Abstract
A method is described for the detection of CFTR chloride channel openers (ClCOs) and blockers. Murine colonic epithelia were used throughout, but the method is applicable to other epithelia and biopsy material.
The principle was to render the epithelial basolateral membranes electrically transparent so that the apical membrane alone could be voltage clamped. This was achieved by potassium depolarization on the basolateral side. Imposition of an apical to basolateral chloride gradient allowed the effects of ClCOs on an outward chloride current and on apical membrane conductance to be measured.
1-ethyl-2-benzimidazolone (EBIO), forskolin, chlorzoxazone, and genistein all showed ClCO activity. In cystic fibrosis (CF) epithelia, either from CF null or CFΔF508 mice, EBIO showed only a minor effect, indicating that CFTR was the target in wild type tissues.
5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) was shown to block CFTR chloride channels. The blockade was pH and voltage-dependent and indicated that while the charged form was the active moiety, movement into the cell depended on the unionized drug. It is concluded NPPB blocks CFTR from the cytosolic side and that the agent preferentially blocks at potentials opposing the inflow of chloride ions. No significant blockade was seen with either N-phenylanthranilic acid (DPC) or with glibenclamide, under standard conditions.
The method described can be used to examine compounds reported to increase the trafficking of ΔF508 CFTR to the membrane or those capable of opening ΔF508 CFTR chloride channels and to differentiate between them. Further, the method distinguishes between chloride channel openers and those acting indirectly to increase the flux through CFTR chloride channels by indirect means, for example, hyperpolarization.
Keywords: CFTR, ΔF508CFTR, CFTR chloride channel openers, CFTR chloride channel blockers
Introduction
In chloride secreting epithelia the apical and basolateral membranes act in concert to effect transport. Chloride ions are raised above their electrochemical equilibrium concentration, inside the cell, by the basolaterally sited NaK2Cl cotransporter. Chloride ions then exit the cell via the apically located cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel (Frizzell et al., 1979). The active transport of chloride can be variously influenced by other activities at apical or basolateral membranes. For example, activation apical or basolateral potassium channels hyperpolarizes the apical membrane and increases the electrical gradient for chloride efflux (Cuthbert, 1999). Some agents, for example EBIO, activate chloride secretion by actions at both apical CFTR channels as well as cyclic AMP-sensitive and Ca2+-sensitive potassium channels on the basolateral side (Devor et al., 1996; Cuthbert et al., 1999). If processes at either the apical or basolateral side are disrupted then so is chloride secretion. In cystic fibrosis (CF) no functional CFTR is present, so chloride secretion is absent, at least in intestinal epithelia where no alternative chloride channels exist (Cuthbert et al., 1994).
Agents that activate CFTR chloride channels may be useful in adjunct therapy in CF, that is to compliment gene therapy, or in strategies designed to promote trafficking of ΔF508 CFTR to the apical membrane. Thus far, however, gene therapy has provided only modest and transient recovery of function in human trials. In the presence of a CFTR channel opener the effectiveness of the meagre number of CFTR channels can be maximized. Appropriate physiological stimuli acting on basolateral chloride uptake could then have a more profound effect on transport.
The purpose of this study is to provide a robust technique for examining CFTR chloride channel openers in intestinal epithelia and which may be applied to other epithelia, including human biopsy material. Here all the experiments have been made with colonic epithelium from wild type or CF mice. The model, which is developed in the next section, is designed to detect an inward chloride movement across the apical membrane and to measure the changes in current and conductance caused by CFTR chloride channel openers (ClCOs). Other methods for monitoring chloride channel activity are available, such as the release of radioactive chloride from pre-loaded cells or fluorescence methods using halide sensitive dyes (Verkman, 1990). Agents causing halide release or fluorescence change are then taken to indicate increased activity at chloride channels. However indirect effects can cause the same result without alteration to the state of the channels. For example, agents increasing Ca2+i, such as carbachol, increase chloride secretion, yet its actions are entirely at another location (Mall et al., 1998) and result from the increased electrical driving force on chloride efflux. This results from the activation of calcium sensitive K-channels. In the model developed here the basolateral membranes of the epithelial cells are essentially eliminated by making them electrically silent. In sweat duct epithelium the same effect was achieved using staphylococcus alpha toxin (Reddy et al., 1999) but was only occasionally possible in the colon. The reason may be the syncitial nature of the sweat duct (Quinton, personal communication). Specifically, therefore, the model can unequivocally detect agents that have a direct action on CFTR chloride channels, either to open or close them. Futhermore, agents which increase the trafficking of ΔF508 CFTR or G551D CFTR to the membrane can be revealed by agents, such as genistein (Hwang et al., 1997), which activates the mutant channels.
Methods
The model
The principle of the model is to convert the epithelium into a functional apical membrane, while the series basolateral membrane remains electrically transparent. The basolateral membranes of epithelia are essentially potassium permselective and with much greater areas than the apical membranes. Consequently, if the basolateral face is bathed in a high K+ containing solution it will have near zero potential and high conductance. Furthermore, if the apical bathing solution is normal (Krebs Henseleit Solution, KHS) and the basolateral solution has only impermeant anions (e.g. gluconate), the epithelium will be polarized in the opposite way to normal (i.e. apical face positive with respect to the basolateral face). Under short circuit conditions the apical membrane will be clamped at zero potential (Figure 1). Addition of ClCOs will cause an inward flow of chloride ions and an increase of conductance. Basolateral chloride channels, for which there is increasing evidence (Schultheiss & Diener, 1998), are needed to allow chloride to exit from the cell into the basolateral medium. Experimentally it may prove necessary to block non-chloride conductances in the apical membrane (for example, epithelial sodium channels (ENaCs) or K+-channels) to ensure the epithelium behaves as an isolated apical membrane containing specifically CFTR chloride channels.
