Abstract
To determine which mediators are involved in antigen-induced bronchospasm and microvascular leakage in the airways of ovalbumin sensitised Brown Norway rats we investigated the effect of a histamine H1 receptor antagonist, mepyramine, a 5-HT receptor antagonist, methysergide, and a cys-leukotriene-1 receptor antagonist, montelukast.
Ovalbumin at 1 mg kg−1 i.v. caused a significant increase in microvascular leakage in the airways and at 3 mg kg−1 i.v. caused a significant increase in airways resistance.
Histamine (1 mg kg−1 i.v.), 5-HT (0.1 mg kg−1 i.v.) and leukotriene D4 (LTD4, 50 μg kg−1 i.v.) caused a significant increase in microvascular leakage in the airways.
Mepyramine (1 mg kg−1 i.v.), methysergide (0.1 mg kg−1 i.v.), or montelukast (30 mg kg−1 i.v.) inhibited histamine, 5-HT or LTD4 -induced microvascular leakage respectively.
Methysergide (0.1 mg kg−1 i.v.) reduced ovalbumin-induced microvascular leakage in the trachea and at 0.3 mg kg−1 i.v. inhibited bronchospasm (38 and 58%, respectively). Montelukast (30 mg kg−1 p.o.) reduced ovalbumin-induced microvascular leakage in airway tissue to basal levels (78%) and inhibited ovalbumin-induced bronchospasm (50%). Mepyramine (3 mg kg−1 i.v.) had no effect on ovalbumin-induced leakage or bronchospasm.
A combination of all three compounds (mepyramine, methysergide and montelukast) reduced ovalbumin-induced microvascular leakage in airway tissue to basal levels (70 – 78%) and almost completely inhibited bronchospasm (92%).
Antigen-induced bronchospasm appears to equally involve the activation of 5-HT and cys-leukotriene-1 receptors whereas ovalbumin-induced microvascular leakage appears to be predominantly mediated by cys-leukotriene-1 receptors.
Keywords: Antigen; bronchospasm; microvascular leakage; histamine; 5-HT, LTD4; cys-leukotriene-1 receptors; airways
Introduction
Bronchospasm and the leakage of plasma proteins from the microvasculature into airways tissue are seen as important consequences of allergic airways inflammation in man (Chung et al., 1990). These phenomena have been modelled in sensitized animals challenged with the appropriate inhaled allergen and the effect of novel anti-asthma compounds assessed. Intravenously administered ovalbumin in the antigen-sensitized Brown Norway rat causes bronchospasm (Nagase et al., 1996) and microvascular leakage into the airways (Taylor et al., 1997). The intravenous route confers the advantage of a reliable reproducible model using small numbers of animals per group. Furthermore, antigen given to sensitized Brown Norway rats via the inhaled route gives a notoriously variable response with some animals not responding at all (Sapienza et al., 1990).
The mechanism of the acute effects of ovalbumin on vascular integrity and bronchospasm are not fully understood but are thought to involve the release and actions of one or more inflammatory mediators. The early response to antigen in sensitized animals is thought to involve inflammatory mediators released from mast cells (Holgate et al., 1985; Kaliner et al., 1989). Evans et al. (1988) have examined the role of various inflammatory mediators on microvascular leakage in guinea-pig airways but mediator involvement in responses to antigen administration in the Brown Norway rat, an animal model which has been frequently used for airway studies and in which there is a pronounced early response to antigen (Kips et al., 1992), has not yet been fully characterized. Nagase et al. (1996) have demonstrated a role for 5-HT and LTD4 in the increase in tissue resistance seen in the airways after antigen administration in sensitised Brown Norway rats. In this study we have investigated the effects of ovalbumin, histamine, 5-HT and LTD4 on leakage in three different regions of the airways, the trachea, bronchi and the intra-pulmonary airways. Furthermore, we have attempted to elucidate which mediators drive both the bronchospasm and microvascular leakage responses to antigen in the airways of sensitized Brown Norway rats by the use of a histamine H1 receptor antagonist, mepyramine, a 5-HT receptor antagonist, methysergide and a cys-leukotriene-1 receptor antagonist, montelukast (Jones et al., 1995). This is the first study in which selective receptor antagonists have been used to define a role for all three mediators in antigen-induced leakage at different airway levels throughout the respiratory tract. Preliminary data from this study has previously been presented in abstract form (Hele et al., 1999; 2000).
Methods
Animals
Male Brown Norway rats (150 – 180 g) were purchased from Harlan-Olac (Bicester, U.K.) and housed at 21±1°C under a 14 : 10 h light – dark cycle and kept for at least 5 days before use. Animals were allowed free access to standard laboratory chow and water.
Antigen sensitization
Rats were sensitized on days 0, 12 and 21 with antigen (ovalbumin at 100 μg i.p., administered with aluminium hydroxide adjuvant at 100 mg i.p.) and were ready to use from day 28.
