Abstract
The signal transduction mechanism of ginsenosides, the active ingredients of ginseng, was studied in Xenopus oocytes using two-electrode voltage-clamp technique. Ginseng total saponin (GTS), i.e., an unfractionated mixture of ginsenosides produced a large outward current at membrane potentials more positive than −20 mV when it was applied to the exterior of oocytes, but not when injected intracellularly. The effect of GTS was concentration-dependent (EC50: 4.4 μg ml−1) and reversible.
Certain fractionated ginsenosides (Rb1, Rb2, Rc, Rf, Rg2 and Ro) also produced an outward current in a concentration-dependent manner with the order of potency of Rf>Ro>Rb1=Rb2>Rg2>Rc. Other ginsenosides (Rd, Re and Rg1) had little or no effect.
The GTS effect was completely blocked by bath application of the Ca2+-activated Cl− channel blocker niflumic acid and by intracellular injection of the calcium chelator BAPTA or the IP3 receptor antagonist heparin. Also, the effect was partially blocked by bath-applied U-73122, a phospholipase C (PLC) inhibitor and by intracellularly injected GTPγS, a non-hydrolyzable GTP analogue. Whereas, it was not altered by pertussin toxin (PTX) pretreatment.
These results indicate that: (1) interaction of ginsenosides with membrane component(s) at the extracellular side leads to Ca2+-activated Cl− channel opening in Xenopus oocyte membrane; and (2) this process involves PLC activation, the release of Ca2+ from the IP3-sensitive intracellular store and PTX-insensitive G protein activation.
Keywords: Ginseng, ginsenosides, PLC, Ca2+-activated Cl− channels, Xenopus oocytes
Introduction
Ginseng, the root of Panax ginseng C.A. Meyer, is a well-known folk medicine that has been shown to produce a variety of medicinal effects. Recent studies showed that ginseng saponins, which are also called ginsenosides, were the main molecular components responsible for the actions of ginseng. Ginsenosides have a four-ring, steroid-like structure with sugar moieties attached, and about 30 different ginsenosides have been isolated and identified from the root of Panax ginseng (Nah, 1997).
Several studies have demonstrated that ginsenosides can act on neuronal cells. They inhibit voltage-dependent Ca2+ channels in chromaffin cells (Kim et al., 1998) and in sensory neurons via PTX-sensitive G proteins (Nah & McCleskey, 1994). They also inhibit acetylcholine (ACh)-stimulated catecholamine release from chromaffin cells via inhibition of Na+ influx through nicotinic receptor-gated cation channels (Tachikawa et al., 1995; Kudo et al., 1998).
There is evidence that ginsenosides can act on non-neuronal cells as well. Chronic treatment with ginsenosides in vivo was shown to increase 32PO4 incorporation into inositol phospholipids and to stimulate PLC activity in the mouse liver (Rim et al., 1997). Furthermore, ginsenosides was shown to increase intracellular Ca2+ concentration in non-neuronal cells such as macrophages and NIH3T3 cells (Shin et al., 1996; Hong et al., 1998).
Despite numerous reports on the action of ginsenosides as described above, our understanding of their signal transduction mechanisms is relatively limited. It is particularly so in non-neuronal cells. In the present study we investigated the signalling pathway for ginsenosides using Xenopus oocytes, a convenient in vitro model which allows intracellular injection of putative second messengers or agents that interrupt the action of second messengers and thus substantially facilitates investigations of intermediate steps in signalling pathways (Parker & Yao, 1994; Hartzell, 1996). Here we present results suggesting that ginsenosides leads to the endogenous Ca2+-activated Cl− channel opening via a well-defined signalling pathway, which involves PLC activation and Ca2+ mobilization from IP3-sensitive intracellular store.
Methods
Oocyte preparation
Xenopus laevis were obtained from Xenopus I (Ann Arbor, MI, U.S.A.). Their care and handling were in accordance with the highest standards of institutional guidelines. To isolate oocytes, frogs were operated on under anaesthesia with an aerated solution of 3-amino benzoic acid ethyl ester. Oocytes were separated by treatment with collagenase and agitation for 2 h in a Ca2+-free medium containing NaCl (82.5 mM), KCl (2 mM), MgCl2 (1 mM), HEPES (5 mM), sodium pyruvate (2.5 mM), 100 units ml−1 penicillin and 100 μg ml−1 streptomycin. Stage V – VI oocytes were collected and stored in ND96 in mM: NaCl 96, KCl 2, MgCl2 1, CaCl2 1.8 and HEPES 5, pH 7.5) supplemented with 0.5 mM theophylline and 50 μg ml−1 gentamycin. This oocyte-containing solution was maintained at 16°C with continuous gentle shaking and changed everyday. Electrophysiological experiments with oocytes were performed within 4 days after their isolation.
