Abstract
Decarbomethoxyllated JW062 (DCJW), the active component of a new oxadiazine insecticide DPX-JW062 (Indoxacarb), was tested on action potentials and the inward sodium current recorded from short-term cultured dorsal unpaired median neurones of the cockroach Periplaneta americana.
Under whole-cell current-clamp conditions, 100 nM DCJW reduced the amplitude of action potentials and induced a large hyperpolarization of the resting membrane potential associated with a 41% increase in input resistance.
In voltage-clamp, DCJW resulted in a dose-dependent inhibition (IC50 28 nM) of the peak sodium current. Based on IC50 values, the effect of DCJW was about 10 fold less potent than tetrodotoxin (TTX) but 1000 fold more potent than the local anaesthetic lidocaine. DCJW (100 nM) was without effect on activation properties of the sodium current, reversal potential, voltage dependence of sodium conductance and on both fast and slow steady-state inactivations.
TTX (2 nM) resulted in 48% inhibition of the peak inward sodium current. Co-application of TTX (2 nM) with various concentrations of DCJW produced an additional inhibition of the peak inward current, indicating that the blocking actions of DCJW and TTX were distinct. Co-application of lidocaine (IC50 30 μM) with various concentrations of DCJW produced a reduction of the apparent potency of DCJW, suggesting that DCJW and lidocaine acted at the same site.
DCJW (100 nM) did not affect inward calcium or outward potassium currents.
This study describes, for the first time, the action on insect neuronal voltage-dependent sodium channels of Indoxacarb, a new class of insecticides.
Keywords: Insect, DUM neurones, oxadiazine, insecticide, local anaesthetic, neuronal sodium channels
Introduction
The insect voltage-dependent sodium channel is an important site of action for a variety of neuroactive compounds such as poisons of plant origin (Pelhate & Sattelle, 1982; Benson, 1992), neurotoxins (Gordon, 1997; Pelhate et al., 1998; Zlotkin, 1999) and insecticides including DDT, dihydropyrazoles and pyrethroïds (Sattelle & Yamamoto, 1988; Soderlund & Bloomquist, 1989; Salgado, 1992; Bloomquist, 1996; Narahashi et al., 1998; Zlotkin, 1999; Narahashi, 2000). At present, synthetic pyrethroïd insecticides (Gammon et al., 1981) which are based on naturally occuring plant chemicals (Elliott, 1977), are among the most widely used commercial insecticides owing to their potency and rapid insect intoxication (ataxia, convulsions, loss of locomotion and coordination), their low toxicity for mammals and limited soil persistence. However, intensive insecticide use has led to the development of insect resistance to DDT, pyrethroïds and other compounds involving alterations at the site of action, increased detoxication and metabolism (Sawicki, 1978; Oppenoorth, 1985; Bloomquist, 1993). This has fuelled the search for new insect chemical control agents.
The latest generation of insecticides includes an oxadiazine insecticide DPX-JW062 (Indoxacarb) which is active against lepidopteran pests (Wing et al., 1998). A previous study has shown that, in insects, this compound is rapidly cleaved to its decarbomethoxyllated JW062 metabolite DCJW which, from studies with extracellular recording electrodes, appears to be a potent blocker of sodium-dependent action potentials in lepidopteran (Manduca sexta) larval motor nerve preparation (Wing et al., 1998). However, as the detailed mechanism of action of DCJW on insect voltage-dependent sodium channels is unknown, it has been tested on the well-characterized sodium channels of cockroach (Periplaneta americana) dorsal unpaired median (DUM) neurones using the whole-cell patch-clamp technique (Lapied et al., 1989; 1990). We demonstrate that both background and voltage-dependent sodium channels are major targets for this insecticide and that its mode of action is quite distinct from the actions of the other well-known sodium channel-active commercial insecticides.
Methods
Preparation and cell isolation of dorsal unpaired median (DUM) neurones
Adult male cockroaches (Periplaneta americana) reared at 29°C under a 12 h light – dark cycle were used. Electrophysiological recordings were made from the somata of dorsal unpaired median (DUM) neurones. Animals were immobilized dorsal side up on a dissection dish. The cuticle, gut and some dorsolongitudinal muscles were removed to allow access to the ventral nerve cord. A preparation containing the abdominal nerve cord and its terminal abdominal ganglion (TAG) was dissected carefully under a binocular microscope and transferred to cockroach saline. Isolation of neuronal cell bodies was performed under sterile conditions using enzymatic digestion and mechanical dissociation of the median parts of the TAG, as previously described (Lapied et al., 1989; Grolleau & Lapied, 1995). Briefly, the dorsal median regions of six ganglia were incubated for 40 min at 29°C in sterile cockroach saline containing collagenase (CLS1, 1.5 mg ml−1, Worthington Biochemical Corporation, Coger, Paris, France). The ganglia were then rinsed twice in normal saline and dissociated mechanically by repetitive gentle suction using fire-polished Pasteur pipettes. The DUM neurone cell bodies were allowed to settle on poly-D-lysine hydrobromide (MW 70,000 – 150,000, Sigma Chemicals, L'isle d'Abeau Chesnes, France) coating the bottom of a Petri dish. The DUM neurones used in the present study were identified as previously described (Lapied et al., 1989).