Antigen-induced microvascular leakage in the airways
Plasma leakage was measured as described by Bernareggi et al. (1997). Briefly, sensitized male Brown Norway rats were anaesthetized with isofluorane (3.5% in oxygen) and given Evans blue dye (20 mg kg−1 i.v.). One minute later while still anaesthetized they received ovalbumin (0.1, 0.3, 1, or 3 mg kg−1 i.v.) or vehicle (sterile saline, 0.9%). They were allowed to recover and 15 minutes after ovalbumin administration were killed by pentobarbitone overdose (200 mg kg−1 i.p.). The chest was opened and an incision made in the left ventricle, a cannula was inserted through the left ventricle and into the ascending aorta and approximately 150 ml of sterile saline (0.9%) perfused at a pressure of 100 mmHg. The heart and lungs were removed en bloc. The trachea, bronchi and lungs were dissected free and the parenchyma was scraped from the intra-pulmonary airways. The trachea, bronchi and intra-pulmonary airways were each placed in 2 ml of formamide for 18 h at 37°C to facilitate the extraction of Evans blue dye. The absorbances of the resulting extracts were determined against standard concentrations of Evans blue at 620 nm using a Cecil 2000 spectrophotometer. Results are expressed as concentration of Evans Blue dye (ng mg−1 of tissue).
Antigen-induced changes in airway resistance
Sensitized male Brown Norway rats were anaesthetized (sodium pentobarbitone, 105 mg kg−1 i.p.) and instrumented. Briefly, the trachea was cannulated and the animal artificially respired. A cannula was also placed in the oesophagus via the mouth to facilitate the measurement of airways resistance. Animals received vehicle (sterile saline 0.9%) or ovalbumin (3 mg kg−1 i.v.) via the tail vein. Changes in airways resistance (cmH2O m−1 s−1) were measured over a 10 min period after vehicle or ovalbumin administration using a Buxco LS20 system.
Histamine, 5-HT or LTD4-induced microvascular leakage in the airways
The experimental protocol for the mediator studies was as for the ovalbumin study described above. Histamine (0.3 – 30 mg kg−1 i.v.), 5-HT (0.03 – 3 mg kg−1 i.v.), LTD4 (1, 10 or 50 μg kg−1 i.v.) or vehicle were administered via the tail vein in isofluorane anaesthetized rats. The vehicle for histamine and 5-HT was sterile saline (0.9%) but for LTD4 the vehicle was 65% ethanol. The effects of this vehicle on microvascular leakage did not differ from those of sterile saline, however, as LTD4 was supplied at a concentration of 50 μg ml−1 in 65% ethanol this determined the highest dose we could reasonably give by the intravenous route as 50 μg kg−1. Animals were killed 15 min after mediator administration and tissues removed for Evans blue dye extraction.
Effect of mepyramine, methysergide or montelukast alone or in combination on mediator or antigen-induced microvascular leakage in the airways
Plasma leakage was assessed as described above. Sensitized male Brown Norway rats received mepyramine (1 mg kg−1 i.v.), methysergide (0.1 mg kg−1 i.v.) or vehicle (sterile saline, 0.9%, 1 ml kg−1 i.v.) 5 min prior to ovalbumin (1 mg kg−1 i.v.), histamine (3 mg kg−1 i.v.), 5-HT (1 mg kg−1 i.v.), LTD4 (0.05 mg kg−1 i.v.) or vehicle (sterile saline, 0.9%) challenge or montelukast (30 mg kg−1 p.o.) or vehicle (0.5% methyl cellulose plus 0.2% Tween 80 at 2 ml kg−1 p.o.) 90 min prior to ovalbumin, histamine, 5-HT or LTD4 challenge. The doses and timings for mepyramine, methysergide (Church, 1975) and montelukast (Jones et al., 1995) were those shown to be effective in the literature. In a separate experiment a further group of animals received a combination of all three antagonists at the same doses and timings outlined above prior to exposure to ovalbumin, histamine, 5-HT or LTD4. The combination was profiled against each of the antagonists. Animals were killed 15 min after ovalbumin or mediator administration and tissues removed for Evans blue dye extraction.
Effect of mepyramine, methysergide or montelukast alone or in combination on antigen-induced changes in airway resistance
Changes in airways resistance were measured as described above. Sensitized male Brown Norway rats received mepyramine (3 mg kg−1 i.v.), methysergide (0.3 mg kg−1 i.v.) or vehicle (sterile saline 0.9%, 1 ml kg−1 i.v.) 5 min prior to ovalbumin challenge (1 mg kg−1 i.v.) or montelukast (30 mg kg−1 p.o.) or vehicle (0.5% methyl cellulose plus 0.2% Tween 80, 2 ml kg−1 p.o.) 90 min prior to ovalbumin challenge. A further group of animals received a combination of the three antagonists, at the doses and timings outlined above, prior to intravenously administered ovalbumin, histamine, 5-HT or LTD4.
Data analysis
The leakage results are presented as data minus basal leak at time 0 and expressed as mean±s.e.mean of the concentration of Evans blue dye (ng mg of tissue−1). The changes in airways resistance are expressed as mean±s.e.mean (cmH2O m−1 s−1). Statistical analysis was carried out using one way analysis of variance with a correction for multiple comparisons using Dunnett's critical values. All experiments were conducted in accordance with Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986.