Oocyte recording
Two-electrode voltage-clamp recordings were obtained from single oocytes placed in a small Plexiglas net chamber (0.5 ml), which was continuously superfused with the bathing medium (i.e., ND96). The microelectrodes were filled with 3 M KCl and had a resistance of 0.2 – 0.7 MΩ. The electrophysiological experiments were performed at room temperature with Geneclamp 500 amplifier (Axon Instruments, CA, U.S.A.) or Oocyte Clamp (OC-725C, Warner Instrument, CT, U.S.A.). Linear leak and capacitance currents were corrected with leak subtraction procedure.
Drugs
The drugs used in this study were either bath-applied or injected into oocytes with a Nanoject Automatic Oocyte Injector (Drummond Scientific, PA, U.S.A.). The injection pipette was pulled from glass capillary tubing, and its tip was broken to an outer diameter of about 20 μm. GTS, BAPTA, heparin and GTPγS solutions (23 – 50 nl each) were injected into oocytes to give calculated intracellular concentrations of about 1 μg μl−1, 1 mM, 1 μg μl−1, and 150 μM, respectively.
Figure 1 shows the structures of the five representative ginsenosides. These ginsenosides and GTS were kindly obtained from Korea Ginseng and Tobacco Research Institute (Taejon, Korea). GTS contained Rb1 (17.1%), Rb2 (9.07%), Rc (9.65%), Rd (8.26%), Re (9%), Rf (3%), Rg1 (6.4%), Rg2 (4.2%), Rg3 (3.8%), Ro (3.8%), Ra (2.91%) and other minor ginsenosides. BAPTA, heparin, niflumic acid and GTPγS were obtained from Sigma. U-73122 (active PLC inhibitor) and U-73343 (inactive PLC inhibitor) were purchased from Calbiochem, and PTX was from List Biological Laboratories.
Figure 1.
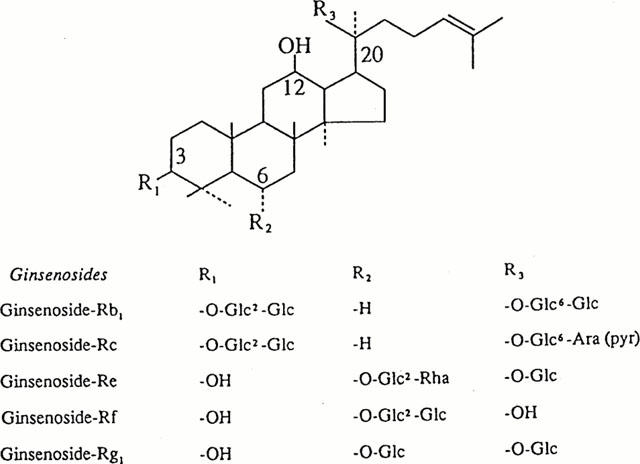
Structures of the five representative ginsenosides. They differ at three side chains attached the common steroid ring. Abbreviations for carbohydrates are as follows: Glc, glucopyranoside; Ara (pyr), arabinopyranoside; Rha, rhamnopyranoside. Superscripts indicate the carbon in the glucose ring that links the two carbohydrates.
Data analysis
All values are presented as mean±s.e.mean. The differences between means of control and treatment data were analysed using unpaired t-test. P<0.05 was considered significant.
Results
Enhancement of presumed Ca2+-activated Cl− current by ginsenosides
The oocytes recorded in this study had a resting membrane potential (Vr) of about −30 mV. Clamping these cells at a potential more negative than Vr made an inward current flow. Whereas, clamping at a potential less negative than Vr produced an outward current. Bath application of GTS increased these currents. To characterize the currents increased by GTS, the current-voltage (I – V) relationship was studied using the following protocol: at a holding potential of −80 mV, 500-ms voltage steps were applied from −60 to +60 mV in 20-mV increments. In the absence of GTS, the inward current at −60 mV was <0.1 μA and the outward current at +60 mV was 0.1 – 0.4 μA (Figure 2A). The addition of GTS to the bathing medium resulted in a slight increase in the inward current at −40 and −60 mV (Figure 2B). In contrast, at a potential more positive than −20 mV, GTS led to a large, voltage-dependent increase in outward current (Figure 2B). Typically, several microamperes of currents were recorded at +60 mV after treatment with 10 μg ml−1 GTS. Figure 2C illustrates schematically the I – V relationship in the absence and presence of GTS. In both cases, the relationship was non-linear and the reversal potential was about −30 mV close to the calculated reversal potential of Cl− current. The analysis of the tail current (Figure 2D) elicited in the presence of GTS showed that the reversal potential of this current is also −30 mV, pointing to the possibility that the in- and outward currents increased by GTS were Ca2+-activated Cl− current reported to be present in Xenopus oocytes (Barish, 1983; Miledi & Parker, 1984).
