Abstract
Little is known about the intrinsic enteric reflex pathways associated with migrating motor complex (MMC) formation. Acetylcholine (ACh) mediates the rapid component of the MMC, however a non-cholinergic component also exists. The present study investigated the possible role of endogenous tachykinins (TKs) in the formation of colonic MMCs and the relative roles of excitatory and inhibitory pathways.
MMCs were recorded from the circular muscle at four sites (proximal, proximal-mid, mid-distal and distal) along the mouse colon using force transducers.
The tachykinin (NK1 and NK2) receptor antagonists SR-140 333 (250 nM) and SR-48 968 (250 nM) reduced the amplitude of MMCs at all recording sites, preferentially abolishing the long duration contraction. Residual MMCs were abolished by the subsequent addition of atropine (1 μM).
The neuronal nitric oxide synthase inhibitor, Nωnitro-L-arginine (L-NOARG, 100 μM), increased MMC amplitude in the distal region, whilst reducing the amplitude in the proximal region. In preparations where MMCs did not migrate to the distal colon, addition of L-NOARG resulted in the formation of MMCs. Subsequent addition of apamin (250 nM) or suramin (100 μM) further increased MMC amplitude in the distal region, whilst suramin increased MMC amplitude in the mid-distal region. Apamin but not suramin reduced MMC amplitude in the proximal region. Subsequent addition of SR-140 333 and SR-48 968 reduced MMC amplitude at all sites. Residual MMCs were abolished by atropine (1 μM).
In conclusion, TKs, ACh, nitric oxide (NO) and ATP are involved in the neural mechanisms underlying the formation of MMCs in the mouse colon. Tachykinins mediate the long duration component of the MMC via NK1 and NK2 receptors. Inhibitory pathways may be involved in determining whether MMCs are formed.
Keywords: Colon, enteric nervous system, migrating motor complex, tachykinins, motility, intestinal pacemakers
Introduction
The enteric nervous system exerts control over various intestinal motor behaviours and comprises neurons whose processes and cell bodies lie within the wall of the gastrointestinal tract. Two such motility patterns are peristalsis and migrating motor complexes (MMCs). The enteric reflex pathways mediating peristalsis are well known (Waterman et al., 1994; Tonini et al., 1996; Smith & Robertson, 1998) and are evoked by chemical or mechanical stimulation of intrinsic primary afferent neurons (see Kunze & Furness, 1999 for review). However, despite the importance of colonic MMCs in normal and abnormal conditions (see Coulie & Camilleri, 1999; Di Lorenzo, 1999 for reviews), comparatively little is known about the neural pathways mediating MMCs.
Recently, spontaneously occurring MMCs (Fida et al., 1997) or myoelectric complexes (Wood, 1973; Bywater et al., 1989; Lyster et al., 1992; 1995; Fida et al., 1997; Spencer et al., 1998a,1998b,1998c) have been recorded in vitro in isolated mouse colon. The contractile or electrical forms of MMCs are separated by periods of quiescence and consist of rapid contractions or rapid oscillations in membrane potential superimposed on a long duration contraction or long duration depolarization, which typically lasts about 30 s (Bywater et al., 1989; Fida et al., 1997).
Colonic MMCs are thought to be neural in origin since neuronal blockade with tetrodotoxin (TTX) abolishes MMC activity, whilst causing a significant increase in membrane potential (Lyster et al., 1992; 1995; Fida et al., 1997; Spencer et al., 1998a,1998b,1998c). Addition of the nitric oxide donor, sodium nitroprusside, to TTX-containing solutions restores membrane potential but not MMC activity, suggesting MMC formation is not dependent upon membrane potential alone, in the mouse (Spencer et al., 1998c). MMCs also require nicotinic ganglionic transmission, as hexamethonium abolishes MMC activity whilst causing a significant increase in membrane potential (Bywater et al., 1989; Lyster et al., 1992; 1995; Spencer et al., 1998b). The effect of hexamethonium on membrane potential is smaller than that of TTX, suggesting the possibility of non-nicotinic activation of motor neurons.
Both excitatory and inhibitory motor neurons are involved in MMC formation. Acetylcholine (ACh) release from excitatory motor neurons mediates the rapid component of the MMC; however a non-cholinergic component exists (Bywater et al., 1989). Interestingly, peristaltic contractions in the guinea-pig ileum also have cholinergic and non-cholinergic components, the latter being mediated by endogenous tachykinin (TK) release from excitatory motor neurons acting via NK1 and NK2 receptors on the circular muscle (see Holzer & Holzer-Petsche, 1997 for review). Recent immunohistochemical studies in the mouse colon have demonstrated the presence of substance P-immunoreactive circular muscle motor neurons (Sang & Young, 1996; 1998; Sang et al., 1997). These neurons also contain choline acetyltransferase and the vesicular acetylcholine transporter (Sang & Young, 1998), suggesting that they are likely to be excitatory motor neurons and may use ACh and substance P as co-transmitters. Whether TKs also mediate the non-cholinergic component of the MMC is unknown.
Between MMCs, inhibitory motor neurons maintain the circular smooth muscle under tonic inhibition predominantly via the release of nitric oxide (NO), but also via an apamin-sensitive mechanism (Lyster et al., 1995; Spencer et al., 1998a,1998b). However, there are discrepancies as to the role of inhibitory motor neurons in the formation of the MMC itself. The nitric oxide synthase inhibitor, L-NOARG, in the presence of atropine and nifedipine, depolarizes the membrane, abolishes the repolarization period between MMCs and significantly reduces the amplitude of electrophysiologically recorded MMCs (Lyster et al., 1995; Spencer et al., 1998a,1998b). These effects are accentuated by subsequent addition of apamin; however a non-cholinergic, non-nitrergic, apamin-resistant residual MMC remains (Spencer et al., 1998a,1998b). By contrast, L-NOARG in control solutions has no effect on MMC amplitude or the long duration (non-cholinergic) contraction associated with mechanically recorded MMCs (Fida et al., 1997).
Therefore, the aims of the present study were to (i) investigate whether endogenous TKs are involved in colonic MMC formation; (ii) determine the relative roles of excitatory and inhibitory pathways in the formation of MMCs; and (iii) investigate the involvement of nicotinic and non-nicotinic activation of motor neurons.
Methods
Tissue preparation
This study was approved by the University of Adelaide Ethics Committee. Methods were modified from Fida et al. (1997). Male Swiss mice weighing 25 – 40 g were killed by cervical dislocation or raised atmospheric CO2 followed by cervical dislocation. The entire colon was quickly excised and placed in a Sylgard (Dow Corning, U.S.A.) covered petri dish containing Krebs' solution and continuously gassed with 95% O2 / 5% CO2, pH: 7.4 at 37°C. The composition of the Krebs' solution was (mM): NaCl 118, KCl 4.7, KH2PO4 1.2, MgSO4 1.5, NaHCO3 25.0, D-Glucose 11 and CaCl2 2.5.
