Abstract
Human GABAA receptors containing different α and β subunits with or without the γ2S or γ2L subunits were expressed in Xenopus oocytes and the effects of the insecticides γ- and δ-hexachlorocyclohexane (γ-HCH and δ-HCH, respectively) on these receptor subunit combinations were examined using two electrode voltage-clamp procedures.
γ-HCH produced incomplete inhibition of GABA responses on all receptor combinations examined with affinities in the range of 1.1 – 1.9 μM. Affinity was not dependent on subunit composition but the maximum percentage of inhibition was significantly reduced in β1-containing receptors.
δ-HCH both potentiated GABAA receptors and activated them in the absence of GABA at concentrations higher than those producing potentiation. Allosteric enhancement of GABAA receptor function by δ-HCH was not affected by the subunit composition of the receptor, By contrast the GABA mimetic actions of δ-HCH were abolished in receptors containing either α4, β1 or γ2L subunits.
Sensitivity to the direct actions were not restored in receptors containing the mutant β1(S290N) subunit, but α1β2γ2L receptors became sensitive to the direct actions of δ-HCH when oocytes were treated for 24 h with the protein kinase inhibitor isoquinolinesulphonyl-2-methyl piperazine dihydrochloride (H-7).
We have shown the influence of various α, β and γ subunits on the inhibitory, GABA mimetic and allosteric effects of HCH isomers. The data reveal that neither the inhibitory actions of γ-HCH nor the allosteric effects δ-HCH has a strict subunit dependency. By contrast, sensitivity to the direct actions of δ-HCH are abolished in receptors containing α4, β1 or γ2L subunits.
Keywords: GABAA receptor, picrotoxin effects, barbiturate effects, Xenopus oocytes, hexachlorocyclohexane, barbiturate site, convulsant site, insecticides
Introduction
The γ-aminobutyric acid type A (GABAA) receptor is the major inhibitory receptor in the central nervous system, and is involved in the control of many neurological states such as vigilance, anxiety, wakefulness and seizures. Molecular biological studies have established that GABAA receptors are hetero-oligomeric proteins composed of combinations of different subunit peptides. In mammals, 20 GABAA receptor subunits (α1 – 6, β1 – 4, γ1 – 3, δ, ε, π, θ and ρ 1 – 3) have been cloned (Barnard et al., 1998, Bonnert et al., 1999). In addition, alternative splice isoforms have been found for some subunits, most notably for the γ2 subunit (γ2S and γ2L) (Whiting et al., 1990).
GABAA receptors have binding sites for diverse allosteric modulators including GABA, benzodiazepines, volatile and intravenous anaesthetics, loreclezole, divalent ions and neuroactive steroids. Studies of recombinant GABAA receptors have revealed the critical importance of subunit composition for a number of pharmacological properties of the GABAA receptor such as sensitivity to loreclezole, etomidate, benzodiazepines (Whiting et al., 1995) and furosemide (Korpi et al., 1995). For example, the type of α subunit present determines whether GABAA receptor display Type I or Type II benzodiazepine pharmacology (Pritchett et al., 1989a; Wafford et al., 1993). The α subunit also contributes to the GABA binding site (Ebert et al., 1994), influences the efficacy of barbiturate receptor activation (Thompson et al., 1996; Wafford et al., 1996) and sensitivity to furosemide (Thompson et al., 1999). The β subunit contributes to the GABA binding site (Amin & Weiss, 1993; Hadingham et al., 1993) and determines the effects of loreclezole (Wafford et al., 1994; Wingrove et al., 1994) and etomidate (Uchida et al., 1995; Hill-Venning et al., 1997; Belleli et al., 1997). In addition, the β subunit probably contributes to both barbiturate and propofol action (Amin, 1999; Pistis et al., 1999). The γ subunit confers benzodiazepine sensitivity (Pritchett et al., 1989b) and has a significant effect on the affinity for GABA (Sigel et al., 1990).
Insecticides such as the cyclodienes (Nagata & Narahashi, 1994), hexachlorocyclohexane (Nagata & Narahashi, 1995; Aspinwall et al., 1997) and avermectins (Payne & Sunderland, 1993) also bind to the GABAA receptor, which leads to receptor blockade and convulsions. Hexachlorocyclohexanes are of particular interest because they can either inhibit or enhance the action of GABA depending on the spatial orientation of the chloride atoms (Pomés et al., 1992; Woodward et al., 1992; Nagata & Narahashi, 1995; Aspinwall et al., 1997). For example, γ-hexachlorocyclohexane (γ-HCH, Lindane) inhibits the GABAA receptor, an effect that is probably mediated by binding at or near the picrotoxin site (Aspinwall et al., 1997; Wafford et al., 1999). In contrast the δ-hexachlorocyclohexane (δ-HCH) isomer is a positive allosteric modulator of the GABAA receptor and at concentrations greater than 10 μM it can directly activate GABAA receptors in a manner resembling the GABA mimetic effects of barbiturate and propofol (Aspinwall et al., 1997). Interestingly, γ-HCH behaves as a partial inverse agonist of the picrotoxin site and δ-HCH appears to interact with the barbitutate site or a site overlapping this site (Aspinwall et al., 1997; Wafford et al., 1999). Little is known about the influence of receptor subunit on the effects of insecticides on GABAA receptors, which would aid the elucidation of the binding sites of these substances on the GABAA receptor and their relationship with the picrotoxin and barbiturate sites. In this paper, we describe the influence of various receptor subunits to the inhibitory, allosteric modulatory and GABA mimetic effects of the HCH isomers.