Figure 2.
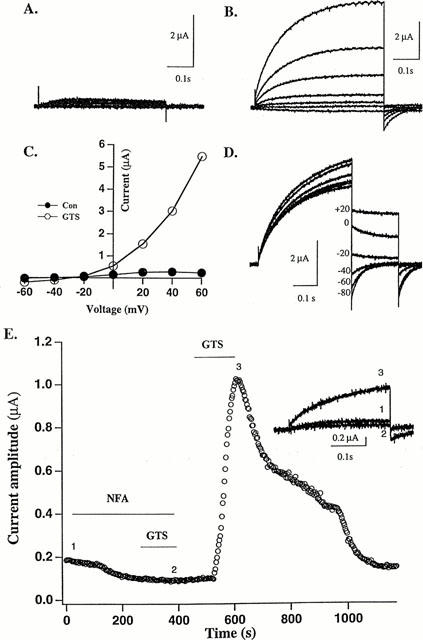
Current produced by GTS and its blockade by the Ca2+-activated Cl− channel blocker niflumic acid (NFA). (A) Current recorded in the absence of GTS. (B) Currents recorded in the presence of GTS (10 μg ml−1) in the same cell in (A). (C) Current-voltage relationships. (D) Tail currents of the responses evoked in the presence of GTS (30 μg ml−1). These currents were generated by first stepping the voltage to +40 mV from a holding potential of −80 mV and re-stepping the voltage to a less positive potential ranging from +20 to −80 mV with 20-mV decrements. Note that the reversal potential of the tail currents is somewhere between −20 and −40 mV. (E) Effect of NFA on GTS-evoked current. The amplitude of the outward current evoked every 5 s by a voltage step to +40 mV from a holding potential of −80 mV is plotted against time. Bars denote drug application (NFA: 100 μM, GST: 10 μg ml−1). Inset: Traces of the current recorded in the presence of NFA (1), GTS (3) or both (2) are superimposed; the time points of the recording are indicated on the graph as 1, 2 and 3. This graph is representative of data obtained from six oocytes from three different frogs.
To provide further evidence for the notion above, we examined the effect of niflumic acid (NFA), a reversible blocker of Ca2+-activated Cl− channels (White & Aylwin, 1990), on outward current. As expected, NFA (100 μM) slightly attenuated the baseline current, and it completely prevented GTS from increasing outward current in a reversible fashion (Figure 2E).
The effect of GTS on the presumed Ca2+-activated Cl− current was concentration-dependent; the EC50 was 4.4±0.5 μg ml−1, and the maximal effect was obtained at about 10 μg ml−1 (Figure 3A). Certain fractionated ginsenosides (Rb1, Rb2, Rc, Rf, Rg2 and Ro) also had a concentration-dependent effect on the presumed Cl− current with the order of potency of Rf>Ro>Rb1=Rb2>Rg2>Rc at 100 μM (Figure 3B). Whereas, other ginsenosides (Rd, Re and Rg1 even at 100 μM-concentration) had little or no effect (data not shown).
Figure 3.
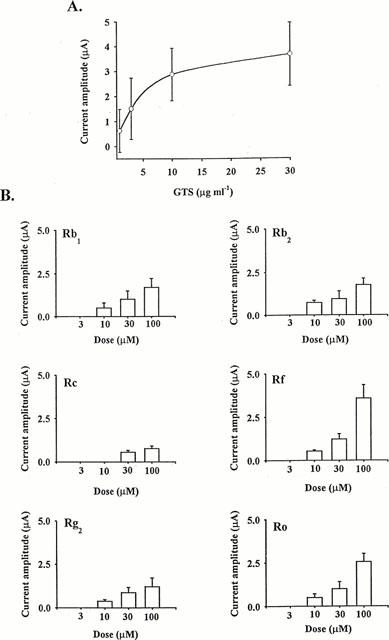
Concentration dependence of the GTS (A) and fractionated ginsenoside (B) effects on outward current. In (A), a continuous curve was fitted according to the following equation: I=Imax [GTS]/([GTS])+EC50, where Imax, the maximum current amplitude and EC50, the concentration producing half-maximum activation, are equal to 4.6 μA and 4.4 μg ml−1, respectively. In (B), the number of oocytes tested ranged from 7 – 10 for each ginsenoside.
