Abstract
Paracetamol (5 mmol kg−1, i.p.) caused liver damage in rats as indicated by increased plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT) and glutamate dehydrogenase (GDH) activities. No change in plasma bilirubin or creatinine was noted. An equimolar dose of nitroparacetamol (a nitric oxide (NO)-releasing derivative of paracetamol) did not alter plasma levels of any of the markers of liver/kidney damage. No difference in plasma or liver paracetamol was apparent in animals injected with paracetamol or nitroparacetamol. These results indicate that NO released from nitroparacetamol exhibits hepatoprotective activity in these animals and suggest that nitroparacetamol may therefore be considered as a safer alternative to paracetamol in the clinic.
Keywords: Nitroparacetamol, paracetamol, liver, kidney
Introduction
Paracetamol (acetaminophen) is a potent analgesic and anti-pyretic agent but is relatively devoid of anti-inflammatory activity. We have recently reported that a nitric oxide (NO)-releasing derivative of paracetamol (i.e. nitroparacetamol, NCX-701) exhibits potent antinociceptive and anti-inflammatory activity in the rat (al-Swayeh et al., 2000). In these experiments, nitroparacetamol was some 20 times more potent than paracetamol. Unlike aspirin and related nonsteroidal anti-inflammatory drugs (NSAID), paracetamol administration is not associated with gastrointestinal haemorrhage or ulceration. Indeed, paracetamol pretreatment has actually been reported to protect against aspirin and ethanol-induced gastric mucosal damage in man (Stern et al., 1984). The high potency and lack of gastrointestinal side effects of this drug have led to the widespread use of paracetamol which is regarded as a safer alternative to NSAID for mild to moderate analgesia. However, high doses of paracetamol do cause severe and often fatal acute liver failure in man (e.g. Plevris et al., 1998). The recent finding that nitroaspirin (but not aspirin) reduces concanavalin A-induced liver damage in mice (Fiorucci et al., 2000) suggests that NO-NSAID exhibit hepatoprotective activity. Whether NO released from nitroparacetamol also affects liver function in vivo is not known. In order to examine this possibility, we have evaluated the effect of equimolar doses of paracetamol and nitroparacetamol on liver function in the intact rat
Methods
Drug treatments
Rats (male, Sprague-Dawley, 100 – 130 g) were used in this study. All experiments were undertaken in accordance with the European Community Council Directive 86/609 (OJL 358, 1, December 12, 1987) on the use of animals in the laboratory. Animals received either no treatment (i.e. ‘naïve'), paracetamol, nitroparacetamol (both 5 mmol kg−1, i.p.) or an appropriate volume (10 ml kg−1, i.p.) of vehicle (0.9% w v−1 NaCl containing 20% v v−1 Tween-20). After an interval of 6 h, animals were killed by cervical dislocation, trunk blood collected into heparinised tubes (500 u ml−1) and centrifuged (2000×g, 5 min, room temperature). Plasma was aspirated and stored at −70°C until assayed as described below. The liver was also removed and stored at −70°C until required.
Measurement of plasma markers of liver and kidney function
Plasma aspartate aminotransferase (AST), alaninine aminotransferase (ALT), glutamate dehydrogenase (GDH), creatinine and bilirubin were assayed spectrophotometrically using commercially available kits (Sigma Ltd, U.K.) according to standard laboratory techniques (Horder et al., 1981). Plasma and liver paracetamol concentrations were determined after deproteinization of samples with trichloroacetic acid (180 mM) again using a commercially available kit (Sigma Ltd, U.K.) as described elsewhere (Waldberg, 1977).
Preparation of liver homogenate
Livers were thawed, weighed and homogenized (Ultra-Turrax, type 18/2N) in five volumes of Tris-HCl buffer (5 mM containing 2 mM EDTA, pH 7.4). Homogenates were centrifuged (2000×g, 5 min, room temperature) and the supernatant used immediately for the assays described below.
Measurement of plasma and liver nitrate/nitrite concentrations
Nitrate/nitrite concentration in samples of plasma and liver homogenates was estimated spectrophotometrically using the Greiss reagent essentially as described by Szabo et al. (1996). Briefly, aliquots of plasma or liver homogenate (100 μl) were incubated (37°C, 30 min) with nitrate reductase (60 mU) in the presence of NADPH (25 μM) to reduce nitrate to nitrite. Thereafter, nitrite was assayed by addition of an equal volume of Greiss reagent (composition: 5% v v−1 H3PO4 containing 1% w v−1 sulphanilic acid and 0.1% w v−1 N-1-napthylenediamine) and absorbance determined at 550 nm using an Anthos Labtech HT3 plate reader. Results are expressed as nitrite concentration in μM. The limit of detection of the assay in our hands is 0.5 μM. Nitrite concentration was determined against a standard curve of sodium nitrite (0 – 50 μM).