Figure 1.
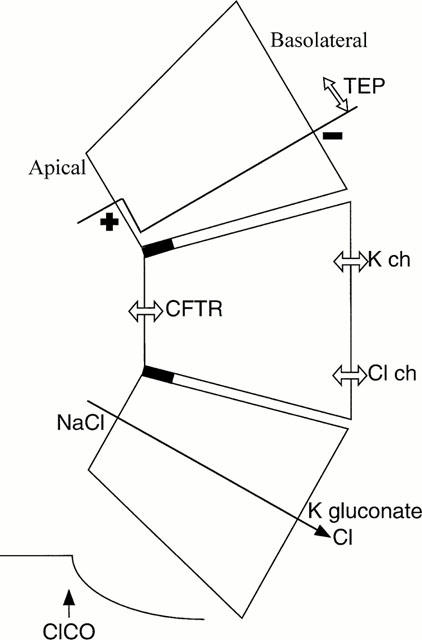
Model epithelium consisting of a functional apical membrane. The inner face is bathed with a K+ rich solution with an impermeant anion, while the apical surface is bathed in a NaCl containing saline, plus other agents to block conductances other than CFTR. The epithelium is polarized such that the apical surface is positively charged with respect to the basolateral surface, the potential drop being exclusively across the apical membrane. Under short circuit conditions addition of CFTR chloride channel openers (ClCOs) will cause a chloride current to flow into the cell through apical CFTR and outward through a basolateral Cl conductance. TEP indicates transepithelial potential. Inset shows the expected effect on SCC of an ClCO.
Experimental procedures
All experiments were made with the colonic epithelia of mice in which the muscle layers had been dissected away. The epithelia were mounted in Ussing chambers (window area 20 mm2) and voltage clamped using a WPI Dual Voltage Clamp (New Haven, CT, U.S.A.); as described elsewhere (Cuthbert et al., 1999). Data acquisition was with a MacLab, used in either Chart or Scope mode. On the apical side the tissues were bathed in 20 ml KHS, bubbled with 95%O2/5% CO2 and maintained at 37°C and pH 7.6. On the basolateral side the tissue was bathed in 20 ml Potassium Gluconate Solution (PGS), again oxygenated and maintained at the same temperature and pH as the apical solution. Throughout all experiments the apical solution contained amiloride, 100 μM to block ENaCs. The composition of the bathing solutions is given below; KHS (mM): NaCl 117, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaHCO3 24.8, KH2PO4 1.2 and glucose 11.1. PGS (mM): K gluconate 117, KCl 4.7, CaCl2 5.0, MgCl2 1.2, KHCO3 24.8, KH2PO4 1.2 and glucose 11.1.
Recording of SCC was straightforward using the Chart mode of MacLab. For measuring changes in both current and conductance the Scope mode was used with staircase changes in transepithelial voltage applied, each step having a duration of 1 s. A high sampling rate was used to record the current (2512 Hz) to avoid distortion. The resulting data allowed the construction of I/V curves and calculation of conductance. Although the I/V curves will be curved, according to the GHK formulation, the voltage range used was small, so that the curves were essentially linear and the conductance was calculated from the slope. Identical methods were applied to colonic epithelia from wild type, CF null (Ratcliff et al., 1993) and CF ΔF508 (Colledge et al., 1995) mice.
Statistical comparisons were made using a unpaired students t-test, P values less than 0.05 were considered significant. EBIO was supplied by Aldrich, forskolin by Calbiochem, N-phenylanthranilic acid (DPC) by Lancaster Synthesis Ltd. and chlorzoxazone, genistein, 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB), glibenclamide, TEA chloride and potassium gluconate were all from Sigma.
Results
Effects of EBIO and forskolin on SCC
Figure 2 gives two examples of SCC recording in colonic epithelia subjected to basolateral depolarization in the presence of impermeant anions. In Figure 2a the colon was from a wild type mouse, while that in Figure 2b was from a CF null mouse. On this occasion only the effect of amiloride is also shown, causing a small change in current due to blockage of ENaCs. Throughout the rest of the study the effects of amiloride are not shown, but the drug was always present in the apical bathing solution. Thus none of the current changes recorded throughout this study can be due to changes in electrogenic sodium transport. Both forskolin and EBIO are known to activate CFTR chloride channels, the former, through protein kinase A, by activation of adenylate cyclase and cyclic AMP production. In accordance with the predictions of the model both EBIO and forskolin caused an increase in the negative SCC in the wild type tissue, but had little or no effect on the tissue from a CF mouse.
Figure 2.
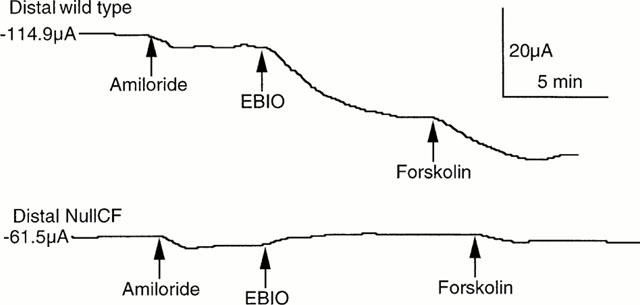
SCC records from depolarized colonic epithelia. In (a) from a wild type and in (b) from a CF null mouse. To each were added, in sequence, amiloride (100 μM, apically), EBIO (600 μM, both sides) and forskolin (10 μM, both sides). The epithelial area was 20 mm2. Initial SCCs are given at the beginning of each trace.