Whole-cell recording and data analysis
The whole-cell patch-clamp recording configuration (Hamill et al., 1981) was used to record membrane currents (voltage-clamp mode) and action potentials (current-clamp mode). Signals were recorded using an Axopatch 200A amplifier (Axon Instruments Inc., Foster City, CA, U.S.A.). Patch pipettes were pulled from borosilicate glass capillary tubes (Clark Electromedical Instruments, Reading, U.K.) with a PP-83 electrode puller (Narishige, Japan) and had resistances of 0.5 – 0.9 MΩ when filled with the pipette solution (see composition below). The liquid junction potential between bath and internal solution was always compensated before the formation of a gigaohm seal (>2 GΩ).
For voltage-clamp studies of the inward sodium current, step voltage pulses were generated by a programmable stimulator (SMP 310, Biologic, Claix, France) or an IBM pentium 100 computer with pClamp software control (pClamp version 6.03, Axon Instruments, U.S.A.). The computer was connected to a 125 kHz labmaster DMA data acquisition system (TL-1-125 interface, Axon Instruments, U.S.A.). Unless otherwise indicated, cells were clamped at a holding potential of −90 mV, and test pulses of 30 ms duration were applied at 0.3 Hz. Although most of the capacitance and leak current were compensated electronically at the beginning of each experiment, subtraction of residual capacitance and leak current were performed on-line using the P/4 protocol provided by pClamp software. By this means, the computer generated four subpulse voltage waveforms prior to the application of the main test pulse. Currents evoked by the subpulses were added together to compute capacitance and leak current and were subtracted from currents evoked by the main pulses to yield the final leak-subtracted currents. It was possible to compensate for up to 85% of the series resistance without introducing oscillations into the recorded currents. Data were displayed on a digital oscilloscope (310, Nicolet Instrument, Madison, WI, U.S.A.) and stored on the hard disk of the computer (sampling frequency 30.3 kHz) for subsequent off-line analysis. Inward calcium currents and outward potassium currents were recorded as described in detail elsewhere (Grolleau & Lapied, 1995; 1996).
For current-clamp experiments, depolarizing current pulses were elicited at 0.5 Hz with a programable stimulator (SMP 310, Biologic). Evoked action potentials were displayed and stored on the hard disk of the computer using pClamp as described above.
Solutions and chemicals
The extracellular solution superfusing the cell used to record inward sodium current contained (mM): NaCl, 100; Tetraethylammonium-chloride (TEA-Cl), 100; KCl, 3.1; CaCl2, 2; MgCl2, 7; CdCl2, 0.5; 4-aminopyridine (4-AP), 3; HEPES, 10; pH 7.4. Patch-clamp electrodes were filled with an internal solution containing (mM): CsCl, 80; CsF, 80; NaCl, 15; MgCl2, 1; ATP-Mg, 2; EGTA, 5; HEPES, 10; pH 7.4. The superfusing solutions and the internal pipette solutions used to record inward calcium and outward potassium currents were designed to eliminate any interference from sodium currents as previously described (Grolleau & Lapied, 1995; 1996).
For current-clamp experiments, the bathing solution contained (mM): NaCl, 200; KCl, 3.1; MgCl2, 4; CaCl2, 5; HEPES, 10; pH 7.4. The recording electrode was filled with the following solution (mM): K-aspartate, 150; KF, 10; NaCl, 10; MgCl2, 1; ATP-Mg, 3; CaCl2, 0.5; EGTA, 10; HEPES, 10; pH 7.4. All compounds were purchased from Sigma Chemicals (L'isle d'Abeau Chesnes, France). DCJW stock solution (10 mM) was prepared in dimethylsulphoxide (DMSO). Final dilution contained at most 0.1% DMSO. These concentrations of solvent were found to be without effect on the electrical activity of DUM neurones. Experiments were carried out at room temperature (20°C). Data were expressed as mean±s.e.mean. The Student's t-test was used for statistical analysis.
Results
Effects of DCJW on membrane potential and triggered DUM neurone action potentials
The somata of cockroach terminal abdominal ganglion (TAG) DUM neurones maintained in short-term culture generate spontaneous overshooting sodium-dependent action potentials (Grolleau & Lapied, 2000) and are characterized by a resting membrane potential depending on the external concentration of both potassium and sodium (Lapied et al., 1989; 1999). When the isolated cell body was superfused with DCJW (Figure 1) at 100 nM, the amplitude of the action potential elicited by a depolarizing current pulse (0.8 nA for 40 ms) was reduced (Figure 2A). This blocking effect of DCJW was associated with a hyperpolarization (from −53.5±0.7 to −72.5±2.8 mV; n=5) of the resting membrane potential (Figure 2B,D). Artificial depolarization imposed upon the DUM neurone cell body to bring its resting potential back to the control value in the continued presence of DCJW failed to reverse action potential attenuation (Figure 2C). Moreover, an increase in DUM neurone input resistance (by 41±3%; n=5) in response to a hyperpolarizing current pulse (150 ms in duration) was observed after application of 100 nM DCJW (Figure 2D). This indicated that the hyperpolarization was due to the loss of a depolarizing conductance. The findings demonstrate that voltage-dependent sodium channels involved in the depolarizing phase of the action potential (Lapied et al., 1990) are blocked by DCJW. The membrane hyperpolarization and conductance changes observed in the presence of DCJW could most simply be accounted by block of sodium channels previously identified as background sodium channels involved in the maintenance of the resting potential (Lapied et al., 1989; 1999). The remaining part of this present study is only focused on the mode of action of DCJW on the properties of the voltage-dependent inward sodium current, under voltage-clamp conditions.