Statistical analysis
Results show mean±s.e.mean. Statistical significance of differences between groups was determined by ANOVA followed by post-hoc Dunnett's test or by unpaired Student's t-test as appropriate. A probability (P) value of less than 0.05 was taken to indicate statistical significance.
Drugs and chemicals
Nitroparacetamol (4-(nitroxy) butanoic acid 4-acetylaminophenyl ester; NCX-701) was the generous gift of Dr P. del Soldato (NicOx Ltd, Sophia-Antipolis. France). All other chemicals and reagents were purchased from Sigma Ltd., U.K.
Results
Plasma markers of organ damage
No difference in plasma AST, ALT or glutamate dehydrogenase (GDH) enzyme activity or bilirubin or creatinine concentration was detected in plasma from vehicle-injected and naïve (uninjected) animals indicating that the vehicle used in these experiments was devoid of hepatotoxic or nephrotoxic activity (Figure 1). However, significant (P<0.05) increases in plasma aspartate aminotransferase (3.3 fold), alanine aminotransferase (2.0 fold) and glutamate dehydrogenase (2.3 fold) enzyme activity but no change in bilirubin (2.4±1.2 μM c.f. 1.38±0.32 μM, n=8, P>0.05) or creatinine (15.9±2.7 μM c.f. 22.7±5.7 μM, n=8, P>0.05) concentration were apparent in animals administered paracetamol. In contrast, nitroparacetamol administration did not alter plasma levels of any of the markers of liver or kidney injury measured in this study (Figure 1) and did not significantly affect plasma bilirubin (0.71±0.18 μM, n=8, P>0.05) or creatinine (20.8±2.2 μM, n=8, P>0.05) concentration.
Figure 1.
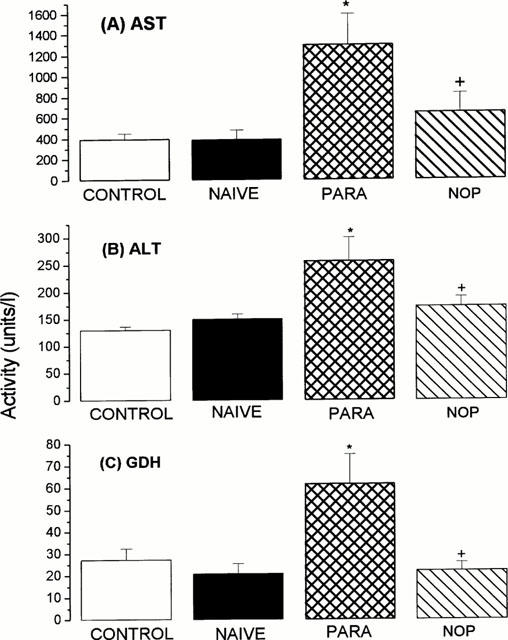
Activity (units l−1) of aspartate aminotransferase (AST; A), alaninine aminotransferase (ALT; B) and glutamate dehydrogenase (GDH; C) in plasma removed 6 h after i.p. injection of vehicle (CONTROL), paracetamol (PARA), nitroparacetamol (NOP) or from untreated animals (NAÏVE). Results show mean±s.e.,mean, n=8 – 10, *P<0.05 (ANOVA followed by post-hoc Dunnett's test).
Measurement of plasma and liver nitrate/nitrite and paracetamol
Plasma nitrate/nitrite concentration was significantly (P<0.05) increased in animals killed 6 h after nitroparacetamol injection (88.6±5.6 μM, n=8) compared with control, vehicle-injected animals (40.8±1.6 μM, n=10) or with animals administered paracetamol (42.4±5.8 μM, n=8). In contrast, very low concentrations of nitrate/nitrite concentration were detected in homogenates of livers from control animals (0.9±0.1 μM, n=8) and values were significantly (P<0.05) increased both in paracetamol (16.0±4.1 μM, n=8) and nitroparacetamol (36.5±13.0 μM, n=7) injected animals.
In separate experiments, paracetamol was undetectable in either plasma or liver homogenate from control animals. The concentration of paracetamol present in plasma (104.8±32.4 μM, n=8) and liver (465.0±40.0 μM, n=8) from paracetamol-pretreated animals was not significantly different (P>0.05) from values obtained in animals injected with an equimolar amount of nitroparacetamol (165.5±12.6 μM and 493.0±11.0 μM, n=8) respectively.