Methods
Expression of human GABAA receptors in Xenopus oocytes
Stage V and VI oocytes were isolated from adult female Xenopus laevis and the theca and epithelial cell layer were removed mechanically with fine watchmaker forceps. Follicle cells were removed by 8 min incubation in Type IA collagenase (Sigma, U.K.) (0.5 mg ml−1) dissolved in modified Barth's solution (MBS) of the following composition: (in mM): NaCl 88; KCl 1; MgSO4 0.82; Ca(NO3)2 0.33; CaCl2 0.41; NaHCO3 2.5; N-2-Hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 10, pH 7.5) Oocyte nuclei were directly injected with 20 nl of sterile water containing different combinations of human GABAA subunit cDNAs (20 ng μl−1) engineered into expression vectors pcDM8 or pcDNAI/Amp. Oocytes were incubated for 24 to 48 h in MBS supplemented with 10 international units per ml penicillin; 10 μg ml−1 streptomycin; 50 μg ml−1 gentamycin and 90 μg ml−1 theophylline. For recording, oocytes were placed in a 50 μl bath and perfused with frog ringer solution (in mM: NaCl 115; KCl 2.5; CaCl2 1.8; HEPES 10, pH 7.6). Cells were impaled with two 0.6 – 2.5 MΩ agarose-cushioned electrodes containing 3 M KCL and were voltage clamped at −60 mV. Drugs were applied to the perfusate and GABA was applied to the peak of the response, which for most oocytes was 30 s or less. After establishing a maximal response to GABA using a 3 mM concentration, constant responses to an EC20/50 were obtained. An EC20/50 was the concentration of GABA that produced 20 or 50% of the maximum response obtained for GABA. Inhibition of the GABA response by γ-HCH or picrotoxin was investigated using an EC50 concentration, whereas the positive allosteric effect of δ-HCH on GABAA receptors was examined with an EC20 GABA concentration. At least a 3 min wash-out period was allowed between each drug application to prevent desensitization. Concentration-response curves were fitted using a non-linear fitting protocol (Prism 2.01, GraphPad, U.S.A.). The data were fitted to the logistic equation f(χ)=Bmax/[1+EC50/X)n], where Bmax is the response at the saturating concentration. EC50, the concentration of ligand producing half-maximal response; X, the concentration of ligand; and n, the Hill coefficient. In the case of γ-HCH, reversal of inhibition was observed at concentrations greater than 10 μM. Data points showing reversal of inhibition were ignored in the analysis; fitting such data is beyond the limits of the logistic equation. Data is presented as arithmetic means or geometric means, which were calculated from data obtained from a number (n) of different oocytes. The statistical significance of differences between mean values was assessed by Student's unpaired two-tailed t-test or one-way analysis of variance (ANOVA), wherever appropriate. A P value of <0.05 was considered statistically significant.
Drugs
Drugs used were GABA (Sigma, U.K.), picrotoxin (Sigma, U.K.), γ- and δ-HCH (Sigma, U.K.) and pentobarbital (Sigma, U.K.) Solutions of GABA and pentobarbital were made in saline. Picrotoxin and HCH isomers were prepared as 10−1 M stocks in DMSO. The highest concentration of DMSO vehicle perfusing the oocyte was 0.1%, which had no effects on GABA induced currents. γ- and δ-HCH were soluble ⩽100 μM in frog ringer solution.
Results
Inhibition of recombinant GABAA receptors by γ-HCH
The effect of γ-HCH on GABA EC50 responses of oocytes injected with various combinations of GABAA receptor subunits (Table 1) was examined using two-electrode voltage clamp techniques. As shown in Figures 1 and 2, γ-HCH caused partial inhibition of GABA EC50 responses in all receptors tested. Inhibition by γ-HCH was dose-dependent, giving a maximum inhibition that ranged from 31 to 64%, and gave no further inhibition at concentrations greater than 10 μM. The inhibitory effect of γ-HCH was reversed at concentrations greater than 10 μM in all of the receptor combinations studied except α1β1γ2S and α1β2γ2L. (Figures 1 and 2). The potency of γ-HCH was similar on all subunits tested (between 1.1 to 1.9 μM; Table 1). By contrast, the maximum percentage of inhibition of GABA EC50 responses was significantly affected by the type of β subunit present, varying from 31.2% on α1β1γ2S receptors to 46.3% on α1β2γ2S and 64.4% on α1β3γ2S receptors, respectively (Table 1). Maximum inhibition was not affected significantly by the type of α subunit present (45% on α4β2γ2s and 46.3% on α1β2γ2S receptors, respectively) or by presence or type of γ (between 46 to 64%; Table 1).
Table 1.
Summary of the data obtained with γ-HCH on the inhibition of GABA EC50 responses on oocytes expressing various human GABAA receptors
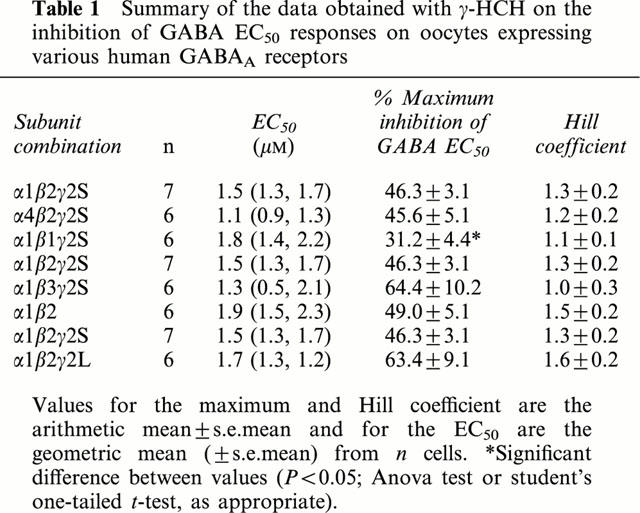
Figure 1.