Figure 1.
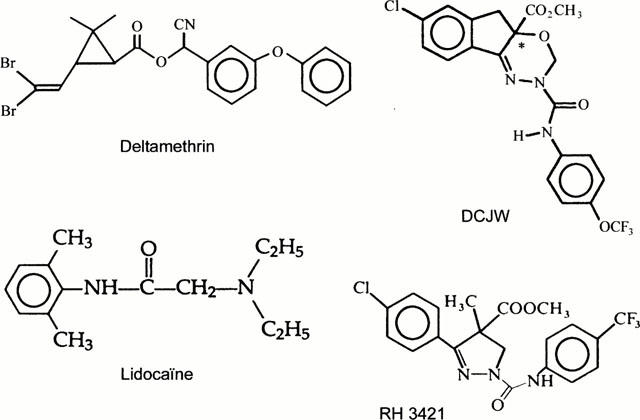
The chemical structure of the oxadiazine insecticide DCJW is shown together with structures of the local anaesthetic lidocaine, the dihydropyrazole RH 3421 and the well-known sodium channel active insecticide the pyrethroïd deltamethrin. *Denotes chiral center.
Figure 2.
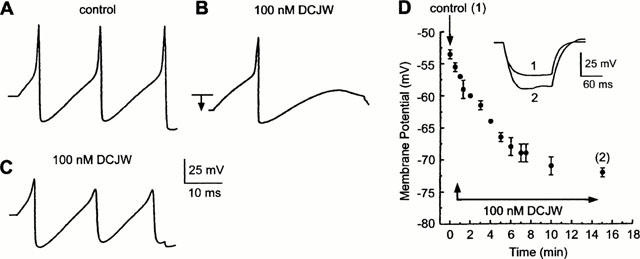
Using the whole cell current-clamp recording configuration, the effect of DCJW on membrane potential and triggered action potentials recorded from an isolated DUM neurone cell body are demonstrated. Action potentials elicited by a 40-ms depolarizing current pulse (0.8 nA) were recorded from an isolated cell body held at −52 mV prior to (A, control) and following the application of 100 nM DCJW (B,C). DCJW reduced the action potential amplitude and produced a large hyperpolarization (B, arrow). Artificial depolarization of the neurone to bring its resting potential back to the control value was ineffective in reversing the DCJW blocking effect (C). (D) The amplitude of the DCJW-induced hyperpolarization is plotted as a function of time of application (n=5). Inset: shows the membrane potential recorded in response to a hyperpolarizing current pulse (150 ms in duration) in saline (control 1) and 15 min (2) after the application of 100 nM DCJW.
Effects of DCJW on the DUM neurone voltage-dependent inward sodium current
Figure 3A shows a typical DUM neurone voltage-dependent inward sodium current elicited by a 30 ms depolarizing pulse to −10 mV applied from a holding potential of −90 mV. Application of 100 nM DCJW for 6 min reduced the maximum amplitude of the sodium current by about 60% without affecting either time-to-peak, or inactivation of the inward current. The peak current-voltage relationship is illustrated in Figure 3B. This shows that the current starts to activate at about −40 mV, reaches a maximum of −10.0±1.1 nA (n=7) and then decreases to an extrapolated reversal potential of +48.6 mV, a value very close to the calculated Nernstian equilibrium potential for sodium ions (+48 mV). The smooth line represents the best fit to the mean data (n=7) according to the equation (Stühmer, 1988):
where INa is the maximum current, V is the membrane potential, V0.5=−21.5 mV and K=4.3 mV are the activation parameters, G=196 nS is the conductance and Vrev=+48 mV is the reversal potential. These last two parameters were obtained from the linear regression (correlation coefficient r=0.998) through the data points for potentials more positive than 0 mV. As shown in Figure 3B, 100 nM DCJW reduced the maximum amplitude of the inward current at all potentials tested. The smooth line corresponding to the best fit through the data points (n=7) according to equation (1) indicated that the sodium conductance was reduced from 196 to 84 nS whereas the activation parameters (V0.5=−19.8 mV, K=4.5 mV), the potential at which the current was maximum and the reversal potential were all unaffected. To test whether DCJW affected the voltage dependence of the sodium conductance (GNa) underlying the inward sodium current, GNa was calculated from equation (2) using data obtained prior to and after application of 100 nM DCJW (see Figure 3B).
The curves shown in Figure 3C indicated that GNa increased strongly from −40 to +10 mV and then reached a maximum. After DCJW application, the maximum GNa represents only 47% of the control value without changes in the GNa voltage dependence (Figure 3C).
Figure 3.