Site of ginsenoside action
Ginsenosides have steroid-like structure with sugar moieties attached (see Introduction). Therefore, it is unclear whether the effects produced by their bath application were mediated via cell surface binding site, intracellular site or both. To address this issue, we further examined the effects of GTS injected into oocytes. Unlike bath application (10 μg ml−1), the intraoocyte injection (n=6, calculated concentration: 1 μg μl−1) exerted essentially no effect (Figure 4), suggesting that the binding site(s) of ginsenosides is located on the surface of the oocyte.
Figure 4.
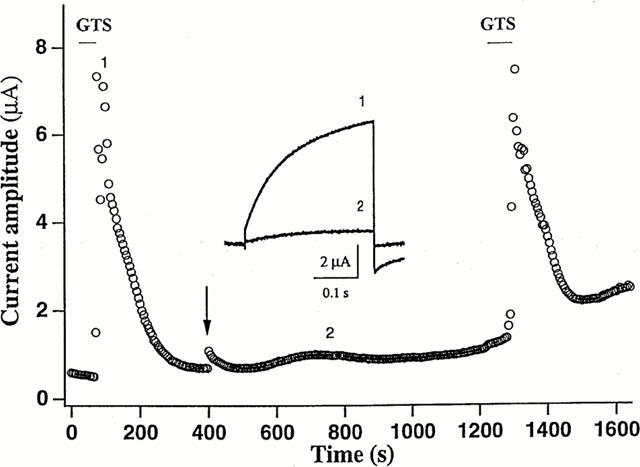
Ginsenoside binding site(s) is located on the external surface of occyte membrane. The amplitude of the outward current evoked every 5 s by a voltage step to +40 mV from a holding potential of −80 mV is plotted against time. Bars denote bath application of GTS (10 μg ml−1), and the arrow indicates intra-oocyte injection of 23 nl of GTS (oocyte concentration: 1 μg μl−1). Inset: Traces of the current recorded at the time points 1 and 2 are superimposed. The graph is representative of data obtained from six oocytes from three different frogs.
Blockade of ginsenosides action by BAPTA and heparin
The signal transduction mechanism underlying the effects of ginsenosides on the presumed Ca2+-activated Cl− current was further studied. First, we examined whether the GTS effect on this current was dependent on external or internal Ca2+. Removal of Ca2+ from the bathing medium did not alter the effect of GTS (data not shown). By contrast, Ca2+ chelation by intraoocyte injection of BAPTA almost completely abolished the effect of GTS (Figure 6). In addition, the BAPTA injection slightly reduced the baseline current recorded in the absence of GTS. Taken together, these observations suggest that GTS induce an elevation of [Ca2+]i by mobilizing Ca2+ from the intracellular store.
Figure 6.
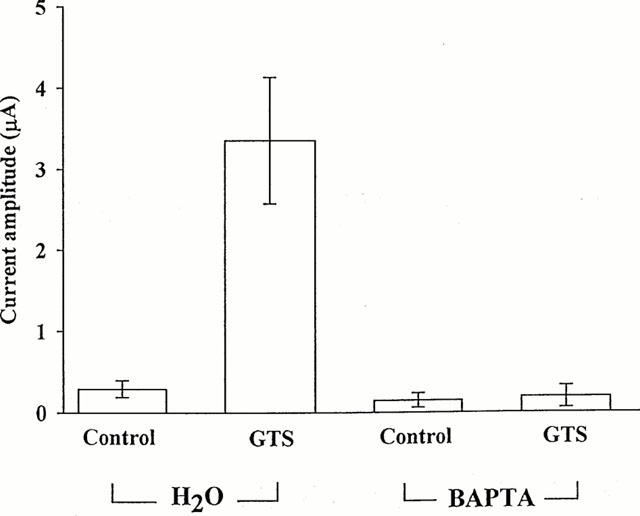
Blockade of GTS action by intraoocyte injection of BAPTA. The mean amplitudes of the outward currents recorded in the absence and presence of GTS (30 μg ml−1) following intra-oocyte injection (23 nl) of BAPTA (oocyte concentration: 1 mM) or vehicle (i.e., H2O) are plotted. A voltage step to +40 mV from a holding potential of −80 mV was used to evoke the outward currents. After BAPTA or H2O injection, oocytes were incubated for 20 min. The numbers in parentheses denote number of oocytes.
