Abstract
This study examines β1-, β2- and β3-adrenoceptor (AR)-mediated responses, mRNA levels and radioligand binding in ileum from β3-AR knock-out (−/−) (KO) and wild type (+/+) (FVB) mice.
In KO and FVB mice, SR59230A (100 nM) (β3-AR antagonist) antagonized responses to (−)-isoprenaline in both KO and FVB mice. (−)-Isoprenaline mediated relaxation of ileum was antagonized weakly by ICI118551 (100 nM) (β2-AR antagonist). Responses to (−)-isoprenaline were more strongly antagonized by CGP20712A (100 nM) (β1-AR antagonist), propranolol (1 μM) (β1-/β2-AR antagonist), carvedilol (100 nM) (non-specific β-AR antagonist), and CGP12177A (100 nM) (β1-/β2-AR antagonist) in ileum from KO than in FVB mice.
Responses to CL316243 (β3-AR agonist) in ileum from FVB mice were antagonized by SR59230A (100 nM) but not by propranolol (1 μM) or carvedilol (100 nM). CL316243 was ineffective in relaxing ileum from KO mice.
CGP12177A had no agonist actions in ileum from either KO or FVB mice.
β1-AR mRNA levels were increased 3 fold in ileum from KO compared to FVB mice. This was associated with an increased maximum number of β1-/β2-AR binding sites (Bmax). β2-AR mRNA levels were unaffected while no β3-AR mRNA was detected in KO mice.
In mouse ileum, β3-ARs and to a lesser extent β1-ARs are the predominant adrenoceptor subtypes mediating relaxation in ileum from FVB mice. In KO mice β1-ARs functionally compensate for the lack of β3-ARs, and this is associated with increased β1-AR mRNA and levels of binding.
Keywords: β3-adrenoceptors, β1- and β2-adrenoceptors, knock-out, ileum, CL316243, (−)-isoprenaline, CGP12177A
Introduction
β3-ARs mediate relaxation in a wide variety of gastrointestinal tissues from various species including guinea-pig, rat, rabbit and man (for review see Manara et al., 1995). A functional role for β3-ARs is supported by studies demonstrating β3-AR mRNA in gastrointestinal tissues (Bensaid et al., 1993; Evans et al., 1996; 1998; Granneman et al., 1991; 1993; Hutchinson et al., 2000; Krief et al., 1993; Roberts et al., 1997; 1999) and radioligand binding studies using (−)-[125I]-cyanopindolol (ICYP) show a site with characteristics of β3-ARs (Hutchinson et al., 2000; Roberts et al., 1995; 1997).
While β3-ARs predominate in gastrointestinal tissues, β1- and β2-ARs may also have roles. The β1-AR agonist Ro363 relaxes rat ileum, and the relaxation is antagonized by CGP20712A (Roberts et al., 1999). Ro363 had a higher intrinsic activity than isoprenaline in rat colon and guinea-pig ileum (Molenaar et al., 1997a), and caused a relaxation in rat ileum which was 60 – 70% of the isoprenaline response (Hoey et al., 1996). Later studies showed responses that were less than 20% of maximum responses with pEC50 values (6.2) (Roberts et al., 1999) intermediate between values at β1- and β3-ARs in rat colon (8.5 and 5.6 respectively) (Molenaar et al., 1997a).
However one of the problems in interpreting some studies is the use of β1-/β2-AR agonists that also act at β3-ARs. Ro363 is a partial agonist at the cloned human β3-AR and on intestinal β3-ARs in the rat and guinea-pig (Molenaar et al., 1997a). Zinterol (β2-AR agonist) causes relaxation in rat ileum, but the response was mediated through β3-ARs since they were antagonized by SR58894A but not ICI118551 (Roberts et al., 1999). Ritodrine (β2-AR agonist) causes relaxation in rat colon, and its responses are antagonized by alprenolol and propranolol but with lower potency than that expected for β2-ARs and it probably also acts at β3-ARs (Bianchetti & Manara, 1990).
The aim of the present study was to examine the importance of β3-ARs in mouse ileum by comparing responses of ileum from β3-AR KO and FVB mice. Relaxation of ileal smooth muscle to (−)-isoprenaline was performed to assess the relative roles of all three β-AR subtypes in mediating smooth muscle relaxation. This study demonstrates that β1- and β3-ARs mediate smooth muscle relaxation in mouse ileum and that β1-ARs compensate for the lack of β3-ARs in β3-AR KO mice, suggesting that β3-ARs are important in gastrointestinal function.
Methods
Animals and genotyping
FVB mice (female, ∼4 months old) were obtained from the Animal Resources Centre, Canning Vale, Western Australia. β3-AR KO mice (male, ∼1 year old) were the offspring of a previously described strain (Susulic et al., 1995). Genomic DNA analysis was conducted on mouse tails to determine the genotype of all mice used for breeding and experimental studies. Genomic DNA was isolated by proteinase K digestion overnight followed by phenol-chloroform extraction (Miller et al., 1988). PCR was performed on 0.5 μg DNA (62°C, 32 cycles) using primers designed to indicate the presence/absence of neomycin disruption to the β3-AR allele (see Table 1). Products were run on a 1.3% agarose gel and the bands photographed. In all experiments conducted, 8 – 14 week old male mice were used.
Table 1.
Oligonucleotides used as PCR primers and hybridization probes
Analysis of β-AR mRNA levels in ileum
Tissue was obtained from mice anaesthetized with 80% CO2/20% O2 and decapitated. Tissue was prepared as previously described (Roberts et al., 1999). Total RNA was extracted by homogenization in Trizol reagent (Gibco BRL, Life Technologies) using a PRO200 homogenizer. The yield and quality of RNA was assessed by measuring absorbance at 260 and 280 nm and by electrophoresis on 1.3% agarose gels. cDNAs were synthesized by reverse transcription of 1 μg of each total RNA using oligo (dT)15 (Gibco BRL, Life Technologies) as a primer (Roberts et al., 1999). PCR amplification was carried out on cDNA equivalent to 100 ng of starting RNA using primers specific for β1-, β2- or β3-AR and the internal standard actin (Gibco BRL, Life Technologies; see Table 1). For β1-AR PCR, PCR mixes contained 0.5 U Platinum® Pfx DNA polymerase (Life Technologies), 1× AMP buffer and 1× Enhancer solution (as supplied by Life Technologies), dNTPs (130 μM) (Amersham Pharmacia Biotech), MgSO4 (1.5 mM), 5.8 pmol forward primer and 5.8 pmol reverse primer. β2-, β3-AR and actin PCR has been described elsewhere (Roberts et al., 1999). The actin reverse primer was labelled prior to PCR with [γ-33P]-ATP (Evans et al., 1998). The annealing temperature for all PCR reactions was 64°C except for β1-AR PCR where the annealing temperature was 60°C. For each set of primers the log (PCR product) versus cycle number was plotted and a cycle number chosen within the linear portion of the graph (data not shown). Cycle numbers were 16 for actin, 26 for β1- and β2-AR, and 30 for β3-AR. Following amplification, PCR products were electrophoresed on 1.3% agarose gels and transferred onto Hybond N+ membranes by Southern blotting in 0.4 M NaOH/1 M NaCl. For detection of β1-, β2-, and β3-AR products, membranes were hybridized with a probe specific for each product expected (Table 1). Probes were end labelled with 50 μCi [γ-33P]-ATP and T4 polynucleotide kinase (Amersham Pharmacia Biotech) (Roberts et al., 1999). Radioactivity was detected with a Molecular Dynamics SI phosphorimager, and bands quantitiated using ImageQuantNT Software (Molecular Dynamics). β-AR levels were normalized for actin levels (β-AR/actin) and expressed as a percentage of the mean±s.e.mean value of n animals from FVB tissue. Student's unpaired t-test (2-tailed) was used to determine significance of differences. Probability values less than or equal to 0.05 were considered significant.