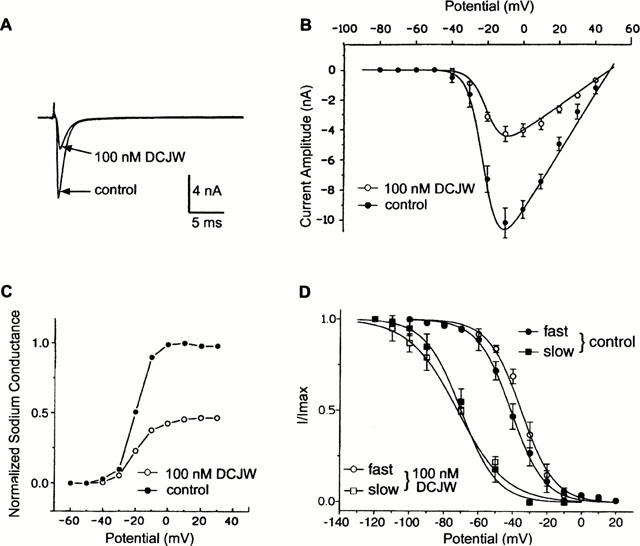
Effects of DCJW on the DUM neurone voltage-dependent inward sodium current. (A) Sodium inward current traces obtained by a 30-ms depolarizing pulse to −10 mV from a holding potential of −90 mV, in the absence (control) and presence of 100 nM DCJW. Currents are leak- and capacity-corrected. (B) Effect of DCJW on the current-voltage relationship of the inward sodium current. The maximum peak current amplitude was plotted versus membrane potential before (control) and after application of 100 nM DCJW. (C) Voltage dependence of the normalized sodium conductance of the inward current was calculated according to equation 2, in normal saline (control) and after the application of 100 nM DCJW. (D) Superimposed voltage dependence of the fast and slow steady-state inactivation curves of the inward sodium current in normal saline (control) and in the presence of 100 nM DCJW. The smooth lines are fitted through the mean data points using the single Boltzmann distribution (equation 3). Data are means±s.e.mean.
The effects of DCJW on the voltage dependence of both fast and slow steady-state inactivation were also tested. A conventional two-pulse voltage-clamp protocol was used. Inactivation properties were examined by applying a conditioning pulse between −120 and +20 mV (in 10-mV increments, each pulse being 110 ms and 1 min in duration for fast and slow inactivation, respectively). The membrane potential was then stepped back to the holding potential (−90 mV) for 2 ms and a 30-ms test pulse was applied to −10 mV, a potential at which the inward sodium current was maximum. Inactivation curves (Figure 3D) were obtained by plotting the amplitude of the inward current, measured at different conditioning potentials, in normal saline and following addition of 100 nM DCJW. The smooth curves were fitted through the mean data points (n=7) using the single Boltzmann distribution:
where V0.5, is the potential at which half the sodium channels are inactivated and K corresponds to the slope factor. Under the control condition (Figure 3D), fast inactivation had a V0.5 at −41 mV and a slope factor K of 9.7 mV (correlation coefficient r=0.998; n=7) whereas slow inactivation measured at steady-state had a V0.5 at −70 mV and K of 10.5 mV (correlation coefficient r=0.997; n=8). After treatment with 100 nM DCJW, no significant shifts of both inactivation curves were observed (P>0.05; Figure 3D). Thus DCJW affected the peak amplitude of the inward sodium current whereas both activation and inactivation were unaffected by this compound.
Dose-response curves for DUM neurone sodium channel block by DCJW, TTX and Lidocaine
Isolated DUM neurones were exposed to various concentrations of DCJW (Figure 4A). Mean values for percentage inhibition of peak inward sodium current amplitude were plotted against the logarithm of the non-cumulative concentration of DCJW (Figure 4B). The sigmoïd curve corresponded to the best fit (correlation coefficient r=0.998) according to the Hill equation. The IC50 value estimated for DCJW (i.e., the concentration of DCJW that produces 50% inhibition of the peak inward sodium current) was 28 nM. The data for DCJW were compared with the dose-dependent actions of tetrodotoxin (TTX) and the local anaesthetic lidocaine (Figure 4B,F). The TTX concentration-response curve was shifted significantly to the left in a parallel manner with no significant change in the maximum blocking effect. The estimated IC50 (2 nM) was about an order of magnitude lower than that for DCJW. An additional set of experiments was performed involving co-application of DCJW and TTX. As shown in Figure 4C, the fraction of the residual inward sodium current measured following application of 2 nM TTX (the estimated IC50 value) was further reduced when 28 nM DCJW (the estimated IC50 value) was co-applied with 2 nM TTX. The simplest explanation is that DCJW acts on a site different from that of TTX. To address this hypothesis, the effect of co-application of TTX and DCJW was tested. TTX (2 nM) applied alone produced 48.3±2.6% (n=6) inhibition of the peak inward sodium current. As illustrated in Figure 4D, co-application of TTX (2 nM) with various concentrations of DCJW produced an additional DCJW-induced dose-dependent inhibition of the maximum peak inward current. In other words, TTX did not prevent the blocking effect of DCJW. These results suggested that the voltage-dependent sodium channel site with which TTX interacts seems to be distinct from the DCJW site. By contrast, as illustrated in Figure 4B, DCJW was a more potent blocker of the inward sodium current than the local anaesthetic lidocaine. The threshold concentration of lidocaine inhibiting the inward sodium current was about 50 nM. The inhibitory action was enhanced with increasing lidocaine concentration (Figure 4B). The IC50 was 30 μM (about 1000 fold higher than the IC50 value estimated for DCJW) and the complete inhibition of the inward sodium current was observed in all DUM neurones at a concentration of 10 mM. As shown in Figure 4E, the fraction of the residual inward sodium current remaining after application of 30 μM lidocaine (the estimated IC50 value) was not further reduced when 28 nM DCJW (the estimated IC50 value) was co-applied with 30 μM lidocaine. When lidocaine (30 μM) was co-applied with various concentrations of DCJW (Figure 4F), the concentration-response curve was shifted to the right on the logarithm of concentration axis with a same maximum reached as compared with the DCJW curve. These results indicated that the presence of lidocaine reduced the apparent potency of DCJW. This could suggest, among other possibilies that DCJW and lidocaine act similarly.