To see if IP3 was involved in the action of ginsenosides, the IP3 receptor antagonist heparin (Yao & Parker, 1993; Callamaras & Parker, 1994) was injected into oocytes. The heparin injection greatly attenuated the GTS effect (Figure 5). However, vehicle (distilled water) injection did not significantly alter the oocyte response to GTS. These results indicate that IP3 plays a key role in the ginsenoside action.
Figure 5.
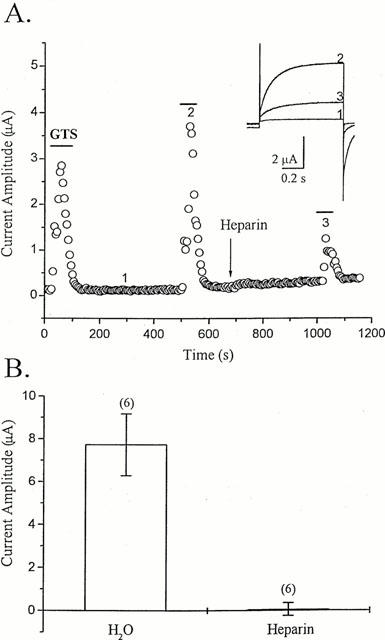
Blockade of GTS action by intra-oocyte injection of heparin. (A) The amplitude of the outward current evoked every 5 s by a voltage step to +40 mV from a holding potential of −80 mV is plotted against time. Bars denote bath application of GTS (30 μg ml−1), and the arrow indicates intra-oocyte injection of heparin (1 μg final). Inset: Traces of the current recorded at the time points 1, 2 and 3 are superimposed. (B) The mean amplitudes of the outward currents recorded in the presence of GTS (100 μg ml−1) following intra-oocyte injection (50 nl) of heparin (1 μg final) or vehicle (i.e., H2O) are plotted. A voltage step to +40 mV from a holding potential of −80 mV was used to evoke the outward currents. After heparin or H2O injection, oocytes were incubated for 20 min. The numbers in parentheses denote number of oocytes.
Blockade of ginsenoside action by PLC inhibitor
Since the ginsenoside action involves the participation of IP3, it may require PLC activation for the production of IP3. To test this possibility, the effects of the active PLC inhibitor U-73122 and its inactive analogue U-73343 (Thompson et al., 1991) were examined on GTS action. Bath application of U-73122, but not U-73343, significantly depressed the action of GTS: the GTS-induced current in U-73122 was 48.0±11.7% of the control, whereas the current in U-73343 was 78.6±6.4% of the control (Figure 7). These results indicate that the PLC inhibitor partially blocked the GTS action.
Figure 7.
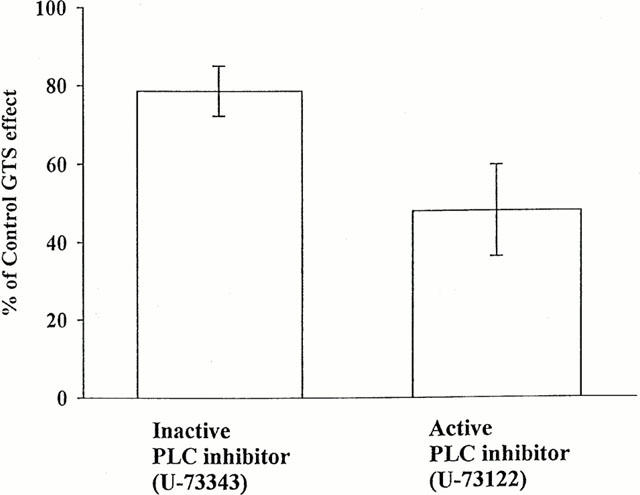
Effects of active and inactive PLC inhibitors on GTS-induced outward current. The amplitudes of the currents recorded in GTS (30 μg ml−1) before and during bath application of either the active (U-73122) or inactive inhibitor (U-73343) of PLC (1 μM) were measured, and their ratios (during/before) are plotted. The application of the PLC inhibitors preceded the GTS application by 5 min. The numbers in parentheses denote number of oocytes. Asterisk denotes statistical difference between the two data sets in the graph.