Receptor binding assay
Membranes were prepared as previously described (Hutchinson et al., 2000). Saturation experiments were performed at room temperature in a total volume of 100 μl in microcentrifuge tubes. Homogenate (approximately 20 – 40 μg protein) was incubated with (−)-[125I]-cyanopindolol (ICYP) (5 – 100 pM) for 60 min at room temperature in the absence or presence of (−)-propranolol (1 μM) to define non-specific binding. Tubes were centrifuged briefly after incubation, supernatants discarded and the pellet resuspended in 100 μl binding buffer (50 mM Tris pH 7.4 room temperature, 5 mM MgCl2, 1 mM EDTA, 10 mg ml−1 bacitracin, 10 mg ml−1 leupeptin, 10 mg ml−1 pepstatin A, 0.5 mg ml−1 aprotinin) for 30 min to minimize any low affinity binding. Reactions were terminated by rapid filtration through GF/B filters using a Packard Cell Harvester. Filters were washed four times with buffer (50 mM Tris pH 7,4 room temperature), dried, 30 μl Microscint-O (Packard) added and radioactivity measured using a Packard Top Count. Experiments were performed in duplicate with tissues from n animals. Results are expressed as mean±s.e.mean of n. Data was analysed using non-linear curve fitting (GraphPad PRISM version 2.0) using a one-site fit to obtain KD and Bmax values. Two-way ANOVA tests were used to determine significance of variations between curves. Probability values less than or equal to 0.05 were considered significant.
Organ bath studies
Mice were anaesthetized with 80% CO2/20% O2 and decapitated. Approximately 10 cm of small intestine was removed 2 cm above the ileocaecal junction. Segments of approximately 2 cm were mounted on tissue hooks and suspended in jacketed organ baths containing 6 ml Krebs-Henseleit solution (composition (mM): NaCl 118.4, KCl 4.7, MgSO4.7H2O 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11, CaCl2 2.5) containing ascorbic acid (0.1 mM) and EDTA (0.04 mM) maintained at 37°C and bubbled with 95% O2/5% CO2 (pH 7.4) under 4 mN force. Responses were measured with a UgoBasile isotonic transducer connected to a MacLab system and an Apple IIci computer. Tissues were allowed to equilibrate with or without antagonists (as specified) for 30 min. Following equilibration, tissues were contracted with carbachol (1 μM) (approximately 80% maximal response; data not shown) until responses maintained plateau (10 – 15 min). Control experiments were conducted and showed that the carbachol-evoked contraction was stable for the whole period of the experiment (data not shown). Cumulative concentration-response (c-r) curves to specified agonists were constructed with increments of 0.5 log units until a stable state was observed. At the end of each c-r curve, tissues were maximally relaxed with papaverine (10 μM) and all responses were expressed as a percentage of this papaverine response. Non-linear regression was used to fit sigmoid c-r curves to the data (GraphPad PRISM version 2.0) and to determine pEC50 values. Values are expressed as mean±s.e.mean of n individual experiments, with each n value referring to the number of individual animals used. In experiments where antagonists were used, pKB values were calculated according to the method of Furchgott (1972). Student's t-test was used to determine statistical significance where P<0.05 was considered to be significant.
Drugs and reagents
CL316243 (Dr T. Nash), SR59230A (Dr L. Manara) and carvedilol (Dr R.R. Ruffolo III) were gifts from Wyeth-Ayerst, Sanofi-Midi and SB Pharmaceuticals respectively. The drugs and reagents used were as follows: [γ-33P]-ATP (2000 Ci mmol−1, Geneworks, Adelaide, SA, Australia); (−)-[125I]-CYP (2200 Ci mmol−1, NEN Life Science Products, Boston, MA, U.S.A.); ICI118551 (Imperial Chemical Industries, Wilmslow, Cheshire, U.K.); CGP12177A (Research Biochemicals Inc., MA, U.S.A.); CGP20712A (Ciba-Geigy AG, Australia); aprotinin, bacitracin, carbachol (carbamylcholine chloride), (−)-isoprenaline bitartate, papaverine hydrochloride, (−)-propranolol (Sigma Chemical Company, St. Louis, MO, U.S.A.); leupeptin, pepstatin A, (Gibco BRL, Life Technologies, Gaithersburg, MD, U.S.A.); L(+)-ascorbic acid (Merck, Frankfurt, Germany); EDTA (AJAX Chemicals, Melbourne, VIC, Australia). All other chemicals were of analytical grade.
Stock solutions of SR59230A were prepared in 50% distilled water, 25% ethanol and 25% DMSO, pepstatin A in DMSO, (−)-isoprenaline bitartate, CGP20712A, ICI118551 and CGP12177A in 10 mM HCl, carvedilol in 0.1 mM ascorbic acid, and the remaining drugs in distilled water.
Results
Detection of β1-, β2- and β3-AR mRNA in FVB and β3-AR KO ileum
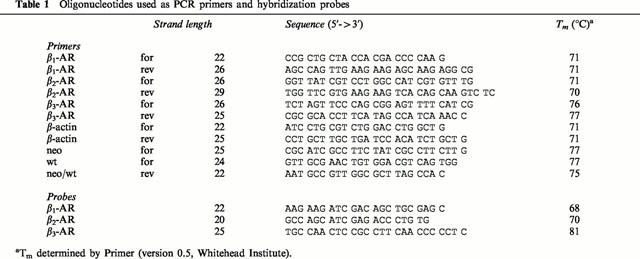
RT – PCR detected all three β-AR subtypes and actin mRNA in ileal smooth muscle from FVB mice. Direct comparison of the levels of β-AR mRNA in KO compared to FVB samples showed that β1-AR mRNA levels were increased 3 fold in the β3-AR KO ileum (FVB 100±37.8%, KO 350.9±92.5%; n=6; Student's t-test *P<0.05), β2-AR mRNA levels were not significantly altered (FVB 100±12.2%, KO 95.2±9.0%, n=6), and as expected no β3-AR mRNA was detected in KO samples (FVB 100±15.5%, KO 2.4±1.1%, n=6; Student's t-test ***P<0.001) (Figure 1).