Figure 4.
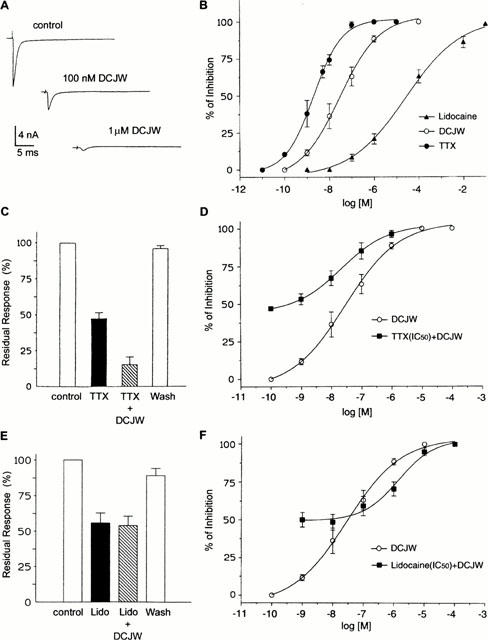
Effects of TTX, DCJW and lidocaine on DUM neurone voltage-dependent inward sodium current. (A) Inward sodium currents evoked by 30-ms depolarizing steps from −90 to −10 mV before (control) and following application of 100 nM and 1 μM DCJW. (B) Semi-logarithmic dose-response curves for the blockade of inward sodium current by TTX, DCJW and lidocaine. The percentage inhibition of the peak inward sodium current was plotted as a function of log [TTX], log [DCJW] and log [lidocaine]. The smooth line represents the best fit to the mean data according to the Hill equation. (C) Comparative histograms of the percentage of residual inward current measured after application of 2 nM TTX and a solution containing 2 nM TTX+28 nM DCJW. These concentrations correspond to the IC50 values calculated from the semi-logarithmic dose-response curves shown in (B). (D) Effects of co-application of 2 nM TTX (corresponding to the IC50) with various concentrations of DCJW. Data plotted are mean values±s.e.mean. (n=6). (E) Comparative histograms of the percentage of residual response measured after application of 30 μM lidocaine and a solution containing 30 μM lidocaine and 28 nM DCJW. These concentrations are the IC50 values obtained from the semi-logarithmic dose response curves shown in (B). (F) Effects of co-application of 30 μM lidocaine (IC50) with various concentrations of DCJW. Data are mean values±s.e.mean (n=7).
DCJW fails to block DUM neurone inward calcium currents and outward potassium currents
To check whether or not DCJW acted selectively on voltage-dependent sodium channels, voltage-clamp experiments were also performed on both inward calcium and outward potassium currents previously characterized in the same preparation (Grolleau & Lapied, 1995; 1996). The inward calcium current evoked by a 100-ms depolarizing pulse to −10 mV from a holding potential of −100 mV is shown in Figure 5Aa. This calcium current was electrophysiologically and pharmacologically identified as high-voltage activated (HVA) current in experiments described in detail elsewhere (Grolleau & Lapied, 1996). A biphasic curve was obtained when the amplitude of the peak calcium current was plotted as a function of membrane potentials (Figure 5Ab). The first part of the curve described an inward current appearing at a membrane potential above −70 mV which gradually plateaued between −60 and −45 mV before rising steeply again and peaking at −10 mV. This biphasic aspect of the current-voltage relationships was shown to result from activation of both low-voltage activated (LVA) and high-voltage-activated (HVA) calcium currents (Grolleau & Lapied, 1996). As illustrated in Figure 5Ac application of 100 nM DCJW was without effect on the amplitude and voltage dependence of either LVA or the HVA inward calcium currents (Figure 5Ab,c).
Figure 5.
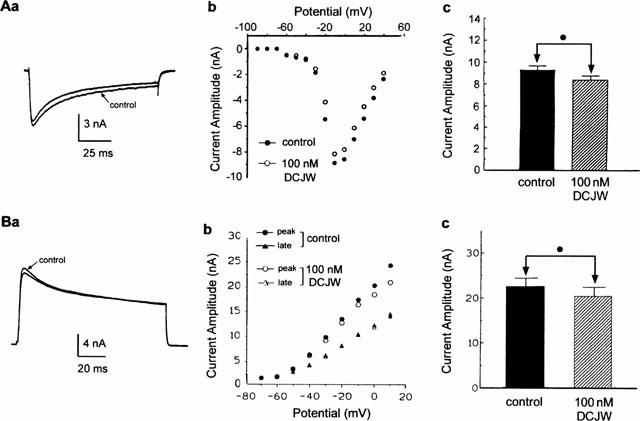
Absence of blocking effect of DCJW on inward calcium and outward potassium currents. Whole-cell high-voltage activated (HVA) calcium current (Aa) elicited by 100-ms depolarizing voltage pulse to −10 mV from a holding potential of −100 mV, before (control) and after application of 100 nM DCJW. (b) Current-voltage relationships constructed from values of peak current plotted as a function of test potentials, in control and after application of 100 nM DCJW. (c) Histogram comparing the peak HVA current recorded before (control) and after bath application of DCJW (n=4). (Ba) Superimposed global outward potassium current traces recorded in saline containing 100 nM TTX (control) and following application of 100 nM DCJW. Outward currents were evoked by depolarization to +10 mV (100 ms in duration) from a holding potential of −80 mV. (b) Current-voltage relationships of both peak and late outward potassium current before and after application of 100 nM DCJW. (c) Histogram comparing the effect of 100 nM DCJW on the peak outward current measured at +10 mV. In both cases, the Student's t-test (•) was used to indicate that the difference was not significant (P>0.05, n=4).
