Abstract
Because changes 5-HT1A receptor number do not occur following repeated agonist treatment, we hypothesized that the basis for 5-HT1A receptor desensitization involves changes in receptor-G protein coupling. We measured the effect of repeated agonist administration on 5-HT1A receptor-stimulated [35S]-GTPγS binding in forebrain areas, (i.e. anterior cingulate cortex, lateral septum, hippocampus, entorhinal cortex), and serotonergic cell body areas, the dorsal and median raphe nuclei.
Following treatment of rats with (±)8-OH-DPAT (1 mg kg−1, s.c.) for 7 or 14 days, 5-HT1A receptor-stimulated [35S]-GTPγS binding was significantly attenuated in both the dorsal and median raphe nuclei.
5-HT1A receptor-stimulated [35S]-GTPγS binding was significantly attenuated in the CA1 region of the hippocampus after 7, but not 14 days of 8-OH-DPAT administration. 5-HT1A receptor-stimulated [35S]-GTPγS binding was not altered in other forebrain areas examined.
The binding of [3H]-MPPF to 5-HT1A receptor sites was not altered in any brain region examined following repeated agonist administration, suggesting that the observed changes in (±)8-OH-DPAT-stimulated [35S]-GTPγS binding were not due to changes in 5-HT1A receptor number.
Our data indicate that in serotonergic cell body areas the regulation of presynaptic 5-HT1A receptor function following repeated agonist administration occurs at the level of receptor-G protein interaction. In forebrain areas, however, the regulation of postsynaptic 5-HT1A receptor sensitivity appears not to be at the level of receptor-G protein coupling.
Keywords: GTPgammaS, quantitative autoradiography, 5-HT1A receptor, 8-OH-DPAT
Introduction
In the central nervous system, cell bodies of serotonergic neurons are localized to discrete groups or nuclei in the brainstem. Serotonergic cell bodies send a dense plexus of serotonergic processes throughout the brain. The majority of ascending serotonergic processes to the forebrain arise from cell bodies in the dorsal and median raphe nuclei (see Molliver, 1987).
The serotonin-1A (5-HT1A) receptor is present in high density in serotonergic cell body areas, in particular the dorsal and median raphe nuclei, as well as in cortical and limbic areas (i.e. frontal cortex, entorhinal cortex, hippocampus, septum) (Vergé et al., 1986; Hensler et al., 1991). In serotonergic cell body areas the 5-HT1A receptor is located on serotonergic cell bodies and dendrites (Sotelo et al., 1990), and functions as the somatodendritic autoreceptor (de Montigny et al., 1984; see Aghajanian et al., 1990). In terminal field areas of serotonergic innervation, the 5-HT1A receptor is located postsynaptically (Vergé et al., 1986; Hensler et al., 1991). 5-HT1A receptors are coupled via pertussis toxin-sensitive G proteins to the inhibition of adenylyl cyclase, or to the opening of potassium channels (De Vivo & Maayani, 1986; Andrade et al., 1986; Markstein et al., 1986; Clarke et al., 1987). In the hippocampus, the 5-HT1A receptor is coupled to both effector systems. By contrast, in the dorsal raphe 5-HT1A receptors are not coupled to the inhibition of adenylyl cyclase (Clarke et al., 1996).
The 5-HT1A receptor has been implicated in psychiatric illnesses, such as anxiety disorders and major depression. The azapirone compounds, such as buspirone and its analogues gepirone and ipsapirone, comprise a new class of psychoactive agents with both anxiolytic and antidepressant effects. These agents, which are agonists with high affinity for the 5-HT1A receptor (Gozlan et al., 1983; Traber & Glaser, 1987), have anxiolytic and antidepressant effects in animal models (Traber & Glaser, 1987; Lucki, 1991; File et al., 1996) and are currently used in the treatment of anxiety disorders (Keppel Hesselink, 1992). Furthermore, antagonists of the 5-HT1A receptor have been suggested to improve the efficacy of certain antidepressant drugs (Artigas et al., 1996; Blier & Bergeron, 1998). 5-HT1A receptor knockout mice display decreased exploratory activity and increased fear of aversive environments (open or elevated spaces), and exhibit decreased immobility in the forced swim test, an effect commonly associated with antidepressant treatment (Ramboz et al., 1998). Because therapeutic effects are not attained in patients until 2 – 3 weeks after the beginning of treatment, adaptive changes in the serotonergic system may underlie the therapeutic effectiveness of antidepressants and anxiolytics. Thus, studies of the regulation of the 5-HT1A receptor may have important implications for our understanding the role of this receptor in the action of antidepressant drugs or azapirone anxiolytics.