Colonic faecal material was gently flushed using a Krebs' filled syringe. The colon was pinned at the oral and caudal ends and the mesentery dissected free. The colonic mesenteric border was secured by cotton thread to a stainless steel rod (100 mm length and 3 mm diameter) and secured in a 70 ml organ bath. The mechanical activity of the circular muscle was recorded by cotton thread attached to the serosa at the anti-mesenteric border and connected to four force-displacement transducers (Grass Medical Instruments, FT03, MA, U.S.A.). At each recording site the preparation was placed under an initial tension of 6 mN and allowed to equilibrate for a minimum of 60 min before the addition of drugs. Transducer output was fed into a Quad bridge amplifier (ADInstruments, Sydney, Australia) and contraction of the circular smooth muscle recorded and stored on a Power Macintosh G3 computer via Chart v 3.6.1/s software and MacLab/8s data acquisition system (ADInstruments).
Experimental protocol
Following the equilibration period, drugs were added cumulatively at 30 min intervals. A number of drug protocols were employed to block the effects of specific neurotransmitters. To determine the role of neurotransmitter release from excitatory motor neurons, the muscarinic receptor antagonist atropine (1 μM) was added to block the cholinergic contractions and the NK1 and NK2 receptor antagonists, SR-140 333 (250 nM) and SR-48 968 (250 nM), were added to block TK-mediated contractions. These concentrations are at least 30 times in excess of the equilibrium dissociation constants which have previously been determined in intestinal preparations using subtype-selective agonists (Emonds-Alt et al., 1993; Croci et al., 1994). To determine the role of neurotransmitter release from inhibitory motor neurons, the neuronal nitric oxide synthase inhibitor, L-NOARG (100 μM) was added to block the effects mediated by NO and apamin (250 nM) or suramin (100 μM) added to block the effects mediated by ATP. An apamin-sensitive, possibly ATP-mediated mechanism has been demonstrated in maintaining the murine circular smooth muscle under tonic inhibition (Spencer et al., 1998a,1998b). However, apamin has previously been shown to inhibit the release of inhibitory neurotransmitters, including NO (Grider & Makhlouf, 1987). Therefore, apamin (250 nM) was added after the addition of L-NOARG. The P2-purinoceptor antagonist, suramin (100 μM), was added to confirm the role of ATP in MMC formation. Suramin was added after the addition of L-NOARG to allow comparison with the results of apamin obtained in the presence of L-NOARG.
Drugs
Apamin, atropine hydrochloride, hexamethonium, Nωnitro-L-arginine (L-NOARG), suramin and tetrodotoxin (TTX) were purchased from Sigma, St Louis, MO, U.S.A. SR-140 333 or (S)-1-{2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenyl-acetyl) piperidin-3-yl ] ethyl }- 4 -phenyl-1- azoniabicyclo [2.2.2]- octane chloride; SR-48 968 or (−)-N-methyl-N[4-acetylamino-4-phenyl-piperidino-2-(3,4-dichlorophenyl)butyl]-benzamide were a kind gift from Dr Carlo A. Maggi, Florence, Italy. Stock solutions of apamin and SR-140 333 were prepared in 0.05 M acetic acid and 100% dimethylsulphoxide, respectively. Stock solutions of all other drugs were prepared in distilled water.
Data analysis and statistics
Colonic MMCs were defined as regularly occurring spontaneous contractions, which alternated with periods of relative quiescence, and were observed to occur at more than one site along the colon (Fida et al., 1997). MMCs in each region of the colon were quantitatively characterized by measuring several parameters: maximum amplitude, integral, middle duration (at 50% amplitude), lower duration (at 20% amplitude) and frequency. After equilibration the above parameters were measured in five control MMCs immediately before the addition of any drugs. After the addition of each drug the same parameters were measured in five MMCs immediately prior to the conclusion of the 30 min incubation period. In preparations where MMCs were abolished by the addition of a drug, the integral and maximum amplitude of the remaining short duration spontaneous contractile activity was measured over a period of 1 min. The maximum amplitude and integral and the frequency of MMCs were calculated using Datapad Chart v 3.6.1/s software (ADInstruments). MMC frequency was calculated at 50% amplitude of the rising phase. The middle and lower duration of MMCs were calculated using Peak Parameters Chart v 3.6.1/s software (ADInstruments) at 50 and 20% amplitudes respectively with low pass digital filters applied at a frequency of 0.2 Hz.
All data are expressed as mean±standard error of the mean (s.e.mean). Student's two-tailed paired t-tests or repeated measures ANOVA were carried out on raw data where appropriate. Raw data for drug treatments were converted to proportions of control. Repeated measures (RM) ANOVA was followed by Tukey/Kramer post hoc test to compare MMC parameters in the presence and absence of drugs. A probability of less than 0.05 (P<0.05) was considered significant throughout.
Results
General characteristics of MMCs
Spontaneously occurring MMCs, separated by periods of relative quiescence, were observed in all regions of the colon. MMCs occurred every 2.8±0.2 min in the proximal colon, 2.9±0.2 min in the proximal-mid colon, 2.9±0.3 min in the mid-distal colon and 2.7±0.2 min in the distal colon. There was no significant difference in the frequency of the MMCs between each region of the colon (ANOVA, P>0.05, n=33).
The duration, amplitude and integral of MMCs varied along the length of the colon. The mean middle duration of MMCs in the proximal colon was 28.7±1.2 s, 21.8±0.7 s in the proximal-mid colon, 18.7±0.8 s in the mid-distal colon and 19.3±1.4 s in the distal colon. The middle duration of MMCs in the proximal region was significantly longer than in all other regions (ANOVA, P<0.05, n=33), whilst MMCs in the proximal-mid region were significantly longer (middle duration) than in the mid-distal and distal regions (ANOVA, P<0.05, n=33). MMCs in the distal region were significantly longer (middle duration) than in the mid-distal region (ANOVA, P<0.05, n=33).
The mean lower duration of MMCs in the proximal colon was 39.7±1.5 s, 35.7±1.6 s in the proximal-mid colon, 37.6±2.5 s in the mid-distal colon and 37.0±2.7 s in the distal colon. The average amplitude and integral of MMCs in the proximal and proximal-mid regions of the colon were significantly greater than in the mid-distal and distal regions of the colon (ANOVA, P<0.05, n=33). Furthermore, the amplitude of MMCs in the mid-distal region was significantly greater than in the distal region (ANOVA, P<0.05, n=33). There was no significant difference in the integral of MMCs between mid-distal and distal regions (ANOVA, P>0.05, n=33).