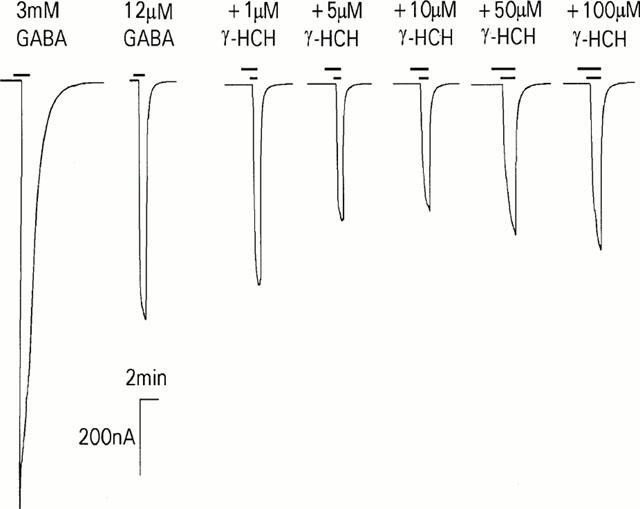
The effects of GABAA receptor subtypes on the inhibition of GABA EC50 responses by γ-HCH. Typical GABA responses on oocytes expressing human α1β2γ2S recombinant GABAA receptors voltage-clamped with two-electrodes. A maximum GABA response is followed by an approximate EC50 concentration, subsequent responses show the effect of increasing concentrations of γ-HCH on control GABA EC50 responses. Drugs were applied as indicated by the bars.
Figure 2.
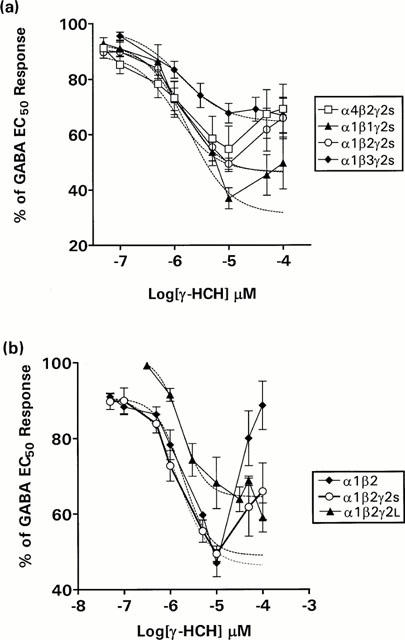
Concentration-response curve for the effects of γ-HCH on GABA EC50 responses on oocytes expressing α1β1γ2S, α1β2γ2S, α1β3γ2S and α4β2γ2S (a) and α1β2, α1β2γ2s, and α1β2γ2L GABAA receptors (b). Each data point represents the arithmetic mean±s.e.mean of 6 – 8 experiments. Data were calculated as a percentage of GABA EC50 responses. Data points showing reversal of inhibition (typically at concentrations of γ-HCH>10 μM) were omitted for the curve fitting procedure; however, the data points are shown in the plots (solid lines). Dashed lines show the fitted curve.
The role of α, β and γ2S subunits on inhibition of GABA responses by picrotoxin
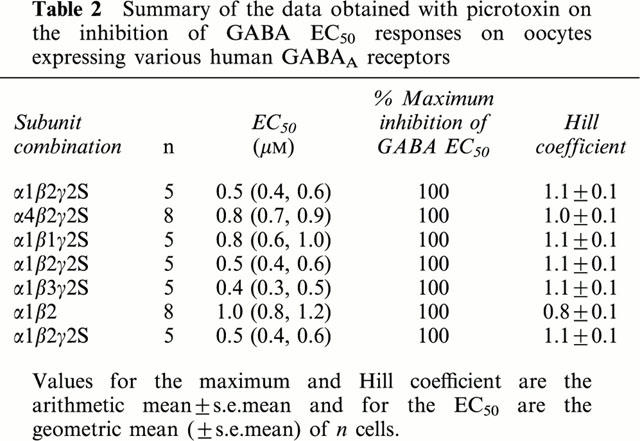
In order to compare the subunit dependency of the inhibition of the GABAA receptors by γ-HCH directly to that of picrotoxin, we investigated the effect of the α1, α4, β1-3 and γ2S subunits on the inhibition of GABA EC50 responses by picrotoxin. The affinity for the inhibition of GABA EC50 responses by picrotoxin was similar on all receptor combinations tested (mean 0.4 μM, 0.4 – 1 μM) (Table 2). Although picrotoxin appeared to have more affinity for α1β3γ2S receptors (EC50 0.43 μM) the difference was not statistically significant. In all receptors studied, picrotoxin gave maximum inhibition at 100 μM. These results are in accord with studies of murine GABAA receptors that have shown that picrotoxin action is unaffected by the subunit composition of GABAA receptors (Krishek et al., 1996).
Table 2.
Summary of the data obtained with picrotoxin on the inhibition of GABA EC50 responses on oocytes expressing various human GABAA receptors
Potentiation of the GABA response by δ-HCH
The positive allosteric effects of δ-HCH were studied on α(1,4)β2γ2S, α1β(1,2,3)γ2S, α1β2γ2L and α1β2 receptors. Measurement of potentiation of GABA EC20 responses included the direct activation component. As shown in Figure 3, δ-HCH caused a dose dependent potentiation of GABA EC20 responses on all receptor tested. The type of α subunit present did not influence the EC50 or efficacy of δ-HCH allosteric effects. Table 3 shows the range of EC50 and maximum inhibition values determined on α1β2γ2S (EC50 16 μM, maximum potentiation 248%) and α4β2γ2S (EC50 19.5 μM and maximum potentiation 231±%) receptors. The presence or type of γ subunit present did not influence the positive allosteric effect of δ-HCH on GABA EC20 responses. Both the EC50 and maximum potentiation of GABA EC20 responses were similar on α1β2γ2S, α1β2γ2L receptors and α1β2 receptors (16.2 μM and 248%, 13.6 μM and 281%, 19.9 μM and 254%, respectively). By contrast, the β subunit significantly affected affinity such that δ-HCH was less potent on receptors containing the β1 subunit (Figure 3, Table 3). However, the alteration of the β-subunit had no effect on the efficacy of δ-HCH (Table 3). The rank order of potency was α1β1γ2s <α1β2γ2s=α1β3γ2s. Neither the significance pattern nor the rank order of potency changed by omitting the direct action of δ-HCH from the measurement of potentiation of GABA EC20 responses (data not shown).