Chemicals
Mepyramine was synthesized at Rhône-Poulenc Rorer (Dagenham, U.K.), methysergide was obtained from ICN Pharmaceuticals Ltd (Basingstoke, U.K.) and montelukast from A.A.H. Ltd (Romford, U.K.). Isofluorane was purchased from Abbot Laboratories (Queenborough, Kent, U.K.), leukotriene-D4 from Cayman Chemical Company (Ann Arbor, MI, U.S.A.), sodium pentobarbitone from Rhône Mérieux Ltd, (Harlow, U.K.), aluminium hydroxide from Rhone Poulenc Ltd (Manchester, U.K.) and Sterile saline (0.9%) from Fresenius Kabi Ltd (Warrington, U.K.). All other materials were purchased from Sigma (Poole, U.K.).
Results
Effect of antigen administration on microvascular leakage in the airways
Intravenous administration of ovalbumin (0.3, 1 or 3 mg kg−1 i.v.) caused significant increases in microvascular leakage. At a dose of 0.1 mg kg−1 i.v. ovalbumin was without significant effect. At 1 mg kg−1 i.v. ovalbumin caused leakage into the trachea, bronchi and intrapulmonary airways (0.2±0.78 increased to 34.11±4.55; 0.99±0.72 increased to 41.89±3.36 and 3.18±0.79 increased to 41.26±5.11 ng mg−1 of tissue, respectively, P<0.001, see Figure 1).
Figure 1.
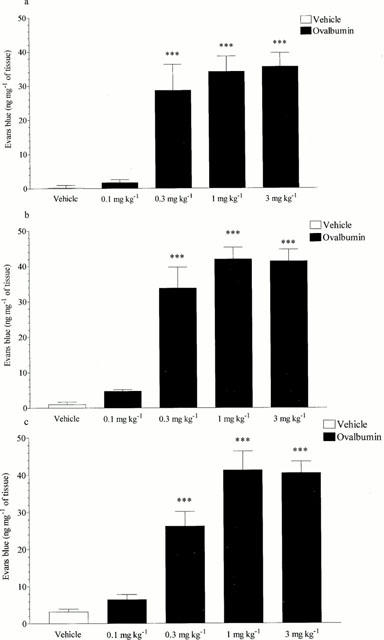
Effect of ovalbumin (0.1 – 3 mg kg−1 i.v.) on microvascular leakage into the (a) trachea, (b) bronchi or (c) intra-pulmonary airways of antigen-sensitized Brown Norway rats. Rats were anaesthetised with isofluorane (3.5% in oxygen) and received Evans blue (20 mg kg−1 i.v.), 1 min later they received ovalbumin or vehicle i.v. The rats were killed 15 min after ovalbumin or vehicle administration and tissues removed and placed in formamide for Evans blue dye extraction. Values are presented as data minus basal leak at time 0 and expressed as mean±s.e.mean of the concentration of Evans blue dye (ng mg−1 of tissue). n=4.*** P<0.001.
Effect of antigen administration on airways resistance
Intravenously administered ovalbumin (3 mg kg−1, i.v.) evoked a significant increase in airways resistance (0.42±0.06 cmH2O ml−1 s−1, P<0.01, see Figure 2).
Figure 2.
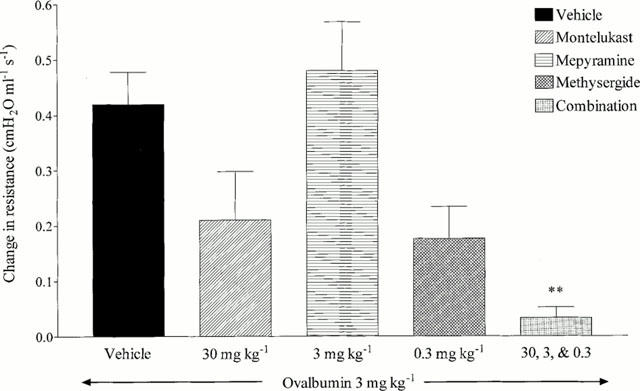
Effect of mepyramine (3 mg kg−1 i.v.), methysergide (0.3 mg kg−1 i.v), montelukast (30 mg kg−1 p.o.) or a combination of all three on antigen-induced changes in airways resistance in antigen-sensitized Brown Norway rats. Rats were anaesthetized with sodium pentobarbitone (105 mg kg−1 i.v.) and the trachea and oesophagus cannulated to facilitate the recording of airways resistance. Mepyramine or methysergide were administered 5 min (i.v.), or montelukast 90 min (p.o.), prior to vehicle (saline 0.9%) or ovalbumin (3 mg kg−1 i.v). Changes in airways resistance (cmH2 0 m−1 s−1) were measured over a 10 min period and expressed as mean±s.e.mean. n=4. **P<0.01.