Discussion
Administration of paracetamol in the rat causes 6 h thereafter an increase in plasma aspartate and alanine aminotransferase and glutamate dehydrogenase activity without affecting bilirubin or creatinine concentrations. Similar results have previously been reported in the rat, and other species, over the same or longer time courses (e.g. Tolman, 1998) and have been interpreted as evidence of ongoing hepatocellular toxicity in the absence of significant cholestasis or renal damage. The most important feature of the present study is the finding that administration of nitroparacetamol (a nitric oxide releasing derivative of paracetamol) did not elevate plasma aspartate or aspartate aminotransferase or glutamate dehydrogenase and, as such, may reasonably be assumed to be devoid of hepatotoxic activity.
Previous research using related NO-NSAID such as nitroaspirin (Tagliaro et al., 1996) have shown that plasma levels of salicylate increase rapidly following parenteral administration in the rat. Whilst similar studies have yet to be undertaken with nitroparacetamol it seems likely that rapid esteratic cleavage of this compound also occurs liberating free paracetamol into the bloodstream. Certainly, in the present study, plasma and liver concentrations of paracetamol were not significantly different in the two treatment groups. Accordingly, it would appear unlikey that differences in the pharmacokinetic profile of paracetamol and nitroparacetamol account for the dramatic difference in their ability to cause liver injury.
With this in mind the possibility that NO, released from nitroparacetamol, may account for the hepatotoprotective effect of paracetamol should be considered. Elevated plasma nitrate/nitrite concentration was apparent 6 h after nitroparacetamol injection in these animals suggesting that substantial release of NO from nitroparacetamol had taken place over the period of the experiment. Furthermore, nitrate/nitrite concentration was also increased in livers prepared from nitroparacetamol-injected rats implying that liver NO concentrations were also raised in these animals.
Despite much study, the precise role of NO in liver damage induced either by paracetamol injection or by other mechanisms (e.g. septic shock) remains unclear with evidence in the literature for both a hepatotoxic and a hepatoprotective effect of NO. For example, paracetamol injection in the rat is associated with induction of the inducible isoform of nitric oxide synthase whilst pretreatment of such animals with aminoguanidine (a relatively selective inhibitor of this enzyme) reduces the resulting hepatic necrosis and associated increases in serum aspartate and alanine aminotransferase activity (Gardner et al., 1998). These authors conclude that NO derived from inducible nitric oxide synthase is hepatotoxic perhaps as a consequence of the formation of damaging free radical species such as peroxynitrite (e.g. Michael et al., 1999). Interestingly, we also observed an increase in nitrate/nitrite concentration in homogenates of liver from paracetamol-injected animals (c.f. control animals) which may perhaps indicate induction of liver inducible nitric oxide synthase in these animals. In marked contrast, NO has also been proposed as an endogenous hepatoprotective mechanism to ‘offset' oxidative injury (e.g. Kuo et al., 1997). Furthermore, ‘classical' NO donors such as S-nitroso-N-acetylpenicillamine (SNAP) have been reported to preserve mitochondrial function in hepatocytes exposed to tert-butyl hydroperoxide to trigger oxidative injury Farghali et al., 1999). Thus, it is not inconceivable that NO exhibits both hepatoprotective and hepatotoxic activity. The precise effect observed may depend on a number of factors including, perhaps, the concentration of NO achieved, the degree of liver damage, the timing of exposure to NO and the cell source and distribution of NO within the liver.
We have previously reported that nitroflurbiprofen, and alternative NO-NSAID, also reduce liver damage associated with administration of E. coli lipopolysaccharide in the rat (McLoughlin et al., 1999). Taken together with the recent report of a hepatoprotective effect of nitroaspirin in the mouse (Fiorucci et al., 2000) it would appear that inhibition of liver damage may well be a feature common to several NO-NSAID.
We propose that the greater anti-inflammatory and analgesic effect of nitroparacetamol coupled with its lack of hepatotoxic activity renders this compound a potentially useful alternative to paracetamol in the clinic.