To confirm that a neural component contributes to MMC formation, tetrodotoxin (TTX, 0.6 μM) was added to control solutions. Tetrodotoxin abolished MMC activity in all regions of the colon and caused an increase in resting tone, suggesting tonic inhibition of the muscle.
Effects of NK1 and NK2 receptor antagonists on MMCs
To investigate whether TKs, acting via NK1 and NK2 receptors, participate in the contractile component of MMCs in the mouse colon, the NK1 (SR-140 333: 250 nM) and NK2 (SR-48 968: 250 nM) receptor antagonists were added to control solutions. Addition of SR-140 333 and SR-48 968 significantly reduced the amplitude and integral of MMCs in all regions of the colon (t-test, P<0.05, n=7; Figure 1). Neurokinin1 and NK2 receptor antagonism abolished the long duration contraction associated with the MMCs, with the rapid contractions remaining, suggesting that the long duration contractions are mediated by TKs (Figure 2a). Furthermore, SR-140 333 and SR-48 968 significantly reduced the middle duration (t-test, P<0.05, n=7) of MMCs in the proximal and proximal-mid regions and significantly reduced the lower duration of MMCs in the proximal colon (t-test, P<0.05, n=7). The addition of SR-140 333 and SR-48 968 had no significant effect on the other MMC parameters (t-test, P>0.05, n=7), including frequency.
Figure 1.

Effect of NK1 and NK2 receptor antagonists, SR-140 333 and SR-48 968, on MMC amplitude and integral. Addition of the NK1 and NK2 receptor antagonists SR-140 333 (250 nM) and SR-48 968 (250 nM) significantly reduced MMC amplitude (a) and integral (b) in all regions of the colon (t-test, P<0.05, n=7). The effects of SR-140 333 and SR-48 968 are expressed as percentages of control MMCs.
Figure 2.

Typical recordings demonstrating the effects of NK1 and NK2 and muscarinic receptor antagonists SR-140 333 and SR-48 968 and atropine on MMC characteristics. (a) Upper trace: Recording of a control MMC from the proximal-mid region of the colon consisting of rapid contractions with an underlying long duration contraction. Lower trace: Addition of SR-140 333 (250 nM) and SR-48 968 (250 nM) abolished the long duration contraction with the rapid contractions remaining. Recordings were from the same preparation. (b) Upper trace: Recording of a control MMC from the proximal-mid region of the colon consisting of rapid contractions with an underlying long duration contraction. Lower trace: Addition of atropine (1 μM) results in abolition of the rapid contractions with the underlying long duration contraction remaining. Recordings were from the same preparation. Effects of SR-140 333 and SR-48 968 and atropine are shown 15 min after their addition.
Effects of muscarinic receptor antagonist on MMCs
To confirm the role of ACh release from excitatory motor neurons in the formation of MMCs, the muscarinic receptor antagonist, atropine (1 μM), was added to control solutions. Muscarinic receptor antagonism abolished the rapid contractions associated with the MMCs, with the long duration component remaining, suggesting that ACh mediates the rapid contractions (Figure 2b). Furthermore, atropine significantly reduced the amplitude, integral, middle and lower duration of MMCs in the proximal region and reduced the amplitude of MMCs in the proximal-mid region (t-test, P<0.05, n=6). However, atropine had no significant effect (t-test, P>0.05, n=6) on the other parameters associated with the MMCs, including frequency.
Effects of the sequential addition of NK1, NK2 and muscarinic receptor antagonists on MMC characteristics
To determine the relative contribution of NK1 and NK2 receptors to the formation of the long duration contraction, SR-140 333 and SR-48 968 were added sequentially. Addition of the NK1 receptor antagonist, SR-140 333 (250 nM), significantly decreased MMC amplitude and integral in all regions of the colon, in addition to significantly reducing MMC middle duration in the proximal-mid region (ANOVA, P<0.05, n=8; Figure 3). SR-140 333 had no significant effect on any of the other MMC parameters (ANOVA, P>0.05, n=8), including MMC frequency. Addition of the NK2 receptor antagonist, SR-48 968 (250 nM), in the presence of SR-140 333, caused a further significant reduction in MMC amplitude and integral in all regions of the colon, whilst significantly reducing MMC middle and lower duration in the proximal-mid colon (ANOVA, P<0.05, n=8; Figure 3). SR-48 968 had no significant effect on any of the other MMC parameters, including MMC frequency (ANOVA, P>0.05, n=8). Residual MMCs, consisting of rapid contractions, were abolished by the subsequent addition of atropine (1 μM; Figure 3).
Figure 3.
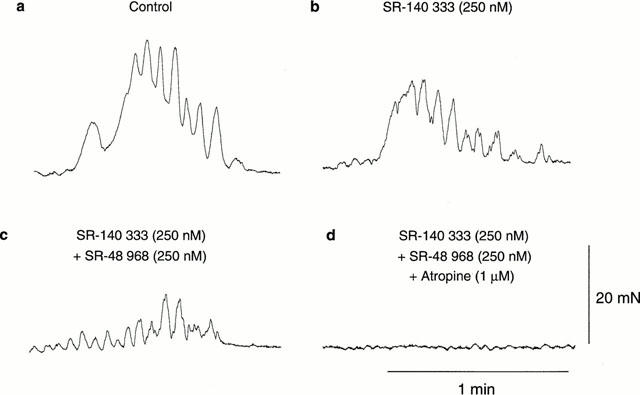
Recordings demonstrating the effects of NK1, NK2 and muscarinic receptor antagonists, SR-140 333 and SR-48 968 and atropine, added sequentially on MMC characteristics. (a) Recording of a control MMC from the proximal-mid region of the colon consisting of rapid contractions with an underlying long duration contraction. (b) Addition of the NK1 receptor antagonist SR-140 333 (250 nM) significantly reduced the amplitude and integral of the long duration contraction associated with the MMC. (c) Addition of the NK2 receptor antagonist SR-48 968 (250 nM), in the presence of SR-140 333, abolished the residual long duration contraction associated with the MMC. (d) Addition of the muscarinic receptor antagonist atropine (1 μM), in the presence of SR-140 333 and SR-48 968 abolished the residual MMC composed of rapid contractions. Recordings were from the same preparation. Effects of SR-140 333, SR-48 968 and atropine are shown 15 min after their addition. Scale bars apply throughout.
Effects of L-NOARG and apamin or suramin on MMC characteristics
To clarify the role of inhibitory pathways in the formation of MMCs, a combination of drugs was added. The nitric oxide synthase inhibitor, L-NOARG (100 μM), was used to determine the role of NO in MMC formation, whilst apamin (250 nM) and the P2-purinoceptor antagonist, suramin (100 μM), were added to confirm the role of ATP in MMC formation.
