et al., 1996), it follows that the position of the pCa curve is in dynamic equilibrium. Thus, Rho-kinase inhibitors will shift the curve to the right and this presumably explains why Y27632 inhibited resting tone in the human LIMA. It also follows that Rho-kinase inhibitors may be effective against contraction evoked by any endogenous vasoconstrictor in human LIMA. We have shown that Y27632 inhibits contraction evoked by the thromboxane A2 agonist U46619 (Figures 3, 4 and 5), endothelin-1 (Figure 6) and phenylephrine (T Batchelor & D Beech, unpublished observation, 2000). Even if other agonists were to exert their effects principally via Ca2+ influx or Ca2+ release from the stores, inhibition of Rho-kinase should still be effective in inhibiting contraction.
Spasm of arterial grafts used for coronary artery surgery is a factor in determining the survival of both the graft and the patient. Our findings provide the first direct evidence that Rho-kinase inhibitors may be effective in preventing human vasospasm. It remains to be seen whether Rho-kinase inhibitors can be employed clinically. However, we speculate that they may be useful if injected into the mammary bed at the time of surgery, incubated with radial artery grafts prior to implantation, or administered systemically. In the future, methods for inhibiting Ca2+-sensitization may include viral transfer of dominant negative constructs of RhoA or Rho-kinase. Effective adenoviral gene transfer of nitric oxide synthase has been achieved in human radial arteries with an incubation period of 1 h (Cable et al., 1998). While our interests lie primarily with the prevention of arterial graft vasospasm, other groups have suggested that Rho-kinase inhibitors may be useful in the treatment of hypertension (Uehata et al., 1997), coronary artery spasm (Shimokawa et al., 1999; Kandabashi et al., 2000) and cerebral vasospasm following subarachnoid haemorrhage (Tachibana et al., 1999). Inhibition of Rho-kinase in a rat model also suppresses neointimal proliferation in arteries subjected to injury by balloon angioplasty (Sawada et al., 2000).
Rho-kinase inhibitors are very effective at preventing agonist-induced vasospasm in human left internal mammary artery, a vessel commonly used as a graft in coronary artery bypass surgery. They prevent spontaneous contraction as well as contraction evoked by several potent endogenous vasoconstrictor substances. The apparent dominance of the Rho-kinase pathway in mediating contraction of human conduit artery suggests this will be an important target for further drug development. The combination of the relaxant and antiproliferative effects of Rho-kinase inhibitors should confer significant therapeutic benefit.
Acknowledgments
The National Heart Research Fund, U.K. provided financial support. Y27632 was a gift from the Welfide Corporation, Japan.
Abbreviations
- ET-1
endothelin-1
- LIMA
left internal mammary artery
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- ROCC
receptor-operated Ca2+ channel
- SOCC
store-operated Ca2+ channel
- TXA2
thromboxane A2;
- VOCC
voltage-operated Ca2+ channel
References
- ASANO T., SUZUKI T., TSUCHIYA M., SATOH S., IKEGAKI I., SHIBUYA M., SUZUKI Y., HIDAKA H. Vasodilator actions of HA1077 in vitro and in vivo putatively mediated by the inhibition of protein kinase. Br. J. Pharmacol. 1989;98:1091–1100. doi: 10.1111/j.1476-5381.1989.tb12652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRITT G.J. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem. J. 1999;337:153–169. [PMC free article] [PubMed] [Google Scholar]
- CABLE D.G., CACCITOLO J.A., PEARSON P.J., O'BRIEN T., MULLANY C.J., DALY R.C., ORSZULAK T.A., SCHAFF H.Z. New approaches to prevention and treatment of radial artery graft vasospasm. Circulation. 1998;98:II-15–II-22. [PubMed] [Google Scholar]