Chronic administration of 5-HT1A receptor agonists, including the azapirone anxiolytics, results in the desensitization of behavioural responses mediated by postsynaptic 5-HT1A receptors (Larsson et al., 1990; Wieland et al., 1993). Desensitization of 5-HT1A somatodendritic autoreceptor function in the dorsal raphe has been demonstrated in electrophysiological studies to follow chronic administration of 5-HT1A receptor agonists (Blier & de Montigny, 1987; Schechter et al., 1990; Dong et al., 1997). In vivo microdialysis studies have confirmed these observations. The ability of 5-HT1A autoreceptors of the dorsal raphe to modulate serotonin release in the striatum is attenuated following 7 days of 8-OH-DPAT administration (Kreiss & Lucki, 1997). In general, changes in 5-HT1A receptor number have not been observed following chronic 5-HT1A receptor agonist administration (Larsson et al., 1990; Schechter et al., 1990; Wieland et al., 1993), although decreased receptor density has been reported by some investigators (Fanelli & McMonagle-Strucko, 1992).
Because changes in the sensitivity of 5-HT1A receptor-mediated responses following chronic administration of 5-HT1A receptor agonists do not appear to be mediated by changes in 5-HT1A receptor binding, we hypothesized that the basis for changes in 5-HT1A receptor function or sensitivity involves post-receptor events, specifically changes in receptor-G protein coupling. Receptor-stimulated [35S]-GTPγS binding is a direct assay of receptor activation of G proteins, as it measures the exchange of GDP for GTPγS. The development of [35S]-GTPγS autoradiography to identify receptor-activated G proteins in brain sections (Sim et al., 1995) allows the demonstration of functional activity at the level of receptor-G protein coupling with anatomical resolution. This approach offers a unique opportunity to examine regional differences in the regulation of the 5-HT1A receptor at the level of receptor-G protein interaction. In the current study we have examined the effect of repeated agonist administration on 5-HT1A receptor-stimulated [35S]-GTPγS binding using quantitative autoradiography. This analysis was performed for post-synaptic 5-HT1A receptors in forebrain areas, which serve as terminal field areas of serotonergic innervation, and presynaptic 5-HT1A receptors located on the soma and dendrites of serotonergic cell bodies in the dorsal and median raphe nuclei. The present study is the first to report the use of this technique to study the regulation of 5-HT1A receptor function in brain following repeated agonist administration.
Methods
Animals
Male Sprague-Dawley rats (250 – 300 g; Harlan, Indianapolis, IN, U.S.A.) were group-housed and maintained on a 14 : 10 h day/night cycle, with constant access to food and water. These studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Drug treatment
In some experiments, rats were injected once daily with vehicle (n=8) or the 5-HT1A receptor agonist (±)8-OH-DPAT (1 mg kg−1, s.c.) (n=8) for 7 days. In a second set of experiments, rats were injected once daily with vehicle (n=8) or the 5-HT1A receptor agonist (±)8-OH-DPAT (1 mg kg−1, s.c.) (n=8) for 14 days. Animals were injected at the same time each day, specifically between 10 : 00 and 11 : 00 h. Animals were sacrificed 24 h after the last injection.
Tissue preparation
Rat brains were rapidly removed and frozen on powdered dry ice. Brains were stored at −80°C until sectioning. Coronal sections of 20 μm thickness were cut at −17°C in a cryostat microtome and thaw-mounted onto gelatin-coated glass slides. The sections were desiccated at 4°C for 18 h under vacuum and then stored at −80°C until use.
[35S]-GTPγS autoradiography
Autoradiography of agonist-stimulated [35S]-GTPγS binding in brain sections was performed as described by Dupuis et al. (1999) with some modifications. Slide-mounted sections were thawed quickly at room temperature for 5 min and then equilibrated in HEPES buffer (50 mM, pH 7.4), supplemented with (mM): MgCl2 3, EGTA 0.2, NaCl 100 and dithiothreitol 0.2, at room temperature for 10 min. Sections were then pre-incubated in HEPES buffer containing GDP (2 mM) for 20 min. Sections were subsequently incubated in pre-warmed HEPES assay buffer containing GDP (2 mM) and 80 pM [35S]-GTPγS either in the absence or in the presence of agonist for 1 h at 30°C. Basal [35S]-GTPγS binding was defined in the absence of agonist. Nonspecific [35S]-GTPγS binding was defined in the absence of agonist and in the presence of 10 μM GTPγS. The incubation was stopped by two washes for 2 min each in ice-cold 50 mM Tris-HCl buffer (pH 7.4), followed by a brief immersion in ice-cold deionized water. Sections were dried on a slide-warmer and exposed to Kodak Biomax MR film (Amersham) for 24 h.
[3H]-MPPF autoradiography
Autoradiography of the binding of [3H]-MPPF to 5-HT1A receptors in brain sections was performed as described by Clarke et al. (2001). Briefly, slide-mounted sections were thawed in a dessicator at 4°C for 30 min. Sections were preincubated for 30 min at room temperature in assay buffer (170 mM Tris-HCl, pH 7.6 at room temperature). Sections were subsequently incubated in assay buffer containing 10 nM [3H]-MPPF for 90 min at room temperature. Nonspecific binding was defined by incubating adjacent sections in the presence of 10 μM WAY-100635. Incubation was terminated by two washes for 5 min each in ice-cold 170 mM Tris-HCl buffer (pH 7.6), followed by a dip in ice-cold deionized water. Sections were dried on a slide warmer and exposed to [3H]-sensitive Hyperfilm film (Amersham) for a period of 3 weeks to generate autoradiograms.
