Abstract
Glibenclamide, a sulphonylurea widely used for the treatment of non-insulin-dependent diabetes mellitus, has been shown to inhibit the activities of various ATP-binding cassette (ABC) transporters. In the present study, its effects towards multidrug resistance protein 1 (MRP1), an ABC efflux pump conferring multidrug resistance and handling organic anions, were investigated.
Intracellular accumulation of calcein, an anionic dye substrate for MRP1, was strongly increased by glibenclamide in a dose-dependent manner in MRP1-overexpressing lung tumour GLC4/Sb30 cells through inhibition of MRP1-related calcein efflux. By contrast, glibenclamide did not alter calcein levels in parental control GLC4 cells. Another sulphonylurea, tolbutamide, was however without effect on calcein accumulation in both GLC4/Sb30 and GLC4 cells.
Glibenclamide used at 12.5 μM was, moreover, found to strongly enhance the sensitivity of GLC4/Sb30 cells towards vincristine, an anticancer drug handled by MRP1.
Efflux of carboxy-2′,7′-dichlorofluorescein, an anionic dye handled by the ABC transporter MRP2 sharing numerous substrates with MRP1 and expressed at high levels in liver, was also strongly inhibited by glibenclamide in isolated rat hepatocytes.
In summary, glibenclamide reversed MRP1-mediated drug resistance likely through inhibiting MRP1 activity and blocked organic anion efflux from MRP2-expressing hepatocytes. Such effects associated with the known inhibitory properties of glibenclamide towards various others ABC proteins suggest that this sulphonylurea is a general inhibitor of ABC transporters.
Keywords: Multidrug resistance protein, glibenclamide, ATP binding cassette transporters, sulphonylurea, anticancer drug resistance, hepatocytes
Introduction
Glibenclamide is a second-generation sulphonylurea which is commonly used for the treatment of non-insulin-dependent diabetes mellitus. Glibenclamide binds to the sulphonylurea receptor (SUR1) expressed by pancreatic β-cells and it subsequently blocks ATP-sensitive potassium channels, leading to the release of insulin (Babenko et al., 1998). SUR1 belongs to the ATP-binding cassette (ABC) protein superfamily that comprises other members involved in membrane transport of ions such as ABC1 also involved in cholesterol metabolism (Becq et al., 1997; Ordovas, 2000) and cystic fibrosis transmembrane conductance regulator (CFTR) acting as a chloride channel (Riordan, 1993). The ABC protein superfamily also includes a biliary acid transporter termed bile salt export pump (BSEP) (Gerloff et al., 1998) and the efflux pump P-glycoprotein (P-gp) known to confer resistance to a wide range of structurally unrelated anticancer drugs (Ambudkar et al., 1999). Glibenclamide has been recently shown to be a potent inhibitor of CFTR and ABC1 (Sheppard & Robinson, 1997; Hamon et al., 1997); this agent also inhibits BSEP activity (Stieger et al., 2000) and blocks P-gp-mediated transport (Golstein et al., 1999). Such data therefore favour the idea that glibenclamide may be a general inhibitor of ABC transporters.
Multidrug resistance proteins (MRPs) constitute an ABC transporter subfamily recently identified and containing at least six members (Borst et al., 1999). MRP1 was the first member described; it is a 190 kDa membrane protein mediating cellular export of various anticancer drugs including anthracyclines and vincristine (Lautier et al., 1996). Overexpression of MRP1, like that of P-gp, therefore confers multidrug resistance to tumour cells (Borst et al., 1999). Although the spectrum of resistance linked to MRP1 is closed to that related to P-gp, some differences have been reported. Indeed, paclitaxel is handled by P-gp but not by MRP1 whereas the latter pump, unlike the former, mediates the outwardly-directed transport of some heavy metals such as antimony (Vernhet et al., 1999) and of anionic compounds, including glutathione S-conjugates. Such anionic agents are also substrates for MRP2, a member of the MRP family predominantly expressed at the biliary pole of hepatocytes (Paulusma et al., 1996).
Several compounds have been shown to inhibit MRP1 and also, at least for some of them, MRP2 activity (Norman, 1998; Chen et al., 1999). Such agents, termed modulators or reversing agents, are likely important to identify, especially since they can sensitize cancerous cells to chemotherapeutic drugs by increasing intracellular drug accumulation through inhibition of cellular efflux. Similarly, various drugs inhibiting P-gp activity have been described and some of them have now entered clinical trials (Bradshaw & Arceci, 1998). Some P-gp modulators, such as the antiprogestatin compound RU486 and the antituberculosis compound rifampicin, also block MRP1 activity (Payen et al., 1999b; Courtois et al., 1999). In the present study, in order to identify new blockers of MRP1 activity and owing to the general inhibitory properties of glibenclamide towards ABC transporters including P-gp, we have investigated the effects of this sulphonylurea on MRP1-mediated transport in human lung cancer cells. We present evidence that glibenclamide can most likely block MRP1 activity and appears therefore as a general inhibitor of multidrug resistance efflux pumps.
Methods
Chemicals
Glibenclamide, tolbutamide, vincristine, potassium antimony tartrate (PAT) and probenecid were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Calcein acetoxymethyl ester (calcein AM) and carboxy-2′,7′-dichlorofluorescein (CF) diacetate were provided by Molecular Probes (Eugene, OR, U.S.A.) whereas [3H] vincristine (7.9 Ci mmol−1) was obtained from Amersham (Les Ulis, France).