The results of the complete series of experiments, such as those of Figure 2, are given in Figure 3. With wild type tissues responses to EBIO and forskolin varied with the order of addition. The same total response to the two agonists was obtained independent of the order in which they were added. The additive nature of the responses to these two agents suggests they are acting by the same mechanism with neither producing a maximal effect at the concentrations used. In both CF null and CF ΔF508 tissues the responses to EBIO were significantly reduced compared to wild type. In wild type the response to EBIO, 600 μM, was 75.0±5.0 μA cm−2 compared to 7.9±6.3 μA cm−2 in CF ΔF508 (P<0.008) and to 2.5±8.3 μA cm−2 in CF null colons (P<0.02), supporting the notion that the model behaves as a CFTR containing, single membrane preparation.
Figure 3.
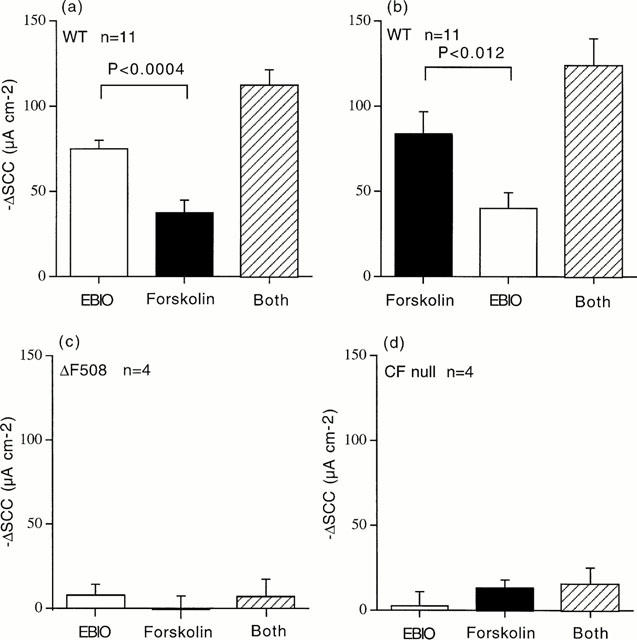
Cumulative SCC responses in depolarized epithelia. In (a) responses are shown to EBIO (600 μM) followed forskolin (10 μM), whereas in (b) the order was reversed. The total responses to both agents was not different in (a) and (b). Data for 11 separate experiments are given in (a) and (b). In (c) and (d) data are shown for experiments comparable to those in (a) for colonic epithelia from CF ΔF508 mice (c) and CF null mice (d), n=4 for all CF tissues. Comparing the responses to EBIO in wild type colons (a) (75.0±6.3 μA cm−2), the responses in CFΔF508 (c) (7.9±6.3 μA cm−2) and CF null colons (d) (2.5±8.3 μA cm−2) were significantly smaller by P<0.02 and P<0.008 respectively.
Effects of EBIO on conductance
Figure 4a,b shows the currents recorded from a wild type and CF null colon when subjected to a voltage staircase of −8 to +8 mV, every minute, for 10 min, after EBIO was added. Thus the current at the beginning, middle and end of each trace, when V was 0, is the SCC. It is seen that in the wild type tissue EBIO causes a rapid initial response, which then slows, until a steady state is reached at around 10 min. The inset shows the I/V relationship, which is linear over the range used, and the increase in slope after EBIO indicates the conductance increase. The change in current caused by EBIO is, as expected, greater at negative potentials than positive ones, since the electrical gradient for chloride influx is increased. However, negative potentials also accelerate the loss of K+ ions into the apical solution, especially given the large potassium gradient present. While the SCC responses to EBIO in CF null colon was less than one-third of that of the wild type it is apparent, in Figure 4b, that the associated conductance change in the CF colon was over 50% of that of the wild type. It therefore appeared crucial to reduce the K+-permeability of the apical membrane if the model is to be a reliable indicator of CFTR function. This was achieved using protocols illustrated in Figure 5 where the effects of EBIO on SCC and conductance are shown for three different conditions. First, in Figure 5a the only addition to the apical bathing solution was amiloride, 100 μM. Secondly, in Figure 5b both amiloride and Ba2+, 5 mM, were added to the apical solution, the latter to block K+ channels. Finally, in Figure 5c amiloride and tetraethyl ammonium chloride, 30 mM (replacing 30 mM NaCl) were used, the latter again used as a K-channel blocker. It is clear that significant reductions in both SCC and conductance following EBIO in CF tissues were obtained only if blockade of apical K-channels was present. Therefore throughout the rest of this study amiloride, 100 μM, and Ba2+, 5 mM, were present in the apical bathing solutions. This combination was shown to produce satisfactory data with either CF null and CF ΔF508 colonic epithelia.
Figure 4.
