Abstract
This study investigated the mechanism of prolonged relaxation to ATP in the rat isolated perfused mesenteric arterial bed.
In methoxamine pre-constricted preparations, ATP elicited dose-dependent, endothelium-dependent, rapid relaxation at 5 pmol – 0.05 μmol (Rmax 76±5.6%, pD2 9.2±0.2), and contraction, followed by prolonged endothelium-independent vasorelaxation at 0.05, 0.5 and 5 μmol (56±3.0, 87±2.9 and 85±4.6%). Suramin (100 μM), attenuated rapid (pD2 7.8±0.1) and prolonged relaxation to ATP. The selective P2 receptor antagonist PPADS (10 μM) reduced prolonged, but not rapid relaxation. Neither phase of relaxation was affected by 8-sulphophenyltheophylline (1 μM) or indomethacin (10 μM).
α,β-methylene ATP (α,β-meATP; 10 μM) attenuated prolonged relaxation to ATP (relaxations at 0.05 and 0.5 μmol were 25±8.3 and 48±9.0%, respectively). α,β-meATP blocked contractions and revealed rapid relaxation to ATP at 0.05 – 5 μmol.
Capsaicin pre-treatment did not affect either phase of vasorelaxation to ATP. α,β-meATP (10 μM) had no effect on vasorelaxation mediated by electrical stimulation of capsaicin-sensitive sensory nerves.
High K+ (25 mM) attenuated prolonged relaxation to ATP (21±2.6 and 64±5.8%, at 0.05 and 0.5 μmol, respectively), but had no effect on rapid relaxation. Ouabain (1 mM), an inhibitor of Na+/K+-ATPase, and glibenclamide (10 μM), an inhibitor of KATP channels, also attenuated prolonged relaxation to ATP. Charybdotoxin (100 nM), a selective inhibitor of KCa channels, and tetraethylammonium (10 mM) had no effect on rapid or prolonged relaxations.
These results show that the prolonged phase of vasorelaxation to ATP in the rat isolated mesenteric arterial bed, which may be mediated by P2Y receptors, is endothelium-independent, involves activation of Na+/K+-ATPase and KATP channels, and is inhibited by α,β-meATP. Neither prolonged nor rapid vasorelaxation to ATP involves capsaicin-sensitive sensory nerves, adenosine P1 receptors, prostanoids or KCa channels.
Keywords: P2 purine receptors; ATP; α,β-methylene ATP; primary afferent neurotransmission; rat mesenteric arterial bed; capsaicin
Introduction
P2 receptors for purine and pyrimidine nucleotides are widely distributed in the cardiovascular system and play an important role in the regulation of vascular tone (Olsson & Pearson, 1990; Boarder & Hourani, 1998; Ralevic & Burnstock, 1998). There are two main classes of P2 receptors; P2X receptors, which are ligand-gated cation channels, and P2Y receptors, which couple to G proteins (Abbracchio & Burnstock, 1994; Fredholm et al., 1994; Ralevic & Burnstock, 1998). In most blood vessels P2X receptors are present on the smooth muscle and mediate a rapidly desensitizing vasocontractile response. P2X1 is the principal isoform of P2X receptor present in vascular smooth muscle (Collo et al., 1996), but there is also evidence for the expression of mRNA for P2X2 and P2X4 (Nori et al., 1997). P2Y1 and P2Y2 receptors are present on the endothelium and mediate vasorelaxation, and P2Y2, P2Y4 and P2Y6 receptors are expressed on the vascular smooth muscle and mediate vasoconstriction (von Kügelegen & Starke, 1990; Erlinge et al., 1998; Ralevic & Burnstock, 1998). The subtype identity of the vasorelaxant P2Y-like receptor expressed on some vascular smooth muscle (see Ralevic & Burnstock, 1998) has not been determined.