Acknowledgments
We would like to thank NicOx SA, Sophia-Antipolis, France for financial support.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GDH
glutamate dehydrogenase
- NO-NSAID
nitro non-steroidal anti-inflammatory drug
- NSAID
non-steroidal anti-inflammatory drug
References
- AL-SWAYEH O.A., FUTTER L.E., CLIFFORD R.H., DEL SOLDATO P., MOORE P.K. Nitroparacetamol exhibits anti-inflammatory and antinociceptive activity. Br. J. Pharmacol. 2000;130:1453–1456. doi: 10.1038/sj.bjp.0703509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARGHALI H., MARTINEK J., MASEK K. The amelioration of hepatocyte oxidative stress injury by nitric oxide released from S-nitroso-N-acetyl penicillamine: a study in immobilized perfused hepatocytes. Methods Fund. Exp. Clin. Pharmacol. 1999;21:395–402. doi: 10.1358/mf.1999.21.6.541919. [DOI] [PubMed] [Google Scholar]
- FIORUCCI S., SANTUCCI L., ANTONELLI E., DISTRUTTI E., DEL SERO G., MORELLI O., ROMANI L., FEDERICI N., DEL SOLDATO P., MORELLI A. NO-aspirin protects from T cell-mediated liver injury by inhibiting caspase-dependent processing of Th1-like cytokines. Gastroenterology. 2000;118:404–421. doi: 10.1016/s0016-5085(00)70223-x. [DOI] [PubMed] [Google Scholar]
- GARDNER C.R., HECK D.E., YANG C.S., THOMAS P.E., ZHANG X.J. , DEGEORGE G.E., LASKIN J.D., LASKIN D.L. Role of nitric oxide in acetaminophen-induced hepatotoxicity in the rat. Hepatology. 1998;27:748–754. doi: 10.1002/hep.510270316. [DOI] [PubMed] [Google Scholar]
- HORDER H., GERHARDT W., HARKONEN M., MAGID E., POTKANEN E., STROMME J.H., THEODORSEN L., WALDENSTROM J. Experiences with the Scandinavian recommended methods for determinations of enzymes in blood. A report by the Scandinavian Committee on Enzyme. Scan. J. Clin. Lab. Invest. 1981;41:107–116. doi: 10.3109/00365518109092022. [DOI] [PubMed] [Google Scholar]
- KUO P.C., SCHROEDER R.A., LOSCALZO J. Nitric oxide and acetaminophen-mediated oxidative injury: modulation of interleukin-1-induced nitric oxide synthesis in cultured rat hepatocytes. J. Pharm. Exp. Ther. 1997;282:1072–1083. [PubMed] [Google Scholar]
- MCLOUGHLIN C.M., KEEBLE J., MOORE P.K. Effect of nitroflurbiprofen on lipopolysaccharide induced sepsis. Mediators of Inflammation. 1999;8:146. [Google Scholar]
- MICHAEL S.L., PUMFORD N.R., MAYEUX P.R., NIESMAN M.R., HINSON J.A. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 1999;30:186–195. doi: 10.1002/hep.510300104. [DOI] [PubMed] [Google Scholar]
- PLEVRIS J.N., SCHINA M., HAYES P.C. The management of acute liver failure. Aliment. Pharmacol. Ther. 1998;12:405–418. doi: 10.1046/j.1365-2036.1998.00320.x. [DOI] [PubMed] [Google Scholar]
- STERN A.I., HOGAN D.L., KAHN L.H., ISENBERG J.I. Protective effect of acetaminophen against aspirin- and ethanol-induced damage to the human gastric mucosa. Gastroenterology. 1984;86:728–733. [PubMed] [Google Scholar]
- SZABO C., SOUTHAN G.J., ZINGARELLI B., SOUTHAN J.B., GAHLMAN T.C., BHAT V., SALZMAN A.L., WOLFF D.J. Pharmacological characterization of guanidinoethyldisulphide (GED), a novel inhibitor of nitric oxide synthase with selectivity towards the inducible isoform. Br. J. Pharmacol. 1996;118:1659–1668. doi: 10.1111/j.1476-5381.1996.tb15589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGLIARO F., CUZZOLIN L., ADAMI A., SCARCELLA D., CRIVELLENTE F., BENONI F. Pharmacokinetics of a new nitroderivative of acetylsalicylic acid after a single dose in rats. Life Sci. 1996;60:101–106. doi: 10.1016/s0024-3205(96)00599-1. [DOI] [PubMed] [Google Scholar]
- TOLMAN K.G. Hepatotoxicity of non-narcotic analgesics. Am. J. Med. 1998;27:13S–19S. doi: 10.1016/s0002-9343(98)00070-9. [DOI] [PubMed] [Google Scholar]
- WALDBERG C.B. Determination of acetaminophen in serum. J. Anal. Toxicol. 1977;1:79–87. [Google Scholar]