Cell culture
The human lung cancer cell line GLC4 (kindly provided by Dr E.G.E. de Vries,) and its antimony-resistant subline GLC4/Sb30 (obtained by step-wise selection with antimony in our laboratory) were cultured in RPMI medium supplemented with 10% foetal calf serum at 37°C in a 5% CO2 atmosphere. GLC4/Sb30 cells overexpress MRP1 but not P-gp and are cross-resistant to anticancer drugs such as doxorubicin and vincristine when compared to parental GLC4 cells (Vernhet et al., 1999); they were routinely maintained in the presence of 30 μg ml−1 PAT, which was, however, withdrawn for at least 3 days before the use of the cells for experiments.
Hepatocytes from adult male Sprague Dawley rats weighing 180 – 200 g were isolated by liver perfusion as previously described (Fardel et al., 1992) except that liberase was used instead of collagenase. Hepatocytes were then maintained at 37°C in suspension in a defined medium containing (mM) NaCl 136, KCl 5.3, KH2PO4 1.1, MgSO4 0.8, CaCl2 1.8, glucose 11 and HEPES 10 and adjusted to pH 7.4.
Calcein accumulation and efflux assays
Calcein is an anionic fluorescent probe substrate for MRP1 (Hollo et al., 1996) and possibly for MRP2 (Evers et al., 2000) whereas it is handled by P-gp only under its esterified form calcein AM (Homolya et al., 1993). Analysis of calcein cellular accumulation and efflux has however been shown to permit evaluation of MRP1 activity in MRP1-positive cells that do not overexpress other multidrug resistance transporter (Feller et al., 1995; Versantvoort et al., 1995).
For calcein accumulation experiments, GLC4 and GLC4/Sb30 cells were incubated with 0.5 μM calcein AM for 90 min in the absence or presence of glibenclamide, tolbutamide or probenecid. The non-fluorescent esterified form calcein AM freely diffuses into the cells where it is cleaved by intracellular esterases to give the fluorescent anionic dye calcein (Haugland, 1996) which can be exported out of the cells through MRP1 activity (Feller et al., 1995). Cells were then washed three times with ice-cold phosphate-buffered saline (PBS) and lysed in distilled water by ultrasonication. Amounts of intracellular calcein were next determined by fluorimetry using a Spectra Max Gemini spectrofluorimeter (Molecular Devices, Sunnyvale, CA, U.S.A.); excitation and emission wavelengths were 485 nm and 538 nm, respectively. An aliquot of cell lysate was used in parallel to determine cellular protein content by the Bio-Rad assay (Bradford, 1976). Results were expressed as fluorescence arbitrary units after normalization to cellular protein content.
For calcein efflux assays, calcein-preloaded cells were incubated in calcein AM-free medium for 90 min in the absence or presence of glibenclamide. Cells were then washed three times with PBS and intracellular calcein retained was then determined by fluorimetry as described above. Results were expressed as percentages of initial calcein staining.
CF efflux studies
CF is an anionic fluorescent probe substrate for MRP1 and MRP2 (Kitmura et al., 1990; Laupeze et al., 1999). Its cellular efflux from hepatocytes has however been postulated to be related rather to MRP2 activity; indeed, hepatocytes display very low, if any, expression of MRP1 whereas they highly express MRP2, thus making negligible the role of MRP1 in CF secretion out of such cells (Payen et al., 2000).
Freshly isolated rat hepatocytes were first loaded with 4 μM CF diacetate for 30 min. This non-fluorescent esterified form of CF freely diffuses into cells where it is cleaved by intracellular esterases to give the fluorescent anionic dye CF (Haugland, 1996) which can be exported out of cells through MRP2 activity (Kitmura et al., 1990). After washing with ice-cold PBS, isolated hepatocytes were re-incubated in CF diacetate-free medium in the presence or absence of glibenclamide, tolbutamide or probenecid. Cells were then washed three times with PBS and intracellular CF retained was then determined by fluorimetry as described above. Results were expressed as percentages of initial CF staining.
Intracellular vincristine accumulation
GLC4 and GLC4/Sb30 cells were incubated with 20 nM [3H]-vincristine for 90 min in the presence or absence of glibenclamide. Cells were then washed three times with ice-cold PBS and lysed in 0.1 M NaOH. Radioactivity in cell extracts was then determined by scintillation counting and normalized to cellular protein content.
Drug-sensitivity assays
The effects of cytotoxic compounds on GLC4 and GLC4/Sb30 cell proliferation was evaluated using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay as previously described (Carmichael et al., 1987). Briefly, cells were cultured with various concentrations of vincristine or PAT for 96 h in the presence or absence of 12.5 μM glibenclamide in 96-well microplates. 100 μl of a 1 mg ml−1 MTT solution was then added to each well for 120 min. The medium was next discarded and replaced by 100 μl of dimethyl sulphoxide. The blue formazan product formed was further quantified by its absorbance at 540 nm using a Titertek Multiskan MCC/340 (Flow Laboratories, Puteaux, France). Growth inhibition was evaluated as IC50, i.e. the drug concentration providing a 50% reduction in absorbance as compared to controls cultured in parallel without cytotoxic compound.
RNA isolation and analysis
Total RNA was extrated from GLC4 and GLC4/Sb30 cells and from isolated hepatocytes by a guanidinium-thiocyanate/cesium chloride method (Chirgwin et al., 1979). RNA (10 μg) was subjected to electrophoresis in a denaturing formaldehyde/agarose gel and transferred onto Hybond-N+ sheets (Amersham). The sheets were then hybridized with human or rat MRP1 and MRP2 cDNA probes as previously described (Payen et al., 1999a; 2000), washed, dried and autoradiographed at −80°C. Equal gel loading and efficiency of transfer were checked using an 18S rRNA probe.