In addition, there is evidence for P2X receptor expression on primary afferent nerves. Primary afferent nerves are widely distributed in the cardiovascular system and have a role in relaying information concerning the periphery to the central nervous system. Their peripheral terminals can also be activated directly by a variety of physical and chemical stimuli, which evoke release of sensory neurotransmitter and elicit a motor response (Maggi & Meli, 1988). It is known that P2X receptors (principally P2X2 and P2X3 isoforms) are expressed in dorsal root ganglia neurones (Guo et al., 1999; Ueno et al., 1999) and that ATP can excite primary afferents, via P2X receptors, indicating a role in nociception (Burnstock & Wood, 1996; Cook et al., 1997). Nociceptive effects mediated by P2X receptors on capsaicin-sensitive primary afferent neurones have been observed in a number of preparations including rat hindpaw (Bland-Ward & Humphrey, 1997) and knee joint (Dowd et al., 1998). Activation of P2X receptors expressed on primary afferent nerves of rat mesenteric arteries mediates an increase in neuronal activity (Kirkup et al., 1999).
ATP, at high doses, has been observed to evoke biphasic vasorelaxation in the rat isolated mesenteric arterial bed, comprising an initial rapid relaxation, followed by a second slow and prolonged response (Stanford & Mitchell, 1998). Rapid relaxation to ATP in this preparation is mediated by activation of P2Y receptors on the endothelium (Ralevic & Burnstock, 1988; 1996) but the mechanism underlying prolonged vasorelaxation to ATP is unclear. Interestingly, the profile of the prolonged phase of ATP vasorelaxation is similar to that of calcitonin gene-related peptide (CGRP), the principal sensory motor neurotransmitter in many blood vessels including rat mesenteric arteries (Kawasaki et al., 1988). This raises the possibility that ATP activation of P2X receptors on primary afferent nerves can mediate an efferent function involving CGRP release and prolonged vasorelaxation.
The present study investigated the mechanism of prolonged vasorelaxation to ATP in the rat isolated mesenteric arterial bed. A possible role of capsaicin-sensitive primary afferent nerves was investigated in mesenteric arterial beds pre-treated with capsaicin in order to cause selective desensitization and/or depletion of sensory neurotransmitter (Ralevic et al., 2000). In addition, the selective P2X receptor agonist and desensitizing agent, α,β-methylene ATP (α,β-meATP) was used in order to test the possible involvement of neuronal and/or smooth muscle P2X receptors in mediation/modulation of vasorelaxation to ATP, and in modulation of neurogenic vasorelaxation to electrical stimulation of sensory nerves. Finally, the possible involvement of adenosine P1 receptors, prostanoids, K+ channels and Na+/K+-ATPase in vasorelaxation to ATP was investigated. A preliminary account of some of these findings has been reported to the British Pharmacological Society (Ralevic, 2000).
Methods
Male Wistar rats (250 – 300 g) were killed by exposure to CO2 and decapitation. Mesenteric beds were isolated and perfused, via the superior mesenteric artery, as described previously (Ralevic et al., 1996). In brief, the abdomen was opened and the superior mesenteric artery exposed and cannulated with a hypodermic needle. The superior mesenteric vein was cut, blood flushed from the preparation with 0.5 ml of Krebs' solution and the gut dissected carefully away from the mesenteric vasculature. The preparation was mounted on a stainless steel grid (7×5 cm) in a humid chamber and perfused at a constant flow rate of 5 ml min−1 using a peristaltic pump (model 7554-30, Cole-Parmer Instrument Co., Chicago, IL, U.S.A.). The perfusate was Krebs'-Bülbring solution of the following composition (mM): NaCl 133, KCl 4.7, NaH2PO4 1.35, NaHCO3 16.3, MgSO4 0.61, CaCl2 2.52 and glucose 7.8, gassed with 95% O2 – 5% CO2 and maintained at 37°C. Preparations were allowed to equilibrate for 30 min prior to experimentation. Responses were measured as changes in perfusion pressure (mmHg) with a pressure transducer (model P23XL, Viggo-Spectramed, Oxnard, CA, U.S.A.) on a side arm of the perfusion cannula, and recorded on a polygraph (model 7D, Grass Instrument Co., Quincy, MA, U.S.A.).