Effect of histamine, 5-HT or LTD4 on microvascular leakage
Histamine at doses from 1 – 30 mg kg−1, i.v. caused significant increases in microvascular leakage in the bronchi and intra pulmonary airways (3 mg kg−1, i.v. increased leakage from 0.99±0.72 to 22.36±2.27, and 3.18±0.79 to 28.43±2.76 ng mg−1 of tissue respectively, P<0.001), at doses of 3 – 30 mg kg−1 i.v. it also caused significant leakage in the trachea (3 mg kg−1, i.v. increased leakage from 0.20±0.78 to 19.26±5.61 ng mg−1 of tissue, P<0.01). 5-HT predominantly caused microvascular leakage in the trachea and bronchi with doses from 0.1 – 3 mg kg−1, i.v. evoking significant responses in the trachea (1 mg kg−1, i.v. increased leakage from 0.20±0.78 to 47.99±2.45 ng mg−1 of tissue, P<0.001) and at doses from 0.3 – 3 mg kg−1, i.v. evoking responses in the bronchi (1 mg kg−1, i.v. increased leakage from 0.99±0.72 to 32.02±2.55 ng mg−1 of tissue, P<0.001). 5-HT only reached significance at 3 mg kg−1, i.v. in the intra-pulmonary airways (3.18±0.79 increased to 14.41±1.64 ng mg−1 of tissue, P<0.05) and with a much reduced response when compared to the other tissues. LTD4 (0.05 mg kg−1, i.v.), in contrast to 5-HT, induced significant increases in microvascular leakage mainly in the intra-pulmonary airways (3.18±0.79 increased to 37.53±3.69 ng mg−1 of tissue, P<0.001) with small but significant effects being seen in the trachea and bronchi at this dose (0.20±0.78 increased to 6.10±2.34 and 0.99±0.72 increased to 14.83±1.89 ng mg−1 of tissue respectively, P<0.05) (See Figure 3).
Figure 3.
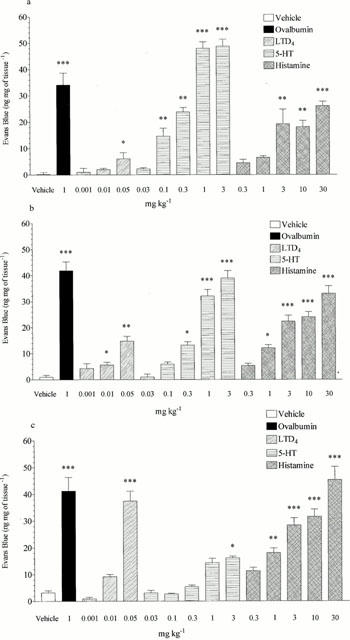
Effect of ovalbumin (1 mg kg−1 i.v.), LTD4 (0.001 – 0.05 mg kg−1 i.v.), 5-HT (0.03 – 3 mg kg−1 i.v.) or histamine (0.3 – 30 mg kg−1 i.v.) on microvascular leakage into the (a) trachea, (b) bronchi or (c) intra-pulmonary airways of antigen-sensitized Brown Norway rats. Rats were anaesthetized with isofluorane (3.5% in oxygen) and received Evans blue (20 mg kg−1 i.v.), 1 min later they received ovalbumin, LTD4, 5-HT, histamine or vehicle i.v. The rats were killed 15 min after ovalbumin or mediator administration and tissues removed and placed in formamide for Evans blue dye extraction. Values are presented as data minus basal leak at time 0 and expressed as mean±s.e.mean of the concentration of Evans blue dye (ng mg−1 of tissue). n=4. *P<0.05, **P<0.01, ***P<0.001.
Effect of mepyramine, methysergide or montelukast alone or in combination on mediator or antigen-induced microvascular leakage in the airways
Mepyramine (1 mg kg−1, i.v.) reduced histamine-induced microvascular leakage to near basal levels in the trachea, bronchi and intra-pulmonary airways (15.12±1.28 reduced to 0.00, 20.26±1.44 reduced to 0.00 and 25.45±1.27 reduced to 3.77±1.24 ng mg−1 of tissue respectively, P<0.001, see Figure 4). Mepyramine had no significant effect on leakage responses to 5-HT or LTD4 and also had no significant effect on ovalbumin-induced microvascular leakage in any of the lung tissues studied (see Figure 4). Methysergide (0.1 mg kg−1, i.v.) reduced 5-HT-induced microvascular leakage in the trachea, bronchi and intrapulmonary airways to near basal levels (33.81±4.53 reduced to 0.64±0.46, P<0.001, 17.20±5.89 reduced to 0.00, P<0.05 and 7.49±1.83 reduced to 1.97±0.92 ng mg−1 of tissue, P<0.05, respectively, see Figure 5). Methysergide did not significantly reduce the leakage responses to histamine or LTD4, however it did slightly enhance the response to LTD4 in the bronchi and intra-pulmonary airways (P<0.05, see Figure 5). Methysergide did reduce the leakage response to ovalbumin but this only reached statistical significance in the bronchi (52.44±7.73 reduced to 29.46±4.28 ng mg−1 of tissue, P<0.05, see Figure 5). Montelukast (30 mg kg−1, p.o.) reduced LTD4-induced microvascular leakage to near basal levels in the bronchi and intra-pulmonary airways (13.5±1.25 reduced to 4.54±0.66 and 36.12±4.09 reduced to 5.15±0.84 ng mg−1 of tissue, respectively, P<0.001, see Figure 6). Montelukast was without effect in the trachea but as seen above LTD4 induced very little leakage in this tissue. Montelukast had no significant effect on 5-HT or LTD4-induced leakage but did significantly reduce ovalbumin-induced microvascular leakage to near basal levels in the trachea, bronchi and intra-pulmonary airways (31.48±5.38 reduced to 3.34±0.87, 36.50±5.43 reduced to 7.73±1.41 and 40.98±4.57 reduced to 8.00±1.33 ng mg−1 of tissue respectively, P<0.01, see Figure 6). When the three antagonists were given in combination ovalbumin-induced microvascular leakage was reduced to near basal levels in the trachea, bronchi and intra-pulmonary airways (31.48±5.38 reduced to 2.54±0.45, 36.5±5.43 reduced to 6.94±1.07 and 40.98±4.57 reduced to 8.34±0.77 ng mg−1 of tissue respectively, P<0.01, see Figure 7). The combined treatment was profiled against the three antagonists given alone, this produced similar results to those shown above. Mepyramine (1 mg kg−1 i.v.) was without effect, methysergide (0.1 mg kg−1 i.v.) caused a small reduction in leakage in the trachea whereas montelukast (30 mg kg−1 p.o.) reduced ovalbumin-induced microvascular leakage to near basal levels in all three tissues, a similar effect to that achieved with the combined treatment (see Figure 7).