Statistical analysis
Data were analysed by the student's t-test or by two-way ANOVA followed by a Dunnett's test. The criterion of significance was P<0.05.
Results
The GLC4/Sb30 and GLC4 cells used in the study were firstly characterized with respect to expression of MRP1 and MRP2 transporters. In agreement with previous immunoblotting data (Vernhet et al., 1999), GLC4/Sb30 cells were found to display a marked MRP1 gene overexpression when compared to GLC4 cells (Figure 1). By contrast, MRP2 gene expression was not detected in both GLC4 and GLC4/Sb30 cells whereas it was clearly found in isolated human hepatocytes used here as positive controls (Figure 1).
Figure 1.
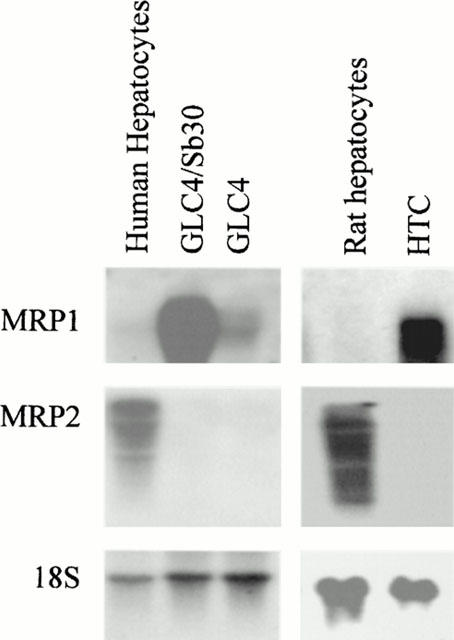
MRP1 and MRP2 gene expression in GLC4/Sb30 and GLC4 cells and in isolated hepatocytes. Each well contains 10 μg total RNA isolated from GLC4 and GLC4/Sb30 cells, from rat and human hepatocytes and from MRP1-overexpressing rat hepatoma HTC cells. RNA was transferred onto Hybond-N+ sheets and hybridized with MRP1, MRP2 and 18S probes.
The effects of several concentrations of glibenclamide on the accumulation of the MRP1 substrate calcein were then investigated in GLC4/Sb30 cells and in their parental counterparts GLC4 cells. As indicated in Figure 2, the sulphonylurea increased calcein levels in GLC4/Sb30 cells in a dose-dependent manner. The effect of the sulphonylurea started for a concentration of 3.12 μM and the use of higher concentrations (50 – 100 μM) allowed to increase calcein levels by approximately 4 fold and thus to reach the dye levels observed in parental GLC4 cells. By contrast, lower doses of glibenclamide (0.39 – 1.56 μM) were found to have no effect (Figure 2). None of the glibenclamide concentrations tested over the 90-min incubation period affected cell viability as assessed by MTT assays and determination of cellular protein content (data not shown). In contrast to GLC4/Sb30 cells, GLC4 cells showed no or only minimal alteration in calcein-related fluorescence in response to glibenclamide used at 25, 50 or 100 μM (Figure 2).
Figure 2.
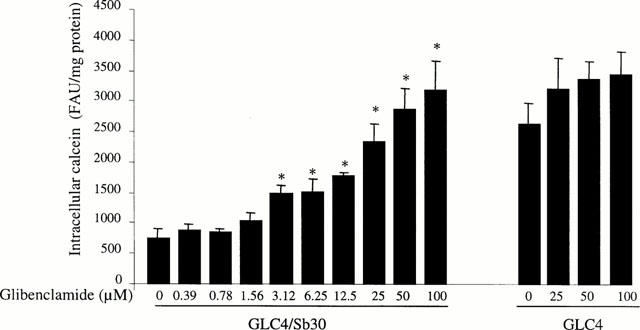
Effects of various concentrations of glibenclamide on calcein accumulation in drug-resistant GLC4/Sb30 and drug-sensitive GLC4 cells. Cells were incubated with 0.5 μM calcein AM in the presence of various concentrations of glibenclamide (from 0 to 100 μM). Intracellular calcein accumulation was then determined by fluorimetry. The values are expressed as fluorescence arbitrary units (FAU) normalized to protein content and are the mean±s.d.mean of at least three independent experiments in triplicate. *P<0.05 when compared to cells not treated by glibenclamide.
Glibenclamide was then compared to tolbutamide, another sulphonylurea interacting with SUR1, and to probenecid, used here as a reference compound inhibiting activity of MRP proteins (Bakos et al., 2000), with respect to their effects on calcein accumulation in GLC4/Sb30 cells. As shown in Figure 3, 50 μM glibenclamide and 5 mM probenecid enhanced cellular dye accumulation in the MRP1-overexpressing cells in a similar manner. By contrast, tolbutamide used at 500 μM failed to alter cellular levels of calcein (Figure 3).
Figure 3.
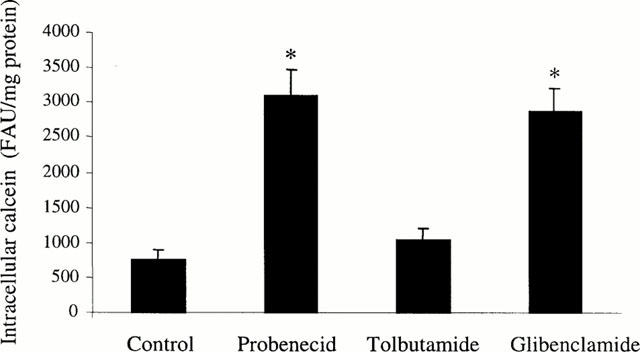
Effect of glibenclamide, tolbutamide and probenecid on calcein accumulation in MRP1-overexpressing GLC4/Sb30 cells. Cells were incubated with 0.5 μM calcein AM in the absence or presence of 50 μM glibenclamide, 500 μM tolbutamide or 5 mM probenecid. Intracellular calcein accumulation was then determined by fluorimetry. The values are expressed as fluorescence arbitrary units (FAU) normalized to protein content and are the mean±s.d.mean of at least three independent experiments in triplicate. *P<0.05 when compared to control cells.