Image analysis
Analysis of the digitized autoradiograms was performed using the image analysis program NIH Image, version 1.47 (NIH, Bethesda, MD, U.S.A.). Tissue sections were stained with thionin and the brain areas identified using the atlas of the rat brain of Paxinos & Watson (1986). Autoradiograms of [3H]-MPPF binding were quantified by the use of simultaneously exposed [3H] standards (ART-123, American Radiochemicals, St. Louis, MO, U.S.A.) which had been calibrated using brain-mash sections according to the method of Geary & Wooten (Geary & Wooten 1983; Geary et al., 1985). The amount of ligand bound was determined by converting optical density measurements to femtomoles per milligram of protein. Specific binding was calculated by subtracting nonspecific binding from total binding on adjacent sections.
Autoradiograms of 5-HT1A receptor-stimulated [35S]-GTPγS binding were quantified by the use of simultaneously exposed [14C] standards (ARC-146, American Radiochemicals, St. Louis, MO, U.S.A.). Standard curves were fit to pixel data obtained from [14C] standards and tissue equivalent values (nCi g−1) provided by American Radiochemicals, and were used to transform the actual regional densitometric values into relative radioactivity measures. Nonspecific binding of [35S]-GTPγS was subtracted from basal binding and from binding in the presence of agonist. Specific, agonist-stimulated binding was expressed as per cent above basal.
Data analysis
Individual dose-response curves for specific, agonist-stimulated [35S]-GTPγS binding were fit by nonlinear regression using KaleidaGraph software (version 3.0, Synergy Software, Reading, PA, U.S.A.) to the model: E=Emax/(1+(EC50/[A])n, where E is the response at the agonist concentration [A], Emax is the maximal response, EC50 is the concentration of drug producing the half-maximal response and n is the slope factor. Statistical comparisons were made by ANOVA. F values reaching significance (P<0.05) were evaluated further by post hoc analysis using Fisher's Protected Least Significant Difference test. Statistical tests were performed using Statistica software (version 4.1, Statsoft, Tulsa, OK, U.S.A.).
Materials
[35S]-GTPγS (1250 Ci mmol−1) and [3H]-MPPF (66.2 Ci mmol−1) were purchased from Dupont/NEN (Boston, MA, U.S.A.). GDP (disodium salt) was purchased from ICN (Costa Mesa, CA, U.S.A.). (±)8-OH-DPAT hydrobromide and WAY 100635 maleate were purchased from Sigma/RBI (Natick, MA, U.S.A.). GTPγS (tetralithium salt) was purchased from Roche/Boehringer-Mannheim (Indianapolis, IN, U.S.A.).
Results
Figure 1 shows autoradiograms of the binding of [35S]-GTPγS to rat brain sections taken at the level of the lateral septum, dorsal hippocampus or dorsal raphe nucleus. As expected (Waeber & Moskowitz, 1997; Sim et al., 1997; Meller et al., 2000), application of the 5-HT1A receptor agonist (±)8-OH-DPAT (1 μM) resulted in an increase in the binding of [35S]-GTPγS in comparison with the basal condition in many brain regions. Dose-response analyses for (±)8-OH-DPAT-stimulated [35S]-GTPγS binding indicated that the maximal stimulation of [35S]-GTPγS binding by 8-OH-DPAT was greater in forebrain areas (Emax values of 70 – 100% above basal) than in serotonergic cell body areas (Emax values of approximately 44% above basal) (see Table 1). In agreement with previous studies (Meller et al., 2000), the 5-HT1A receptor antagonist WAY 100635 (100 nM) completely blocked the stimulation of [35S]-GTPγS binding by (±)8-OH-DPAT (1 μM) in all areas examined (Table 2).
Figure 1.
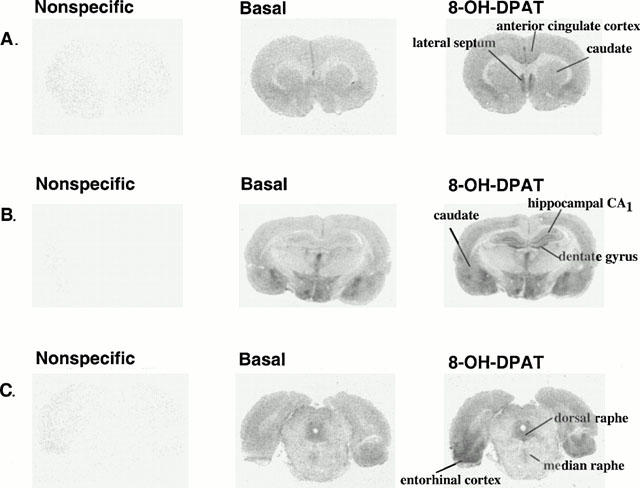
Autoradiograms of [35S]-GTPγS binding to sections of rat brain. Coronal sections at the level of (A) the lateral septum, (B) the dorsal hippocampus, and (C) the dorsal raphe nucleus, were incubated with [35S]-GTPγS (80 pM). Nonspecific binding was defined in the presence of 10 μM GTPγS. The binding of [35S]-GTPγS was stimulated by (±)8-OH-DPAT (1 μM).