L-NOARG significantly decreased the amplitude, integral and lower duration of MMCs in the proximal region of the colon (ANOVA, P<0.05, n=6). By contrast, L-NOARG significantly increased the amplitude, integral and middle duration of MMCs in the distal colon (ANOVA, P<0.05, n=6; Figure 4). In preparations where MMCs did not migrate to the distal region of the colon, addition of L-NOARG (100 μM) resulted in the formation of MMCs in this region (Figure 5). The addition of L-NOARG had no significant effect on the other parameters associated with the MMCs, including frequency (ANOVA, P>0.05, n=6). In the presence of L-NOARG, the rapid and long duration contractions associated with MMCs were still present in all regions of the colon.
Figure 4.

Typical recordings displaying the effects of sequential addition of L-NOARG, apamin, SR-140 333 and SR-48 968 and atropine, on MMCs in the proximal, proximal-mid, mid-distal and distal regions of the colon. L-NOARG (100 μM) caused a significant increase in amplitude and integral of MMCs in the distal region, whilst decreasing amplitude and integral in the proximal region. Subsequent addition of apamin (250 nM) caused a further increase in amplitude and integral of MMCs in the distal region, whilst reducing MMC amplitude in the proximal region. Both the rapid and long duration contractions of the MMCs remain after the addition of L-NOARG and apamin. Subsequent addition of SR-140 333 (250 nM) and SR-48 968 (250 nM) significantly reduced MMC amplitude and integral in all regions of the colon whilst preferentially abolishing the long duration component of MMCs. Residual MMCs were abolished by the addition of atropine (1 μM). L-NOARG, apamin and SR-140 333 and SR-48 968 had no effect on MMC frequency in any of the regions. Recordings were made from the same preparation. Scale bars for each region apply throughout. Addition of each drug occurred at least 15 min before the start of the recording shown.
Figure 5.
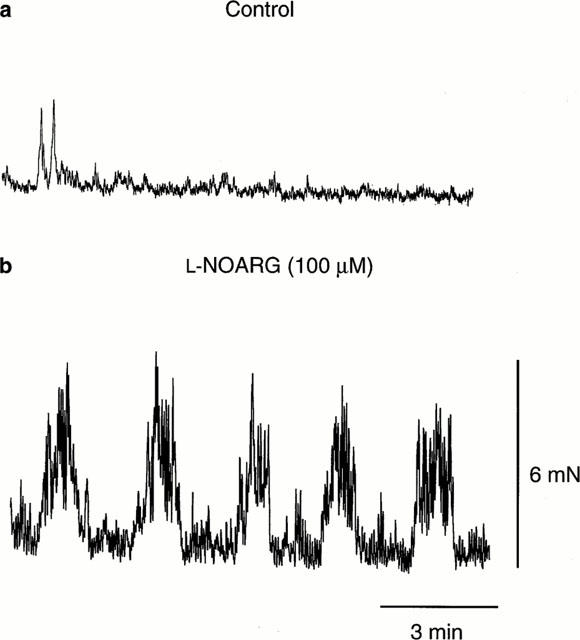
Typical recordings demonstrating the effects of L-NOARG on preparations where MMCs did not propagate to the distal region of the colon. (a) Recording of a preparation lacking MMC activity in the distal region of the colon. (b) Addition of L-NOARG (100 μM; 20 min prior to the start of the recording shown) resulted in MMCs propagating to this region of the colon. Recordings were from the same preparation. Scale bars apply to both (a) and (b).
The addition of apamin (250 nM) in the presence of L-NOARG caused a further significant reduction in the amplitude and middle duration of MMCs at the proximal region (ANOVA, P<0.05, n=6; Figure 4). Conversely, apamin significantly increased the amplitude and integral of MMCs in the distal region. Apamin, however, had no significant effect on the other parameters associated with the MMCs, including frequency (ANOVA, P>0.05, n=6). In the presence of L-NOARG and apamin the rapid or long duration contractions associated with MMCs were still present in all regions of the colon.
In separate preparations, the addition of suramin (100 μM) in the presence of L-NOARG (100 μM) caused a significant increase in MMC amplitude and integral in the mid-distal and distal colon (ANOVA, P<0.05, n=6), but did not have a significant effect in the proximal or proximal-mid colon (ANOVA, P>0.05, n=6; Figure 6). Furthermore, suramin significantly increased the middle and lower duration of MMCs in the distal colon (ANOVA, P<0.05, n=6). Suramin had no significant effect on the other parameters associated with the MMCs, including frequency (ANOVA, P>0.05, n=6). MMC middle duration in the mid-distal colon, amplitude in the proximal and distal colon and integral and lower duration in the mid-distal and distal colon were significantly greater in L-NOARG- and suramin-containing solutions compared with L-NOARG- and apamin-containing solutions (ANOVA, P<0.05, n=12). In the combined presence of L-NOARG and suramin, the rapid and long duration contractions associated with MMCs were still present in all regions of the colon.
Figure 6.
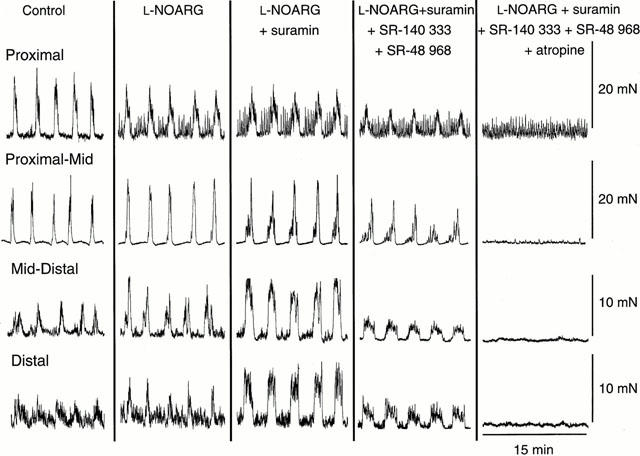
Typical recordings demonstrating the effects of L-NOARG, suramin, SR-140 333 and SR-48 968 and atropine, added sequentially to the organ bath, on MMCs in the proximal, proximal-mid, mid-distal and distal regions of the colon. L-NOARG (100 μM) caused a significant increase in amplitude and integral of MMCs in the distal region, whilst decreasing amplitude and integral in the proximal region. The subsequent addition of suramin (100 μM) caused a further increase in amplitude and integral of MMCs in the distal region, whilst increasing amplitude and integral in the mid-distal region. Both the rapid and long duration contractions of the MMCs were formed after the addition of L-NOARG and suramin. Subsequent addition of SR-140 333 (250 nM) and SR-48 968 (250 nM) significantly reduced MMC amplitude and integral in all regions of the colon whilst preferentially abolishing the long duration component of MMCs. Residual MMCs were abolished by the addition of atropine (1 μM). L-NOARG, suramin and SR-140 333 and SR-48 968 had no effect on MMC frequency in any of the regions. Recordings were made from the same preparation. Scale bars for each region apply throughout. Addition of each drug occurred at least 15 min before the start of the recording shown.