Efflux experiments were thereafter performed in order to establish whether glibenclamide-related changes in calcein accumulation in GLC4/Sb30 cells were due to down-modulation of the cellular export of the dye. As indicated in Figure 4, GLC4/Sb30 cells poorly retained calcein when compared to their parental GLC4 cells and such a loss of dye was found to be strongly inhibited by glibenclamide. By contrast, the sulphonylurea did not, or only marginally, alter calcein retention in GLC4 cells.
Figure 4.
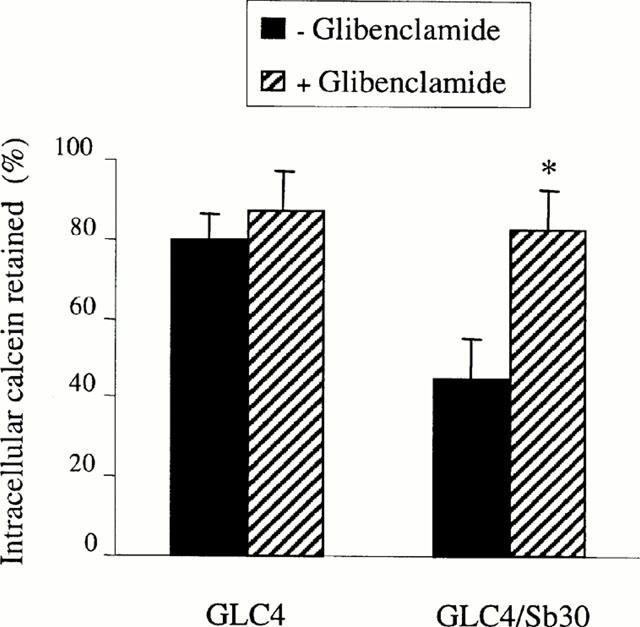
Effect of glibenclamide on calcein retention in GLC4/Sb30 and GLC4 cells. Calcein-loaded cells were incubated in calcein AM-free medium for 90 min in the absence or presence of 50 μM glibenclamide. Intracellular retained calcein was then determined by fluorimetry and expressed as per cent of initial dye staining. The values are the mean±s.d.mean of at least three independent experiments in triplicate. *P<0.05 when compared to cells not treated by glibenclamide.
To determine whether glibenclamide may alter cellular retention of MRP1 substrates distinct from calcein, intracellular accumulation of the anticancer drug vincristine was further determined in the presence or absence of the sulphonylurea. As shown in Figure 5, glibenclamide was found to strongly enhance vincristine accumulation in GLC4/Sb30 cells whereas it had no effect in GLC4 cells. Cellular amounts of the anticancer drug were therefore similar in glibenclamide-treated GLC4/Sb30 cells and in GLC4 cells whereas the resistant cells poorly accumulated vincristine in the absence of the sulphonylurea when compared to their parental drug-sensitive cells (Figure 5).
Figure 5.
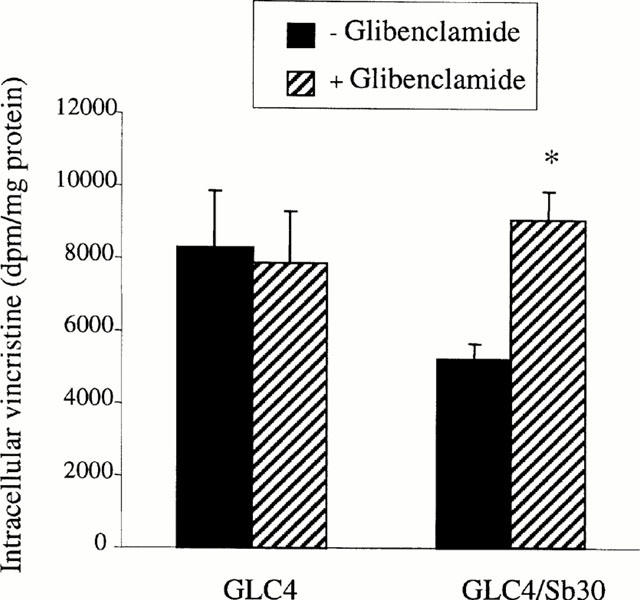
Effect of glibenclamide on [3H]-vincristine accumulation in GLC4/Sb30 and GLC4 cells. Cells were incubated with 20 nM [3H]-vincristine for 90 min in the presence or absence of 50 μM glibenclamide. Intracellular [3H]-vincristine accumulation was then determined by scintillation counting. The values are the mean±s.d.mean of at least three independent experiments in triplicate. *P<0.05 when compared to cells not treated by glibenclamide.