Figure 1.
β1-, β2-, and β3-AR mRNA levels in KO and FVB mice. β1-AR mRNA levels were increased 3 fold in ileum from KO as compared to FVB mice. β2-AR mRNA levels were unaltered and no detectable levels of β3-AR mRNA was observed in KO mice.
[125I]-Cyanopindolol binding in membranes from FVB and β3-AR KO ileum
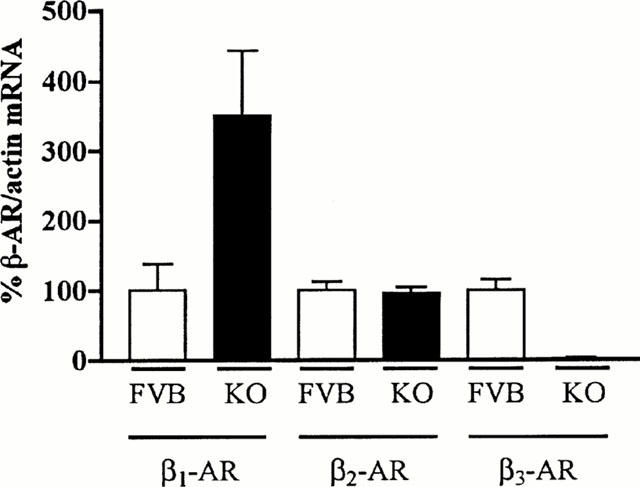
ICYP binding occurred in a saturable manner (Figure 2) to a single population of sites in FVB ileum (KD 44.6±30.4 pM; Bmax 16.7±4.9 fmol mg−1 protein; n=5). ICYP binding levels were increased in KO (KD 57.2±25.5 pM; Bmax 30.6±6.5 fmol mg−1 protein; n=6) compared to FVB samples (2-way ANOVA ***P=0.0001). At the highest concentration of ICYP used (100 pM), approximately 66% of the β-AR binding sites in FVB and KO ileum would be labelled.
Figure 2.
Saturation binding curve of ICYP to KO and FVB ileal membrane preparations. Graph shows specific binding, showing a significant increase in ICYP binding in ileum from KO as compared to FVB mice (2-way ANOVA ***P<0.0001). Points show mean±s.e.mean (n=5 – 6).
Organ bath studies of ileum from β3-AR KO and FVB mice
Effect of CL316243
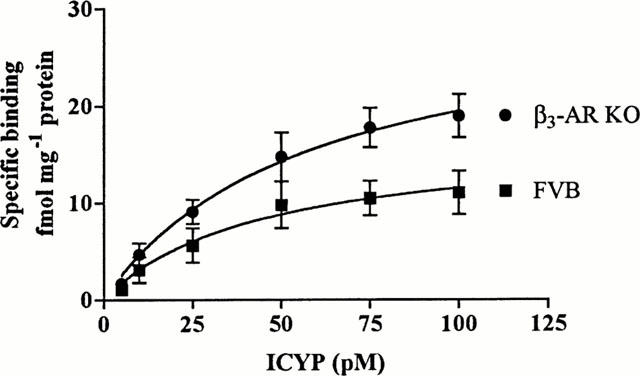
The β3-AR agonist CL316243 relaxed smooth muscle in carbachol precontracted FVB ileum in a dose-dependent manner (pEC50 8.5±0.3, n=7) whereas ileum from KO animals (n=7) was unresponsive to CL316243 (Figure 3a). Responses to CL316243 in FVB ileum were antagonized by SR59230A (100 nM) (control: pEC50 8.8±0.5, n=6; SR59230A (100 nM): pEC50 7.6±0.2, n=6) with a pKB value of 8.3±0.2 (n=6) (Figure 3b). Antagonism of CL316243 responses by both propranolol (100 nM) (control: pEC50 8.8±0.6, n=6; propranolol (100 nM): pEC50 8.3±0.2, n=6) and carvedilol (100 nM) (control: pEC50 8.3±0.3, n=3; carvedilol (100 nM): pEC50 8.1±0.1, n=3) was relatively weak (Figure 3c,d).
Figure 3.
The effect of CL316243 on ileal responses in KO and FVB mice. Graph shows: (a) responses to CL316243 are abolished in ileum from KO as compared to FVB mice (n=7); and responses to CL316243 in FVB mice in the absence and presence of (b) SR59230A (n=6), (c) propranolol (n=6) and (d) carvedilol (n=3). Points show mean and vertical lines indicate s.e.mean.
Effect of (−)-isoprenaline
The non-subtype selective β-AR agonist (−)-isoprenaline caused concentration-dependent relaxation of carbachol-precontracted ileum from both FVB and KO animals (Figure 4).
Figure 4.
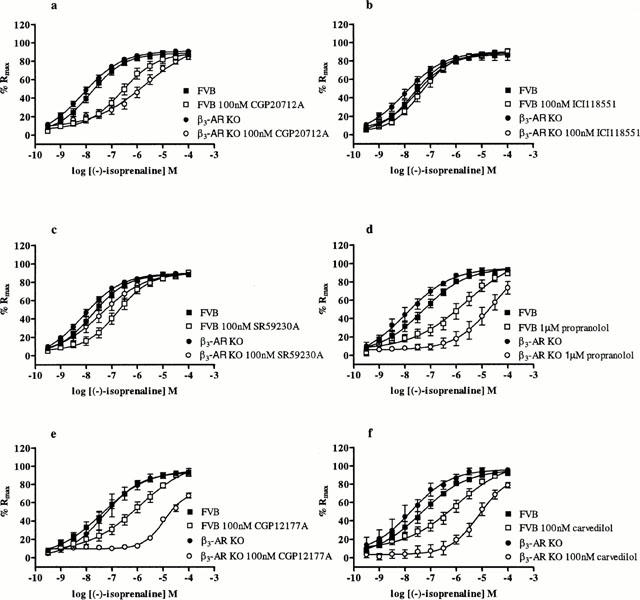
The effect of (−)-isoprenaline on ileal responses in KO and FVB mice. Graph shows responses to (−)-isoprenaline are more strongly antagonized by (a) CGP20712A (n=7 – 9), (d) propranolol (n=6 – 7), (e) CGP12177A (n=3 – 5), and (f) carvedilol (n=3 – 5) in KO as compared to FVB mice. Responses to (−)-isoprenaline are weakly antagonized by (b) ICI118551 in both KO and FVB mice (n=6 – 8) while (c) SR59230A antagonized (−)-isoprenaline responses in FVB mice but was still effective in KO mice but to a lesser degree (n=8 – 9). Points show mean and vertical lines indicate s.e.mean.