Table 1.
Stimulation of [35S]-GTPγS binding by (±)8-OH-DPAT

Table 2.
The binding of [35S]-GTPγS stimulated by (±)8-OH-DPAT (1 μM) in the absence and presence of the 5-HT1A receptor antagonist WAY 100635 (100 nM)

To determine the effect of repeated agonist administration on 5-HT1A receptor-stimulated [35S]-GTPγS binding, rats were treated for 7 or 14 days with (±)8-OH-DPAT (1 mg kg−1, s.c.). For these studies a maximal dose of (±)8-OH-DPAT was used. The binding of [35S]-GTPγS to rat brain sections taken at the level of the lateral septum, dorsal hippocampus or dorsal raphe nucleus was quantitated. The effect of repeated administration of (±)8-OH-DPAT on 5-HT1A receptor-stimulated [35S-GTPγS binding in terminal field areas of serotonergic innervation is shown in Figure 2. (±)8-OH-DPAT-stimulated [35S]-GTPγS binding was significantly attenuated only in the CA1 region of the hippocampus after 7, but not 14 days of agonist administration (Figure 2). (±)8-OH-DPAT-stimulated [35S]-GTPγS binding was not altered in other forebrain areas examined. These data indicate that in forebrain areas the regulation of postsynaptic 5-HT1A receptor sensitivity or function following repeated agonist administration appears not to be at the level of receptor-G protein interaction.
Figure 2.
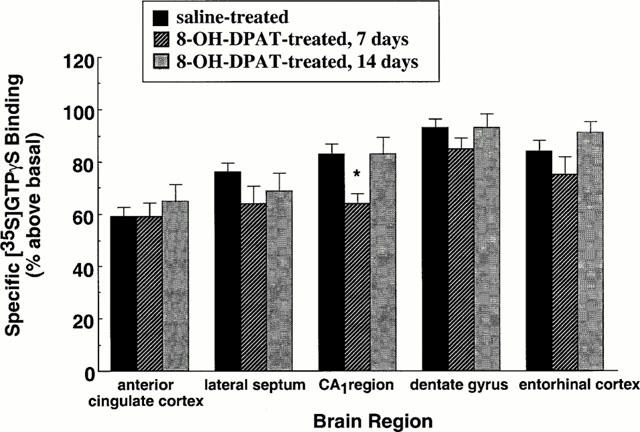
Effect of repeated administration of 8-OH-DPAT on 5-HT1A receptor-stimulated [35S]-GTPγS binding in terminal field areas of serotonergic innervation. Rats were administered either saline vehicle or (±)8-OH-DPAT (1 mg kg−1, once daily, s.c.) for 7 or 14 days. Coronal sections were incubated with [35S]-GTPγS (80 pM). Nonspecific binding was defined in the presence of 10 μM GTPγS. [35S]-GTPγS binding was stimulated by (±)8-OH-DPAT (1 μM). Specific binding of [35S]-GTPγS is expressed as per cent above basal. Shown are the mean±s.e.mean saline-treated, n=16; 8-OH-DPAT-treated, n=8 per experimental group. *P<0.05.
The effect of administration of (±)8-OH-DPAT for 7 or 14 days on 5-HT1A receptor-stimulated [35S]-GTPγS binding in serotonergic cell body areas is shown in Figure 3. (±)8-OH-DPAT-stimulated [35S]-GTPγS binding was significantly attenuated in the dorsal and median raphe nuclei. These data indicate that in serotonergic cell body areas the regulation of presynaptic 5-HT1A receptor sensitivity and function occurs at the level of receptor-G protein interaction.
Figure 3.
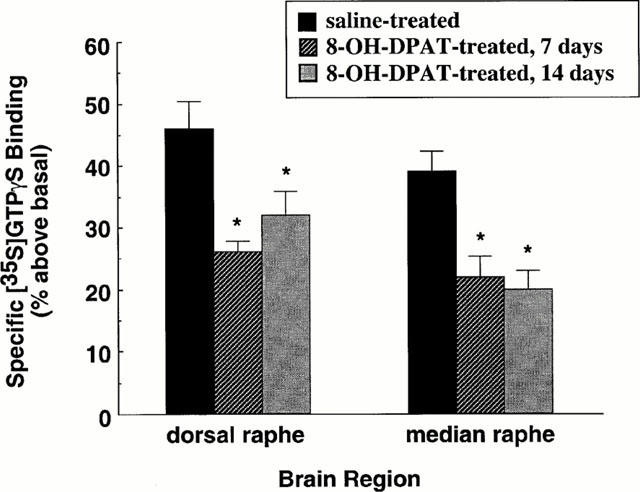
Effect of repeated administration of 8-OH-DPAT on 5-HT1A receptor-stimulated [35S]-GTPγS binding in serotonergic cell body areas. Rats were administered either saline vehicle or 8-OH-DPAT (1 mg kg−1, once daily, s.c.) for 7 or 14 days. Coronal sections were incubated with [35S]-GTPγS (80 pM). Nonspecific binding was defined in the presence of 10 μM GTPγS. [35S]-GTPγS binding was stimulated by 8-OH-DPAT (1 μM). Specific binding of [35S]-GTPγS is expressed as per cent above basal. Shown are the mean±s.e.mean. Saline-treated, n=16; 8-OH-DPAT-treated, n=8 per experimental group. *P<0.05.