The effects of prolonged glibenclamide exposure (96 h) on cell proliferation were then monitored using the MTT assay. Glibenclamide IC50 values were found to be 26±9 μM (n=7) and 22±4 μM (n=7) for GLC4/Sb30 cells and GLC4 cells, respectively. Interestingly, the dose of 12.5 μM, active on MRP1-mediated transport of calcein (Figure 2), was found to not alter growth of both GLC4/Sb30 cells and GLC4 cells and it was further retained for reversing experiments. In such assays, glibenclamide was evidenced to down-modulate resistance of GLC4/Sb30 cells towards PAT and vincristine. Indeed, PAT and vincristine IC50 values for GLC4/Sb30 cells in the presence of glibenclamide were reduced by 10.5 and 5.5 fold, respectively (Table 1). By contrast, the sulphonylurea did not alter the cytotoxicity of PAT and vincristine towards GLC4 cells.
Table 1.
Effects of glibenclamide on PAT and vincristine cytotoxicity

Finally, we examined the effect of glibenclamide on CF efflux from freshly isolated rat hepatocytes. As previously reported (Paulusma et al., 1996; Keppler & König, 1997; Kauffmann et al., 1997), such cells were found to express MRP2 mRNAs, whereas they displayed no or only a very low MRP1 gene expression in contrast to MRP1-overexpressing rat hepatoma HTC cells (Figure 1). As indicated in Figure 6, 50 μM glibenclamide was shown to markedly enhance cellular retention of the anionic dye in hepatocytes; in a similar manner, probenecid, recently found to interact with MRP2 activity (Bakos et al., 2000), increased CF levels, whereas tolbutamide used at 500 μM was uneffective.
Figure 6.
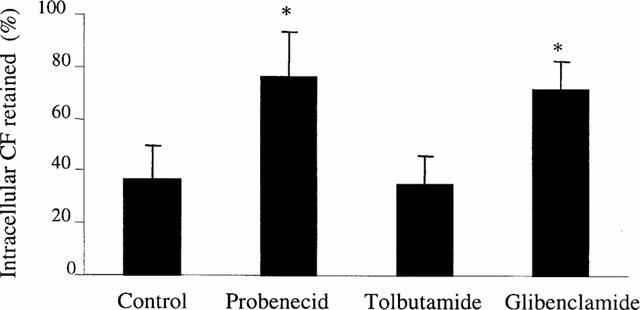
Effect of glibenclamide on CF efflux from isolated rat hepatocytes CF-loaded isolated rat hepatocytes were incubated in CF diacetate-free medium in the presence or absence of 50 μM glibenclamide, 500 μM tolbutamide or 5 mM probenecid for 90 min. Intracellular retained CF was then determined by fluorimetry and expressed as per cent of initial dye staining. The values are the mean±s.d.mean of at least three independent experiments in triplicate. *P<0.05 when compared to cells not treated by glibenclamide.
Discussion
Previous studies have shown that the sulphonylurea glibenclamide blocks activity of numerous ABC transporters, including the P-gp multidrug transporter involved in anticancer drug resistance (Golstein et al., 1999). The results reported in the present study demonstrated that glibenclamide also inhibits activity of MRP1, another ABC transporter conferring multidrug resistance. Indeed, the sulphonylurea was shown to increase intracellular accumulation of calcein and vincristine, two known substrates of MRP1, in MRP1-overexpressing GLC4/Sb30 cells. Such an effect was most likely related to inhibition of MRP1-mediated drug efflux since glibenclamide was demonstrated to down-modulate calcein export out of GLC4/Sb30 cells and since GLC4/Sb30 cells have been found to overexpress neither MRP2 nor P-gp (Vernhet et al., 1999), thus discarding a role for a putative glibenclamide-related inhibition of these ABC transporters in such cells. It is also noteworthy that GLC4/Sb30 cells failed to display increased MRP3 or MRP5 gene expression when compared to parental GLC4 cells (Vernhet et al., unpublished data). In addition, glibenclamide strongly decreased resistance of GLC4/Sb30 cells towards compounds known to be handled by MRP1 such as vincristine and PAT as demonstrated by MTT assays. By contrast, glibenclamide did not obviously alter calcein and vincristine accumulation in parental GLC4 cells; it also did not alter sensitivities of such cells towards PAT and vincristine. Such data likely indicate that the effects of the sulphonylurea in GLC4/Sb30 cells are linked to the MRP1 overexpression displayed by these cells. This conclusion is moreover supported by the fact that glibenclamide also blocked calcein efflux from other MRP1-overexpressing cells such as MRP2-negative GLC4/ADR cells generated by stepwise selection with doxorubicin (Zaman et al., 1993; Kool et al., 1997) and HL60/130 cells obtained by treatment with vincristine (Lehne et al., 1999) (data not shown).
In addition to blocking MRP1 activity, glibenclamide was demonstrated to markedly inhibit efflux of the anionic dye CF from freshly isolated rat hepatocytes. Such cells were found to express at high levels MRP2 that handles CF (Kitmura et al., 1990). MRP1 has also been recently shown to transport CF (Laupeze et al., 1999); however, isolated hepatocytes were demonstrated to display no, or only very low, MRP1 gene expression, in agreement with previous reports (Kauffmann et al., 1997). This makes unlikely a major role of MRP1 in CF efflux in such liver cells; this conclusion is fully supported by the absence of CF secretion from hepatocytes of mutant TR- rats lacking MRP2 expression (Kitmura et al., 1990). In addition, rat hepatocytes have been demonstrated to not, or only very poorly, express the MRP transporter MRP3 (Hirohashi et al., 1998). Taken together, these data strongly support the conclusion that glibenclamide may inhibit CF efflux from rat hepatocytes through blocking MRP2 activity and may therefore be included in the compounds such as cyclosporin A and the leukotriene receptor antagonist MK571 that down-regulate both MRP1- and MRP2-mediated drug transports (Chen et al., 1999). Interestingly, we have recently found that glibenclamide down-modulates CF efflux from primary human hepatocytes, thus suggesting that the sulphonylurea likely also interacts with MRP2 in such human cells (Payen et al., 2000).