The β1-AR selective antagonist CGP20712A (100 nM) (Figure 4a) caused a rightward shift in the (−)-isoprenaline c-r curve in both KO (control: pEC50 8.2±0.1, n=9; CGP20712A (100 nM): pEC50 5.7±0.3, n=9) and FVB ileum (control: pEC50 7.8±0.1, n=7; CGP20712A (100 nM): pEC50 6.6±0.1, n=7), with the larger shift observed in the KO compared to FVB ileum (pKB values 9.4±0.3 (n=9) and 8.1±0.3 (n=7) respectively; Student's t-test ***P<0.001).
The β2-AR antagonist ICI118551 (100 nM) (Figure 4b) was a very weak antagonist in both KO (control: pEC50 8.0±0.2, n=8; ICI118551 (100 nM): pEC50 7.5±0.1, n=8) and FVB (control: pEC50 7.7±0.1, n=6; ICI118551 (100 nM): pEC50 7.4±0.1, n=6) ileum and failed to significantly shift c-r curves to (−)-isoprenaline.
The β3-AR antagonist SR59230A (100 nM) (Figure 4c) significantly shifted the (−)-isoprenaline c-r curve to the right in FVB ileal samples (control: pEC50 7.8±0.1, n=8; SR59230A (100 nM): pEC50 6.8±0.1, n=8; pKB 8.0±0.2 (n=8)). (−)-Isoprenaline responses in KO ileum was antagonised to a weaker degree by SR59230A (100 nM) (control: pEC50 8.1±0.1, n=9; SR59230A (100 nM): pEC50 7.4±0.2, n=9; pKB 7.4±0.3 (n=9)). There was no statistical difference between the pKB values obtained for FVB or KO mice (Student's t-test NS).
The β1-/β2-AR antagonist (−)-propranolol (1 μM) (Figure 4d) caused a rightward shift of the (−)-isoprenaline c-r curve with pKB values of 7.7±0.2 (n=7) and 8.9±0.3 (n=6) (Student's t-test ***P<0.001) in FVB (control: pEC50 7.3±0.1, n=7; propranolol (1 μM): pEC50 5.6±0.4, n=7) and KO (control: pEC50 8.0±0.3, n=6; propranolol (1 μM): pEC50 4.6±0.8, n=6) ileum respectively.
CGP12177A (β1-/β2-AR antagonist) (Figure 4e) antagonized responses to (−)-isoprenaline in both FVB and KO ileum, with responses more strongly antagonized in ileum from KO (control: pEC50 7.3±0.2, n=3; CGP12177A (100 nM): pEC50 5.0±0.1, n=3) as compared to FVB mice (control: pEC50 7.4±0.1, n=5; CGP12177A (100 nM): pEC50 5.9±0.3, n=5) with pKB values of 9.6±0.6 (n=3) and 8.6±0.4 (n=5) respectively (Student's t-test ***P<0.001).
Responses to (−)-isoprenaline were antagonized by carvedilol (100 nM) (non-specific β-AR antagonist) (Figure 4f) more strongly in ileum from KO (control: pEC50 7.8±0.3, n=3; carvedilol (100 nM): pEC50 5.1±0.2, n=3) compared to FVB mice (control: pEC50 7.4±0.1, n=5; carvedilol (100 nM): pEC50 6.0±0.3, n=5) with pKB values of 10.1±0.4 (n=3) and 8.4±0.4 (n=5) respectively (Student's t-test ***P<0.001).
Effect of CGP12177A
CGP12177A failed to exert any significant agonistic actions in both KO and FVB ileum, even at concentrations of 10 μM (Figure 5). Subsequent addition of (−)-isoprenaline (10 μM) failed to produce any relaxation of the ileum, suggesting that CGP12177A acted purely as an antagonist in this preparation. Subsequent additions of CL316243 (1 μM) in FVB mice caused relaxation in the same tissue, suggesting an intact β3-AR response, whereas in KO mice CL316243 had no effect (Figure 5).
Figure 5.
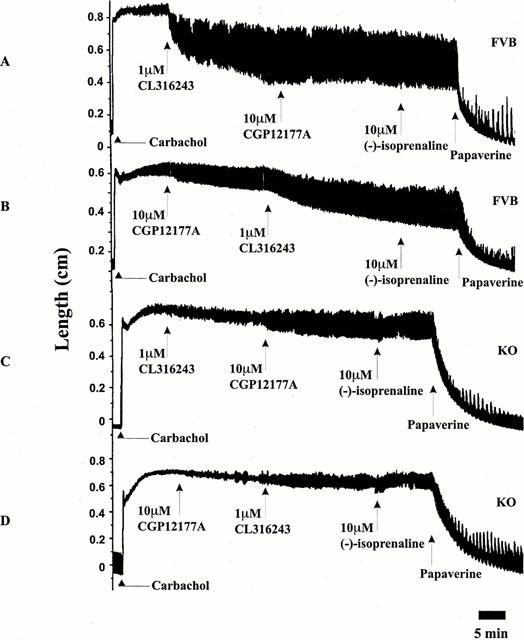
Original traces obtained from (A,B) FVB and (C,D) KO ileum tissues. Tissues were contracted with carbachol (1 μM) and then exposed to CL316243 (1 μM) followed by CGP12177A (10 μM) or, CGP12177A (10 μM) followed by CL316243 (1 μM) as indicated. (−)-Isoprenaline (10 μM) and finally papaverine (10 μM) were added. Arrows indicate administration of drug. CL316243 was effective only in FVB ileum while CGP12177A was ineffective in causing ileal relaxation in both FVB and KO mice. Note the lack of effect of (−)-isoprenaline in FVB and KO ileum following CGP12177A administration. Traces are representative of n=4 experiments.
Discussion
β1-, β2- and β3-AR mediated relaxation of mouse ileum
Both β1- and β3-ARs mediate relaxation of mouse ileal smooth muscle in FVB mice. The relaxant effects of (−)-isoprenaline are antagonized strongly by the selective β1-(CGP20712A) and β3-AR (SR59230A) antagonists but weakly by the β2-AR antagonist (ICI118551), suggesting both β1- and β3-AR involvement in relaxation to (−)-isoprenaline. Responses to (−)-isoprenaline were also antagonized by the β1-/β2-AR antagonist propranolol, the non-selective β-AR antagonist carvedilol and the β1-/β2-AR antagonist (and putative β4-AR agonist) CGP12177A. The degree of blockade caused by CGP20712A and SR59230A was similar, indicating that β1- and β3-ARs contribute to a similar extent to (−)-isoprenaline relaxation.