To confirm that repeated agonist administration did not result in changes in 5-HT1A receptor number, experiments were performed measuring the binding of a single saturating concentration of the 5-HT1A receptor antagonist [3H]-MPPF (10 nM). As shown in Table 3, the binding of [3H]-MPPF to 5-HT1A receptor sites was not altered by repeated agonist administration in any brain region examined. These data indicate that changes in 8-OH-DPAT-stimulated [35S]-GTPγS binding were not due to changes in 5-HT1A receptor number.
Table 3.
Effect of repeated administration of 8-OH-DPAT on the binding of [3H]-MPPF to 5-HT1A receptors

Discussion
We have examined the effect of repeated administration of the agonist (±)8-OH-DPAT on 5-HT1A receptor-stimulated [35S]-GTPγS binding using quantitative autoradiography. The present study is the first to report the use of this technique to study the regulation of 5-HT1A receptor function in brain following repeated agonist administration. Our data indicate that in serotonergic cell body areas the regulation of presynaptic 5-HT1A receptor sensitivity following repeated agonist administration occurs at the level of receptor-G protein interaction. In forebrain areas, however, the regulation of postsynaptic 5-HT1A receptor sensitivity or function following repeated agonist treatment appears not to be at the level of receptor-G protein interaction, but may occur more distally, for example at the level of the effector system or signalling cascade.
The distribution of (±)8-OH-DPAT-stimulated [35S]-GTPγS binding in rat brain as measured by quantitative autoradiography (Waeber & Moskowitz, 1997; Sim et al., 1997; Meller et al., 2000; current study), is in agreement with the distribution of 5-HT1A receptor sites observed with the binding of [3H]-8-OH-DPAT or the 5-HT1A receptor antagonist radioligand [3H]-WAY 100635 (Hensler et al., 1991; Gozlan et al., 1995; Khawaja, 1995). Our experiments using the 5-HT1A receptor antagonist WAY 100635 (100 nM) are in agreement with previous studies (Waeber & Moskowitz, 1997; Sim et al., 1997; Meller et al., 2000) and indicate that (±)8-OH-DPAT stimulated [35S]-GTPγS binding is mediated by activation of 5-HT1A receptors.
Dose-response analyses for (±)8-OH-DPAT-stimulated [35S]-GTPγS binding indicated that the maximal stimulation of [35S]-GTPγS binding by (±)8-OH-DPAT was greater in forebrain areas than in serotonergic cell body areas (Table 1). These data are in agreement with the observations of Meller et al. (2000) that R-(+)8-OH-DPAT is more efficacious to stimulate [35S]-GTPγS binding in hippocampus and lateral septum than in the dorsal raphe. The Emax values of (±)8-OH-DPAT to stimulate [35S]-GTPγS binding in various brain regions in the current study do not necessarily correlate with the density of 5-HT1A receptor sites (Table 3; Hensler et al., 1991; Khawaja, 1995). For example, although the density of 5-HT1A receptors in the dorsal raphe is comparable to that in the lateral septum or CA1 region of hippocampus, the maximal stimulation of [35S]-GTPγS binding in the dorsal raphe is approximately half of that observed in these forebrain areas. We are uncertain as to why (±)8-OH-DPAT is less efficacious to stimulate [35S]-GTPγS binding in the dorsal raphe nucleus. Differences in the efficacy of (±)8-OH-DPAT to stimulate [35S]-GTPγS binding in forebrain areas and the dorsal raphe may be due to regional differences in the ratio of 5-HT1A receptors to G proteins, or in the availability of G protein subunits for coupling.
Following treatment of rats with 8-OH-DPAT (1 mg kg−1, s.c.) for 7 or 14 days, 5-HT1A receptor-stimulated [35S]-GTPγS binding was not altered in anterior cingulate cortex, lateral septum, dentate gyrus or entorhinal cortex. Of the forebrain areas examined, 5-HT1A receptor-stimulated [35S]-GTPγS binding was significantly attenuated only in the CA1 region of the hippocampus after 7, but not 14 days of chronic agonist treatment. The apparent transient decrease in 5-HT1A receptor-stimulated [35S]-GTPγS binding in this region may reflect a region specific regulatory phenomenon and highlights the importance of time-course studies. Repeated administration of 8-OH-DPAT results in the desensitization of behavioural responses mediated by postsynaptic 5-HT1A receptors (Larsson et al., 1990). The regulation of postsynaptic 5-HT1A receptor sensitivity or function following repeated 8-OH-DPAT administration appears not to be at the level of receptor-G protein interaction, but may occur more distally, for example at the level of the effector system or signalling cascade, or in the case of 5-HT1A receptor-mediated behaviours, may involve complex neuronal circuits.