The mechanism by which glibenclamide inhibits MRP1 and MRP2 activity remains to determine. The fact that tolbutamide used at 500 μM failed to alter both calcein accumulation in GLC4/Sb30 cells and CF efflux from rat hepatocytes argues against a general inhibitory effect of sulphonylureas and suggests that structural features restricted to glibenclamide may be required to block MRP1- and MRP2-mediated transport. Sensitivities of GLC4/Sb30 and GLC4 cells towards glibenclamide were quite similar as assessed by MTT assays, suggesting that glibenclamide is not, or only poorly, transported by MRP1. Such data therefore likely indicate that glibenclamide-mediated inhibition of MRP1-related drug efflux is not due to a competitive process between substrates for the ABC transporter. It is possible, however, that glibenclamide may bind to the efflux pump without being effluxed and subsequently block its activity. In this context, it is noteworthy that the sulphonylurea has already been reported to interfere with drug binding sites of P-gp as demonstrated by photoaffinity-labelling experiments of this pump (Golstein et al., 1999). Similarly, a high affinity binding site for glibenclamide has been localized on SUR1 (Ashfield et al., 1999). Further studies are required to validate or not the direct interaction of glibenclamide with MRP1 and/or MRP2 and to identify the incriminated regions of the pumps.
As for many modulators of drug efflux pumps, the concentrations of glibenclamide that inhibit MRP1 activity, namely 3.12 – 100 μM, appear to be rather higher than those usually achievable in patients without adverse effects. In particular, the 12.5 μM concentration of glibenclamide used in the present study in reversing experiments is higher than plasma concentrations usually observed in patients; indeed, such therapeutic concentrations which have hypoglycaemic effects do not overrun 1 μM (Rydberg et al., 1997). These data most likely preclude the clinical use of glibenclamide as a reversing agent inhibiting MRP1 activity. Glibenclamide derivatives blocking MRP1-mediated transport and devoid of hypoglycaemic properties may however be interesting to develop in order to obtain new modulators of MRP1 function potentially acting without inducing toxic effects. Interestingly, the concentrations of glibenclamide interfering with MRP1 are closed to those inhibiting ABC proteins such as P-gp, CFTR, ABC1 and BSEP (Sheppard & Robinson, 1997; Hamon et al., 1997; Stieger et al., 2000); they have also been reported to inhibit peptide transporters (Sawada et al., 1999) and choline influx (Kirk et al., 1993). Such data clearly highlight the multiple interferences of glibenclamide used at rather elevated concentrations with membrane transporters
In summary, our results demonstrated that the sulphonylurea inhibits MRP1-mediated drug transport and enhances the sensitivity of MRP1-overexpressing cells to cytotoxic compounds. It also blocks efflux of organic anion such as the dye CF from MRP2-expressing hepatocytes. These data therefore clearly support the conclusion that glibenclamide, already known to inhibit various ABC proteins such as SUR1, ABC1, CFTR, BSEP and P-gp, is a general inhibitor of ABC transporters, including multidrug resistance efflux pumps.
Acknowledgments
Léa Payen and Arnaud Courtois are in receipt of fellowships from the Ligue Nationale contre le Cancer and the Association pour la Recherche sur le Cancer, respectively. This work was supported by the Ligue Nationale contre le Cancer (Comité d'Ille et Vilaine). We wish to thank Dr V. Lecureur and Dr L. Vernhet for critical reading of the manuscript, Dr E.G.E. de Vries for the gift of GLC4 and GLC4/ADR cells and Dr G. Lehne for the gift of the HL60/130 cells.
Abbreviations
- ABC
ATP-binding cassette
- BSEP
bile salt export pump
- calcein AM
calcein acetomethoxy
- CF
carboxy-2′,7′-dichlorofluorescein
- CFTR
cystic fibrosis transmembrane conductance regulator
- MRP
multidrug resistance protein
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- PAT
potassium antimonyl tartrate
- P-gp
P-glycoprotein
- SUR
sulphonylurea receptor
References
- AMBUDKAR A.S.V., DEY S., HRYCYNA C.A., RAMACHANDRA M., PASTAN I., GOTTESMAN M.M. Biochemical, cellular and pharmcological aspects of the multidrug transporter. Ann. Rev. Pharmacol. Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- ASHFIELD R., GRIBBLE F.M., ASHCROFT S.J., ASHCROFT F.M. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the K(ATP) channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- BABENKO A.P., AGUILAR-BRYAN L., BRYAN J. A view of SUR K1R6.X KATP channels. Ann. Rev. Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- BAKOS E., EVERS R., KO E., VARADI A., BORST P., SARKADI B. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol. Pharmacol. 2000;57:760–768. doi: 10.1124/mol.57.4.760. [DOI] [PubMed] [Google Scholar]