The pKB values observed in ileum from FVB mice with propranolol (7.7) was much higher than the values found in studies in rat distal colon (6.6; McLaughlin & MacDonald, 1990), ileum (6.8; Roberts et al., 1999) and gastric fundus (6.3; McLaughlin & MacDonald, 1991), but not other studies where higher pKB values have been reported (8.4, guinea-pig taenia caecum (Koike et al., 1995); 8.2 and 8.5, rat colon (Bianchetti & Manara, 1990; Croci et al., 1988)). In rat colon, there is a mixture of β-ARs present since in studies where higher pA2 values for propranolol are reported, Schild plot slopes well below unity were found, suggesting the presence of more than one β-AR subtype (Bianchetti & Manara, 1990; Croci et al., 1988; McLaughlin & MacDonald, 1990). Other studies of antagonism of (−)-isoprenaline by propranolol show higher pKB values in guinea-pig atria (β1-AR) (pA2 8.7 – 8.9), trachea (β2-AR) (pA2 8.3) and diaphragm (β2-AR) (pA2 9.2) (Harms et al., 1977), all tissues with predominantly β1- or β2-AR responses. Antagonism with CGP20712A also revealed a pKB value of 8.1 which was lower than that at cardiac β1-ARs (pA2 9.0; Molenaar & Summers, 1987) but higher than that at atypical β-ARs (pA2 4.1 – 4.6; Hollenga & Zaagsma, 1989; Van Liefde et al., 1993). Studies carried out in rat ileum, distal colon and guinea-pig colon (De Ponti et al., 1995; MacDonald & Lamont, 1993; Roberts et al., 1999) demonstrated no significant shift with CGP20712A. Carvedilol antagonized (−)-isoprenaline responses in FVB ileum with a pKB (8.4) similar to that observed in ferret myocardium (pKB 8.1; Lowe et al., 1999). This data taken together suggests that a significant β1-AR component exists in mouse ileum, as evidenced by the higher than expected pKB values obtained from antagonism of (−)-isoprenaline by propranolol and CGP20712A at β3-ARs, and the similarity of the pKB values to those reported in tissues rich in β1-ARs.
The functional role of the β3-AR in mouse ileum was also confirmed in this study, as in FVB mice responses were produced to the β3-AR agonist CL316243 and were antagonized by SR59230A but not by propranolol or carvedilol. Affinity values for antagonism by SR59230A were consistent with previous reports (this study pKB 8.3, in rat colon pA2 8.1 (Manara et al., 1996)). Carvedilol was investigated since it was reported to have high affinity at the cloned human β3-AR (Ki 0.4 nM (Candelore et al., 1999)). However in the present study it had no significant antagonist action against CL316243 in mouse ileum. Propranolol resistance of β3-AR mediated responses has been used in many studies of gastrointestinal smooth muscle to demonstrate the presence of atypical β-ARs (Bianchetti & Manara, 1990; De Ponti et al., 1995; McLaughlin & MacDonald, 1991), and this was confirmed in this study where propranolol failed to antagonize responses to CL316243. In the present study no responses to CL316243 were observed in ileum from KO animals. This is supported by other studies using β3-AR KO animals where responses to CL316243 were abolished in stomach fundus (Cohen et al., 2000), colon (Oostendorp et al., 2000), adipose tissue (Grujic et al., 1997; Preitner et al., 1998; Susulic et al., 1995) and there was also decreased gastrointestinal motility (Fletcher et al., 1998).
A role for β1- and β3-ARs is supported by the presence of mRNA for both receptors in ileum. In contrast β2-AR mRNA was present yet responses to (−)-isoprenaline were only weakly antagonized by the β2-AR antagonist ICI118551 in FVB mice. Despite other in vitro studies showing a minor β2-AR mediated relaxation in mouse colon (Oostendorp et al., 2000), rat distal colon, and jejunum (MacDonald & Lamont, 1993; van der Vliet et al., 1990), and in vivo studies in rat duodenum and jejunum showing (−)-isoprenaline and ritodrine can disrupt migrating myoelectric complexes which are blocked by ICI118551 (Thollander et al., 1996), other studies in rat ileum show that despite β2-AR mRNA being present, no β2-AR mediated relaxation responses could be found (Roberts et al., 1999). It is likely therefore that β2-ARs play roles other than relaxation in mouse ileum. β-AR agonists such as isoprenaline, adrenaline and terbutaline are known to increase glucagon-like peptide-1 and peptide YY secretions in rat ileum and this effect is antagonized by propranolol and likely to be mediated by β2-AR activation (Claustre et al., 1999; Dumoulin et al., 1995).
β1-ARs appear to compensate for the lack of β3-ARs in ileum from β3-AR KO mice. This was supported functionally by the increased antagonism of (−)-isoprenaline mediated relaxation by CGP20712A, propranolol and carvedilol in ileum from KO as compared to FVB mice. The increase in pKB values for these agents suggests an enhanced β1-AR response. There may also be a slight increase in β2-AR function in KO mice since while responses to (−)-isoprenaline were very weakly antagonized by ICI118551 in FVB mice, there was a somewhat greater shift in KO mice, despite the absence of a change in β2-AR mRNA levels. Compensation by β1-ARs was associated with an increase in ICYP binding and β1-AR mRNA levels. Interestingly, this parallels the increase in β1-AR mRNA levels seen in adipose tissue in this strain of KO mice (Grujic et al., 1997; Susulic et al., 1995) where these animals compensate for the lack of β3-ARs in adipose tissue by upregulation of β1-AR gene expression. More recent studies with another strain of KO mice on a 129Sv+C57BL/6 mouse background (Revelli et al., 1997) show that in mouse colon, while β1-ARs also compensate for the lack of β3-ARs functionally, no differences in β1-AR mRNA levels were observed in 129Sv+C57BL/6 and KO ileum (Oostendorp et al., 2000). These authors suggested that in the KO animals, existing β1-ARs may become coupled more strongly to signalling pathways governing gastrointestinal relaxation. Two groups have successfully targeted the inactivation of the mouse β3-AR gene. Both groups used different approaches (for review see Rohrer, 1998) and this may be a factor in the differing results obtained. Susulic et al. (1995) showed that the lack of β3-ARs resulted in upregulation of β1-AR mRNA in adipose tissue, whereas the model established by Revelli et al. (1997) showed downregulation of β1-ARs in adipose tissue. This difference may be a result of the different strain backgrounds of the mice used, the knock-out strategy used or other unknown variables. Nevertheless, both studies show several similar findings, including KO animals being susceptible to a slowly developing mild increase in body fat content, with no associated increase in food intake, and no changes in circulating levels of insulin, glucose or free fatty acids.
Another interesting feature of (−)-isoprenaline mediated relaxation in KO ileum was that antagonism by SR59230A was still observed, although much weaker than that in FVB mice. This would suggest that SR59230A may not be as specific for the β3-AR as previously suggested (Manara et al., 1996). In this study, responses to the β3-AR agonist SR58611A were antagonized by SR59230A with a pA2 value of 8.76 in rat proximal colon, similar to that observed here with either CL316243 or (−)-isoprenaline as the agonist (pKB 8.3 and 8.0 respectively in FVB mice). In KO mice, a pKB value against (−)-isoprenaline of 7.4 was observed, in comparison with a pA2 value of 7.3 observed in guinea-pig atria against (−)-isoprenaline (Manara et al., 1996). A recent study has indicated that at cloned human β-ARs, SR59230A displays little selectivity in binding for the β3-AR compared to β1- or β2-ARs (Candelore et al., 1999). This may suggest SR59230A is not as selective as previously reported and may also interact with β1-ARs but this needs further investigation.