Effects of SR-140 333, SR-48 968 and atropine on MMC characteristics in the presence of L-NOARG and apamin or suramin
After blocking the effects mediated by NO and ATP using L-NOARG and apamin or suramin, SR-140 333 (250 nM), SR-48 968 (250 nM) and atropine (1 μM) were added to block TK-mediated and ACh-mediated contraction of the circular smooth muscle respectively. Addition of the NK1 and NK2 receptor antagonists abolished the long duration component of the MMCs, with the rapid contractions remaining, confirming earlier results (see above) showing that the long duration component of MMCs is TK-mediated. Furthermore, addition of SR-140 333 and SR-48 968 significantly reduced the amplitude and integral of MMCs in all regions of the colon and reduced the middle duration of MMCs in the proximal-mid and distal regions and lower duration in the mid-distal region (ANOVA, P<0.05, n=6; Figures 4 and 6). By contrast, the addition of SR-140 333 and SR-48 968 failed to affect significantly the other parameters associated with the MMCs, including frequency (ANOVA, P>0.05, n=6). Residual MMCs in all regions of the colon were abolished by the subsequent addition of atropine (1 μM), confirming that the rapid contractions are ACh-mediated.
Effects of L-NOARG in the presence of hexamethonium
The nicotinic receptor antagonist, hexamethonium (500 μM), was added to determine the role of nicotinic inputs in the formation of MMCs. The addition of hexamethonium abolished MMC activity including rapid and long duration contractions in all regions of the colon (Figure 7), suggesting that nicotinic inputs activate ACh- and TK-releasing motor neurons. The subsequent addition of L-NOARG (100 μM) resulted in an increase in resting tone of the preparation, suggesting that non-nicotinic inputs are involved in triggering the release of NO from inhibitory motor neurons.
Figure 7.

Typical recording demonstrating the effect of hexamethonium and L-NOARG on MMCs. Addition of hexamethonium (500 μM) abolishes MMC formation in all regions of the colon, as well as causing a small increase in the resting tone of the preparation. The subsequent addition of L-NOARG (100 μM) causes a further increase in the resting tone and the formation of short duration spontaneous contractions.
Effects of hexamethonium in the presence of L-NOARG and atropine
In the presence of L-NOARG (100 μM) and atropine (1 μM) to block NO- and ACh-mediated activity and to reveal TK-mediated long duration contractions, addition of hexamethonium (500 μM) abolished residual MMCs in all regions of the colon (Figure 8).
Figure 8.
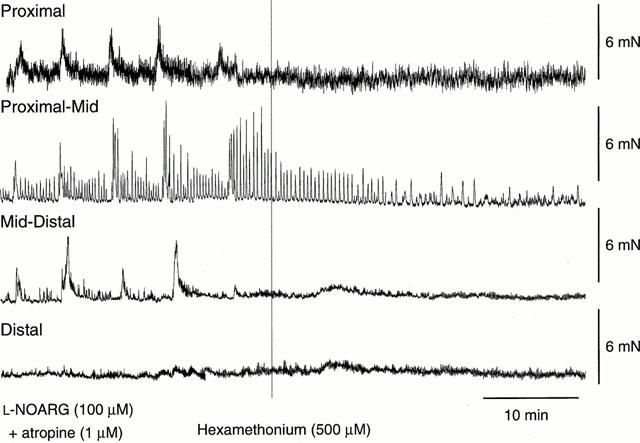
Typical recording demonstrating the effect of hexamethonium on MMCs in the presence of L-NOARG and atropine. In the presence of L-NOARG (100 μM; added 45 min prior to the start of the recording) and atropine (1 μM; added 15 min prior to the start of the recording), the addition of hexamethonium (500 μM) abolished the residual (long duration) MMCs in all regions of the colon.
Discussion
The present study demonstrates for the first time a role for TKs, acting via NK1 and NK2 receptors, in the formation of MMCs. Furthermore, this study has clarified the respective roles of excitatory and inhibitory pathways and shown that TKs, ACh, NO and ATP are the neurotransmitters underlying MMC formation. Release of these transmitters from the final motor neurons involves both nicotinic and non-nicotinic receptor-mediated pathways.
Involvement of tachykinins and acetylcholine in the formation of MMCs
Although excitatory circular muscle motor neurons in mouse colon are immunoreactive for substance P (Sang & Young, 1996; 1998; Sang et al., 1997), and release TKs following electrical field simulation (Nichols & Waterman, unpublished observation), a role for TKs in MMC formation has not previously been investigated. The results from the present study suggest that TKs, acting via NK1 and NK2 receptors, play an important role in the formation of MMCs in isolated mouse colon and demonstrate that neuropeptides can be released under physiological conditions during a spontaneously occurring motor behaviour.
The myoelectric complex comprises rapid oscillations in membrane potential superimposed on a long duration depolarization of the smooth muscle (Bywater et al., 1989; Lyster et al., 1995). Addition of the muscarinic receptor antagonist, atropine, abolishes the rapid oscillations, but has little effect on the long duration depolarization (Bywater et al., 1989; Lyster et al., 1992; 1995; Spencer et al., 1998a,1998b). Furthermore, atropine reduces the amplitude of mechanically recorded MMCs but neither abolishes them nor alters their frequency (Fida et al., 1997; and present study). Together, these studies suggest there are atropine-sensitive (ACh-mediated) and atropine-resistant (non-ACh mediated) components of MMCs. The present study demonstrates that the non-cholinergic component is mediated by TKs, since addition of the NK1 and NK2 receptor antagonists, together and sequentially, significantly reduces the amplitude, integral and duration of MMCs, and abolishes the slow contraction whilst sparing the fast contractions. Thus TKs mediate the long duration contraction of the MMC via NK1 and NK2 receptors, whilst ACh mediates the rapid contractions via muscarinic receptors.