- BECQ F., HAMON Y., BAJETTO A., GOLA M., VERRIER B., CHIMINI G. ABC1, an ATP binding cassette transporter required for phagocytosis of apoptotic cells, generates a regulated anion flux after expression in Xenopus laevis oocytes. J. Biol. Chem. 1997;272:2695–2699. doi: 10.1074/jbc.272.5.2695. [DOI] [PubMed] [Google Scholar]
- BORST P., EVERS R., KOOL M., WIJNHOLDS J. The multidrug resistance protein family. Biochim. Biophys. Acta. 1999;1461:347–357. doi: 10.1016/s0005-2736(99)00167-4. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRADSHAW D.M., ARCECI R.J. Clinical relevance of transmembrane drug efflux as a mechanism of multidrug resistance. J. Clin. Oncol. 1998;16:3674–3690. doi: 10.1200/JCO.1998.16.11.3674. [DOI] [PubMed] [Google Scholar]
- CARMICHAEL J., DEGRAAF W.G., GAZDAR A.F., MINNA J.D., MITCHELL J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- CHEN Z.S., KAWABE T., ONO M., AOKI S., SUMIZAWA T., FURUKAWA T., UCHIUMI T., WADA M., KUWANO M., AKIYAMA S.I. Effect of multidrug reversing agents on transporting activity of human canalicular multispecific organic anion transporter. Mol. Pharmacol. 1999;56:1219–1228. doi: 10.1124/mol.56.6.1219. [DOI] [PubMed] [Google Scholar]
- CHIRGWIN J.M., PRZYBYLA A.E., MACDONALD R.J., RUTTER W.J. Isolation of biologically active ribonucleic acid from source enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- COURTOIS A., PAYEN L., VERNHET L., DE VRIES E.G.E., GUILLOUZO A., FARDEL O. Inhibition of multidrug resistance-associated protein (MRP) activity by rifampicin in human multidrug-resistant lung tumor cells. Cancer Lett. 1999;139:97–104. doi: 10.1016/s0304-3835(99)00024-5. [DOI] [PubMed] [Google Scholar]
- EVERS R., KOOL M., SMITH A.J., VAN DEEMTER L., DE HAAS M., BORST P. Inhibitory effect of the reversal agents V-104, GF120918 and pluronic L61 on MDR1 P-gp-, MRP1- and MRP2-mediated transport. Br. J. Cancer. 2000;83:366–374. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARDEL O., RATANASAVANH D., LOYER P., KETTERER B., GUILLOUZO A. Overexpression of the multidrug resistance gene product in adult rat hepatocytes during primary culture. Eur. J. Biochem. 1992;205:847–852. doi: 10.1111/j.1432-1033.1992.tb16849.x. [DOI] [PubMed] [Google Scholar]
- FELLER N., BROXTERMAN H.J., WAHRER D.C.R., PINEDO H.M. ATP-dependent efflux of calcein by the multidrug resistance protein (MRP): no inhibition by intracellular glutathione depletion. FEBS Lett. 1995;368:385–388. doi: 10.1016/0014-5793(95)00677-2. [DOI] [PubMed] [Google Scholar]
- GERLOFF T., STIEGER B., HAGENBUCH B., MADON J., LANDMANN L., ROTH J., HOFFMANN A.F., MEIER P.J. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J. Biol. Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- GOLSTEIN P.E., BOOM A., VAN GEFFEL J., JACOBS P., MASEREEL B., BEAUWENS R. P-glycoprotein inhibition by glibenclamide and related compounds. Pflügers Arch. 1999;437:652–660. doi: 10.1007/s004240050829. [DOI] [PubMed] [Google Scholar]
- HAMON Y., LUCIANI M.F., BECQ F., VERRIER B., RUBARTELLI A., CHIMINI G. Interleukin-1β secretion is impaired by inhibitors of the Atp binding cassette transporter ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- HAUGLAND R.P.Probes for for live-cell function Handbook of Fluorescent Probes and Research Chemicals 1996Eugene: Molecular Probes; 377–398.Spence, M.T.Z. (ed.) [Google Scholar]
- HIROHASHI T., SUZUKI H., ITO K., OGAWA K., KUME K., SHIMIZU T., SUGIYAMA Y. Hepatic expression of multidrug resistance-associated protein-like proteins maintained in Eisai hyperbilirubinemic rats. Mol. Pharmacol. 1998;53:1068–1075. [PubMed] [Google Scholar]
- HOLLO Z., HOMOLYA L., HEGEDUS T., SARKADI B. Transport properties of the multidrug resistance-associated protein (MRP) in human tumour cells. FEBS Lett. 1996;383:99–104. doi: 10.1016/0014-5793(96)00237-2. [DOI] [PubMed] [Google Scholar]
- HOMOLYA L., HOLLO Z., GERMANN U.A., PASTAN I., GOTTESMAN M.M., SARKADI B. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J. Biol. Chem. 1993;268:21493–21496. [PubMed] [Google Scholar]
- KAUFFMANN H.M., KEPPLER D., KARTENBECK J., SCHRENK D. Induction of cmrp/cMoat gene expression by cisplatin, 2-acetylaminofluorene, or cycloheximide in rat hepatocytes. Hepatology. 1997;26:980–985. doi: 10.1002/hep.510260427. [DOI] [PubMed] [Google Scholar]
- KEPPLER D., KONIG J. Expression and localization of the conjugate export pump encoded by the MRP2 (cMRP/cMOAT) gene in liver. FASEB J. 1997;11:509–516. doi: 10.1096/fasebj.11.7.9212074. [DOI] [PubMed] [Google Scholar]
- KIRK K., HORNER H.A., SPILLET D.J., ELFORD B.C. Glibenclamide and meglitinide block the transport of low molecular weight solutes into malaria-infected eryhtrocytes. FEBS Lett. 1993;323:123–128. doi: 10.1016/0014-5793(93)81462-9. [DOI] [PubMed] [Google Scholar]