Electrophysiological and neurochemical responses mediated by postsynaptic 5-HT1A receptors in hippocampus are not altered following chronic administration of the azapirone anxiolytics (i.e. buspirone, gepirone and ipsapirone) (Blier & de Montigny, 1987; Dong et al., 1997; Schechter et al., 1990; Varrault et al., 1991). Because these agents are partial agonists at postsynpatic 5-HT1A receptors (Smith & Peroutka, 1986; Andrade & Nicoll, 1987; Bockaert et al., 1987; Martin & Mason, 1987; Sprouse & Aghajanian, 1988), we have speculated that the apparent resistance of postsynaptic 5-HT1A receptors to regulation by the azapirone anxiolytics may be related to the low intrinsic efficacy of these agonists at postsynaptic 5-HT1A receptor sites. Postsynaptic 5-HT1A receptors may be more readily desensitized following treatment with the more efficacious agonist 8-OH-DPAT. Data from the current study indicate however that postsynaptic 5-HT1A receptors are resistant to regulation by repeated 8-OH-DPAT treatment.
5-HT1A receptor-stimulated [35S]-GTPγS binding was significantly attenuated in the dorsal and median raphe nuclei following treatment of rats with 8-OH-DPAT (1 mg kg−1) for 7 and 14 days. Desensitization of 5-HT1A somatodendritic autoreceptor function in the dorsal raphe has been demonstrated in electrophysiological studies to follow chronic administration of 5-HT1A receptor agonists gepirone or ipsapirone (Blier & de Montigny, 1987; Schechter et al., 1990; Dong et al., 1997). In vivo microdialysis studies have shown that following 7 days of 8-OH-DPAT administration the ability of 5-HT1A autoreceptors of the dorsal raphe to modulate serotonin release in the striatum is attenuated (Kreiss & Lucki, 1997), an indication of receptor desensitization. In these studies however, 5-HT1A autoreceptors of the median raphe appeared to be more resistant to regulation by 5-HT1A receptor agonists than those of the dorsal raphe. Although the ability of median raphe 5-HT1A autoreceptors to modulate serotonin release in the hippocampus was reduced after 14 days of 8-OH-DPAT administration, this trend was not statistically significant (Kreiss & Lucki, 1997). It is interesting to note that while 5-HT1A receptor – G protein interactions are decreased in both the dorsal and median raphe nuclei by repeated 8-OH-DPAT administration (current study), cells in the median raphe may be functionally less sensitive to this apparent decrease in autoreceptor-G protein coupling.
Following repeated treatment of rats with 8-OH-DPAT, the binding of [3H]-MPPF to 5-HT1A receptor sites was not altered in any brain region examined, suggesting that the observed changes in (±)8-OH-DPAT-stimulated [35S]-GTPγS binding were not due to changes in 5-HT1A receptor number. In general, changes in 5-HT1A receptor number have not been observed following chronic 5-HT1A receptor agonist administration (Larsson et al., 1990; Schechter et al., 1990; Wieland et al., 1993), although decreased receptor density has been reported by some investigators (Fanelli & McMonagle-Strucko, 1992). The data from the present study indicate that in serotonergic cell body areas the regulation of presynaptic 5-HT1A receptor sensitivity and function following repeated agonist administration occurs at the level of receptor-G protein interaction. In many forebrain areas, however, the regulation of postsynaptic 5-HT1A receptor sensitivity or function following repeated agonist administration appears not to be at the level of receptor-G protein coupling. These observations raise interesting questions as to the different cellular processes or mechanisms underlying regional differences in the regulation of 5-HT1A receptor responsiveness.
Acknowledgments
The authors would like to thank Drs Donald Hensler and Irwin Lucki for many helpful discussions. This research was supported by US PHS grant MH 52369 and funds from the South Texas Health Research Center.