The effects of DCJW on DUM neurone outward potassium currents (Grolleau & Lapied, 1995) were also examined. To isolate the voltage-dependent outward potassium current, the inward sodium current was blocked by adding 100 nM TTX. Figure 5Ba shows a typical example of the resulting outward potassium current evoked by depolarization (100 ms in duration) from a holding potential of −80 mV. DCJW (100 nM) resulted in a slight reduction of the peak outward current without affecting either the late component or the voltage dependence of the potassium current, as illustrated in the Figure 5Bb where both peak transient and late outward currents (measured at the end of the pulse) were plotted against various test potentials (in 10-mV increments) from a holding potential of −80 mV. The effects of DCJW on the peak current (Figure 5Bc) were not significant (P>0.05).
Discussion
This study is the first whole-cell patch-clamp analysis of the blocking action of DCJW on the TTX-sensitive insect neuronal voltage-dependent sodium channels. This effect is similar to the blocking action of DPX-JW062 observed on the rat dorsal root ganglion neurone voltage-dependent sodium current (Nagata et al., 1998) but differs strikingly from those reported for other insecticides such as pyrethroïds, the widely employed insecticides also known to affect voltage-dependent sodium channels (Bloomquist, 1996; Zlotkin, 1999; Narahashi, 2000). DCJW induces a hyperpolarization of the membrane potential associated with an increase in input resistance and a reduction in action potential amplitude. The DCJW-induced hyperpolarization reflects an alteration of background sodium channels which are known to be involved in driving the membrane potential to the threshold for action potential generation and in determining the firing pattern of pacemaker DUM neurones (Lapied et al., 1989; 1999). The reduction of the action potential amplitude correlates well with the effects of DCJW observed on the voltage-dependent sodium current. DCJW blocks the DUM neurone inward sodium current amplitude without modification of either inactivation or activation kinetics as is the case for pyrethroïds and DDT (Bloomquist, 1996; Narahashi, 1996; 2000; Narahashi et al., 1998). Although the effect of DCJW on DUM neurones is very similar to that observed when TTX is tested under both current- and voltage-clamp conditions (Lapied et al., 1989), experimental evidences based on the inhibition curve of DCJW obtained in the presence of a partially effective concentration (IC50) of TTX indicate that the blocking actions of DCJW and TTX on voltage-dependent sodium channels are distinct.
The present study also shows that DCJW seems to act at the same site of the local anaesthetic, lidocaine, a class of drugs also known to block voltage-dependent sodium channels (Bean et al., 1983; Catterall, 1987; Kaneda et al., 1989; Hille, 1989; 1992). Co-application of various concentrations of DCJW in the presence of 30 μM lidocaine reveals a shift of the inhibition curve to the right indicating a reduction of the apparent potency of DCJW. Block by local anaesthetics such as procaine, lidocaine or etidocaine is complex, mainly due to the use-dependent (phasic) effect but also due to the tonic block of sodium current (Hille, 1992). It has previously been shown that, in most cases, local anaesthetics have higher affinity for inactivated rather than resting state of the channels (Ulbricht & Stoye-Herzog, 1984; Strichartz & Wang, 1986; Wang et al., 1989). Similar observations have been made with another class of insecticidally-active molecules the dihydropyrazoles (e.g., RH-3421, RH-1211) (Salgado, 1990; 1992). These compounds, though not developed as commercial insecticides, are capable of blocking spike initiation with no change in resting membrane potential or input resistance. Voltage-clamp experiments show that sodium current block by dihydropyrazoles is strongly voltage-dependent. This effect described as a shift of the steady-state sodium current inactivation curve toward hyperpolarization, indicates a selective binding to the inactivated state of the channel (Salgado, 1990; 1992; Bloomquist, 1996). Because dihydropyrazoles bind much more slowly than do the local anaesthetics, it has been proposed that such compounds bind mainly to the slowly inactivated state. However, possible interactions of dihydropyrazoles with other states of voltage-dependent sodium channel are not excluded since some members of this class (e.g., RH-1211) are capable of blocking voltage-dependent sodium channels on trypsin- or N-bromoacetamide-treated neuronal preparations, pointing to interactions with the non-inactivated state (Salgado, 1992). Our study clearly reveals that DCJW did not share a common mode of action with dihydropyrazoles as sodium channel blockers. The study of both fast and slow inactivation properties indicate that, under control condition, the potential range over which slow inactivation occurs is about 30 mV more negative than fast inactivation. DCJW block does not appear as a parallel shift of the steady-state slow inactivation curve in the direction of hyperpolarization where slow inactivation occurs. In fact DCJW does not alter both fast and slow inactivation properties in DUM neurones. From these results, it seems that DCJW does not bind selectively to the slow inactivated state of the channel as previously reported for dihydropyrazole such as RH-3421 (Salgado, 1992).