Figure 3.
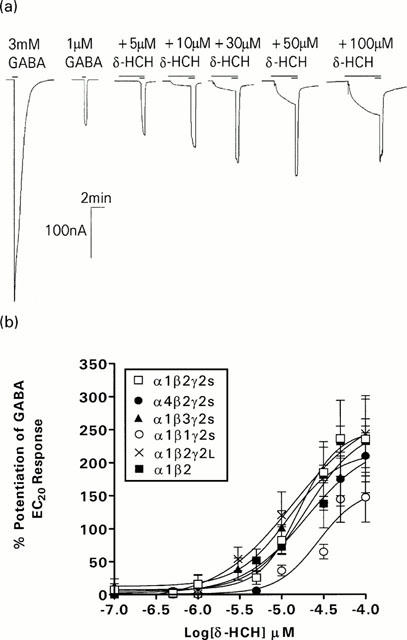
Typical current responses on oocytes expressing human α1β2γ2S recombinant GABAA receptors. (a) A maximum GABA response is followed by an approximate EC20 response, subsequent responses show the effects of increasing concentrations of δ-HCH on the control GABA EC20 response. (b) Concentration-response curve for the effects of δ-HCH on GABA EC20 responses on oocytes α1β2γ2S, α4β2γ2S, α1β1γ2S, α1β3γ2S and α1β2 GABAA receptors. Each point represents the arithmetic mean±s.e.mean of 6 – 7 experiments and was calculated as a percentage increase of the GABA EC20.
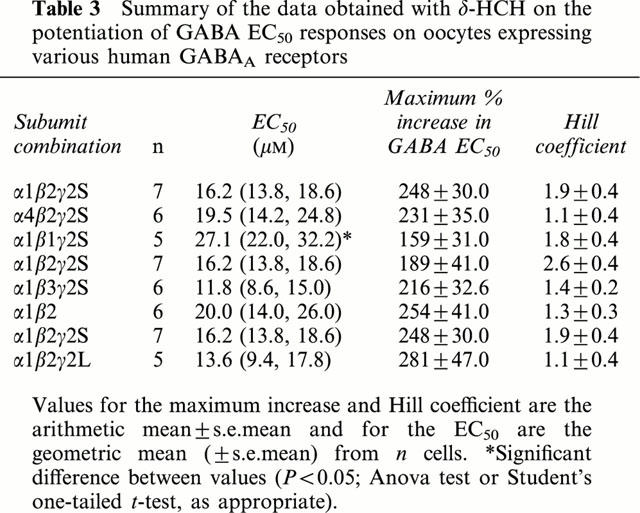
Table 3.
Summary of the data obtained with δ-HCH on the potentiation of GABA EC50 responses on oocytes expressing various human GABAA receptors
Direct activation of the recombinant human GABAA receptor by δ-HCH
Previous studies have shown that the direct effect of δ-HCH is not significantly different in α1β3γ2s or α6β3γ2s receptors (Aspinwall et al., 1997). To further investigate the role of α subunits we investigated the direct effects of δ-HCH on α4β2γ2s receptors. As shown in Figure 4, Table 4, the direct effects of δ-HCH on α1β2γ2S and α4β2γ2S receptors differed significantly. In oocytes expressing α1β2γ2S, δ-HCH evoked dose-dependent currents with an EC50 value of 42.2 μM and efficacy of 30.5%. However, the direct effect of δ-HCH was almost abolished on α4β2γ2s receptors, producing currents that were only 5% of the maximal GABA response. These results are comparable to those reported by Wafford et al., (1996), where α4-containing receptors were not directly activated by pentobarbital or propofol.
Figure 4.
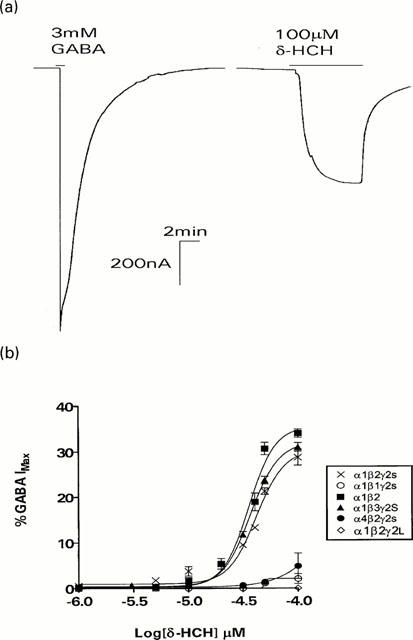
The effect of subunit isoform present within GABAA receptors on the GABA mimetic action of δ-HCH. (a) Traces show typical maximum responses for GABA and δ-HCH of an oocyte injected with α1, β2 and γ2S cDNAs. To elicit maximum responses the oocyte was superfused with 3 mM GABA or 100 μM δ-HCH. (b) Concentration-response curves for the direct effect of δ-HCH on oocytes expressing α1β2γ2S, α4β2γ2S, α1β1γ2S, α1β3γ2S, α1β2γ2L, α1β2 and the mutant α1β1 (S290N) γ2S GABAA receptors. Each point represents the arithmetic mean±s.e.mean of 5 – 7 experiments and was calculated as a percentage of the response obtained with a maximum concentration of GABA (3 mM).