- FAYMONVILLE M.E., DEBY-DUPONT G., LARBUISSON R., DEBY S., BODSON L., LIMET R., LAMY M. Prostaglandin E2, prostacyclin, and thromboxane changes during nonpulsatile cardiopulmonary bypass in humans. J. Thorac. Cardiovasc. Surg. 1986;91:858–866. [PubMed] [Google Scholar]
- FU X., GONG M.C., JIA T., SOMLYO A.V., SOMLYO A.P. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPγS-, and phorbol ester-induced Ca2+ sensitization of smooth muscle. FEBS Lett. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- GONG M.C., IIZUKA K., NIXON G., BROWNE J.P., HALL A., ECCLESTON J.F., SUGAI M., KOBAYASHI S., SOMLYO A.V., SOMLYO A.P. Role of guanine nucleotide-binding proteins – ras-family or trimeric proteins or both – in Ca2+ sensitisation of smooth muscle. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE G.-W. Arterial grafts for coronary artery bypass grafting: biological characteristics, functional classification, and clinical choice. Ann. Thorac. Surg. 1999;67:277–284. doi: 10.1016/s0003-4975(98)01207-7. [DOI] [PubMed] [Google Scholar]
- HE G.-W., ROSENFELDT F.L., BUXTON B.F., ANGUS J.A. Reactivity of human isolated internal mammary artery to constrictor and dilator agents. Implications for treatment of internal mammary artery spasm. Circulation. 1989;80:I-141–I-150. [PubMed] [Google Scholar]
- HE G.-W., YANG C.-G. Effect of thromboxane A2 antagonist GR32191B on prostanoid and nonprostanoid receptors in the human internal mammary artery. J. Cardiovasc. Pharmacol. 1995;26:13–19. doi: 10.1097/00005344-199507000-00003. [DOI] [PubMed] [Google Scholar]
- HE G.-W., YANG C.-G. Comparative study on calcium channel antagonists in the human radial artery: clinical implications. J. Cardiovasc. Surg. 2000a;119:94–100. doi: 10.1016/s0022-5223(00)70222-4. [DOI] [PubMed] [Google Scholar]
- HE G.-W., YANG C.-G. Vasorelaxant effect of phosphodiesterase-inhibitor milrinone in the human radial artery used as coronary bypass graft. J. Thorac. Cardiovasc. Surg. 2000b;119:1039–1045. doi: 10.1016/S0022-5223(00)70102-4. [DOI] [PubMed] [Google Scholar]
- HE G.-W., YANG C.-Q., STARR A. Overview of the nature of vasoconstriction in arterial grafts for coronary operations. Ann. Thorac. Surg. 1995;59:676–683. doi: 10.1016/0003-4975(94)01011-0. [DOI] [PubMed] [Google Scholar]
- HIMPENS B., KITAZAWA T., SOMLYO A.P. Agonist-dependent modulation of Ca2+ sensitivity in rabbit pulmonary artery smooth muscle. Pflügers Arch. 1990;417:21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- IIZUKA K., YOSHII A., TSUKAGOSHI H., TSUKAGOSHI H., ISHIZUKA T., DOBASHI K., NAKAZAWA T., MORI M. A major role for the Rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea. Br. J. Pharmacol. 1999;128:925–933. doi: 10.1038/sj.bjp.0702864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZAKI T., UEHATA M., TAMECHIKA I., KEEL J., NONOMURA K., MAEKAWA M., NARUMIYA S. Pharmacological properties of y-27632, a specific inhibitor of Rho-associated kinases. Mol. Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- KANDABASHI T., SHIMOKAWA H., MIYATA K., KUNIHIRO I., KAWANO Y., FUKATA Y., HIGO T., EGASHIRA K., TAKAHASHI S., KAIBUCHI K., TAKESHITA A. Inhibition of myosin phosphatase by upregulated Rho-kinase plays a key role for coronary artery spasm in a porcine model with interleukin-1b. Circulation. 2000;101:1319–1323. doi: 10.1161/01.cir.101.11.1319. [DOI] [PubMed] [Google Scholar]