Action of CGP12177A
CGP12177A is a high affinity β1-/β2-AR antagonist (Staehelin & Hertel, 1983) which also shows partial agonist activity at β3-ARs in adipose tissues, and has been suggested to have partial agonist actions at another site termed the putative β4-AR. It has been used to describe a putative β4-AR in the heart of several species including man and mouse (Kaumann, 1996; Kaumann & Molenaar, 1997; Molenaar et al., 1997b), as well as in mouse and human adipocytes (Galitzky et al., 1997; Preitner et al., 1998). In cardiac tissues CGP12177A and other non-conventional partial agonists cause antagonism of responses mediated by β1- and β2-ARs but at higher concentrations have agonist effects that are resistant to blockade by propranolol but not by bupranolol. These effects cannot be mediated by β3-ARs since the heart does not express this subtype (Evans et al., 1996) and CGP12177A still produces these effects in the heart from β3-AR KO mice (Kaumann et al., 1998). In tissues that do express β3-ARs such as rat colon or guinea-pig taenia caecum, CGP12177A acts as an agonist (Kaumann & Molenaar, 1996; Molenaar et al., 1997a; Sennitt et al., 1998; Koike et al., 1995; 1996). Support for the β4-AR concept came from studies showing that CGP12177A activated brown adipose tissue and cardiac responses in β3-AR KO mice (Kaumann et al., 1998; Preitner et al., 1998). In β3-AR KO mice, CGP12177A has agonist actions in atria (Cohen et al., 2000), adipose tissue (Preitner et al., 1998) and oesophageal and colonic smooth muscle (Oostendorp et al., 2000). However it is clear that CGP12177A effects at the putative β4-AR only occur in tissues (heart and adipose) that express high levels of β1-AR, and also in cultured cells overexpressing the β1-AR (Pak & Fishman, 1996). It is now clear from a recent study in β1- or β3-AR KO mice, that β1-ARs mediate most, if not all, of the β3-AR independent effects of CGP12177A on brown adipocyte adenylate cyclase activity (Konkar et al., 2000). In the present study in mouse ileum, no agonist actions of CGP12177A were observed in either KO or FVB ileum, even with concentrations up to 10 μM, indicating no agonist actions at either β3- or β1-ARs. Instead, CGP12177A acted as a potent antagonist of (−)-isoprenaline mediated relaxations with pKB values of 9.6 in KO and 8.6 in FVB ileum. These values are much higher than those reported in rat ileum (7.6; Roberts et al., 1999) and consistent with those in guinea-pig taenia caecum (9.3; Koike et al., 1996). These findings are in accord with an action of CGP12177A to block β1-ARs which are expressed at higher levels in KO ileum.
In conclusion, in mouse ileum, β3-ARs and to a lesser extent β1-ARs mediate smooth muscle relaxation, with no β2-AR involvement. In KO mice, β1-ARs functionally compensate for the lack of β3-ARs, and these animals have increased β1-AR mRNA and levels of binding. CGP12177A acts as an antagonist in this preparation with no agonist actions and in mice SR59230A appears not to be as selective for the β3-AR as previously reported.
Acknowledgments
We would like to acknowledge Dr Bradford Lowell for kindly supplying us with β3-AR KO mice. This work was supported by the National Health and Medical Research Council of Australia. D.S. Hutchinson is a Monash University Postgraduate Scholar.
Abbreviations
- β-AR
β-adrenoceptor
- Bmax
maximum number of binding sites
- CGP12177A
(±)-4-(3-t-butylamino-2-hydroxypropoxy)benzimidazol-2-one
- CGP20712A
2-hydroxy-5(2-((2-hydroxy-3-(4-((1-methyl-4-trifluoromethyl)1H-imidazole-2-yl)-phenoxy)propyl)amino)ethoxy)-benzamide monomethane sulfonate, CL316243, (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-dicarboxylate
- c-r
concentration-response
- ICI118551
erythro-DL-1(7-methylindian-4-yloxy)-3-isopropylaminobutan-2-ol
- ICYP
(−)-[125I]-cyanopindolol
- KO
knock-out
- Rmax
maximal relaxation
- RT – PCR
reverse transcrition-polymerase chain reaction
- SR59230A
3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate
References
- BENSAID M., KAGHAD M., RODRIGUEZ M., LE FUR G., CAPUT D. The rat β3-adrenergic receptor gene contains an intron. FEBS Lett. 1993;318:223–226. doi: 10.1016/0014-5793(93)80516-w. [DOI] [PubMed] [Google Scholar]
- BIANCHETTI A., MANARA L. In vitro inhibition of intestinal motility by phenylethanolaminotetralines: evidence of atypical β-adrenoceptors in rat colon. Br. J. Pharmacol. 1990;100:831–839. doi: 10.1111/j.1476-5381.1990.tb14100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANDELORE M.R., DENG L., TOTA L., GUAN X.M., AMEND A., LIU Y., NEWBOLD R., CASCIERI M.A., WEBER A.E. Potent and selective human β3-adrenergic receptor antagonists. J. Pharmacol. Exper. Ther. 1999;290:649–655. [PubMed] [Google Scholar]
- CLAUSTRE J., BRECHET S., PLAISANCIE P., CHAYVIALLE J.A., CUBER J.C. Stimulatory effect of β-adrenergic agonists on ileal L cell secretion and modulation by alpha-adrenergic activation. J. Endocrin. 1999;162:271–278. doi: 10.1677/joe.0.1620271. [DOI] [PubMed] [Google Scholar]
- COHEN M.L., BLOOMQUIST W., ITO M., LOWELL B.B. β3 receptors mediate relaxation in stomach fundus whereas a fourth β receptor mediates tachycardia in atria from transgenic β3 receptor knockout mice. Rec. Channels. 2000;7:17–23. [PubMed] [Google Scholar]
- CROCI T., CECCHI R., TARANTINO A., AUREGGI G., BIANCHETTI A., BOIGEGRAIN R., MANARA L. Inhibition of rat colon motility by stimulation of atypical β-adrenoceptors with new gut-specific agents. Pharmacol. Res. Commun. 1988;20:147–151. doi: 10.1016/s0031-6989(88)80007-9. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., GIBELLI G., CREMA F., LECCHINI S. Functional evidence for the presence of β3-adrenoceptors in the guinea pig common bile duct and colon. Pharmacology. 1995;51:288–297. doi: 10.1159/000139338. [DOI] [PubMed] [Google Scholar]