Figure 4.
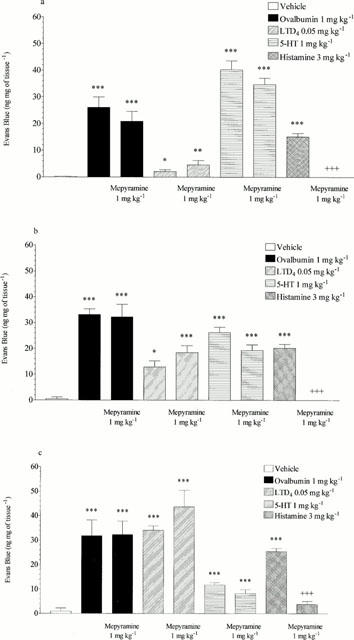
Effect of mepyramine (1 mg kg−1 i.v.) on ovalbumin (1 mg kg−1 i.v.), LTD4 (0.05 mg kg−1 i.v.), 5-HT (1 mg kg−1 i.v.) or histamine (3 mg kg−1 i.v.) induced microvascular leakage into the (a) trachea, (b) bronchi or (c) intra-pulmonary airways of antigen-sensitized Brown Norway rats. Rats were anaesthetized with isofluorane (3.5% in oxygen) and received Evans blue (20 mg kg−1 i.v.), 1 min later they received ovalbumin, LTD4, 5-HT, histamine or vehicle i.v. The rats were killed 15 min after ovalbumin or mediator administration and tissues removed and placed in formamide for Evans blue dye extraction. Values are presented as data minus basal leak at time 0 and expressed as mean±s.e.mean of the concentration of Evans blue dye (ng mg−1 of tissue). n=4. *P<0.05, **P<0.01, ***P<0.001 when compared to vehicle group. +++P<0.001 when compared to relevant control.
Figure 5.
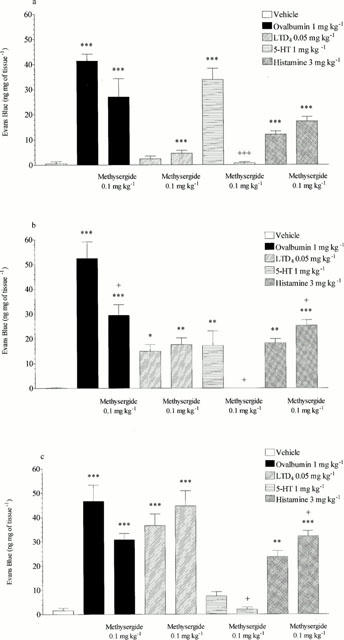
Effect of methysergide (0.1 mg kg−1 i.v.) on ovalbumin (1 mg kg−1 i.v.), LTD4 (0.05 mg kg−1 i.v.), 5-HT (1 mg kg−1 i.v.) or histamine (3 mg kg−1 i.v.) induced microvascular leakage into the (a) trachea, (b) bronchi or (c) intra-pulmonary airways of antigen-sensitized Brown Norway rats. Rats were anaesthetized with isofluorane (3.5% in oxygen) and received Evans blue (20 mg kg−1 i.v.), 1 min later they received ovalbumin, LTD4, 5-HT, histamine or vehicle i.v. The rats were killed 15 min after ovalbumin or mediator administration and tissues removed and placed in formamide for Evans blue dye extraction. Values are presented as data minus basal leak at time 0 and expressed as mean±s.e.mean of the concentration of Evans blue dye (ng mg−1 of tissue). n=4. *P<0.05, **P<0.01, ***P<0.001 when compared to vehicle group, +P<0.05, +++ P<0.001 when compared to relevant control.
Figure 6.