Table 4.
Summary of the data obtained for the direct effect of δ-HCH on oocytes expressing various GABAA receptors
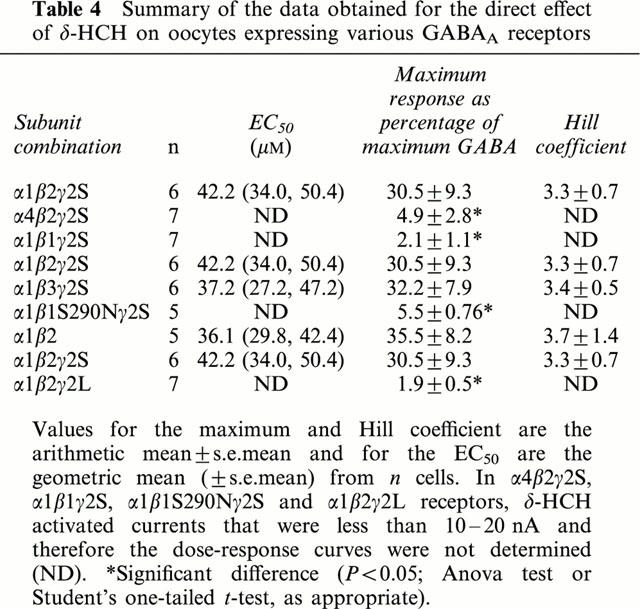
Effect of the β subunit on the direct actions of δ-HCH
The influence of β subunits on the direct effect of δ-HCH was studied on α1βγ2s receptors containing β1, β2, or β3 subunits. δ-HCH induced dose-dependent currents in both α1β2γ2S and α1β3γ2S receptors but not in β1 containing receptors. Table 4 shows that there were no significant differences between the direct effects of δ-HCH on α1β2γ2S (30.5% of the maximum GABA response; EC50 42.2 μM) or α1β3γ2S (32.2% of the maximum GABA response; EC50 37.2 μM). However, on β1-containing (α1β1γ2S) receptors, δ-HCH had no significant direct effects (efficacy 2.1%) (Figure 4, Table 4).
The data generated suggested that the presence of the β1 subunit abolished sensitivity to the GABA mimetic actions of δ-HCH. The exchange of a serine residue (S290) in the β1 subunit for an asparagine, which is found in the corresponding positions of the β2 and β3 subunits (N289 and N290, respectively), confers sensitivity to both loreclezole (Wingrove et al., 1994) and etomidate (Belleli et al., 1997) on receptors containing the mutant β1 subunit and influences direct activation by etomidate (Belleli et al., 1997). We investigated therefore whether S290 also influenced sensitivity to the direct actions of δ-HCH by testing the effect of δ-HCH on α1β1S290Nγ2S mutant receptors. δ-HCH did not activate currents on α1β1(S290N)γ2S mutant receptors in the absence of GABA, suggesting that this amino acid does not confer direct activation by δ-HCH (Table 4).
The γ subunit and the direct effects of δ-HCH
The role of the γ2S-subunit on the direct effect of δ-HCH was investigated by comparing α1β2 and α1β2γ2S recombinant human GABAA receptors. δ-HCH directly activated α1β2 with and efficacy of 35.5% and EC50 36.1 μM. These values were not significantly different from the affinity and efficacy observed on α1β2γ2S receptors (42.2 μM and 30.5%, respectively). By contrast, when the γ2S subunit was replaced by the γ2L subunit the direct effects of δ-HCH were abolished.
The effect of the γ2L subunit on the GABA mimetic effects of δ-HCH suggested that phosphorylation by protein kinase C may be important for the activation of GABAA receptors by δ-HCH. To test that possibility we modified the state of phosphorylation of oocytes expressing α1β2γ2L receptors. After 48 h post-injection of cDNAs oocytes were incubated overnight in MBS medium containing 0.2 mM of the protein kinase C inhibitor isoquinolinesulphonyl-2-methyl piperazine dihydrochloride (H-7). As shown in Figure 5a, 100 μM (a concentration that activates 30.5% of maximal GABA responses on α1β2γ2S receptors) δ-HCH elicited responses in oocytes treated with H-7 that were approximately 20% of maximal GABA responses, restoring direct activation by δ-HCH. Treatment with H-7 did not modify the potentiating effect of γ-HCH on α1β2γ2L receptors (Figure 5), which suggests that protein kinase C-dependent phosphorylation affects only the GABA mimetic actions of δ-HCH.
Figure 5.
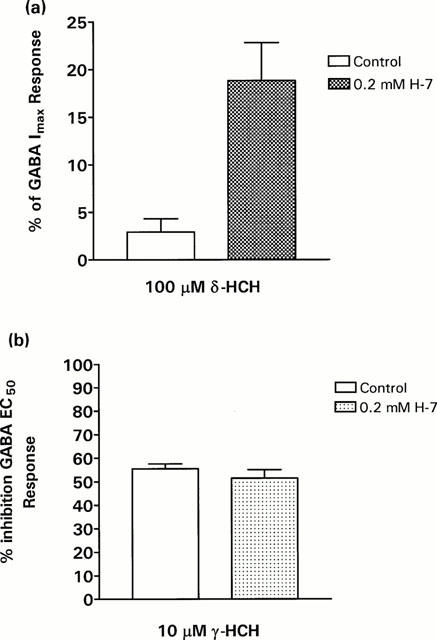
Effects of phosphorylation on receptor activation by δ-HCH in oocytes expressing α1β2γ2L GABAA receptors. In (a) the direct action of 100 μM δ-HCH is shown in either untreated oocytes or those which have been incubated for 24 h in 0.2 mM isoquinolinesulphonyl-2-methyl piperazine dihydrochloride (H-7), a protein kinase C inhibitor. In (b) blockade of α1β2γ2 receptors by 10 μM γ-HCH is shown in both untreated oocytes and H-7 preincubated oocytes. Each bar represents the mean±s.e.mean of 8 – 10 oocytes tested. Oocytes were from at least three different donor frogs.