- KITMURA T., JANSEN P., HARDENBROOK C., KAMIMOTO Y., GATMAITAN Z., ARIAS I.M. Defective ATP-dependent bile canalicular transport of organic anions in mutant (TR-) rats with conjugated hyperbilirubinemia. Proc. Natl. Acad. Sci. U.S.A. 1990;87:3557–3561. doi: 10.1073/pnas.87.9.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOL M., DE HAAS M., SCHEFFER G.L., SCHEPER R.J., Van EIJK M.J.T., JUIJN J.A., BAAS F., BORST P. Analysis of expression of cMOAT(MRP2), MRP3, MRP4 and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1) in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- LAUPEZE B., AMIOT L., COURTOIS A., VERNHET L., DRENOU B., FAUCHET R., FARDEL O. Use of the anionic dye carboxy-2′-dichlorofluorescein for sensitive flow cytometric detection of multidrug resistance-associated protein activity. Int. J. Oncol. 1999;15:571–576. doi: 10.3892/ijo.15.3.571. [DOI] [PubMed] [Google Scholar]
- LAUTIER D., CANITROT Y., DEELEY R.G., COLE S.P.C. Multidrug resistance mediated by the multidrug resistance protein (MRP) gene. Biochem. Pharmacol. 1996;52:227–233. doi: 10.1016/0006-2952(96)00450-9. [DOI] [PubMed] [Google Scholar]
- LEHNE G., DE ANGELIS P., DEN BOER M., RUGSTAD H.E. Growth inhibition, cytokinesis failure and apoptosis of multidrug-resistant leukemia cells after treatment with P-glycoprotein inhibitory agents. Leukemia. 1999;13:768–778. doi: 10.1038/sj.leu.2401392. [DOI] [PubMed] [Google Scholar]
- NORMAN B.H. Inhibitors of MRP1-mediated multidrug resistance. Drugs Fut. 1998;23:1001–1013. [Google Scholar]
- ORDOVAS J.M. ABC1: the gene for Tangier disease and beyond. Nutr. Rev. 2000;58:76–79. doi: 10.1111/j.1753-4887.2000.tb01843.x. [DOI] [PubMed] [Google Scholar]
- PAULUSMA C.C., BOSMA P.J., ZAMAN G.J., BAKKER C.T., OTTER M., SCHEFFER G.L., SCHEPER R.J., BORST P., OUDE ELFERINK R.P. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science. 1996;271:1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- PAYEN L., COURTOIS A., CAMPION J.P., GUILLOUZO A., FARDEL O. Characterization and inhibition by a wide range of xenobiotic of organic anion excretion by primary human hepatocytes. Biochem. Pharmacol. 2000;60:1967–1975. doi: 10.1016/s0006-2952(00)00496-2. [DOI] [PubMed] [Google Scholar]
- PAYEN L., COURTOIS A., VERNHET L., GUILLOUZO A., FARDEL O. The multidrug resistance-associated protein (MRP) is over-expressed and functional in rat hepatoma cells. Int. J. Cancer. 1999a;81:479–485. doi: 10.1002/(sici)1097-0215(19990505)81:3<479::aid-ijc24>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- PAYEN L., DELUGIN L., COURTOIS A., TRINQUART Y., GUILLOUZO A., FARDEL O. Reversal of MRP-mediated multidrug resistance in human lung cancer cells by the antiprogestatin drug RU486. Biochem. Biophys. Res. Commun. 1999b;258:513–518. doi: 10.1006/bbrc.1999.0671. [DOI] [PubMed] [Google Scholar]
- RIORDAN J.R. The cystic fibrosis transmembrane conductance regulator. Ann. Rev. Physiol. 1993;55:609–630. doi: 10.1146/annurev.ph.55.030193.003141. [DOI] [PubMed] [Google Scholar]
- RYDBERG T., JONSSON A., KARLSSON M.O., MELANDER A. Concentration effect relations of glibenclamide and its active metabolites in man: modelling of pharmacokinetics and pharmacodynamics. Br. J. Clin. Pharmacol. 1997;43:373–381. doi: 10.1046/j.1365-2125.1997.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWADA K., TERADA T., SAITO H., HASHIMOTO Y., INUI K.I. Effects of glibenclamide on glycylsarcosine transport by the rat peptide transporters PEPT1 and PEPT2. Br. J. Pharmacol. 1999;128:1159–1164. doi: 10.1038/sj.bjp.0702895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPPARD D.N., ROBINSON K.A. Mechanism of glibenclamide inhibition of cystic fibrosis transmembrane conductance regulator Cl- channels expressed in a murine cell line. J. Physiol. 1997;503:333–346. doi: 10.1111/j.1469-7793.1997.333bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STIEGER B., FATTINGER K., MADON J., KULLAK-UBLIK G.A., MEIER P.J. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422–430. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- VERHNET L., COURTOIS A., ALLAIN N., PAYEN L., ANGER J.P., GUILLOUZO A., FARDEL O. Overexpression of the multidrug resistance-associated protein (MRP1) in human heavy metal-selected tumor cells. FEBS Lett. 1999;443:321–325. doi: 10.1016/s0014-5793(98)01716-5. [DOI] [PubMed] [Google Scholar]
- VERSANTVOORT C.H.M., BAGRIJ T., WRIGHT K.A., TWENTYMAN P.R. On the relationship between the probenecid-sensitive transport of daunorubicin or calcein and the glutathione status of cells overexpressing the multidrug resistance-associated protein (MRP) Int. J. Cancer. 1995;63:855–862. doi: 10.1002/ijc.2910630617. [DOI] [PubMed] [Google Scholar]
- ZAMAN G.J.R., VERTSANTVOORT C.H.M., SMIT J.J.M., EIJDEMS E.W.H.M., de HAAS M., SMITH A.J., BROXTERMAN H.J., MULDER N.H., de VRIES E.G.E., BAAS F., BORST P. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines. Cancer Res. 1993;53:1747–1750. [PubMed] [Google Scholar]