Role of inhibitory pathways in MMC formation
Previous studies have shown that NO (Lyster et al., 1995; Fida et al., 1997; Spencer et al., 1998a,1998b) and a transmitter acting via an apamin-sensitive mechanism, possibly ATP (Lyster et al., 1995; Spencer et al., 1998a,1998b), are involved in maintaining smooth muscle membrane potential between MMCs. In the present study, L-NOARG, apamin and suramin enhanced MMC amplitude and integral in the distal colon and reduced quiescence between MMCs, consistent with NO and ATP mediating transmission from inhibitory motor neurons in this tissue. However, removal of NO- and ATP-mediated presynaptic inhibition of excitatory pathways could also contribute to these effects. Nitric oxide mediates presynaptic inhibition of slow excitatory postsynaptic potentials (Tamura et al., 1993), whilst addition of NO synthase (NOS) inhibitors facilitates the release of ACh and substance P from enteric neurons (Knudsen & Tøttrup, 1992; Wiklund et al., 1993; Kilbinger & Wolf, 1994; Yunker & Galligan, 1996). In addition, ATP can inhibit cholinergic transmission in the myenteric plexus via an action on presynaptic suramin-sensitive P2-purinoceptors (Barajas-Lopez et al., 1995; Kamiji et al., 1994; LePard et al., 1997). Thus, the effects of L-NOARG and suramin on the distal colon in the present study could also be explained by removal of ongoing presynaptic inhibition of transmitter release from excitatory motor neurons or neurons in excitatory pathways.
Spencer et al. (1998a,1998b) have proposed that the long-duration depolarization associated with MMCs is due to suppression of inhibitory transmission only, since both L-NOARG and apamin depolarized the circular muscle whilst reducing the amplitude of MMCs. However, these experiments were performed in the presence of atropine; therefore the MMCs were partly inhibited before the roles of NO and ATP were investigated. Indeed, the authors were unable to measure accurately the frequency and duration of the residual MMCs after the addition of L-NOARG and apamin to atropine-containing solutions. As discussed above, our results suggest that the long duration contraction is mediated by TKs, rather than by inhibition of inhibitory motor neuron activity. Furthermore, both rapid and long duration components of the MMC were formed after blocking inhibitory neurotransmission, whilst in separate preparations, both the long and rapid components of the MMC were abolished by the addition of neurokinin and muscarinic receptor antagonists respectively.
This raises the question of the role of inhibitory pathways in the development of MMCs. The present study has demonstrated that in preparations where MMCs did not migrate to the distal region of the colon, addition of L-NOARG resulted in the formation of MMCs (Figure 5). Thus NO can inhibit MMC propagation into the distal colon. Furthermore, sequential addition of apamin or suramin enhanced MMC amplitude and integral in the distal colon, suggesting that ATP-mediated inhibitory transmission may also have contributed to the failure of MMC propagation. Thus inhibitory pathways may be involved in determining whether MMCs are formed. Nitric oxide and ATP may act presynaptically to inhibit excitatory pathways and thereby inhibit MMC propagation (see above). Moreover, release of NO and ATP from inhibitory motor neurons may not be disinhibited, a process proposed by Spencer et al. (1998b) to be crucial to MMC formation. In light of the present findings, such a mechanism would require activation of excitatory motor neurons to release ACh and TKs and simultaneous inhibition of inhibitory motor neurons and suppression of the release of NO and ATP. Disinhibition may be mediated by presynaptic inhibition of transmitter release from inhibitory motor neurons by ACh acting on nicotinic receptors or by transmitters at other receptors (Spencer et al., 1998b).
The addition of L-NOARG and apamin or suramin had no significant effect on MMC frequency, suggesting that inhibitory motor neurons do not determine the frequency of MMCs. This result is in agreement with Spencer et al. (1998b), but differs from other studies which found that L-NOARG significantly increases the frequency of MMCs (Fida et al., 1997; Spencer et al., 1998a). The discrepancy between the results may be due to the time allowed for drug equilibration; in the present study five MMCs were analysed at least 15 min after the addition of a drug, whereas the previous studies analysed the effects of drugs on three MMCs immediately after their addition, at which stage the drugs may not have equilibrated. Spencer et al. (1998b) noted an initial transient increase in MMC frequency with L-NOARG addition, which we were unable to detect (results not shown). Nevertheless, if NO were to play an important role in setting MMC frequency, one might predict a sustained rather than transient effect of L-NOARG, just as L-NOARG produces a sustained effect on the frequency of peristaltic contractions in guinea-pig ileum (Waterman & Costa, 1994). Since MMC frequency is not altered by blocking the effects of either ACh, TKs, NO and ATP, frequency of MMCs may thus be determined by a non-neural mechanism or by a transmitter other than those investigated. Fida et al. (2000) have recently suggested that MMC frequency in the mouse colon may be under the influence of endogenously released 5-hydroxytryptamine (5-HT) acting mainly via neural 5-HT3 and possibly 5-HT2 receptors.
Regional differences in neuronal mechanisms underlying MMC formation
In the present study, L-NOARG, apamin and suramin enhanced MMC amplitude and integral in the distal colon, confirming an important role for NO- and ATP-mediated inhibitory neurotransmission in MMC formation. However in the proximal colon, L-NOARG reduced MMC amplitude and integral, suggesting that NO caused a net excitatory effect in this region of the colon. Nitric oxide-mediated excitation has not previously been described in mouse colon; however an indirect excitatory effect of NO mediated by stimulation of excitatory motor neurons has been reported in guinea-pig ileum longitudinal muscle (Holzer et al., 1997). Direct excitation of longitudinal muscle has been shown in rat ileum (Lefebvre & Barthó, 1997) whilst inhibition of distension-evoked descending inhibitory reflexes to the circular muscle by nitric oxide has been reported in guinea-pig ileum (Yuan et al., 1995). Further experiments will be required to determine whether any of these mechanisms are responsible for the net excitation produced by nitric oxide in the mouse proximal colon circular muscle. Apamin, but not suramin, also decreased MMC amplitude in mouse proximal colon. This suggests that blockade of small conductance calcium-activated potassium channels that are coupled to suramin-resistant receptors decreases MMC activity in the proximal colon. Apamin-sensitive and suramin-resistant P2-purinoceptors have been demonstrated in guinea-pig ileum and colon (Zagorodnyuk & Maggi, 1998; Heinemann et al., 1999). However, these receptors are extrajunctional and not likely to be activated by endogenous transmitter, and produced inhibition rather than the excitation observed in the present study. Further experiments will be required to determine the mechanisms responsible for the decrease in MMC amplitude in the proximal colon caused by apamin in this study.
The reasons underlying the regional differences in the effects of L-NOARG, apamin and suramin are presently unclear. In rat colon however, regional differences have been demonstrated in the number of NOS-containing neurons and NOS activity (Takahashi & Owyang, 1998). Moreover, it has been demonstrated that anatomical and neuro-chemical differences occur between the proximal and distal colon of the guinea-pig (Messenger, 1993). Differences in the relative density of excitatory versus inhibitory motor neurons as well as regional differences in synaptic inputs and receptor localization along the colon may thus be important factors contributing to the regional variations identified in the present study.