Discussion
The present study shows that using a wide range of receptor GABAA receptor subtypes, γ-HCH and δ-HCH isomers interact with GABAA receptors in an opposite fashion, to either partially inhibit GABA-induced currents (γ-HCH), or enhance sub-maximal GABA responses (δ-HCH). δ-HCH also directly activates the receptor in a subtype dependent manner. This study also reveals that at high concentrations of γ-HCH, the inhibition reverses, potentiating GABA induced currents, which is again a subtype dependent phenomenon. This potentiation may well be related to that observed with the δ-isomer.
The potency of γ-HCH and picrotoxin appear to be relatively unaffected by both α and γ2S subunits. The type of β subunit present does not affect picrotoxin action, although it influences maximum inhibition of GABA EC50 responses by γ-HCH. However, this effect, as discussed in the previous paragraph, may be a consequence of potentiation of GABAA receptor function by γ-HCH, which is influenced by the type of β subunit present, rather than from a direct effect on the inhibitory action of γ-HCH. Thus, neither picrotoxin nor γ-HCH block of GABAA receptors appear to be affected by receptor subunit composition, which support our early view that γ-HCH and picrotoxin interact with the same site on GABAA receptors. Additional evidence for this hypothesis comes from electrophysiological studies that showed that γ-HCH induces a dose-dependent rightward shift in the picrotoxin dose-response curve (Aspinwall et al., 1997). Furthermore γ-HCH also displaces 35S-tert-butylbycyclophosphorothionate (35S-TBPS) binding to mouse fibroblast cell lines expressing human GABAA receptors (Aspinwall et al., 1997) and neuronal membranes (Pomés et al., 1992). In addition, mutations in the TM2 residue A302 in the Drosophila Rdl receptor reduces sensitivity to both picrotoxin and γ-HCH (Zhang et al., 1994; Belleli et al., 1995). Thus, overall the inverse agonist model of activity seems firmly supported by empirical evidence.
Similar to several other allosteric modulators, in addition to enhancing GABA responses, δ-HCH possess GABA-mimetic activity at concentrations greater than those required for potentiation of sub-maximal GABA responses. A range of structurally unrelated compounds also displays both allosteric and GABA mimetic actions, including barbiturates (Thompson et al., 1996), etomidate (Uchida et al., 1995; Hill-Venning et al., 1997), loreclezole (Wafford et al., 1994), propofol (Sanna et al., 1995) and alphaxalone (Belleli et al., 1996). It is still unclear whether this mimetic effect is mediated via a distinct locus, or through the same binding site. Evidence for a separate locus comes from studies of α4β1γ2S receptors (Wafford et al., 1994), which are enhanced by pentobarbitone but lack sensitivity to the direct actions of the anaesthetic. Moreover, in the Drosophila Rdl the exchange of a methionine residue (M314) in TM2 for a serine (the equivalent position in GABAA β1 subunit) confers sensitivity to the direct actions of barbiturates (Pistis et al., 1999). This study provides additional support for separate allosteric and GABA mimetic sites because receptor combinations that were insensitive to the GABA mimetic effects of δ-HCH (α4β2γ2S, α1β1γ2S and α1β2γ2L) were sensitive to the positive allosteric effects of δ-HCH.
The type of α and β subunits present in recombinant GABAA receptors influence the potency and efficacy of the GABA mimetic action of δ-HCH. There is no significant difference in the sensitivity of α1 (this study) or α6 (Aspinwall et al., 1997) containing βγ2S receptors, but α4 containing β2/3γ2S receptors are insensitive to the direct actions of δ-HCH. The α4β1γ2S receptor combination is also insensitive to the direct actions of both pentobarbital and propofol (Wafford et al., 1996). The α4 subunit is most closely related to the α6 subunit, which is, however, highly sensitive to the direct actions of barbiturates (Thompson et al., 1996; Wafford et al., 1996) and propofol (Wafford et al., 1996). Residues in TM2 (Belleli et al., 1999; Pistis et al., 1999) and TM3 (Amin, 1999) have been found to confer sensitivity to the GABA mimetic barbiturate. It is however unlikely that TM2 residues in the α4 subunit influence sensitivity to the direct actions of barbiturates, propofol or δ-HCH because the TM2 domain in α4 and α6 subunits are identical (Wafford et al., 1996).
β1-containing receptors were insensitive to the GABA mimetic effects of δ-HCH, although on β2- and β3-containing receptors δ-HCH activated currents with comparable potency and efficacy. This is in contrast to the GABA activation by pentobarbital, which is not abolished in the presence of the β1 subunit, although the affinity and efficacy of barbiturate direct actions is lower at α1β1γ2S receptors than those containing β2 or β3 subunits (Thompson et al., 1996). Thus, δ-HCH may still bind to a site for direct activation on β1-containing GABAA receptors and activate the transduction mechanism but with a significantly reduced affinity and efficacy.
Residue S290 in the β1 subunit reduces sensitivity to the anti-convulsant loreclezole (Wafford et al., 1994; Wingrove et al., 1994) and the anaesthetic etomidate (Belleli et al., 1997). S290N did not affect the GABA mimetic effects of barbiturates (Pistis et al., 1999), and in this report does not affect the direct effect of δ-HCH. These data support our view of a related GABA-mimetic barbiturate/δ-HCH site.