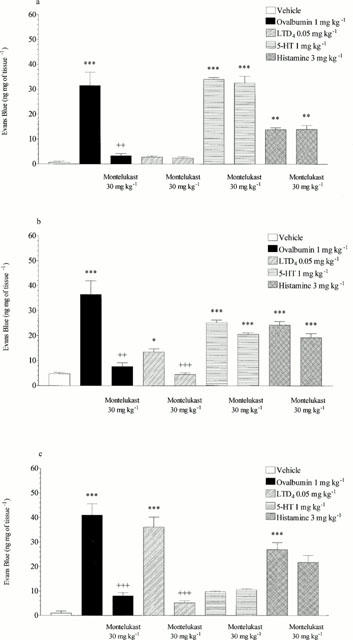
Effect of montelukast (30 mg kg−1 p.o.) on ovalbumin (1 mg kg−1 i.v.), LTD4 (0.05 mg kg−1 i.v.), 5-HT (1 mg kg−1 i.v.) or histamine (3 mg kg−1 i.v.) induced microvascular leakage into the (a) trachea, (b) bronchi or (c) intra-pulmonary airways of antigen-sensitized Brown Norway rats. Rats were anaesthetized with isofluorane (3.5% in oxygen) and received Evans blue (20 mg kg−1 i.v.), 1 min later they received ovalbumin LTD4, 5-HT, histamine or vehicle i.v. The rats were killed 15 min after ovalbumin or mediator administration and tissues removed and placed in formamide for Evans blue dye extraction. Values are presented as data minus basal leak at time 0 and expressed as mean±s.e.mean of the concentration of Evans blue dye (ng mg−1 of tissue). n=4. *P<0.05, **P<0.01, ***P<0.001 when compared to vehicle group. ++ P<0.01, +++ P<0.001 when compared to relevant control.
Figure 7.
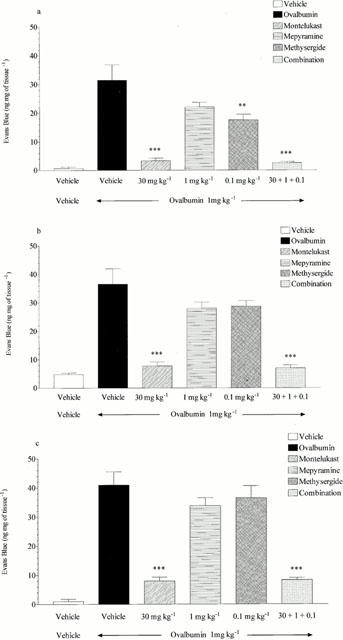
Effect of mepyramine (1 mg kg−1 i.v.), methysergide (0.1 mg kg−1 i.v.), montelukast (30 mg kg−1 p.o.) or a combination of all three on ovalbumin (1 mg kg−1 i.v.) induced microvascular leakage into the (a) trachea, (b) bronchi or (c) intra-pulmonary airways of antigen-sensitized Brown Norway rats. Rats were anaesthetized with isofluorane (3.5% in oxygen) and received Evans blue (20 mg kg−1 i.v.), 1 min later they received ovalbumin i.v. The rats were killed 15 min after ovalbumin administration and tissues removed and placed in formamide for Evans blue dye extraction. Values are presented as data minus basal leak at time 0 and expressed as mean±s.e.mean of the concentration of Evans blue dye (ng mg−1 of tissue). n=4. **P<0.01, ***P<0.001 when compared to vehicle/ovalbumin group.
Effect of mepyramine, methysergide or montelukast alone or in combination on antigen-induced changes in airway resistance
Mepyramine (3 mg kg−1 i.v.) had no significant effect on ovalbumin-induced bronchospasm. Methysergide (0.3 mg kg−1 i.v.) caused a reduction in bronchospasm which did not reach statistical significance (0.419±0.059 reduced to 0.176±0.058 cmH2O ml−1 s−1, 58% inhibition). Montelukast (30 mg kg−1 p.o.) also inhibited ovalbumin-induced bronchospasm (0.419±0.059 reduced to 0.21±0.088 cmH2O ml−1 s−1, 50% inhibition) but did not reach statistical significance. However, when the three antagonists where given in combination they produced a statistically significant and near maximal reduction in antigen-induced bronchospasm (0.419±0.059 reduced to 0.033±0.019 cmH2O ml−1 s−1, 92% inhibition, P<0.01) (see Figure 2).
Discussion
Bronchospasm and the leakage of plasma proteins from the microvasculature into airways tissue are seen as important consequences of allergic airways inflammation. The exposure to antigen of sensitized individuals and its interaction with specific immunoglobulin-E (IgE) elicits the release of inflammatory mediators from mast cells (Kaliner et al., 1989). The Brown Norway rat is known to produce IgE in large quantities after sensitization (Murphy et al., 1974). In this study we have shown that ovalbumin, when administered intravenously to antigen-sensitized Brown Norway rats, causes bronchospasm and microvascular leakage into the airways tissue. The leakage observed was evenly distributed throughout the trachea, bronchi and intra-pulmonary airways. Ovalbumin-induced leakage and bronchospasm are thought to involve several different mast cell derived inflammatory mediators of which 5-HT, histamine (Saria et al., 1983) and LTD4 (Bel et al., 1987; Dahlen et al., 1981), have been shown to be prime candidates.