Neuro-neuronal transmission in MMC formation
The present study has demonstrated that nicotinic ganglionic transmission is crucial for the activation of excitatory motor neurons releasing ACh and TKs during MMCs, since hexamethonium abolished both the rapid cholinergic and the slow tachykininergic components of the MMC. By contrast, significant NO- and ATP-mediated transmission occurred in the absence of nicotinic transmission, suggesting either that there are non-nicotinic inputs to these inhibitory motor neurons or that the neurons are spontaneously active. Furthermore, the increase in circular muscle membrane potential following addition of hexamethonium (Lyster et al., 1995; Spencer et al., 1998a,1998b) suggests that there is also nicotinic activation of inhibitory motor neurons. Studies in guinea-pig ileum demonstrate that non-nicotinic neuro-neuronal transmission in mucosal- and distension-evoked ascending and descending reflexes can be mediated by TKs acting at NK3 receptors, by ACh acting at muscarinic receptors and by ATP acting at P2-purinoceptors (Clark et al., 1996; Johnson et al., 1996; Spencer et al., 2000). Roles for these transmitters and receptors in neuro-neuronal transmission in MMC formation of the mouse colon remain to be investigated.
In conclusion, this study demonstrates that TKs, ACh, NO and ATP are the neurotransmitters required for formation of MMCs in mouse isolated colon. The release of TKs from excitatory motor neurons mediates the long duration contraction of the MMC via NK1 and NK2 receptors, whilst ACh mediates the rapid contractions via muscarinic receptors. The release of NO and ATP from inhibitory motor neurons appears primarily to be involved in maintaining quiescence between MMCs. Disinhibition of inhibitory motor neurons alone is insufficient to cause MMC formation, and must be accompanied by simultaneous activation of excitatory motor neurons. However, inhibitory pathways may be involved in determining if MMCs are formed. Nicotinic and non-nicotinic neuro-neuronal pathways appear to be involved in activating excitatory and inhibitory motor neurons. However, nicotinic neuro-neuronal pathways are critical in mediating MMC activity. Finally, regulation of MMC frequency may be controlled by an independent pacemaker system.
Acknowledgments
This work is supported by a project grant from the NH&MRC of Australia. S.M. Brierley is the recipient of an Australian Postgraduate Award. K. Nichols is the recipient of an NH&MRC Peter Doherty Fellowship. S.A. Waterman is the recipient of an NH&MRC R.D. Wright Fellowship. The authors wish to thank Dr Carlo A. Maggi (Florence, Italy) for the kind gift of the tachykinin receptor antagonists used in this study.
Abbreviations
- ACh
acetylcholine
- ATP
adenosine triphosphate
- 5-hydroxytryptamine (5-HT); L-NOARG
Nωnitro-L-arginine
- MMCs
migrating motor complexes
- NK
neurokinin
- NO
nitric oxide
- NOS
nitric oxide synthase
- SR-140 333
(S)-1-{2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenyl-acetyl)piperidin-3-yl]ethyl}-4 phenyl-1-lazoniabicyclo[2.2. 2]-octane chloride
- SR-48 968
(−)-N-methyl-N[4-acetylamino-4-phenyl-piperidino-2-(3,4-dichlorophenyl)butyl]-benzamide
- TK
tachykinin and TTX, tetrodotoxin
References
- BARAJAS-LÓPEZ C., MULLER M.J., PRIETO-GÓMEZ B., ESPINOSA-LUNA R. ATP inhibits the synaptic release of acetylcholine in submucosal neurons. J. Pharmacol. Exp. Ther. 1995;274:1238–1245. [PubMed] [Google Scholar]
- BYWATER R.A., SMALL R.C., TAYLOR G.S. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J. Physiol. (Lond.) 1989;413:505–519. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK S.R., COSTA M., TONINI M., BROOKES S.J.H. Purinergic transmission is involved in a descending excitatory reflex in the guinea-pig ileum. Proc. Aust. Neurosci. Soc. 1996;7:176. [Google Scholar]
- COULIE B., CAMILLERI M. Intestinal pseudo-obstruction. Annu. Rev. Med. 1999;50:37–55. doi: 10.1146/annurev.med.50.1.37. [DOI] [PubMed] [Google Scholar]
- CROCI T., EMONDS-ALT X., LEFUR G., MANARA L. In vitro characterization of the non-peptide tachykinin NK1 and NK2-receptor antagonists, SR140333 and SR48968 in different rat and guinea-pig intestinal segments. Life Sci. 1994;56:267–275. doi: 10.1016/0024-3205(94)00921-x. [DOI] [PubMed] [Google Scholar]
- DI LORENZO C. Pseudo-obstruction: current approaches. Gastroenterology. 1999;116:980–987. doi: 10.1016/s0016-5085(99)70082-x. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO V., VAN BROECK D., SOUBRIER P., LE FUR G., BRELIERE J.C. In vitro and in vivo biological activities of SR 140,333, a novel potent nonpeptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- FIDA R., BYWATER R.A.R., LYSTER D.J.K., TAYLOR G.S. Chronotropic action of 5-hydroxytryptamine (5-HT) on colonic migrating motor complexes (CMMCs) in the isolated mouse colon. J. Auton. Nerv. Syst. 2000;80:52–63. doi: 10.1016/s0165-1838(00)00074-6. [DOI] [PubMed] [Google Scholar]
- FIDA R., LYSTER D.J., BYWATER R.A., TAYLOR G.S. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol. Motil. 1997;9:99–107. doi: 10.1046/j.1365-2982.1997.d01-25.x. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., MAKHLOUF G.M. Prejunctional inhibition of vasoactive intestinal peptide release. Am. J. Physiol. 1987;253:G7–G12. doi: 10.1152/ajpgi.1987.253.1.G7. [DOI] [PubMed] [Google Scholar]
- HEINEMANN A., SHAHBAZIAN A., BARTHÓ L., HOLZER P. Different receptors mediating the inhibitory action of exogenous ATP and endogenously released purines on guinea-pig intestinal peristalsis. Br. J. Pharmacol. 1999;128:313–320. doi: 10.1038/sj.bjp.0702808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P., HOLZER-PETSCHE U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol. Ther. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- HOLZER P., LIPPE I.T., TABRIZI A.L., LÈNÁRD L.J., BARTHÓ L. Dual excitatory and inhibitory effect of nitric oxide on peristalsis in the guinea pig intestine. J. Pharmacol. Exp. Ther. 1997;280:154–161. [PubMed] [Google Scholar]