Experimental protocol
After equilibration, a submaximal concentration of methoxamine (10 – 50 μM) was added in order to increase the perfusion pressure of the preparations (by 40 – 80 mmHg) above baseline, and relaxant responses to ATP (5 pmol – 5 μmol) were investigated. Antagonists/inhibitors were added to the perfusate during equilibration, 30 min before construction of dose-response curves to ATP. A group of mesenteric beds was pre-treated with capsaicin (10 μM for 1 h, followed by 30 min washout) in order to cause selective desensitization and/or neurotransmitter depletion of sensory nerves (Ralevic et al., 2000). ATP relaxations were additionally evaluated in another group of preparations, time-matched for the longer duration of the capsaicin pre-treatment experiment. In one group of preparations guanethidine (5 μM) was added to the perfusate after 10 min equilibration in order to block sympathetic neurotransmission. Electrical field stimulation (EFS; 2 – 12 Hz, 0.1 ms, 60 V, 30 s) (Grass S9D stimulator) was applied in these preparations in order to stimulate sensory nerves and generate frequency-response curves (Ralevic et al., 1996). Endothelium removal was achieved by perfusing the preparations with water (10 ml, with repeated application as necessary) until the relaxant response to ATP at 0.5 nmol was abolished. In experiments with high K+ Krebs' solution, isotonicity was maintained by reducing the concentration of NaCl.
Drugs
ATP, capsaicin (8-methyl-N-vanillyl-6-nonenamide), glibenclamide, indomethacin, ouabain, methoxamine (hydrochloride), tetraethylammonium acetate and suramin were from Sigma Chemical Co. Guanethidine (Ismelin) was from Alliance Pharmaceuticals, Wiltshire, U.K. 8-(p-sulphophenyl)theophylline was from Research Biochemicals International. Charybdotoxin was from Latoxan, France. Pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) was from TOCRIS Cookson Ltd. Stock solutions of capsaicin (10 mM) and glibenclamide (100 mM) were made up in dimethyl sulphoxide. Indomethacin was dissolved in ethanol. All other drugs were dissolved in distilled water or Krebs' solution.
Data analysis
Vasorelaxant responses of the mesenteric arterial beds were measured as changes in perfusion pressure (mmHg) and expressed as percentage relaxation of the methoxamine-induced increase in tone above baseline. Data are expressed as mean±s.e.m. and analysed by Student's t-test or by analysis of variance followed by Tukey's multiple comparison. A value of P<0.05 was taken to indicate a statistically significant difference. F50 is the stimulation frequency (Hz) required to elicit a response that is half of the maximal relaxation. Rmax is maximal relaxation. pD2 is the negative logarithm of the dose required to elicit 50% relaxation of methoxamine-induced tone, as a maximal response was not always achieved.
Results
Multiphasic response to ATP in the rat mesenteric arterial bed
In methoxamine pre-constricted mesenteric arterial beds ATP (5 pmol – 0.05 μmol) elicited dose-dependent rapid relaxation; Rmax 76±5.6% and pD2 9.2±0.2 (n=8). At doses of 0.05 – 5 μmol ATP elicited an initial contraction that at 0.5 and 5 μmol generally opposed the rapid relaxation (Figure 1a). This was followed by a slow onset, prolonged relaxation of 56±3.0, 87±2.9 and 85±5.6%, at 0.05, 0.5 and 5 μmol ATP, respectively (n=8) (Figure 1a).
Figure 1.