Abbreviations
- GDP
guanosine-5′-diphosphate
- GTPγS
guanosine-5′-O- (3-thio)triphosphate
- HEPES
(N- [2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid)
- [3H]-MPPF
4-(2′-methoxy)-phenyl-1-[2′-(N-2′′-pyridinyl)-p-fluorobenzamido]ethyl-piperzin
- 8-OH-DPAT
8-hydroxy-dipropylaminotetralin hydrobromide: WAY 100635, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinyl-cyclohexanecarboxamide maleate
References
- AGHAJANIAN G.K., SPROUSE J.S., SHELDON P., RASMUSSEN K. Electrophysiology of the central serotonin system: receptor subtypes and transducer mechanisms. Ann. NY Acad. Sci. 1990;600:93–103. doi: 10.1111/j.1749-6632.1990.tb16875.x. [DOI] [PubMed] [Google Scholar]
- ANDRADE R., NICOLL R.A. Novel anxiolytics discriminate between postsynaptic serotonin receptors mediating different physiological responses on single neurons of the rat hippocampus. Naunyn Schmiedebergs Arch. Pharmacol. 1987;336:5–10. doi: 10.1007/BF00177743. [DOI] [PubMed] [Google Scholar]
- ANDRADE R., MALENKA R.C., NICOLL R.A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., ROMERO L., DE MONTIGNY C., BLIER P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- BLIER P., BERGERON R. The use of pindolol to potentiate antidepressant medication. J. Clin. Psychiatry. 1998;59:16–23. [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- BOCKAERT J., DUMUIS A., BOUHELAL R., SEBBEN M., CORY R.N. Piperazine derivatives including the putative anxiolytic drugs, buspirone and ipsapirone, are agonists at 5-HT1A receptors negatively coupled with adenylate cyclase in hippocampal neurons. Naunyn Schmiedebergs Arch. Pharmacol. 1987;335:588–592. doi: 10.1007/BF00169129. [DOI] [PubMed] [Google Scholar]
- CLARKE W.P., BERG K.A., GOULD G., FRAZER A.Serotonin receptor binding Current Protocols in Pharmacology 2001. ed. Enna, S.J. In press [DOI] [PubMed]
- CLARKE W.P., DE VIVO M., BECK S.G., MAAYANI S., GOLDFARB J. Serotonin decreases population spike amplitude in hippocampal cells through a pertussis toxin substrate. Brain Res. 1987;410:357–361. doi: 10.1016/0006-8993(87)90338-6. [DOI] [PubMed] [Google Scholar]
- CLARKE W.P., YOCCA F.D., MAAYANI S. Lack of 5-hydroxytryptamine1A-mediated inhibition of adenylyl cyclase in dorsal raphe of male and female rats. J. Pharmacol. Exp. Ther. 1996;277:1259–1266. [PubMed] [Google Scholar]
- DE MONTIGNY C., BLIER P., CHAPUT Y. Electrophysiologically-identified serotonin receptors in the rat CNS. Effect of antidepressant treatment. Neuropharmacology. 1984;23:1511–1520. doi: 10.1016/0028-3908(84)90095-9. [DOI] [PubMed] [Google Scholar]
- DE VIVO M., MAAYANI S. Characterization of the 5-hydroxytryptaminela receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in guinea pig and rat hippocampal membranes. J. Pharmacol. Exp. Ther. 1986;238:248–253. [PubMed] [Google Scholar]
- DONG J., DE MONTIGNY C., BLIER P. Effect of acute and repeated versus sustained administration of the 5-HT1A receptor agonist ipsapirone: electrophysiological studies in the rat hippocampus and dorsal raphe. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:303–311. doi: 10.1007/pl00005055. [DOI] [PubMed] [Google Scholar]
- DUPUIS D.S., PAUWELS P.J., RADU D., HALL H. Autoradiographic studies of 5-HT1A-receptor-stimulated [35S]GTPgammaS-binding responses in the human and monkey brain. Eur. J. Neurosci. 1999;11:1809–1817. doi: 10.1046/j.1460-9568.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- FANELLI R.J., MCMONAGLE-STRUCKO K. Alteration of 5-HT1A receptor binding sites following chronic treatment with ipsapirone measured by quantitative autoradiography. Synapse. 1992;12:75–81. doi: 10.1002/syn.890120109. [DOI] [PubMed] [Google Scholar]
- FILE S.E., GONZALEZ L.E., ANDREWS N. Comparative study of pre- and postsynaptic 5-HT1A receptor modulation of anxiety in two ethological animal tests. J. Neurosci. 1996;16:4810–4815. doi: 10.1523/JNEUROSCI.16-15-04810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEARY W.A.D., WOOTEN G.F. Quantitative film autoradiography of opiate agonist and antagonist binding in rat brain. J. Pharmacol. Exp. Ther. 1983;225:234–240. [PubMed] [Google Scholar]
- GEARY W.A.D., TOGA A.W., WOOTEN G.F. Quantitative film autoradiography for tritium: methodological considerations. Brain Res. 1985;337:99–108. doi: 10.1016/0006-8993(85)91613-0. [DOI] [PubMed] [Google Scholar]
- GOZLAN H., EL MESTIKAWY S., PICHAT L., GLOWINSKI J., HAMON M. Identification of presynaptic serotonin autoreceptors using a new ligand: 3H-PAT. Nature. 1983;305:140–142. doi: 10.1038/305140a0. [DOI] [PubMed] [Google Scholar]
- GOZLAN H., THIBAULT S., LAPORTE A.M., LIMA L., HAMON M. The selective 5-HT1A antagonist radioligand [3H]WAY 100635 labels both G-protein-coupled and free 5-HT1A receptors in rat brain membranes. Eur. J. Pharmacol. 1995;288:173–186. doi: 10.1016/0922-4106(95)90192-2. [DOI] [PubMed] [Google Scholar]