Through the use of selective antagonists of histamine, 5-HT and LTD4, i.e. mepyramine, methysergide and montelukast respectively, we examined the involvement of each of the putative mediators in the bronchospasm and microvascular leakage responses to intravenously administered ovalbumin. These antagonists were given both alone and as a combination of all three against each of the mediators in the microvascular leakage model only. Firstly dose response studies to histamine, 5-HT and LTD4 were carried out in the leakage model to determine single effective doses of each of the agonists. A dose that produced a near maximal effect was chosen for each agonist for use in the antagonist studies in the leakage model. During these dose response studies in the leakage model it was noted that the agonists produced different degrees of microvascular leakage in different regions of the airways, as seen previously in guinea-pig airways by Evans et al. (1989). However, LTD4 in the Brown Norway rat, unlike in the guinea-pig where it caused a similar degree of microvascular leakage in all regions of the airways, had the most impact on intra-pulmonary airways with small but still significant effects in the bronchi and trachea. Histamine in the Brown Norway rat again differed in response to that seen in the guinea-pig, where it evoked leakage mainly in the trachea and bronchi. In the rat histamine produced leakage throughout the airways with a tendency to a greater effect in the intra-pulmonary airways. In contrast, 5-HT, whose effects were not examined in the aforementioned guinea-pig study, produced the bulk of its leakage response in the trachea and bronchi, only reaching significance at the highest dose in the intra-pulmonary airways.
The doses and times of dosing for mepyramine, methysergide (Church, 1975) and montelukast (Jones et al., 1995) used in the antagonist studies were those previously shown to be effective in the literature. The antagonists were profiled against each of three agonists in the leakage model and these studies demonstrated that each antagonist selectively inhibited their respective agonist and effectively reduced the agonist responses to basal or near basal levels. This also confirmed as correct the selection of dose and time of pre-dosing for each antagonist. The same doses of mepyramine, methysergide or montelukast or a combination of all three were then administered prior to antigen challenge in both the bronchospasm and leakage models. In the bronchospasm model montelukast inhibited approximately half of the antigen response and methysergide also inhibited approximately half of the response. Mepyramine had no effect on antigen-induced bronchospasm in this study, confirming earlier published data where mepyramine has been shown not to inhibit antigen-induced bronchoconstriction in the rat (Farmer et al., 1975) and histamine has been shown not to cause bronchoconstriction also in the rat (Church et al., 1975). The combination of all three antagonists reduced bronchospasm to near basal levels. This data suggests that in Brown Norway rats antigen-induced bronchospasm appears to be mediated equally by the activation of 5-HT and cys-leukotriene-1 receptors with little or no involvement of histamine or its receptors. This is in contrast to studies conducted by Nagase et al. (1996), also in the Brown Norway rat, which demonstrated that antigen-induced changes in airways tissue resistance were largely mediated via the activation of 5-HT receptors with little involvement of cys-leukotriene-1 receptors. However, in the leakage studies, apart from a slight inhibitory effect of methysergide in the trachea, montelukast was the only antagonist to significantly inhibit the antigen-induced response in all regions of the airways and the degree of inhibition, which was near maximal, was not increased by administration of the three antagonists in combination. This suggests that antigen-induced microvascular leakage in the Brown Norway rat appears to be predominantly mediated via activation of cys-leukotriene-1 receptors. This is despite the fact that at the highest dose of LTD4 that we could administer the bulk of the leakage evoked was mainly in the intra-pulmonary airways. This suggests that the local concentration of LTD4 in the airways as a whole after antigen administration, enough to produce a leakage response in all regions of the airways, may be higher than that achieved after exogenous LTD4 administration.
In conclusion these studies suggest that the cys-leukotrienes and 5-HT, but not histamine, may be involved in the early bronchospasm and microvascular leakage responses to antigen in the Brown Norway rat airways and that antigen-induced microvascular leakage in the sensitized Brown Norway rat may provide a robust model in which to study the actions of putative leukotriene antagonists on mast cell driven inflammatory responses.
Abbreviations
- 5-HT
5-Hydroxytryptamine
- IgE
Immunoglobulin E
- i.p.
intraperitoneal
- i.v.