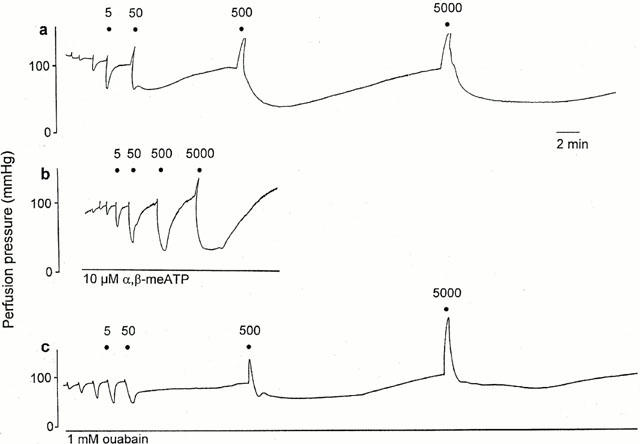
Representative traces showing response to ATP (0.005 – 5000 nmol) of the raised-tone rat isolated mesenteric arterial bed under (a) control conditions; (b) +10 μM α,β-methylene ATP (α,β-meATP); (c) +1 mM ouabain. For clarity only responses at 5 – 5000 nmol ATP are labelled, and the preceding three unlabelled responses are at doses of ATP of 0.005, 0.05 and 0.5 nmol. Note the triphasic response to ATP – rapid relaxation, prolonged relaxation and vasoconstriction – under control conditions. (The peak of the vasoconstriction at 500 and 5000 nmol was not recorded in (a)). Note the different vertical scale for the trace in (c).
There was no significant difference between the different experimental groups with respect to the level of methoxamine-induced tone: control, 51.0±6.8 mmHg (n=8); control for capsaicin pre-treatment, 71±12.0 mmHg (n=5); capsaicin pre-treatment, 75±5.5 mmHg (n=6); 25 mM K+, 58±6.5 mmHg (n=10); suramin, 42±4 mmHg (n=6); PPADS, 63±6.2 mmHg (n=4); 0.1 μM α,β-meATP, 49±10.3 mmHg (n=4); 10 μM α,β-meATP, 46±4.3 mmHg (n=12); glibenclamide, 42±5.8 mmHg (n=6); charybdotoxin, 74±8.3 mmHg (n=6); 8-SPT, 54±6.8 mmHg (n=6); indomethacin, 45±11.2 mmHg (n=6); endothelially-denuded, 78±8.1 mmHg (n=6); ouabain, 76±6.1 mmHg (n=5); TEA, 57±14.9 mmHg (n=4).
Effect of endothelium removal on response to ATP
Endothelium removal virtually abolished rapid relaxations to ATP (n=6; Figure 2a). Endothelium removal attenuated prolonged relaxation to ATP at 0.05 μmol (control 56±3.0%, n=8; endothelium-denuded 14±4.3%; P<0.001), but had no effect on relaxations at 0.5 and 5 μmol (n=6; Figure 2b). Contraction to ATP was unaffected by endothelium removal (n=6; Figure 4c).
Figure 2.
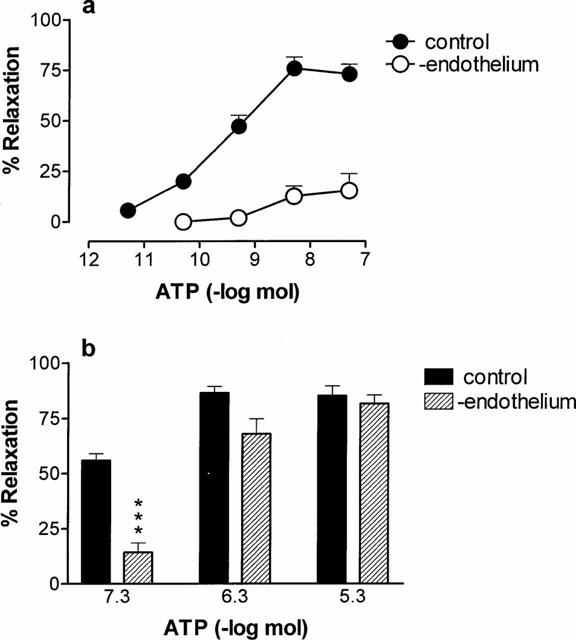
Effect of endothelium denudation on rapid and prolonged phases of relaxation to ATP in the rat isolated mesenteric arterial bed. (a) Rapid relaxation to ATP (5 pmol – 0.05 μmol) without and with endothelium removal (n=6 – 8); (b) prolonged relaxation to ATP (0.05 – 5 μmol) without and with endothelium removal (n=6 – 8).