- HENSLER J.G., KOVACHICH G.B., FRAZER A. A quantitative autoradiographic study of serotonin1A receptor regulation. Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology. 1991;4:131–144. [PubMed] [Google Scholar]
- KEPPEL HESSELINK J.M.Promising anxiolytics? A new class of drugs Serotinin1A Receptors in Depression and Anxiety 1992Raven Press: New York; 171–183.ed. Stahl S.M. pp [Google Scholar]
- KHAWAJA X. Quantitative autoradiographic characterisation of the binding of [3H]WAY-100635, a selective 5-HT1A receptor antagonist. Brain Res. 1995;673:217–225. doi: 10.1016/0006-8993(94)01416-f. [DOI] [PubMed] [Google Scholar]
- KREISS D.S., LUCKI I. Chronic administration of the 5-HT1A receptor agonist 8-OH-DPAT differentially desensitizes 5-HT1A autoreceptors of the dorsal and median raphe nuclei. Synapse. 1997;25:107–116. doi: 10.1002/(SICI)1098-2396(199702)25:2<107::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- LARSSON L.G., RENYI L., ROSS S.B., SVENSSON B., ANGEBY-MOLLER K. Different effects on the responses of functional pre- and postsynaptic 5-HT1A receptors by repeated treatment of rats with the 5-HT1A receptor agonist 8-OH-DPAT. Neuropharmacology. 1990;29:86–91. doi: 10.1016/0028-3908(90)90047-u. [DOI] [PubMed] [Google Scholar]
- LUCKI I. Behavioral studies of serotonin receptor agonists as antidepressant drugs. J. Clin. Psychiatry. 1991;52:24–31. [PubMed] [Google Scholar]
- MARKSTEIN R., HOYER D., ENGEL G. 5-HT1A-receptors mediate stimulation of adenylate cyclase in rat hippocampus. Naunyn Schmiedebergs Arch. Pharmacol. 1986;333:335–341. doi: 10.1007/BF00500006. [DOI] [PubMed] [Google Scholar]
- MARTIN K.F., MASON R. Isapirone is a partial agonist at 5-hydroxytryptamine 1A (5-HT1A) receptors in the rat hippocampus: electrophysiological evidence. Eur. J. Pharmacol. 1987;141:479–483. doi: 10.1016/0014-2999(87)90569-3. [DOI] [PubMed] [Google Scholar]
- MELLER E., LI H., CARR K.D., HILLER J.M. 5-Hydroxytryptamine(1A) receptor-stimulated [(35)S]GTPgammaS binding in rat brain: absence of regional differences in coupling efficiency. J. Pharmacol. Exp. Ther. 2000;292:684–691. [PubMed] [Google Scholar]
- MOLLIVER M.E. Serotonergic neuronal systems: what their anatomic organization tells us about function. J. Clin. Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. Academic Press: New York; 1986. [Google Scholar]
- RAMBOZ S., OOSTING R., AMARA D.A., KUNG H.F., BLIER P., MENDELSOHN M., MANN J.J., BRUNNER D., HEN R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHECHTER L.E., BOLANOS F.J., GOZLAN H., LANFUMEY L., HAJ-DAHMANE S., LAPORTE A.M., FATTACCINI C.M., HAMON M. Alterations of central serotoninergic and dopaminergic neurotransmission in rats chronically treated with ipsapirone: biochemical and electrophysiological studies. J. Pharmacol. Exp. Ther. 1990;255:1335–1347. [PubMed] [Google Scholar]
- SIM L.J., SELLEY D.E., CHILDERS S.R. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIM L.J., XIAO R., CHILDERS S.R. In vitro autoradiographic localization of 5-HT1A receptor-activated G proteins in the rat brain. Brain Res. Bull. 1997;44:39–45. doi: 10.1016/s0361-9230(97)00061-0. [DOI] [PubMed] [Google Scholar]
- SMITH L.M., PEROUTKA S.J. Differential effects of 5-hydroxytryptaminela selective drugs on the 5-HT behavioral syndrome. Pharmacol. Biochem. Behav. 1986;24:1513–1519. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- SOTELO C., CHOLLEY B., EL MESTIKAWY S., GOZLAN H., HAMON M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur. J. Neurosci. 1990;2:1144. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- SPROUSE J.S., AGHAJANIAN G.K. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988;27:707–715. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- TRABER J., GLASER T. 5-HT1A receptor-related anxiolytics. Trends Pharmacol. Sci. 1987;8:432–437. [Google Scholar]
- VARRAULT A., LEVIEL V., BOCKAERT J. 5-HT1A-sensitive adenylyl cyclase of rodent hippocampal neurons: effects of antidepressant treatments and chronic stimulation with agonists. J. Pharmacol. Exp. Ther. 1991;257:433–438. [PubMed] [Google Scholar]
- VERGE D., DAVAL G., MARCINKIEWICZ M., PATEY A., EL MESTIKAWY S., GOZLAN H., HAMON M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J. Neurosci. 1986;6:3474–3482. doi: 10.1523/JNEUROSCI.06-12-03474.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAEBER C., MOSKOWITZ M.A. 5-HydroxytryptaminelA and 5-hydroxytryptamine1B receptors stimulate [35S]guanosine-5′-O-(3-thio)triphosphate binding to rodent brain sections as visualized by in vitro autoradiography. Mol. Pharmacol. 1997;52:623–631. doi: 10.1124/mol.52.4.623. [DOI] [PubMed] [Google Scholar]
- WIELAND S., FISCHETTE C.T., LUCKI I. Effect of chronic treatments with tandospirone and imipramine on serotonin-mediated behavioral responses and monoamine receptors. Neuropharmacology. 1993;32:561–573. doi: 10.1016/0028-3908(93)90052-5. [DOI] [PubMed] [Google Scholar]