- DUMOULIN V., DAKKA T., PLAISANCIE P., CHAYVIALLE J.A., CUBER J.C. Regulation of glucagon-like peptide-1-(7-36) amide, peptide YY, and neurotensin secretion by neurotransmitters and gut hormones in the isolated vascularly perfused rat ileum. Endocrinology. 1995;136:5182–5188. doi: 10.1210/endo.136.11.7588257. [DOI] [PubMed] [Google Scholar]
- EVANS B.A., PAPAIOANNOU M., ANASTASOPOULOS F., SUMMERS R.J. Differential regulation of β3-adrenoceptors in gut and adipose tissue of genetically obese (ob/ob) C57BL/6J-mice. Br. J. Pharmacol. 1998;124:763–771. doi: 10.1038/sj.bjp.0701867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS B.A., PAPAIOANNOU M., BONAZZI V.R., SUMMERS R.J. Expression of β3-adrenoceptor mRNA in rat tissues. Br. J. Pharmacol. 1996;117:210–216. doi: 10.1111/j.1476-5381.1996.tb15176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLETCHER D.S., CANDELORE M.R., GRUJIC D., LOWELL B.B., LUELL S., SUSULIC V.S., MACINTYRE D.E. β3-Adrenergic receptor agonists cause an increase in gastrointestinal transit time in wild-type mice, but not in mice lacking the β3-adrenergic receptor. J. Pharmacol. Exper. Ther. 1998;287:720–724. [PubMed] [Google Scholar]
- FURCHGOTT R.F. Handbook of Experimental Pharmacology. Berlin: Springer Verlag; 1972. The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory; pp. 283–335. [Google Scholar]
- GALITZKY J., LANGIN D., VERWAERDE P., MONTASTRUC J.L., LAFONTAN M., BERLAN M. Lipolytic effects of conventional β3-adrenoceptor agonists and of CGP 12,177 in rat and human fat cells: preliminary pharmacological evidence for a putative β4-adrenoceptor. Br. J. Pharmacol. 1997;122:1244–1250. doi: 10.1038/sj.bjp.0701523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N., CHAUDHRY A. Molecular cloning and expression of the rat β3-adrenergic receptor. Mol. Pharmacol. 1991;40:895–899. [PubMed] [Google Scholar]
- GRANNEMAN J.G., LAHNERS K.N., CHAUDHRY A. Characterization of the human β3-adrenergic receptor gene. Mol. Pharmacol. 1993;44:264–270. [PubMed] [Google Scholar]
- GRUJIC D., SUSULIC V.S., HARPER M.E., HIMMS-HAGEN J., CUNNINGHAM B.A., CORKEY B.E., LOWELL B.B. β3-Adrenergic receptors on white and brown adipocytes mediate β3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J. Biol. Chem. 1997;272:17686–17693. doi: 10.1074/jbc.272.28.17686. [DOI] [PubMed] [Google Scholar]
- HARMS H.H., ZAAGSMA J., DE VENTE J. Differentiation of β-adrenoceptors in right atrium, diaphragm and adipose tissue of the rat, using stereoisomers of propranolol, alprenolol, nifenalol and practolol. Life Sci. 1977;21:123–128. doi: 10.1016/0024-3205(77)90432-5. [DOI] [PubMed] [Google Scholar]
- HOEY A., JACKSON C., PEGG G., SILLENCE M. Atypical responses of rat ileum to pindolol, cyanopindolol and iodocyanopindolol. Br. J. Pharmacol. 1996;117:712–716. doi: 10.1111/j.1476-5381.1996.tb15248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLENGA C., ZAAGSMA J. Direct evidence for the atypical nature of functional β-adrenoceptors in rat adipocytes. Br. J. Pharmacol. 1989;98:1420–1424. doi: 10.1111/j.1476-5381.1989.tb12692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHINSON D.S., EVANS B.A., SUMMERS R.J. β3-Adrenoceptor regulation and relaxation responses in mouse ileum. Br. J. Pharmacol. 2000;129:1251–1259. doi: 10.1038/sj.bjp.0703160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J. (−)-CGP 12177-induced increase of human atrial contraction through a putative third β-adrenoceptor. Br. J. Pharmacol. 1996;117:93–98. doi: 10.1111/j.1476-5381.1996.tb15159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Differences between the third cardiac β-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Modulation of human cardiac function through four β-adrenoceptor populations. Naunyn Schmiedebergs Arch. Pharmacol. 1997;355:667–681. doi: 10.1007/pl00004999. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., PREITNER F., SARSERO D., MOLENAAR P., REVELLI J.P., GIACOBINO J.P. (−)-CGP 12177 causes cardiostimulation and binds to cardiac putative β4-adrenoceptors in both wild-type and β3-adrenoceptor knockout mice. Mol. Pharmacol. 1998;53:670–675. doi: 10.1124/mol.53.4.670. [DOI] [PubMed] [Google Scholar]
- KOIKE K., HORINOUCHI T., TAKAYANAGI I. Signal transduction pathway involved in β3-adrenoceptor-mediated relaxation in guinea pig taenia caecum. Jpn. J. Pharmacol. 1995;68:41–46. doi: 10.1254/jjp.68.41. [DOI] [PubMed] [Google Scholar]
- KOIKE K., TAKAYANAGI I., YAMAZAKI M. Differentiation of binding sites of CGP12177, a β3-adrenoceptor partial agonist, and carteolol, a β1/β2-adrenoceptor partial agonist, to the β-adrenoceptors in guinea-pig taenia caecum. Can. J. Physiol. Pharmacol. 1996;74:928–933. [PubMed] [Google Scholar]
- KONKAR A.A., ZHAI Y., GRANNEMAN J.G. β1-Adrenergic receptors mediate β3-adrenergic-independent effects of CGP 12177 in brown adipose tissue. Mol. Pharmacol. 2000;57:252–258. [PubMed] [Google Scholar]
- KRIEF S., LONNQVIST F., RAIMBAULT S., BAUDE B., VAN SPRONSEN A., ARNER P., STROSBERG A.D., RICQUIER D., EMORINE L.J. Tissue distribution of β3-adrenergic receptor mRNA in man. J. Clin. Invest. 1993;91:344–349. doi: 10.1172/JCI116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE M.D., GRACE A.A., KAUMANN A.J. Blockade of putative β4- and β1-adrenoceptors by carvedilol in ferret myocardium. Naunyn-Schmiedebergs Arch. Pharmacol. 1999;359:400–403. doi: 10.1007/pl00005367. [DOI] [PubMed] [Google Scholar]