Figure 4.
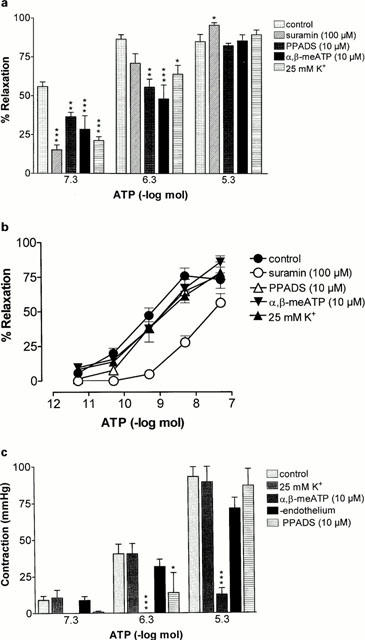
Effect of suramin, PPADS, α,β-methylene ATP (α,β-meATP), high K+ Krebs' solution or endothelial denudation on responses to ATP (0.5 pmol – 5 μmol) in the rat isolated mesenteric arterial bed: (a) prolonged relaxation to ATP; (b) rapid relaxation to ATP; (c) contraction to ATP. Control (n=8); suramin (100 μM; n=6), PPADS (10 μM; n=4) α,β-meATP (10 μM; n=8); 25 mM K+ Krebs' (n=10); endothelium-denuded (n=6). Data are presented as means and bars indicate s.e.mean.
Effect of capsaicin pre-treatment on vasorelaxation to ATP
Capsaicin pre-treatment (10 μM for 1 h) to cause selective desensitization and/or neurotransmitter depletion of sensory nerves had no significant effect on either phase of vasorelaxation to ATP (n=6) (Figure 3a,b). This protocol of capsaicin pre-treatment has been shown to abolish vasorelaxant responses to capsaicin in the rat isolated mesenteric arterial bed (Ralevic et al., 2000). For rapid relaxation to ATP in control preparations the Rmax was 65±6% and the pD2 was 8.6±0.5 (n=5), and after capsaicin pre-treatment the Rmax was 69±7.5% and the pD2 was 9.0±0.1 (n=6).
Figure 3.
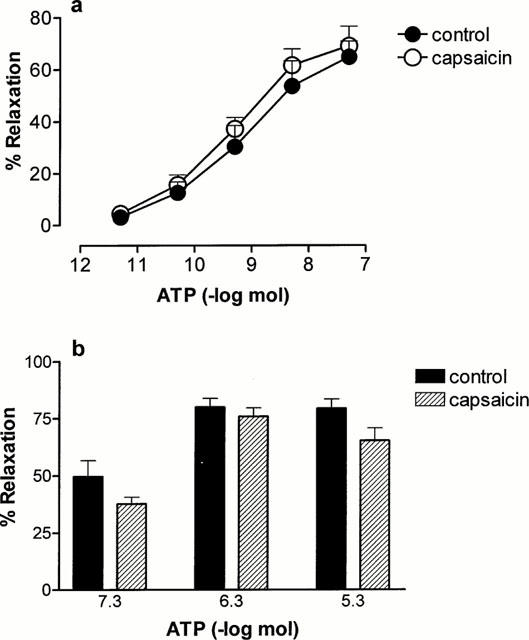
Effect of capsaicin pre-treatment (10 μM, 1 h) on rapid and prolonged relaxation to ATP in the rat mesenteric arterial bed. (a) Rapid relaxation to ATP (5 pmol – 0.05 μmol) in controls (n=5) and after capsaicin pre-treatment (n=6); (b) prolonged phase of relaxation to ATP (0.05 – 5 μmol) in controls (n=5) and after capsaicin pre-treatment (n=6). Data are presented as means and bars indicate s.e.mean.