Effects of PTX and GTPγS on GTS action
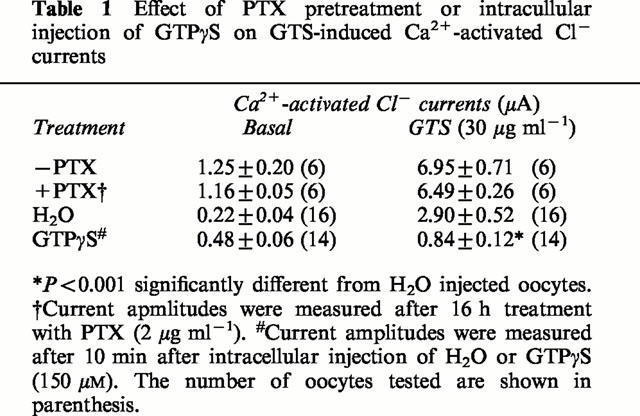
Previously, we demonstrated that ginseng root extract and ginsenoside Rf inhibited voltage-dependent Ca2+ channels through PTX-sensitive G proteins in rat sensory neurons (Nah & McCleskey, 1994; Nah et al., 1995). Hence, we tested the effects of PTX and of the non-hydrolyzable GTP analogue GTPγS on the GTS action in oocyte (Dascal et al., 1984). As summarized in Table 1, pretreatment of PTX (2 μg ml−1, 16 h) did not depress the action of GTS. However, the intra-oocyte injection of GTPγS (calculated concentration: 150 μM) significantly blocked the GTS effect on the oocyte current.
Table 1.
Effect of PTX pretreatment or intracullular injection of GTPγS on GTS-induced Ca2+-activated Cl− currents
Discussion
To investigate the signalling pathway underlying ginsenoside action, we utilized Xenopus oocytes. The reason for employing this model was 2 fold. First, it allows the intracellular injection of putative second messengers or drugs that block the action of second messengers without much damaging the cells under investigation. Second, it exhibits various, well-studied ion channel activities which may be used as signal transduction markers.
In this study, we provided evidence that interaction of ginsenosides with their binding site(s) located at the cell surface increases the endogenous Ca2+-activated Cl− current via PLC activation leading to Ca2+ release from the intracellular store by IP3 and this process involves PTX-insensitive G protein.
The effect of ginsenosides on Ca2+-activated Cl− current was transient in some oocytes tested. The current produced by ginsenosides diminished spontaneously after reaching peak amplitude even in the continued presence of ginsenosides (Figure 5A). Thus, the Cl− channels seemed to be desensitized to ginsenosides. Moreover, the channels showed cross-desensitization to ginsenosides and ACh (data not shown). This finding, coupled with the reports that stimulation of oocyte muscarinic receptors by ACh activates PLC leading to intracellular Ca2+ mobilization and activation of Cl− current (Dascal et al., 1984; Berridge & Irvine, 1989; Lechleiter & Clapham, 1992), suggests that the ginsenosides binding site(s) shares a common signalling pathway with muscarinic receptor. However, this does not indicate that ginsenosides act on muscarinic receptors, since the muscarinic receptor antagonist atropine had no effect on the ginsenoside action (data not shown).
About 30 different ginsenosides have now been isolated and identified from Panax ginseng. Studies have shown that certain ginsenosides are more potent than others (Nah et al., 1995; Tachikawa et al., 1995). In this study, we examined the effects of nine different ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg2 and Ro) and found that there was a clear difference in potency among these fractionated ginsenosides. Interestingly, we noted that GTS, i.e., an unfractionated mixture of ginsenosides, was more potent than any of the fractionated ginsenosides tested in the present study. This seemed to be due to a synergistic effect of the individual ginsenosides contained in GTS. However, other trace ingredients of ginseng such as alkaloids, carbohydrates, lipophilic components, phenolic components and β-sitosterol did not affect Ca2+-activated Cl− channels in Xenopus oocytes (data not shown).
On the other hand, lysophosphatidic acid (LPA), trypsin, or hyaluronan also induces activation of the endogenous Ca2+-activated Cl− channel in Xenopus oocytes in similar manner with ginsenosides (Tigyi & Miledi, 1992; Durieux et al., 1994; Fraser, 1997). However, higher concentration of trypsin or hyaluronan was required for the activation of Ca2+-activated Cl− channels than that used in present study. Recently, the endogenous receptor against LPA has been cloned in Xenopus oocytes (Guo et al., 1996). In addition to Xenopus oocytes, other cell types also express the endogenous Ca2+-activated Cl− channels. For example, lung epithelial cells possess Ca2+-activated Cl− channels and they are involved in airway secretion (Ran & Benos, 1991). Interestingly, NFA inhibits the endogenous Ca2+-activated Cl− channels in oocytes but not in lung epithelial cells (White & Aylwin, 1990; Ji et al., 1998). Moreover, treatment of phorbol ester, a protein kinase C activator, activates the endogenous Ca2+-activated Cl− channels in lung endothelial cells but inhibits the endogenous Ca2+-activated Cl− channels in Xenopus oocytes (Welsh, 1987; Boton et al., 1990). Thus, the regulatory modes on the endogenous Ca2+-activated Cl− might be cell-type specific. Recently, the clonings of Ca2+-activated Cl− channels in various species of tracheal endothelium have been reported (Cumningham et al., 1995; Gandhi et al., 1998; Gruber et al., 1998). However, it seems unlikely that this is the same channel investigated here because the channel kinetics and pharmacology differ (Ji et al., 1998).