- MACDONALD A., LAMONT M. Effects of selective antagonism of β-adrenoceptor sub-types on responses to isoprenaline in rat distal colon in vitro. Br. J. Pharmacol. 1993;110:1551–1555. doi: 10.1111/j.1476-5381.1993.tb14000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., LE FUR G. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANARA L., CROCI T., LANDI M. β3-Adrenoceptors and intestinal motility. Fund. Clin. Pharmacol. 1995;9:332–342. doi: 10.1111/j.1472-8206.1995.tb00507.x. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN D.P., MACDONALD A. Evidence for the existence of ‘atypical' β-adrenoceptors (β3-adrenoceptors) mediating relaxation in the rat distal colon in vitro. Br. J. Pharmacol. 1990;101:569–574. doi: 10.1111/j.1476-5381.1990.tb14122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAUGHLIN D.P., MACDONALD A. Characterization of catecholamine-mediated relaxations in rat isolated gastric fundus: evidence for an atypical β-adrenoceptor. Br. J. Pharmacol. 1991;103:1351–1356. doi: 10.1111/j.1476-5381.1991.tb09792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER S.A., DYKES D.D., POLESKY H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLENAAR P., SARSERO D., ARCH J.R., KELLY J., HENSON S.M., KAUMANN A.J. Effects of (−)-RO363 at human atrial β-adrenoceptor subtypes, the human cloned β3-adrenoceptor and rodent intestinal β3-adrenoceptors. Br. J. Pharmacol. 1997a;120:165–176. doi: 10.1038/sj.bjp.0700850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLENAAR P., SARSERO D., KAUMANN A.J. Proposal for the interaction of non-conventional partial agonists and catecholamines with the ‘putative β4-adrenoceptor' in mammalian heart. Clin. Exper. Pharmacol. Physiol. 1997b;24:647–656. doi: 10.1111/j.1440-1681.1997.tb02107.x. [DOI] [PubMed] [Google Scholar]
- MOLENAAR P., SUMMERS R.J. Characterization of β1- and β2-adrenoceptors in guinea pig atrium: functional and receptor binding studies. J. Pharmacol. Exper. Ther. 1987;241:1041–1047. [PubMed] [Google Scholar]
- OOSTENDORP J., PREITNER F., MOFFAT J., JIMENEZ M., GIACOBINO J.P., MOLENAAR P., KAUMANN A.J. Contribution of β-adrenoceptor subtypes to relaxation of colon and oesophagus and pacemaker activity of ureter in wild-type and β3-adrenoceptor knockout mice. Br. J. Pharmacol. 2000;130:747–758. doi: 10.1038/sj.bjp.0703365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAK M.D., FISHMAN P.H. Anomalous behavior of CGP 12177A on β1-adrenergic receptors. J. Receptor Signal Transduction Res. 1996;16:1–23. doi: 10.3109/10799899609039938. [DOI] [PubMed] [Google Scholar]
- PREITNER F., MUZZIN P., REVELLI J.P., SEYDOUX J., GALITZKY J., BERLAN M., LAFONTAN M., GIACOBINO J.P. Metabolic response to various β-adrenoceptor agonists in β3-adrenoceptor knockout mice: evidence for a new β-adrenergic receptor in brown adipose tissue. Br. J. Pharmacol. 1998;124:1684–1688. doi: 10.1038/sj.bjp.0702007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVELLI J.P., PREITNER F., SAMEC S., MUNIESA P., KUEHNE F., BOSS O., VASSALLI J.D., DULLOO A., SEYDOUX J., GIACOBINO J.P., HUARTE J., ODY C. Targeted gene disruption reveals a leptin-independent role for the mouse β3-adrenoceptor in the regulation of body composition. J. Clin. Invest. 1997;100:1098–1106. doi: 10.1172/JCI119620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS S.J., PAPAIOANNOU M., EVANS B.A., SUMMERS R.J. Functional and molecular evidence for β1-, β2- and β3-adrenoceptors in human colon. Br. J. Pharmacol. 1997;120:1527–1535. doi: 10.1038/sj.bjp.0701056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS S.J., PAPAIOANNOU M., EVANS B.A., SUMMERS R.J. Characterization of β-adrenoceptor mediated smooth muscle relaxation and the detection of mRNA for β1-, β2- and β3-adrenoceptors in rat ileum. Br. J. Pharmacol. 1999;127:949–961. doi: 10.1038/sj.bjp.0702605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS S.J., RUSSELL F.D., MOLENAAR P., SUMMERS R.J. Characterization and localization of atypical β-adrenoceptors in rat ileum. Br. J. Pharmacol. 1995;116:2549–2556. doi: 10.1111/j.1476-5381.1995.tb17206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROHRER D.K. Physiological consequences of β-adrenergic receptor disruption. J. Mol. Med. 1998;76:764–772. doi: 10.1007/s001090050278. [DOI] [PubMed] [Google Scholar]
- SENNITT M.V., KAUMANN A.J., MOLENAAR P., BEELEY L.J., YOUNG P.W., KELLY J., CHAPMAN H., HENSON S.M., BERGE J.M., DEAN D.K., KOTECHA N.R., MORGAN H.K., RAMI H.K., WARD R.W., THOMPSON M., WILSON S., SMITH S.A., CAWTHORNE M.A., STOCK M.J., ARCH J.R. The contribution of classical (β1/2-) and atypical β-adrenoceptors to the stimulation of human white adipocyte lipolysis and right atrial appendage contraction by novel β3-adrenoceptor agonists of differing selectivities. J. Pharmacol. Exper. Ther. 1998;285:1084–1095. [PubMed] [Google Scholar]
- STAEHELIN M., HERTEL C. [3H]CGP-12177, a β-adrenergic ligand suitable for measuring cell surface receptors. J. Receptor Res. 1983;3:35–43. doi: 10.3109/10799898309041921. [DOI] [PubMed] [Google Scholar]
- SUSULIC V.S., FREDERICH R.C., LAWITTS J., TOZZO E., KAHN B.B., HARPER M.E., HIMMS-HAGEN J., FLIER J.S., LOWELL B.B. Targeted disruption of the β3-adrenergic receptor gene. J. Biol. Chem. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- THOLLANDER M., SVENSSON T.H., HELLSTROM P.M. β-Adrenoceptors regulate myoelectric activity in the small intestine of rats: stimulation by β2 and inhibition by β3 subtypes. Neurogastroenterol. Motility. 1996;8:143–151. doi: 10.1111/j.1365-2982.1996.tb00254.x. [DOI] [PubMed] [Google Scholar]
- VAN DER VLIET A., RADEMAKER B., BAST A. A β adrenoceptor with atypical characteristics is involved in the relaxation of the rat small intestine. J. Pharmacol. Exper. Ther. 1990;255:218–226. [PubMed] [Google Scholar]
- VAN LIEFDE I., VAN WITZENBURG A., VAUQUELIN G. Isoproterenol and selective agonists stimulate similar atypical β-adrenoceptors in rat adipocytes. Biochem. Pharmacol. 1993;45:974–977. doi: 10.1016/0006-2952(93)90184-x. [DOI] [PubMed] [Google Scholar]