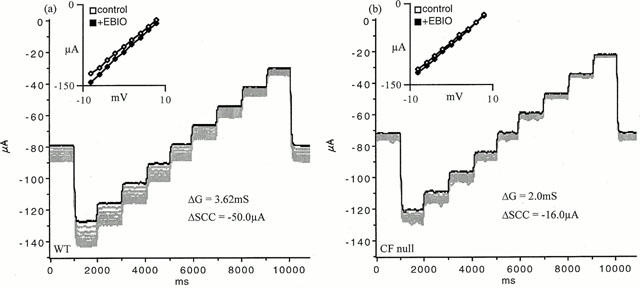
Current voltage relationships in depolarized epithelia. Epithelia were exposed to depolarizing solution on the basolateral side and amiloride, 100 μM, was added to the apical bathing solution. Tissues were initially short circuited and subjected to a voltage staircase of −8 to +8 mV in 2 mV steps, each of 1 s duration. The dark line indicates the control condition, further traces were obtained at minute intervals for 10 min after EBIO (600 μM) was added. The insets show the I/V relationships before EBIO and 10 min after its addition. The change in SCC and conductance is indicated. Examples are for a wild type (a) and CF null epithelium (b).
Figure 5.
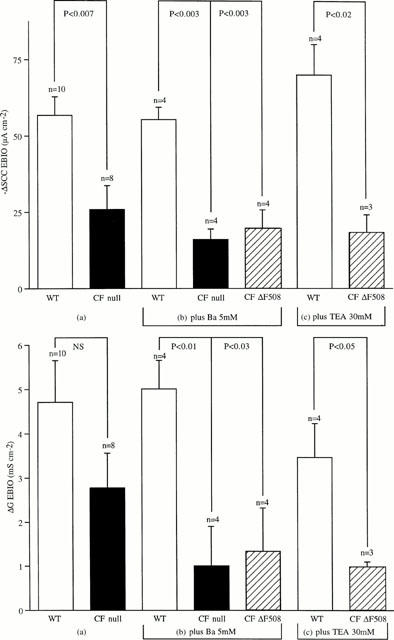
SCC and conductance changes following EBIO. The conditions for these experiments were the same as those described for Figure 4. Results of three different sets of experiments are given in which the additions to the apical bathing solution were different. In (a) amiloride alone, 100 μM, was added. In (b) amiloride, 100 μM plus Ba2+, 5 mM were added, while in (c) amiloride, 100 μM plus TEA chloride, 30 mM were added, with a consequent reduction in the NaCl content of the KHS by 30 mM. Note that when either amiloride and Ba2+ or amiloride and TEA were added both the SCC and conductance were significantly reduced in the CF tissues. n values are indicated. The concentration of EBIO used was 600 μM throughout.
Other CFTR chloride channel openers and blockers
Thus far, EBIO and forskolin, two known CFTR chloride channel openers, have been shown to increase the negative SCC in depolarized colonic epithelia. To further test the reliability of the model two other known ClCOs, genistein (Illeck et al., 1995) and chlorzoxazone (Singh et al., 2000) were examined and the data are presented in Figure 6. Both agents produced results similar to those with EBIO with increases in negative SCC and conductance increases, using concentrations of each agent close to the EC50 for effects with conventional SCC recording. With genistein the voltage range was increased (−20 to +20 mV). At the most negative potential (−20 mV) the current showed some fade during the one second pulse.
Figure 6.
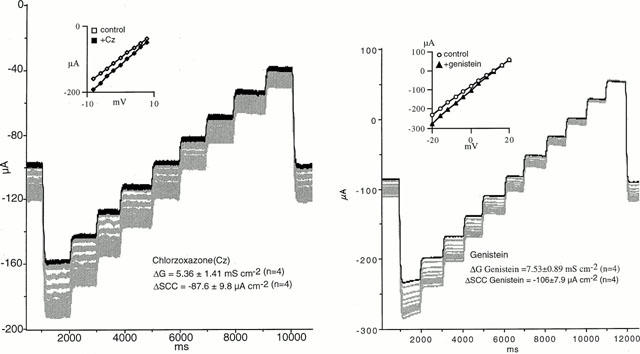
SCC and conductance changes in wild type colonic epithelia with chlozoxazone and genistein. The experiments were conducted with the conditions used in Figure 5b, i.e. the apical bathing solution contained amiloride, 100 μM plus Ba2+, 5 mM. In each instance the dark line shows the control condition in short circuited epithelia subjected to the voltage staircase. Recordings were then made every minute for 10 min after CICOs were added (chlozoxazone, 500 μM, both sides and genistein, 50 μM, apically only). The insets show the linear I/V curves obtained before and 10 min after agents were added. Mean values±s.e. mean are given for n=4. Note the current fade occuring when the clamp potential was −20 mV.