Effect of α,β-meATP on relaxant response mediated by electrical stimulation of capsaicin-sensitive sensory nerves
In the presence of guanethidine to block sympathetic neurotransmission, electrical field stimulation (EFS; 2 – 12 Hz, 60 V, 0.1 ms, 30 s) elicited frequency-dependent sensory neurogenic vasorelaxation (Rmax 71±6.8%, F50 5.8±0.3 Hz (n=4). α,β-methylene ATP (α,β-meATP; 0.1 μM and 10 μM), a selective P2X receptor agonist and desensitizing agent, had no significant effect on vasorelaxation to EFS; 0.1 μM α,β-meATP, Rmax 68±6.6%, F50 5.4±0.3 Hz (n=4); 10 μM α,β-meATP, Rmax 60±5.6%, F50 5.6±0.1 Hz (n=4).
Effect of suramin, PPADS, 8-SPT and indomethacin on response to ATP
Prolonged relaxation to 0.05 μmol ATP was reduced by suramin (100 μM); relaxation was 15.2±3.2% (n=6; P<0.001) (Figure 4a). There was a trend for attenuation of relaxation to 0.5 μmol ATP in the presence of suramin, although this did not reach statistical significance. Suramin also attenuated rapid relaxation to ATP; the pD2 was 7.8±0.1 (n=6; P<0.001) (Figure 4b). PPADS (10 μM) had no effect on rapid relaxation to ATP (Figure 4b), but attenuated the prolonged phase of relaxation to 0.05 and 0.5 μmol ATP (n=4; P<0.01) (Figure 4a). Neither phase of relaxation to ATP was affected by the P1 receptor antagonist 8-sulphophenyltheophylline (1 μM; n=6) or indomethacin (10 μM, n=4) (data not shown).
Effect of α,β-meATP on response to ATP
Prolonged relaxation to ATP was attenuated by α,β-meATP (10 μM); responses to 0.05 and 0.5 μmol ATP were 25±8.3 and 48±9.0%, respectively (P<0.01; n=8) (Figures 1b and 4a). α,β-meATP had no significant effect on the sensitivity of rapid relaxation to ATP (Figure 4b), but revealed rapid relaxation at 0.5 and 5 μmol ATP of 91±2.2 and 93±1.9%, respectively (n=8) (Figure 1b). Contraction to ATP was virtually abolished by α,β-meATP (n=8) (Figures 1b and 4c).
Effect of 25 mM K+ Krebs' solution on response to ATP
In high K+ (25 mM) Krebs' solution prolonged relaxation to ATP was attenuated; responses at 0.05 and 0.5 μmol ATP were 21±2.6 and 64±5.8%, respectively (P<0.01; n=10) (Figure 4a). High K+ Krebs' solution had no effect on rapid relaxation to ATP (Figure 4b). There was also no effect of high K+ solution on contractions to ATP (at 0.05 – 5 μmol) (n=6) (Figure 4c).
Effect of glibenclamide, charybdotoxin and tetraethylammonium on relaxations to ATP
Glibenclamide (10 μM), a selective inhibitor of KATP channels, attenuated prolonged relaxations to ATP at 0.5 and 5 μmol (n=6; Figure 5a). It had no significant effect on the sensitivity (pD2 9.1±0.6) or Rmax (63.2±4.7%) of rapid relaxation to ATP although there was a decrease in the threshold for relaxation (n=6; Figure 5b). Charybdotoxin (100 nM), a selective inhibitor of KCa channels, had no significant effect on prolonged relaxation to ATP (Figure 5a). Charybdotoxin had no effect on the sensitivity of rapid relaxation to ATP (pD2 9.8±0.3) although the Rmax was increased (88±3.2%; P<0.05, n=4) and the threshold decreased (Figure 5b). Contractions to 0.5 μmol ATP were increased by both charybdotoxin and tetraethylammonium (TEA; 10 mM; P<0.001) (Figure 5c). TEA had no significant effect on rapid or prolonged relaxation to ATP (Figures 5a,b).
Figure 5.