- JOHNSON P.J., BORNSTEIN J.C., YUAN S.Y., FURNESS J.B. Analysis of contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. Br. J. Pharmacol. 1996;118:973–983. doi: 10.1111/j.1476-5381.1996.tb15495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMIJI T., MORITA K., KATAYAMA Y. ATP regulates synaptic transmission by pre- and postsynaptic mechanisms in guinea-pig myenteric neurons. Neuroscience. 1994;59:165–174. doi: 10.1016/0306-4522(94)90107-4. [DOI] [PubMed] [Google Scholar]
- KILBINGER H., WOLF D. Increase by NO synthase inhibitors of acetylcholine release from guinea-pig myenteric plexus. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:543–545. doi: 10.1007/BF00169145. [DOI] [PubMed] [Google Scholar]
- KNUDSEN M.A., TØTTRUP A. A possible role of the L-arginine-nitric oxide pathway in the modulation of cholinergic transmission in the guinea-pig taenia coli. Br. J. Pharmacol. 1992;107:837–841. doi: 10.1111/j.1476-5381.1992.tb14533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNZE W.A., FURNESS J.B. The enteric nervous system and regulation of intestinal motility. Annu. Rev. Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., BARTHÓ L. Mechanism of nitric oxide-induced contraction in the rat isolated small intestine. Br. J. Pharmacol. 1997;120:975–981. doi: 10.1038/sj.bjp.0700996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPARD K.J., MESSORI E., GALLIGAN J.J. Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology. 1997;113:1522–1534. doi: 10.1053/gast.1997.v113.pm9352854. [DOI] [PubMed] [Google Scholar]
- LYSTER D.J., BYWATER R.A., TAYLOR G.S. Migrating myoelectric complexes and their neurogenic control. Proc. Aust. Physiol. Pharmacol. Soc. 1992;23:111–119. [Google Scholar]
- LYSTER D.J., BYWATER R.A., TAYLOR G.S. Neurogenic control of myoelectric complexes in the mouse isolated colon. Gastroenterology. 1995;108:1371–1378. doi: 10.1016/0016-5085(95)90684-3. [DOI] [PubMed] [Google Scholar]
- MESSENGER J.P. Immunohistochemical analysis of neurons and their projections in the proximal colon of the guinea-pig. Arch. Histol. Cytol. 1993;56:459–474. doi: 10.1679/aohc.56.459. [DOI] [PubMed] [Google Scholar]
- SANG Q., YOUNG H.M. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat. Rec. 1998;251:185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- SANG Q., YOUNG H.M. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- SANG Q., WILLIAMSON S., YOUNG H.M. Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. J. Anat. 1997;190:209–222. doi: 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH T.K., ROBERTSON W.J. Synchronous movements of the longitudinal and circular muscle during peristalsis in the isolated guinea-pig distal colon. J. Physiol. (Lond.) 1998;506:563–577. doi: 10.1111/j.1469-7793.1998.563bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER N.J., BYWATER R.A., HOLMAN M.E., TAYLOR G.S. Inhibitory neurotransmission in the circular muscle layer of mouse colon. J. Auton. Nerv. Syst. 1998a;70:10–14. doi: 10.1016/s0165-1838(98)00045-9. [DOI] [PubMed] [Google Scholar]
- SPENCER N.J., BYWATER R.A., TAYLOR G.S. Disinhibition during myoelectric complexes in the mouse colon. J. Auton. Nerv. Syst. 1998b;71:37–47. doi: 10.1016/s0165-1838(98)00063-0. [DOI] [PubMed] [Google Scholar]
- SPENCER N.J., BYWATER R.A., TAYLOR G.S. Evidence that myoelectric complexes in the isolated mouse colon may not be of myogenic origin. Neurosci. Lett. 1998c;250:153–156. doi: 10.1016/s0304-3940(98)00461-3. [DOI] [PubMed] [Google Scholar]
- SPENCER N.J., WALSH M., SMITH T.K. Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J. Physiol. (Lond.) 2000;522:321–331. doi: 10.1111/j.1469-7793.2000.t01-1-00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI T., OWYANG C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998;115:1504–1512. doi: 10.1016/s0016-5085(98)70029-0. [DOI] [PubMed] [Google Scholar]
- TAMURA K., SCHEMANN M., WOOD J.D. Actions of nitric oxide-generating sodium nitroprusside in myenteric plexus of guinea pig small intestine. Am. J. Physiol. 1993;265:G887–G893. doi: 10.1152/ajpgi.1993.265.5.G887. [DOI] [PubMed] [Google Scholar]
- TONINI M., COSTA M., BROOKES S.J.H., HUMPHREYS C.M. Dissociation of the ascending excitatory reflex from peristalsis in the guinea-pig small intestine. Neuroscience. 1996;73:287–297. doi: 10.1016/0306-4522(96)00040-1. [DOI] [PubMed] [Google Scholar]
- WATERMAN S.A., COSTA M. The role of enteric inhibitory motoneurons in peristalsis in the isolated guinea-pig small intestine. J. Physiol. (Lond.) 1994;477:459–468. doi: 10.1113/jphysiol.1994.sp020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERMAN S.A., TONINI M., COSTA M. The role of ascending excitatory and descending inhibitory pathways in peristalsis in the isolated guinea-pig small intestine. J. Physiol. (Lond.) 1994;481:223–232. doi: 10.1113/jphysiol.1994.sp020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIKLUND C.U., OLGART C., WIKLUND N.P., GUSTAFSSON L.E. Modulation of cholinergic and substance P-like neurotransmission by nitric oxide in the guinea-pig ileum. Br. J. Pharmacol. 1993;110:833–839. doi: 10.1111/j.1476-5381.1993.tb13888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD J.D. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am. J. Dig. Dis. 1973;18:477–488. doi: 10.1007/BF01076598. [DOI] [PubMed] [Google Scholar]
- YUAN S.Y., BORNSTEIN J.C., FURNESS J.B. Pharmacological evidence that nitric oxide may be a retrograde messenger in the enteric nervous system. Br. J. Pharmacol. 1995;114:428–432. doi: 10.1111/j.1476-5381.1995.tb13244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUNKER A.M., GALLIGAN J.J. Endogenous NO inhibits NANC but not cholinergic neurotransmission to circular muscle of guinea pig ileum. Am. J. Physiol. 1996;271:G904–G912. doi: 10.1152/ajpgi.1996.271.5.G904. [DOI] [PubMed] [Google Scholar]
- ZAGORODNYUK V., MAGGI C.A. Pharmacological evidence for the existence of multiple P2 receptors in the circular muscle of guinea-pig colon. Br. J. Pharmacol. 1998;123:122–128. doi: 10.1038/sj.bjp.0701558. [DOI] [PMC free article] [PubMed] [Google Scholar]