A number of substances are reported to block CFTR ion channels. Examples are NPPB, DPC and glibenclamide (Sheppard & Robinson, 1997; Zhang et al., 2000), but none of these compounds are specific or potent (Doughty et al., 1998). All three were examined on depolarized colonic epithelia. As shown in Figure 7 glibenclamide, 100 μM, had no effect on an EBIO stimulated, wild type epithelium, whereas NPPB, 100 μM, caused a slow reversal of the SCC after EBIO. However as also seen NPPB caused a slow inhibition of the negative SCC in the unstimulated colonic epithelium. Table 1 contains data from a variety of different experiments using the three antagonists. It is clear from the data that NPPB at 100 μM reduces a conductance which is unassociated with CFTR. However the current change caused by NPPB in unstimulated epithelia (22.5±9.1 μA cm−2) was significantly less than when stimulated by EBIO/forskolin (86.1±13.7 μA cm−2, P<0.002) or by EBIO alone (54.5±8.0 μA cm−2, P<0.02). Furthermore a similar change is found in CF epithelia as found with unstimulated wild type tissues. A characteristic of NPPB blockade of CFTR is its voltage dependence and slow onset of action (Zhang et al., 2000). As NPPB was the only inhibitor that reduced the response to EBIO an examination of whether the blockade showed voltage dependence was undertaken. Depolarized epithelia to which amiloride and barium had been added, to block Na+ and K+ conductances, were treated with EBIO, 600 μM, and, after the current had stabilized, NPPB was added to the apical surface. The block of the EBIO generated current was followed over time. Figure 8 shows an experiment with NPPB, 250 μM, and the inhibition of the EBIO response after 10 min, being c. 20% at −8 mV and c. 60% at +8 mV. The results with NPPB were somewhat variable as shown by other data for experiments of this type (Table 2). One problem in the interpretation of the data is the presence of a non-CFTR conductance, sensitive to NPPB, as described above and also seen with CF epithelia. This will cause the percentage inhibition by NPPB to be overestimated. An approach to correct this can be made as follows, using the example given in Figure 8. Here EBIO caused a SCC current change of 132.5 μA cm−2 that was inhibited by NPPB by 41.5 μA cm−2 at 0 mV and after 10 min, representing 31.3% inhibition. Similarly at −8.0 and +8.0 mV the inhibition was 19.3 and 63.1% respectively. The change in current due to NPPB, 100 μM, in the absence of EBIO, for wild type epithelia was 27.8±13.9, 22.5±9.1 and 8.35±5.85 μA cm−2 at −8.0, 0 and +8 mV respectively (all values n=8). Subtracting these mean values from the apparent reduction caused by NPPB indicated that the percentage inhibition of the CFTR current was 5.9, 14.3 and 52.8% respectively at −8, 0 and +8 mV. Nevertheless after making these corrections a clear voltage dependency remained. A further characteristic of NPPB blockade is its slow onset, even when used at high concentration, suggesting the agent needs to penetrate the cell to be effective. The pKa of NPPB is circa 4, which means at physiological pH there will be virtually no unprotonated drug able to penetrate into the cells. For each pH lowering of one unit there will be a 10 fold increase in the amount of uncharged NPPB. To examine the effect of pH on the rate of onset of blockade was compared at pH 7.6 with that at pH 5.6. To do this the solutions bathing both faces of the epithelia had to be altered. Essentially bicarbonate was omitted and replaced with potassium gluconate (for the basolateral solution) or sodium chloride (for the apical solution). The solutions were buffered with Tris at either pH 7.6 (basolateral) or pH 5.6 (apical) and were gassed with 100% O2. Figure 9 shows the results obtained using two colonic preparations from the same mouse. The inhibition of the SCC by NPPB at the relatively modest concentration of 50 μM was rapid at pH 5.6 and slow at pH 7.6. Here no attempt was made to correct for the non-CFTR current inhibition, explaining why, at pH 5.6, inhibition was greater than 100% at 10 min. At pH 5.6 the concentration of the unionized form of NPPB must have been equivalent to 5 mM NPPB at pH 7.6.
Figure 7.
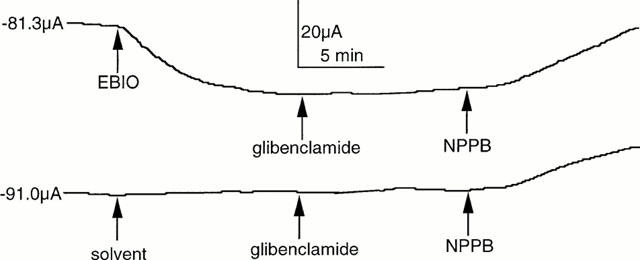
Effect of NPPB and gilbenclamide on SCC responses in depolarized epithelia. SCC responses are shown for wild type colonic epithelia. In (a) the negative SCC was activated by addition of EBIO (600 μM) and, after the effect had stabilized, glibenclamide (100 μM) and then NPPB (100 μM) were added. A second epithelium was treated identically, except EBIO was not added. The SCC value at the start of each trace is indicated.
Table 1.
Effects of putative CFTR chloride channel blockers

Figure 8.
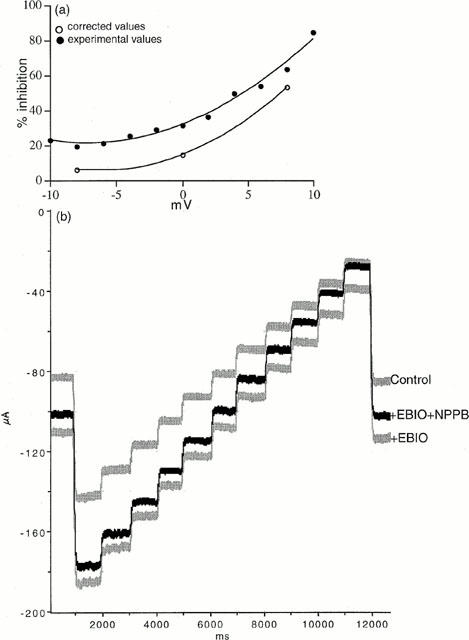
Voltage dependence of the blocking action of NPPB. (a) Relation of the apparent inhibition by NPPB (250 μM at 10 min) of the response to EBIO, 600 μM, versus the clamp potential. Corrected values are shown for the voltage-dependent inhibition by NPPB in which allowance has been made for the non-CFTR actions of NPPB. (b) The experimental conditions were the same as those in Figure 6. Current changes resulting from the application of a voltage staircase are shown for the control condition, 10 min after EBIO, 600 μM was added and 10 min after NPPB, 250 μM had been added to the apical side.
Table 2.