In summary, we have obtained results suggesting that ginsenosides can increase Ca2+-activated Cl− current in Xenopus oocytes via a signalling pathway linked to muscarinic ACh receptor, which involves G protein-coupled PLC activation and Ca2+ mobilization from IP3-sensitive intracellular store.
Acknowledgments
This work was suported by ‘99 KRF (1999-015-DP0347) grant and 2000 MOST NRDP grants (2000-N-NL-01-C-180) to S.Y. Nah and by a grant from the Ministry of Science and Technology, Korea (BK21) (Star Project, ‘98-NQ-07-01-A) to C.S. Park. The authors are grateful to Dr Yang In Kim at Korea University College of Medicine for his constructive comments on the earlier draft of the manuscript and editorial help.
Abbreviations
- ACh
acetylcholine
- GTS
ginseng total saponins
- PLC
phospholipase C
- PTX
pertussis toxin
References
- BARISH M.E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. (Lond.) 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIDGE M.J., IRVINE R.F. Inositol triphosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- BOTON R., SINGER D., DASCAL N. Inactivation of calcium-activated chloride conductance in Xenopus oocytes: roles of calcium and protein kinase C. Pflügers Arch. 1990;416:1–6. doi: 10.1007/BF00370214. [DOI] [PubMed] [Google Scholar]
- CALLAMARAS N., PARKER I. Inositol 1, 4, 5-triphosphate receptors in Xenopus laevis oocyte: localization and modulation by Ca2+ Cell Calc. 1994;15:66–78. doi: 10.1016/0143-4160(94)90105-8. [DOI] [PubMed] [Google Scholar]
- CUMNINGHAM S.A., AWAYDA M.S., BUBIEN J.K., ISMAILOV I.I., ARRATE M.P., BERDIEV B.K., BENOS D.J., FULLER C.M. Cloning of an epithelial chloride channel from bovine trachea. J. Biol. Chem. 1995;270:31016–31026. doi: 10.1074/jbc.270.52.31016. [DOI] [PubMed] [Google Scholar]
- DASCAL N., YEKUEL R., ORON Y. Acetylcholine promotes progesterone-induced maturation of Xenopus oocytes. J. Exp. Zool. 1984;230:131–135. doi: 10.1002/jez.1402300117. [DOI] [PubMed] [Google Scholar]
- DURIEUX M.E., SALAFRANCA M.N., LYNCH K.R. Trypsin induces Ca2+-activated Cl− currents in X. laevis oocytes. FEBS Lett. 1994;337:235–238. doi: 10.1016/0014-5793(94)80198-3. [DOI] [PubMed] [Google Scholar]
- FRASER S.P. Hyaluronan activates calcium-dependent chloride currents in Xenopus oocytes. FEBS Lett. 1997;404:56–60. doi: 10.1016/s0014-5793(97)00083-5. [DOI] [PubMed] [Google Scholar]
- GANDHI R., ELBLE R.C., GRUBER A.D., SCHREUR K.D., JI H.-L., FULLER C.M., PAULI B.U. Molecular and functional characterization of a calcium-sensitive chloride channel from mouse lung. J. Biol. Chem. 1998;273:32096–32101. doi: 10.1074/jbc.273.48.32096. [DOI] [PubMed] [Google Scholar]
- GRUBER A.D., ELBLE R.C., JI H.L., SCHREUR K.D., FULLER C.M., PAULI B.U. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl− channel proteins. Genomics. 1998;54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- GUO Z., LILIOM K., FISCHER D.J., BATHURST I.C., TOMEI L.D., KIEFER M.C., TIGYI G. Molecular cloning of a high-affinity receptor for the growth factor-like lipid mediator lysophosphatidic acid from Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14367–14372. doi: 10.1073/pnas.93.25.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTZELL H.C. Activation of different Cl currents in Xenopus oocytes by Ca liberated from stores and by capacitative Ca influx. J. Gen. Physiol. 1996;108:157–175. doi: 10.1085/jgp.108.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG H.Y., YOO G.S., CHOI J.K. Effects of ginsenosides on pp60c-sre kinase, intracellular calcium and cell proliferation in NIH 3T3 cells. J. Ginseng Res. 1998;22:126–132. [Google Scholar]