In conclusion, this study describes, for the first time, the actions on insect neuronal voltage-dependent sodium channels of the active metabolite DCJW of Indoxacarb, a new class of insecticides (oxadiazines). These results are of particular interest since the widspread use of earlier generations of sodium channel insecticides (e.g., pyrethroïds) has resulted in the development of resistance such as knockdown resistance (kdr, super-kdr) in many insect species (Sawicki, 1978; Williamson et al., 1996; Lee et al., 1999; Zlotkin, 1999). The fact that DCJW alter the voltage-dependent sodium channels in a manner distinct from that of such insecticides, it might be anticipated that a kdr or super-kdr-resistant insect would show low cross-resistance to DCJW.
Acknowledgments
The authors wish to thank Dr K.D. Wing of Dupont Agricultural Products, Newark, DE 19714, U.S.A. for the gift of DCJW.
Abbreviations
- 4-AP
4-aminopyridine
- ATP
adenosine 5′-triphosphate
- DCJW
decarbomethoxyllated JW062
- DDT
1,1,1-trichloro-2,2-bis(ρ-chlorophenyl) ethan
- DMSO
dimethyl-sulphoxide
- EGTA
ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetra-acetic acid
- HEPES
N-2-hydroxyethylpiperazin-N′-2-ethanesulphonic acid
- TEA-Cl
tetraethyl-ammonium chloride
- TTX
tetrodotoxin
References
- BEAN B.P., COHEN C.J., TSIEN R.W. Lidocaine block of cardiac sodium channels. J. Gen. Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON J.A.Natural and synthetic toxins at insect receptors and ion channels: the search for insecticide leads and target sites Molecular basis of drug and pesticide action 1992London, New York: Elsevier Applied Science; 57–70.ed. Duce, I.R. pp [Google Scholar]
- BLOOMQUIST J.R. Neuroreceptor mechanisms in pyrethroïd mode of action and resistance. Rev. Pestic. Toxicol. 1993;2:185–230. [Google Scholar]
- BLOOMQUIST J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996;41:163–190. doi: 10.1146/annurev.en.41.010196.001115. [DOI] [PubMed] [Google Scholar]
- CATTERALL W.A. Common modes of drug action on Na+ channels: local anesthetics, antiarrhythmics and anticonvulsants. TIPS. 1987;8:57–65. [Google Scholar]
- ELLIOTT M.Synthetic pyrethroïds Synthetic pyrethroïds 1977Washington DC: ACS Symposium Series No. 42, American Chemical Society; 1–28.ed. Elliott, M. pp [Google Scholar]
- GAMMON D.W., BROWN M.A., CASIDA J.E. Two classes of pyrethroïd action in the cockroach. Pestic. Biochem. Physiol. 1981;15:181–191. [Google Scholar]
- GORDON D.Sodium channels as targets for neurotoxins: mode of action and interaction of neurotoxins with receptor sites on sodium channels Toxins and signal transduction 1997Amsterdam: Harwood; 119–149.ed. Gutman, Y. & Lazarowici, P. pp [Google Scholar]
- GROLLEAU F., LAPIED B. Separation and identification of multiple potassium currents regulating the pacemaker activity of insect neurosecretory cells (DUM neurons) J. Neurophysiol. 1995;73:160–171. doi: 10.1152/jn.1995.73.1.160. [DOI] [PubMed] [Google Scholar]
- GROLLEAU F., LAPIED B. Two distinct low-voltage-activated Ca2+ currents contribute to the pacemaker mechanism in cockroach dorsal unpaired median neurons. J. Neurophysiol. 1996;76:963–976. doi: 10.1152/jn.1996.76.2.963. [DOI] [PubMed] [Google Scholar]
- GROLLEAU F., LAPIED B. Dorsal unpaired median neurones in the insect central nervous system: Towards a better understanding of the ionic mechanisms underlying spontaneous electrical activity. J. Exp. Biol. 2000;203:1633–1648. doi: 10.1242/jeb.203.11.1633. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp technique for high resolution current recording from cells and cell-free membrane patches. Pflüger Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HILLE B. Local anesthetics: hydrophobic and hydrophilic pathways for the drug-receptor reaction. J. Gen. Physiol. 1989;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLE B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 1992. pp. 390–422. [Google Scholar]
- KANEDA M., OYAMA Y., IKEMOTO Y., AKAIKE N. Blockade of the voltage-dependent sodium current in isolated rat hippocampal neurons by tetrodotoxin and lidocaine. Brain Res. 1989;484:348–351. doi: 10.1016/0006-8993(89)90379-x. [DOI] [PubMed] [Google Scholar]
- LAPIED B., MALECOT C.O., PELHATE M. Ionic species involved in the electrical activity of single aminergic neurones isolated from the sixth abdominal ganglion of the cockroach Periplaneta americana. J. Exp. Biol. 1989;144:535–549. [Google Scholar]