The voltage dependence of blockade by NPPB at pH 7.6
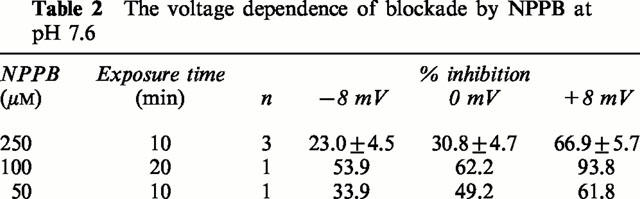
Figure 9.
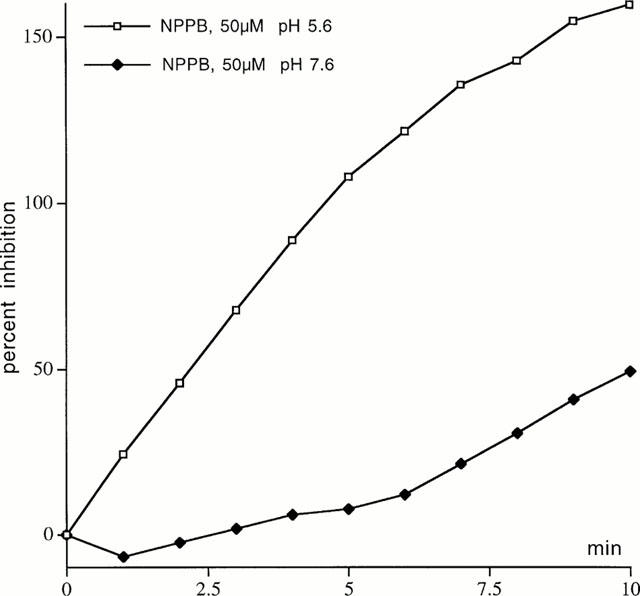
The effect of pH on the blocking action of NPPB. Inhibition of the SCC after EBIO (600 μM) was applied for 10 min by NPPB, 50 μM. NPPB was applied to the apical solution with the pH adjusted to 5.6 (open symbols) or with the pH adjusted to 7.6 (closed symbols). The bathing solutions were modified for these experiments in that bicarbonate was removed and replaced with K gluconate (basolaterally) or NaCl (apically) and the solutions bubbled with 100% O2. The solutions were buffered to the pHs indicated with Tris. No allowance has been made for the non-specific effects on non-CFTR currents. At pH 5.6 EBIO caused an increase in the negative SCC of 88.5 μA cm−2, while at pH 7.6 the corresponding value was 147.5 μA cm−2.
Discussion
The strategy used here to develop the ‘apical membrane only' model of an epithelium owes much to earlier work by Lindemann (Lindemann & van Driessche, 1977), who used a similar approach for examining ENaCs in frog skin. It is also similar to the use of staphylococcus alpha toxin to generate pores in the basolateral membrane, effectively removing it as a barrier, as with perfused sweat gland ducts (Reddy et al., 1999). Essentially the membranes of the basolateral faces of epithelia are of the normal type, with NaKATPase activity and predominantly potassium permselectivity. This latter property, combined with the much greater surface area than for apical membranes allows the basal membrane to become electrically invisible when depolarized. In depolarizing solutions there will be no potassium gradient or potential across the membrane and the high potassium concentration, plus the large area, will ensure the basolateral membrane has a high conductance. By contrast the apical membranes of epithelia are specialized for a variety of absorptive and secretory processes and do not have a predominant potassium conductance. Specifically, in the mouse colon, the apical membrane contains CFTR chloride channels, ENaCs and some K channels. In this study the apical bathing solution contained chloride ions in the presence of amiloride (to block ENaCs) and Ba2+ (to block K+ channels). It is doubtful from earlier studies (Cuthbert et al., 1994) that the concentration of Ba2+ was sufficient to completely block K+-channels, but the Ba2+ levels were restricted to avoid possible toxic effects.
In the configuration used here the prediction is that the epithelium would be polarized with the inner surface negative, as was found. The potential drop must occur across the apical membrane only, as the basolateral surface was depolarized. The transport of chloride, in this situation, is inward and the SCC negative by normal conventions. Experimentally it was found that there was a considerable current under SCC conditions before any stimulus was applied, as shown in Figure 2a,b. Little attention has been paid to this current, although it is to be noted it was significantly less in CF epithelia compared to wild type (Cuthbert, 2000). It is considered that most of this current is associated with intercellular pathways in the presence of a steep ionic gradient. The possibility cannot be ignored that some CFTR channels are open, even in the absence of CICOs, and will contribute to the basal conductance. However as was shown NPPB can reduce the conductance in the absence of agonists, equally well in both wild type and CF colons, arguing against the presence of constitutively active CFTR chloride channels in the colon. Little is known currently about how chloride ions might move out of the cell across the basolateral membrane, although there is increasing evidence for a non-CFTR chloride conductance at this site (Schultheiss & Diener, 1998). Clearly, Cl− can move across this membrane as agonist stimulated chloride secretion is only reduced by 50% in mice in which the cotransporter has been knocked out (Flagella et al., 1999). The steep chloride gradient from inside the cell to the basolateral bathing solution should ensure that the gradient across the apical face is preserved, unless the entry rate is very high. The time dependent fade in the current at large negative potentials, in stimulated epithelia (Figure 6), may represent such a limitation.