- JI H.-L., DUVALL M.D., PATTON H.K., SATTERFIELD C.L., FULLER C.M., BENOS D.J. Functional expression of a truncated Ca2+-activated Cl- channel and activation by phorbol ester. Am. J. Physiol. 1998;274:C455–C464. doi: 10.1152/ajpcell.1998.274.2.C455. [DOI] [PubMed] [Google Scholar]
- KIM H.S., LEE J.H., GOO Y.S., NAH S.Y. Effects of ginsenosides on Ca channels and membrane capacitance in rat adrenal chromaffin cells. Brain Res. Bull. 1998;46:245–251. doi: 10.1016/s0361-9230(98)00014-8. [DOI] [PubMed] [Google Scholar]
- KUDO K., TACHIKAWA E., KASHIMOTO T., TAKAHASHI E. Properties of ginseng saponin inhibition of catecholamine secretion in bovine adrenal chromaffin cells. Eur. J. Pharmacol. 1998;341:139–144. doi: 10.1016/s0014-2999(97)01350-2. [DOI] [PubMed] [Google Scholar]
- LECHLEITER J.D., CLAPHAM D.E. Molecular mechanisms of intracellular calcium excitability in Xenopus laevis oocytes. Cell. 1992;69:283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- MILEDI R., PARKER I. Chloride current induced by injection of calcium into Xenopus oocytes. J. Physiol. (Lond.) 1984;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAH S.Y. Ginseng: Recent advances and trends. Kor. J. Ginseng Sci. 1997;21:1–12. [Google Scholar]
- NAH S.Y., MCCLESKEY E.W. Ginseng root extract inhibits calcium channels in rat sensory neurons through a similar path, but different receptor, as μ-type opioids. J. Ethnopharmacol. 1994;42:45–51. doi: 10.1016/0378-8741(94)90022-1. [DOI] [PubMed] [Google Scholar]
- NAH S.Y., PARK H.J., MCCLESKEY E.W. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8739–8743. doi: 10.1073/pnas.92.19.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER I., YAO Y. Relation between intracellular Ca2+ signals and Ca2+-activated Cl− current in Xenopus oocytes. Cell Calc. 1994;15:276–288. doi: 10.1016/0143-4160(94)90067-1. [DOI] [PubMed] [Google Scholar]
- RAN S., BENOS D.J. Isolation and functional reconstitution of a 38-kDa chloride channel protein from bovine tracheal membranes. J. Biol. Chem. 1991;266:4782–4788. [PubMed] [Google Scholar]
- RIM K.T., CHOI J.S., LEE S.M., CHO K.S. Effect of ginsenosides from red ginseng on the enzymes of cellular signal transduction system. Kor. J. Ginseng Sci. 1997;21:19–27. [Google Scholar]
- SHIN E.K., PARK H.W., KIM S.C., JUNG N.P. The effect of ginseng components on the signal transduction in the activation of murine macrophages. Kor. J. Ginseng Sci. 1996;20:159–167. [Google Scholar]
- TACHIKAWA E., KUDO T., KASHIMOTO T., TAKASHSHI E. Ginseng saponins reduce acetylcholine-evoked Na+ influx and catecholamine secretion in bovine adrenal chromaffin cells. J. Pharmacol. Exp. Ther. 1995;273:629–636. [PubMed] [Google Scholar]
- THOMPSON A.K., MOSTAFAPOUR S.P., DENLIGER L.C., BLEASDALE J.E., FISHER S.K. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. J. Biol. Chem. 1991;266:23856–23862. [PubMed] [Google Scholar]
- TIGYI G., MILEDI R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J. Biol. Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- WELSH M.J. Effect of phorbol ester and calcium ionophore on chloride secretion in canine tracheal epithelium. Am. J. Physiol. 1987;253:C828–C834. doi: 10.1152/ajpcell.1987.253.6.C828. [DOI] [PubMed] [Google Scholar]
- WHITE M.M., AYLWIN M. Niflumic and flufenamic acids are potent reversible blockers of Ca2+-activated Cl− channels in Xenopus oocytes. Mol. Pharmacol. 1990;37:720–724. [PubMed] [Google Scholar]
- YAO Y., PARKER I. Inositol triphosphate-mediated Ca2+ influx into Xenopus oocytes triggers Ca2+ liberation from intracellular stores. J. Physiol. (Lond.) 1993;468:275–296. doi: 10.1113/jphysiol.1993.sp019771. [DOI] [PMC free article] [PubMed] [Google Scholar]