The role of the γ-subunit is intriguing. So far, this subunit has not been found to affect significantly the direct actions of barbiturates or loreclezole. However, a significant increase in the efficacy of propofol has been noted with the removal of the γ2L-subunit (Lam & Reynolds, 1998) and both α3β1γ2L and α6β3γ2L receptors are insensitive to the direct actions of δ-HCH (Pistis et al., 1999). The γ2L and γ2S subunits are identical with the exception of an eight-amino acid segment containing a unique protein kinase C phosphorylation site (Whiting et al., 1990). It has been suggested that this unique phosphorylation site may affect the coupling between allosteric sites (Leidenheimer et al., 1993). In the case of the GABA mimetic effects of barbiturates and δ-HCH, the site may influence affinity and/or efficacy.
Our results confirm that HCH isomers act at distinct sites on GABAA receptors to inhibit, enhance or activate GABAA receptor function. The pattern of subunit dependency for the three effects supports our view that inhibition of GABA function by γ-HCH is mediated via the picrotoxin binding site, whereas the GABA-mimetic and allosteric effects of δ-HCH is mediated via the barbiturate GABA-mimetic and allosteric sites, respectively.
Acknowledgments
The authors would like to thank Paul Whiting and Peter Wingrove for the GABA cDNAs.
Abbreviations
- δ-HCH
δ-hexachlorocyclohexane
- γ-HCH
γ-hexachlorocyclohexane
- HEPES
N-2-Hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- H-7
isoquinolinesulphonyl-2-methyl piperazine dihydrochloride
- MBS
modified Barth's solution
- 35S-TBPS
35S-tert-butylbycyclophosphorothionate
References
- AMIN J. A single hydrophobic residue confers barbiturate sensitivity to γ-aminobutyric acid type C receptor. Mol. Pharmacol. 1999;55:411–423. [PubMed] [Google Scholar]
- AMIN N.J., WEISS D.S. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- ASPINWALL L.S., BERMUDEZ I., KING L.A., WAFFORD K.A. The interactions of hexachlorocyclohexane isomers with human γ-aminobutyric acidA receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1997;282:1557–1564. [PubMed] [Google Scholar]
- BARNARD E.A., SKOLNICK P., OLSEN R.W., MOHLER H., SIEGHART W., BIGGIO G., BRAESTRUP C., BATESON A.N., LANGER S.Z. International union of pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- BELLELI D., CALLACHAN H., HILL-VENNING C., PETERS J.A., LAMBERT J.J. Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors in Xenopus laevis oocytes. Br. J. Pharmacol. 1996;118:563–576. doi: 10.1111/j.1476-5381.1996.tb15439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLELI D., HOPE A.G., CALLACHAN H., HILL-VENNING C., PETERS J.A., LAMBERT J.J. A mutation the putative M2 domain of a Drosophila GABA receptor subunit differentially affects agonist potency. Br. J. Pharmacol. 1995;116:442P. [Google Scholar]
- BELLELI D., LAMBERT J.J., PETERS J.A., WAFFORD K.A., WHITING P.J. The interaction of the general anaesthetic etomidate with the γ-aminobutyric acid type A receptor is influenced by a single amino acid. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLELI D., PAU D., CABRAS G., PETERS J.A., LAMBERT J. A single amino acid confers barbiturate sensitivity upon ρ1 receptor. Br. J. Pharmacol. 1999;127:601–604. doi: 10.1038/sj.bjp.0702611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNERT T.P., MCKERNAN R.M., FARRAR S., LE BOURDELLES B., HEAVENS R.P., SMITH D.W., HEWSON L., RIGBY M.R., SIRINATHSINGHJI D.J., BROWN N., WAFFORD K.A., WHITING P.J. Theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBERT B., WAFFORD K.A., WHITING P.J., KROGSGAARD-LAARSEN P., KEMP J.A. Molecular pharmacology of γ-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different α, β & γ receptor subunit combinations. Mol. Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- HADINGHAM K.L., WINGROVE P.B., WAFFORD K.A., BAIN C., KEMP J.A., PALMER K.J., WILSON A.W., WICOX A.S., SIKELA J.M., RAGAN C.I., WHITING P.J. Role of the β-subunit in determining the pharmacology of human γ-aminobutyric acid type A receptors. Mol. Pharmacol. 1993;44:1211–1218. [PubMed] [Google Scholar]
- HILL-VENNING C., BELLELI D., PETERS J.A., LAMBERT J.J. Subunit-dependant interaction of the general anaesthetic etomidate with the γ-aminobutyric acid type A receptor. Br. J. Pharmacol. 1997;120:749–756. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORPI E.R., KUNER T., SEEBURG P.H., LÜDDENS H. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol. Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- KRISHEK B.J., MOSS S.J., SMART T.G. A functional comparison of the antagonists bicuculline and picrotoxin at recombinant GABAA receptors. Neuropharmacology. 1996;35:1289–1298. doi: 10.1016/s0028-3908(96)00089-5. [DOI] [PubMed] [Google Scholar]
- LAM D.W., REYNOLDS J.N. Modulatory and direct effects of propofol on recombinant GABA(A) receptors expressed in Xenopus oocytes: Influence of alpha- and gamma(2)-subunits. Brain Res. 1998;784:179–187. doi: 10.1016/s0006-8993(97)01334-6. [DOI] [PubMed] [Google Scholar]