It is already known that EBIO stimulates chloride secretion in the colon, by a dual action at CFTR and K+-channels. Similarly forskolin, acting via PKA/cyclic AMP, can activate CFTR and cyclic AMP-sensitive K+-channels (Cuthbert et al., 1999) as well as stimulating the cotransporter indirectly (Haas & Forbush, 2000). Under the depolarizing conditions of this study both forskolin and EBIO increased the negative SCC, the quantitative relations between the two suggesting that both acted on the same mechanism (Figure 3). Furthermore, the responses were significantly less in CF colons from either null or ΔF508 mice. These SCC responses are attributed to a chloride current moving in the apical to basolateral direction, but could include some potassium movement in the opposite direction, given the steep gradient. However the conductance change in CF colons after EBIO, while reduced, was not significantly lower than in wild type. Addition of Ba2+ ions or alternatively TEA Cl−, to the apical solution, to block apical K-channels, however ensured that both conductance and current changes were significantly less in CF tissues compared to control (Figure 5).
Two other agents chlorzoxazone (Singh et al., 2000) and genistein (Illeck et al., 1995) are reported to act as ClCOs and were found to be effective at increasing both current and conductance in depolarized colons. Chlorzoxazone, like EBIO, is a benzimidazolone, while genistein is benzopyranone. Genistein is a tyrosine kinase inhibitor, but its action of CFTR is considered to be independent of this. In summary, EBIO, cyclic AMP, chlorzoxazone and genistein are shown to be chloride channel openers, independently of other effects they might have, for example on K channels.
No specific high affinity blockers of CFTR are known, but there are several compounds which block CFTR in high concentration, including DPC, glibenclamide and NPPB (Sheppard & Robinson, 1997; Walsh et al., 1999; Zhang et al., 2000). None of these have impressive effects on intact epithelia and the model described would be a useful one to test for high affinity antagonist acting from the external face of the apical membrane. All the studies on the nature of the blockade by these agents have been on transfected cells, usually oocytes, using microelectrode methods. In general, it is shown these three agents block from the cytosolic aspect of the cell, that the charged forms are the active moieties and that blockade was voltage-dependent, preferentially for inward currents. In this study an attempt was made to look for voltage dependence in an intact epithelial tissue. The pKa of NPPB is c. 4, which means it will be almost completely charged at pH 7.6 and therefore unable to penetrate the cell, as the slow onset rate suggests. When the pH of the apical solution was lowered to 5.6 current inhibition appeared more quickly. This result is compatible with the idea that NPPB must enter the cell in order to be effective, assuming that the pH change on the apical surface did not influence interaction with the drug. Voltage dependence of NPPB block was demonstrated and was retained after correction was made for the non-specific effects of the drug. Blockade by NPPB was weakest when the epithelium was clamped at negative potentials (i.e. the outside apical face negative with respect to the inner surface, supporting an inward flow of chloride ions or an outward current) and strongest at positive potentials (i.e. the potential opposing the inward flow of chloride ions). This study is therefore consistent with the data from microelectrode studies in transfected oocytes. It suggests that large anions, such as NPPB, compete with Cl− ions for a site approached from the cytoplasmic domain. In the conditions of this study chloride ions are flowing into the cell, along a steep gradient, and only when the electical gradient is lessened does NPPB exert substantial blockade. It is known that lowering the chloride ion concentration promotes the blocking action. Zhang et al. (2000) found that DPC in high concentration was not able to inhibit outward currents. As all the currents in this study were outward the virtual lack of effect of this agent is explained. The same explanation may also apply to the result with glibenclamide.
Pure CFTR channel openers alone, such as NS004, have little or no effect on transepithelial chloride secretion since action at both series barriers is required (Devor et al., 1996). Thus increasing the potential for exit without altering inflow can have no sustained effect on current. In CF the reverse is the case, whatever the Cl− influx through the basolateral aspects of the cell, no apical exit can occur in the absence of CFTR. The most common CF mutation is ΔF508 in which phenylalanine is missing from position 508. ΔF508CFTR fails to be trafficked within the cell and is destroyed without being inserted in the membrane. Currently there is considerable effort to discover ways to increase the trafficking of mutant CFTR to the membrane. Virtually all ΔF508CFTR fails to be released from heat shock proteins and is destroyed in the proteasome. Recently it has been shown that geldanomycin can stabilize ΔF508CFTR to the same extent as wild type CFTR (Fuller & Cuthbert, 2000). Whether the resultant stabilized mutant protein is transferred to the membrane could be detected by the described method using agents such as genistein. Thus the method described in this study could be used to examine agents that increase trafficking or are able to activate membrane inserted ΔF508CFTR and distinguish between the two. Although only the colon has been used in this study to illustrate proof in principle, the method is capable of application to other epithelia, including human biopsy samples. A therapeutic strategy with ClCOs for CF treatment may be valuable either alone or as an adjunct therapy alongside gene therapy.
Acknowledgments
This work was supported by the MRC and the Wellcome Trust. I am grateful to Bill Colledge for the supply of CF mice.
Abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- ClCO
CFTR chloride channel opener
- DPC
N-phenylanthranilic acid
- EBIO
1-ethyl-2-benzimidazolone, ENaC, amiloride sensitive epithelial sodium channel
- NPPB
5-nitro-2-(3-phenylpropylamino)benzoic acid
References
- COLLEDGE W.H., ABELLA B.S., SOUTHERN K.W., RATCLIFF R., JIANG C., CHENG S.H., MACVINISH L.J., ANDERSON J.R., CUTHBERT A.W., EVANS M.J. Generation and characterisation of a Δ508 cystic fibrosis mouse model. Nat. Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- CUTHBERT A.W.Functional role of CFTR chloride channels in airway and gut epithelia Chloride Channels 1999Isis Medical Media Ltd: Oxford; 79–95.ed. Kozlowski, R.Z. pp [Google Scholar]
- CUTHBERT A.W. 1-ethyl-2-benzimidazolone (EBIO) is a cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel opener. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 2000;7:59. [Google Scholar]
- CUTHBERT A.W., HICKMAN M.E., THORN P., MACVINISH L.J. Activation of Ca2+- and cAMP-sensitive K+ channels in murine colonic epithelia by 1-ethyl-2-benzimidazolone. Am. J. Physiol. 1999;277:C111–C120. doi: 10.1152/ajpcell.1999.277.1.C111. [DOI] [PubMed] [Google Scholar]
- CUTHBERT A.W., MACVINISH L.J., HICKMAN M.E., RATCLIFF R., COLLEDGE W.H., EVANS M.J. Ion transporting activity in the murine colonic epithelium of normal and cystic fibrosis animals. Pflugers. Arch. 1994;428:508–515. doi: 10.1007/BF00374572. [DOI] [PubMed] [Google Scholar]
- DEVOR D.C., SINGH A.K., BRIDGES R.J., FRIZZELL R.A. Modulation of chloride secretion by benzimidazolones. II. Co-ordinate regulation of apical GCl and basolateral GK. Am. J. Physiol. 1996;271:L785–L795. doi: 10.1152/ajplung.1996.271.5.L785. [DOI] [PubMed] [Google Scholar]
- DOUGHTY J.M., MILLER A.L., LANGTON P.D. Nonspecificity of chloride channel blockers in rat cerebral arteries:block of the L-type calcium channel. J. Physiol. 1998;507:433–439. doi: 10.1111/j.1469-7793.1998.433bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAGELLA M., CLARKE L.L., MILLER M.L., ERWAY L.C., GIANELLA R.A., ADRINGA A., GAWENIS L.R., KRAMER J., DUFFY J.J., DOETSCHMAN T., LORENZ J.N., YAMOAH E.N., CARDELL E.L., SHULL G.E. Mice lacking basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J. Biol. Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- FRIZZELL R.A., FIELD M., SCHULTZ S.G. Sodium coupled chloride transport in epithelial tissues. Am. J. Physiol. 1979;236:F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- FULLER W., CUTHBERT A.W. Post-translational disruption of the ΔF508 CFTR-molecular chaperone complex with geldanomycin stabilises ΔF508 CFTR in the rabbit reticulocyte lysate. J. Biol. Chem. 2000;275:37462–37468. doi: 10.1074/jbc.M006278200. [DOI] [PubMed] [Google Scholar]
- HAAS M., FORBUSH B. The Na-K-Cl cotransporter of secretory epithelia. Annu. Rev. Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- HWANG T.-C., WANG F., YANG IC-H., REENSTRA W. Genistein potentiates the wild-type and ΔF508CFTR channel activity. Am. J. Physiol. 1997;273:C988–C998. doi: 10.1152/ajpcell.1997.273.3.C988. [DOI] [PubMed] [Google Scholar]
- ILLECK B., FISCHER H., SANTOS G.F., WIDDICOMBE J.H., MACHEN T.E., REENSTRA W.W. cAMP-independent activation of CFTR Cl− channels by the tyrosine kinase inhibitor genistein. Am. J. Physiol. 1995;268:C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- LINDEMANN B., VAN DRIESSCHE W. Sodium specific membrane channels in frog skin are pores. Current fluctuations reveal a high turnover. Science. 1977;195:292–294. doi: 10.1126/science.299785. [DOI] [PubMed] [Google Scholar]
- MALL M., BLEICH M., SCHURLEIN J., KUHR J., SEYDEWITZ H.H., BRANDIS M., GREGER R., KUNZELMANN K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am. J. Physiol. 1998;275:G1274–G1281. doi: 10.1152/ajpgi.1998.275.6.G1274. [DOI] [PubMed] [Google Scholar]
- RATCLIFF R., EVANS M.J., CUTHBERT A.W., MACVINISH L.J., FOSTER D., ANDERSON J.R., COLLEDGE W.H. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nat. Genet. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- REDDY M.M., LIGHT M.J., QUINTON P.M. Activation of epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- SCHULTHEISS G., DIENER M. K+ and Cl− conductances in the distal colon of the rat. Gen. Pharmacol. 1998;3:337–342. doi: 10.1016/s0306-3623(97)00458-8. [DOI] [PubMed] [Google Scholar]
- SHEPPARD D.N., ROBINSON K.A. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl− channels expressed in a murine cell line. J. Physiol. 1997;503:333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH A.K., DEVOR D.C., GERLACH A.C., GONDOR M., PILEWSKI J.M., BRIDGES R.J. Stimulation of Cl− secretion by chlorzoxazone. J. Pharmacol. Exp. Ther. 2000;292:778–787. [PubMed] [Google Scholar]
- VERKMAN A.S. Development and biological applications of chloride-sensitive fluorescent indicators. Am. J. Physiol. 1990;259:C375–C388. doi: 10.1152/ajpcell.1990.259.3.C375. [DOI] [PubMed] [Google Scholar]
- WALSH K.B., LONG K.J., SHEN X. Structural and ionic determinants of 5-nitro-2-(3-phenylpropylamino)-benzoic acid block of the CFTR chloride channel. Br. J. Pharmacol. 1999;127:369–376. doi: 10.1038/sj.bjp.0702562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Z.-R., ZELTWANGER S., McCARTY N.A. Direct comparison of NPPB and DPC as probes of CFTR expressed in Xenopus oocytes. J. Memb. Biol. 2000;175:35–52. doi: 10.1007/s002320001053. [DOI] [PubMed] [Google Scholar]