- KIMURA K., ITO M., AMANO M., CHIHARA K., FUKATA Y., NAKAFUKU M., YAMAMORI B., FENG J., NAKANO T., OKAWA K., IWAMATSU A., KAIBUCHI K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- LEWIS R.S. Store-operated calcium channels. Advances in second messenger and phosphoprotein research. 1999;33:279–307. doi: 10.1016/s1040-7952(99)80014-7. [DOI] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.A., MOORES K.E., RINK T.J. SK&F 96365, a novel inhibitor of receptor-stimulated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL N., GODFRAIND T.Regulation of calcium channels in vascular smooth muscle Calcium antagonists 1993Dordrecht, The Netherlands: Kluwer Academic Publishers; 25–30.ed. Godfraind, T., Govoni, S., Paoletti, R. & Vanhoutte, P.M. pp [Google Scholar]
- NAGUMO H., SASAKI Y., ONO Y., OKAMOTO H., SETO M., TAKUWA Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am. J. Physiol. Cell. Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- ROSENFELDT F.L., HE G.-W., BUXTON B.F., ANGUS J.A. Pharmacology of coronary artery bypass grafts. Ann. Thorac. Surg. 1999;67:878–888. doi: 10.1016/s0003-4975(98)01299-5. [DOI] [PubMed] [Google Scholar]
- SADABA J.R., MATHEW K., MUNSCH C.M., BEECH D.J. Vasorelaxant effects of nicorandil on human radial artery. Eur. J. Cardiothorac. Surg. 2000;17:319–324. doi: 10.1016/s1010-7940(00)00325-0. [DOI] [PubMed] [Google Scholar]
- SALMENPERÄ M., LEVY J.H. The in vitro effects of phosphodiesterase inhibitors on the human internal mammary artery. Anesth. Analg. 1996;82:954–957. doi: 10.1097/00000539-199605000-00011. [DOI] [PubMed] [Google Scholar]
- SARABU M.R., MCCLUNG J.A., FASS A., REED G.E. Early postoperative spasm in left internal mammary artery bypass grafts. Ann. Thorac. Surg. 1987;44:199–200. doi: 10.1016/s0003-4975(10)62041-3. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE JEUNE H., CARIO-TOUMANIANTZ C., SMOLENNSKI A., LOHMANN S.M., BERTOGLIO J., CHARDIN P., PACAUD P., LOIRAND G. Cyclic GMP-dependent protein kinase signalling pathway inhibits RhoA-induced Ca2+ sensitisation of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- SAWADA N., ITOH H., UEYAMA K., YAMASHITA J., DOI K., CHUN T.-H., INOUE M., MASATSUGO K., SAITO T., FUKUNAGA Y., SAAKAGUCHI S., ARAI H., OHNO N., KOMEDA M., NAKAO K. Inhibition of Rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- SHIMOKAWA H., SETO M., KATSUMATA N., AMANO M., KOZAI T., YAMAWAKI T., KUWATA K., KANDABASHI KT., EGASHIRA K., IKEGAKI I., ASANO T., KAIBUCHI K., TAKESHITA A. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc. Res. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522.2:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACHIBANA E., HARADA T., SHIBUYA M., SAITO K., TAKAYASU M., SUZUKI Y., YOSHIDA J. Intra-arterial infusion of fasudil hydrochloride for treating vasospasm following subarachnoid haemorrhage. Acta Neurochir. 1999;141:13–19. doi: 10.1007/s007010050260. [DOI] [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- WATKINS W.D., PETERSON M.B., KONG D.L., KONO K., BUCKLEY M.J., LEVINE F.H., PHILBIN D.M. Thromboxane and prostacyclin changes during cardiopulmonary bypass with and without pulsatile flow. J. Thorac. Cardiovasc. Surg. 1982;84:250–256. [PubMed] [Google Scholar]
- YOSHII A., IIZUKA K., DOBASHI K., HORIE T., HARADA T., NAKAZAWA T., MORI M. Relaxation of contracted rabbit trachel and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+-sensitisation. Am. J. Resp. Cell. Mol. Biol. 1999;20:1190–1200. doi: 10.1165/ajrcmb.20.6.3441. [DOI] [PubMed] [Google Scholar]
- ZHANG X.-F., IWAMURO Y., ENOKI T., OKAZAWA M., LEE K., KOMURO T., MINOWA T., OKAMOTO Y., HASEGAWA H., FURUTANI H., MIWA S., MASAKI T. Pharmacological characterization of Ca2+ entry channels in endothelin-1-induced contraction of rat aorta using LOE 908 and SK&F 96365. Br. J. Pharmacol. 1999;127:1388–1398. doi: 10.1038/sj.bjp.0702661. [DOI] [PMC free article] [PubMed] [Google Scholar]