- LEIDENHEIMER N.J., WHITING P.J., HARRIS A.R. Activation of calcium-phospholipid-dependent protein kinase enhances benzodiazepine and barbiturate potentiation of the GABAA receptor. J. Neurochem. 1993;60:1972–1975. doi: 10.1111/j.1471-4159.1993.tb13432.x. [DOI] [PubMed] [Google Scholar]
- NAGATA K., NARAHASHI T. Dual action of the cyclodiene insecticide dieldrin on the γ-aminobutyric acid-chloride channel complex of rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 1994;269:164–171. [PubMed] [Google Scholar]
- NAGATA K., NARAHASHI T. Differential effects of hexachlorocyclohexane isomers on the GABA receptor-chloride channel complex in rat dorsal root ganglionic neurones. Brain Res. 1995;704:85–91. doi: 10.1016/0006-8993(95)01108-0. [DOI] [PubMed] [Google Scholar]
- PAYNE G.T., SUNDERLAND D.M. Actions of avermectin analogues on γ-aminobutyric acid (GABA)-sensitive and GABA-insensitive chloride channels in mouse brain. Pesticide Biochem. Physiol. 1993;47:178–184. [Google Scholar]
- PISTIS M., BELLELI D., MCGURK K., PETERS J.A., LAMBERT J.J. Complementary regulation of anaesthetic activation of human α6β3γ2L and Drosophila RDL GABA receptors by a single amino acid residue. J. Physiol. 1999;515:3–18. doi: 10.1111/j.1469-7793.1999.003ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POMÉS A., RODRIGUEZ-FARRE E., SUÑOL C. Inhibition of t-[35S] butylbicyclophosphorothionate binding by convulsant agents in primary cultures of cerebellar neurons. Dev. Brain. Res. 1992;73:85–90. doi: 10.1016/0165-3806(93)90049-g. [DOI] [PubMed] [Google Scholar]
- PRITCHETT D.B., LUDDENS H., SEEBURG P.H. Type I and type II GABAA benzodiazepine receptor produced in transfected cells. Science. 1989a;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- PRITCHETT D.B., SONTHEIMER H., SHIVERS B.H., YMER S., KETTENMANN H., SCHOFIELD P.H., SEEBURG P.H. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989b;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- SANNA E., MASCIA M.P., KLEIN R.L., WHITING P.J., BIGGO G., HARRIS R.A. Actions of general anaesthetic propofol on recombinant human GABAA receptors: influence of receptor subunits. J. Pharmacol. Exp. Ther. 1995;274:353–360. [PubMed] [Google Scholar]
- SIGEL E., BAUR R., KELLENBERGER S., MALHERBE P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- THOMPSON S.A., ARDEN S.A., MARSHALL G., WINGROVE P.B., WHITING P.J., WAFFORD K.A. Residues in transmembrane domain I and II determine γ-aminobutyric acid type A receptor subtype-selective antagonism by furosemide. Mol. Pharmacol. 1999;55:993–999. doi: 10.1124/mol.55.6.993. [DOI] [PubMed] [Google Scholar]
- THOMPSON S.A., WHITING P.J., WAFFORD K.A. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br. J. Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UCHIDA I., KAMACHI G., BURT D., YANG J. Etomidate potentiation of GABAA receptor gated currents depends on subunit composition. Neurosci. Lett. 1995;185:203–206. doi: 10.1016/0304-3940(95)11263-v. [DOI] [PubMed] [Google Scholar]
- WAFFORD K.A., BAIN C.J., QUIRK K., MCKERNAN R.M., WINGROVE P.B., WHITING P.J., KEMP J.A. A novel allosteric modulatory site on the GABAA receptor β-subunit. Neuron. 1994;12:775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- WAFFORD K.A., MASKELL P.D., ASPINWALL L.A., BEADLE D.J., BERMUDEZ I.The effects of hexachlorocyclohexane isomers on human GABAA receptors Neurotox '98 1999100–105.ed. Beadle, D.J. Royal Society of Chemistry pp
- WAFFORD K.A., THOMPSON S.A., THOMAS D., SIKELA J., WILCOX A.S., WHITING P.J. Functional characterisation of human γ-aminobutyric acidA receptors containing the α4 subunit. Mol. Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- WAFFORD K.A., WHITING P.J., KEMP J.A. Differences in affinity and efficacy of benzodiazepine receptor ligands at recombinant γ-aminobutyric acidA receptor subtypes. Mol. Pharmacol. 1993;43:240–244. [PubMed] [Google Scholar]
- WHITING P., MCKERNAN R.M., WAFFORD K.A. Structure and pharmacology of vertebrate GABAA receptor subtypes. Int. Rev. Neurobiol. 1995;38:95–138. doi: 10.1016/s0074-7742(08)60525-5. [DOI] [PubMed] [Google Scholar]
- WHITING P., MCKERNAN R.M., IVERSEN L.L. Another mechanism for creating diversity in γ-aminobutyrate type A receptors: RNA splicing directs expression two forms of the γ2 subunit, one of which contains a protein kinase C phosphorylation site. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINGROVE P.P., WAFFORD K.A., BAIN C., WHITING P.J. The modulatory action of loreclezole at the γ-aminobutyric acid type A receptor is determined by a single amino acid in the β2- and β3-subunit. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4569–4573. doi: 10.1073/pnas.91.10.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODWARD R.M., POLENZANI L., MILEDI R. Effects of Hexachlorocyclohexane on γ-aminobutyric acid receptors expressed in Xenopus oocytes by RNA from mammalian brain and retina. Mol. Pharmacol. 1992;41:1107–1115. [PubMed] [Google Scholar]
- ZHANG H-G., FFRENCH-CONSTANT R.H., JACKSON M.B. A unique amino acid of the Drosophila GABA receptor with influence on drug sensitivity by two mechanisms. J. Physiol. 1994;479:65–75. doi: 10.1113/jphysiol.1994.sp020278. [DOI] [PMC free article] [PubMed] [Google Scholar]