- LAPIED B., MALECOT C.O., PELHATE M. Patch-clamp study of the properties of the sodium current in cockroach single isolated adult aminergic neurones. J. Exp. Biol. 1990;151:387–403. [Google Scholar]
- LAPIED B., STANKIEWICZ M., GROLLEAU F., ROCHAT H., ZLOTKIN E., PELHATE M. Biophysical properties of scorpion α-toxin-sensitive background sodium channel contributing to the pacemaker activity in insect neurosecretory cells (DUM neurons) Eur. J. Neurosci. 1999;11:1449–1460. doi: 10.1046/j.1460-9568.1999.00554.x. [DOI] [PubMed] [Google Scholar]
- LEE S.H., SMITH T.J., KNIPPLE D.C., SODERLUND D.M. Mutations in the house fly Vssc1 sodium channel gene associated with super-kdr resistance abolish the pyrethroid sensitivity of Vssc1/tipE sodium channels expressed in Xenopus oocytes. Insect Biochem. Mol. Biol. 1999;29:185–194. doi: 10.1016/s0965-1748(98)00122-2. [DOI] [PubMed] [Google Scholar]
- NAGATA K., IKEDA T., HONDA H., SHONO T. Suppression of voltage-gated sodium currents by the dihydropyrazole insecticide DPX-JW062 in rat dorsal root ganglion neurons. J. Pestic. Sci. 1998;23:62–64. [Google Scholar]
- NARAHASHI T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 1996;78:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T. Neuroreceptors and ion channels as the basis for drug action: Past, present and future. J. Pharmacol. Exp. Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- NARAHASHI T., GINSBURG K.S., NAGATA K., SONG J.H., TATEBAYASHI H. Ion channels as targets for insecticides. NeuroToxicology. 1998;19:581–590. [PubMed] [Google Scholar]
- OPPENOORTH F.J.Biochemistry and genetics of insecticide resistance Comprehensive Insect Physiology, Biochemistry and Pharmacology 1985vol. 12New York: Pergamon Press; 731–773.ed. Kerkut, G.A. & Gilbert, L.I. pp [Google Scholar]
- PELHATE M., SATTELLE D.B. Pharmacological properties of insect axons: A review. J. Insect Physiol. 1982;28:889–903. [Google Scholar]
- PELHATE M., STANKIEWICZ M., BEN KHALIFA R. Anti-insect scorpion toxins: historical account, activities and prospects. C. R. Soc. Biol. 1998;192:463–484. [PubMed] [Google Scholar]
- SALGADO V.L. Mode of action of insecticidal dihydropyrazoles: selective block of impulse generation in sensory nerves. Pestic. Sci. 1990;28:389–411. [Google Scholar]
- SALGADO V.L. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol. Pharmacol. 1992;41:120–126. [PubMed] [Google Scholar]
- SATTELLE D.B., YAMAMOTO D. Molecular targets of pyrethroïd insecticides. Adv. Insect Physiol. 1988;20:147–213. [Google Scholar]
- SAWICKI R.M. Unusual response of DDT-resistant houseflies to carbinol analogues of DDT. Nature. 1978;275:443–444. doi: 10.1038/275443a0. [DOI] [PubMed] [Google Scholar]
- SODERLUND D.M., BLOOMQUIST J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989;34:77–96. doi: 10.1146/annurev.en.34.010189.000453. [DOI] [PubMed] [Google Scholar]
- STRICHARTZ G.R., WANG G.K.The kinetic basis for phasic local anesthetic blockade of neuronal sodium channels Molecular and Cellular Mechanisms of Anaesthesia 1986New York: Plenum Publishing Corp; 217–226.ed. Miller, K. & Roth, S. pp [Google Scholar]
- STÜHMER W.Electrical recordings from cloned sodium channels expressed in Xenopus oocytes Molecular Biology of Ionic Channels 1988San Diego: Academic Press Inc; 271–276.ed. Agnew, W.S., Claudio, T. & Sigworth, F.J. pp [Google Scholar]
- ULBRICHT W., STOYE-HERZOG M. Distinctly different rates of benzocaine action on sodium channels of Ranvier nodes kept open by chloramine-T and veratridine. Pflügers Arch. 1984;402:439–445. doi: 10.1007/BF00583945. [DOI] [PubMed] [Google Scholar]
- WANG G.K., BRODWICK M.S., EATON D.C., STRICHARTZ G.R. Inhibition of sodium currents by local anesthetics in chloramine-T-treated squid axons. J. Gen. Physiol. 1989;89:645–667. doi: 10.1085/jgp.89.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON M.S., MARTINEZ-TORRES D., HICK C.A., DEVONSHIRE A.L. Identification of mutations in the houseflyes para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroïd insecticides. Mol. Gen. Genet. 1996;252:51–60. doi: 10.1007/BF02173204. [DOI] [PubMed] [Google Scholar]
- WING K.D., SCHNEE M.E., SACHER M., CONNAIR M. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Arch. Insect Biochem. Physiol. 1998;37:91–103. [Google Scholar]
- ZLOTKIN E. The insect voltage-gated sodium channel as target of insecticides. Annu. Rev. Entomol. 1999;44:429–455. doi: 10.1146/annurev.ento.44.1.429. [DOI] [PubMed] [Google Scholar]