intravenous
- LTD4
Leukotriene D4
References
- BEL E.H., VAN DER VEEN H., KRAMPS J.A., DIJKMAN J.H., STERK P.J. Maximal airway narrowing to inhaled leukotriene D4 in normal subjects. Comparison and interaction with methacholine. Am. Rev. Respir. Dis. 1987;136:979–984. doi: 10.1164/ajrccm/136.4.979. [DOI] [PubMed] [Google Scholar]
- BERNAREGGI M., MITCHELL J.A., BARNES P.J., BELVISI M.G. Dual action of nitric oxide on airway plasma leakage. Am. J. Respir. Crit. Care Med. 1997;155:869–874. doi: 10.1164/ajrccm.155.3.9117019. [DOI] [PubMed] [Google Scholar]
- CHUNG K.F., ROGERS D.F., BARNES P.J., EVANS T.W. The role of increased airway microvascular permeability and plasma exudation in asthma. Eur. Resp. J. 1990;3:329–337. [PubMed] [Google Scholar]
- CHURCH M.K. Response of rat lung to humoral mediators of anaphylaxis and its modification by drugs and sensitisation. Br. J. Pharmacol. 1975;55:423–430. doi: 10.1111/j.1476-5381.1975.tb06947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLEN S.E., BJORK J., HEDQVIST P., ARFORS K.E., HAMMARSTROM S., LINDGREN J.A., SAMUELSSON B. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 1981;78:3387–3391. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS T.W., ROGERS D.F., AURSUDKIJ B., CHUNG K.F., BARNES P.J. Inflammatory mediators involved in antigen-induced airway microvascular leakage in guinea-pigs. Am. Rev. Respir. Dis. 1988;138:395–399. doi: 10.1164/ajrccm/138.2.395. [DOI] [PubMed] [Google Scholar]
- EVANS T.W., ROGERS D.F., AURSUDKIJ B., CHUNG K.F., BARNES P.J. Regional and time-dependent effects of inflammatory mediators on airway microvascular permeability in the guinea-pig. Clin. Sci. 1989;76:479–485. doi: 10.1042/cs0760479. [DOI] [PubMed] [Google Scholar]
- FARMER J.B., RICHARDS I.M., SHEARD P., WOODS A.M. Mediators of passive lung anaphylaxis in the rat. Br. J. Pharmacol. 1975;55:57–64. doi: 10.1111/j.1476-5381.1975.tb07610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELE D.J., BIRRELL M.A., FOSTER M., WEBBER S.E., BELVISI M.G. Regional variation in microvascular leakage induced by ovalbumin, 5-HT, histamine and leukotriene D4 in the airways of the sensitised Brown Norway rat. Eur. Resp. J. 1999;14 Suppl. 30:158s. [Google Scholar]
- HELE D.J., BIRRELL M.A., FOSTER M., WEBBER S.E., BELVISI M.G. Mediator involvement in antigen-induced airway microvascular leakage and bronchospasm in ovalbumin sensitised Brown Norway rats. Br. J. Pharmacol. 2000;129:223P. doi: 10.1038/sj.bjp.0703847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLGATE S.T., BENYON R.C., HOWARTH P.H., AGIUS R., HARDY C., ROBINSON C., DURHAM S.R., KAY A.B., CHURCH M.K. Relationship between mediator release from human lung mast cells in vitro and in vivo. Int. Arch. Allergy Appl. Immunol. 1985;77:47–56. doi: 10.1159/000233752. [DOI] [PubMed] [Google Scholar]
- JONES T.R., LABELLE M., BELLEY M., CHAMPION E., CHARETTE L., EVANS J., FORD-HUTCHINSON A.W., GAUTHIER J.-Y., LORD A., MASSON P., MCAULIFFE M., MCFARLANE C.S., METTERS K.M., PICKETT C., PIECHUTA H., ROCHETTE C., RODGER I.W., SAWYER N., YOUNG R.N., ZAMBONI R., ABRAHAM W.M. Pharmacology of montelukast sodium (Singulair™), a potent and selective leukotriene D4 receptor antagonist. Can. J. Physiol. Pharmacol. 1995;73:191–201. doi: 10.1139/y95-028. [DOI] [PubMed] [Google Scholar]
- KALINER M. Asthma and mast cell activation. J. Allergy Clin. Immunol. 1989;83:510–520. doi: 10.1016/0091-6749(89)90031-6. [DOI] [PubMed] [Google Scholar]
- KIPS J., CUVELIER C.A., PAUWELS R.A. Effect of acute and chronic antigen inhalation on airway morphology and responsiveness in actively sensitised rats. Am. Rev. Respir. Dis. 1992;145:1306–1310. doi: 10.1164/ajrccm/145.6.1306. [DOI] [PubMed] [Google Scholar]
- MURPHY S.M., BROWN S., MIKLOS N., FIREMAN P. Reagin synthesis in inbred strains of rats. Immunology. 1974;27:245–252. [PMC free article] [PubMed] [Google Scholar]
- NAGASE T., DALLAIRE M.J., LUDWIG M.S. Airway and tissue behaviour during early response in sensitised rats: role of 5-HT and LTD4. J. Appl. Physiol. 1996;80:583–590. doi: 10.1152/jappl.1996.80.2.583. [DOI] [PubMed] [Google Scholar]
- SAPIENZA S., EIDELMAN D.H., RENZI P.M., MARTIN J.G. Role of leukotriene D4 in the early and late pulmonary responses of rats to allergen challenge. Am. Rev. Respir. Dis. 1990;142:353–358. doi: 10.1164/ajrccm/142.2.353. [DOI] [PubMed] [Google Scholar]
- SARIA A., LUNDBERG J.M., SKOFITSCH G., LEMBECK F. Vascular protein leakage in various tissues is induced by substance P, capsaicin, bradykinin, serotonin, histamine, and by antigen challenge. Arch. Pharmacol. 1983;324:212–218. doi: 10.1007/BF00503897. [DOI] [PubMed] [Google Scholar]
- TAYLOR B.M., KOLBASA K.P., CHIN J.E., RICHARDS I.M., FLEMING W.E., GRIFFIN R.L., FIDLER S.F., SUN F.F. Roles of adhesion molecules ICAM-1 and alpha4 integrin in antigen-induced changes in microvascular permeability associated with lung inflammation in sensitised Brown Norway rats. Am. J. Respir. Cell Mol. Biol. 1997;17:757–766. doi: 10.1165/ajrcmb.17.6.2697. [DOI] [PubMed] [Google Scholar]