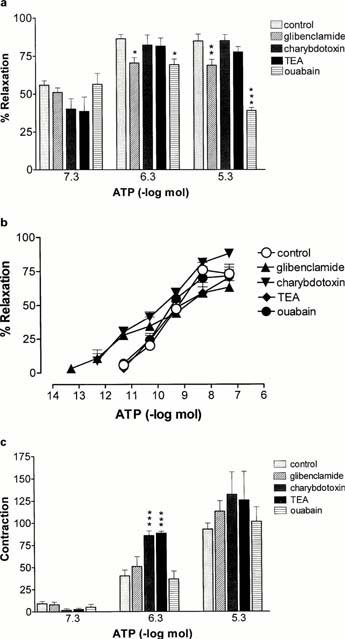
Effect of inhibitors of K+ channels and Na+/K+ ATPase on responses to ATP in the rat isolated mesenteric arterial bed. (a) prolonged relaxation to ATP; (b) rapid relaxation to ATP; (c) contraction to ATP. Control (n=8); glibenclamide (10 μM; n=6); charybdotoxin (0.1 μM; n=4); tetraethylammonium (TEA, 10 mM; n=4); ouabain (1 mM; n=5). Data are presented as means and bars indicate s.e.mean.
Effect of ouabain on responses to ATP
Ouabain (1 mM; n=5), an inhibitor of Na+/K+-ATPase, attenuated prolonged relaxation to 0.5 (P<0.05) and 5 (P<0.001) μmol ATP (Figures 1c and 5a) but had no effect on rapid relaxation or contraction to ATP (Figure 5b,c). Ouabain was most effective against prolonged relaxation to the highest dose of ATP, which may indicate a greater effectiveness of inhibition of Na+/K+-ATPase activity with time.
Discussion
The present study shows that the prolonged phase of relaxation to ATP of the rat isolated mesenteric arterial bed, which may involve P2Y receptors, is endothelium-independent, involves activation of Na+/K+-ATPase and KATP channels, and is inhibited by α,β-meATP. In addition, these data indicate that although P2X receptors are expressed on capsaicin-sensitive primary afferent nerves in rat mesenteric arteries (Kirkup et al., 1999), they neither mediate nor modulate an efferent function of sensory nerves.
The relaxant response to high doses of ATP in the mesenteric arterial bed was biphasic as reported by Stanford & Mitchell (1998). The initial rapid phase of relaxation was endothelium-dependent, and suramin-sensitive, but PPADS-insensitive, as shown previously (Ralevic & Burnstock, 1988; 1996). It is likely to be mediated by a UTP/ATP preferring endothelial P2Y2 receptor, as opposed to the PPADS-sensitive ADP preferring P2YI receptor that is also expressed on the endothelium in this vascular bed (Ralevic & Burnstock, 1996). In contrast, the prolonged phase of ATP-induced relaxation is largely independent of the endothelium. In the representative trace in Figure 1a it can be seen that the initial rapid relaxation to 50 nmol ATP dictates the starting level of tone for prolonged relaxation, which likely explains why the prolonged response to this dose of ATP appears to be attenuated when the endothelium is removed (i.e. rapid relaxation is abolished and prolonged relaxation starts at a higher level of tone, so the response is smaller when expressed as a percentage of tone). Prolonged relaxation to ATP does not involve adenosine P1 receptors and vasodilator prostanoids as it is unaffected by 8-SPT and indomethacin. The response is, however, reduced by suramin and PPADS indicating possible actions of ATP at a vasorelaxant P2Y receptor on the smooth muscle. This may be equivalent to the vasorelaxant smooth muscle P2Y-like receptor that has been observed in a variety of blood vessels including rabbit hepatic artery (Brizzolara & Burnstock, 1991) and rabbit mesenteric resistance arteries (Brayden, 1991).
Prolonged vasorelaxation to ATP was attenuated by a high K+ depolarizing Krebs' solution, and by inhibition of Na+/K+-ATPase with ouabain, indicating a likely involvement of smooth muscle hyperpolarization. The response was also reduced by glibenclamide, which indicates an involvement of hyperpolarizing KATP channels. Purinergic endothelium-independent, glibenclamide-sensitive, relaxation and hyperpolarization, has also been reported for ADP in rabbit mesenteric and skeletal muscle resistance arteries (Brayden, 1991). It is noteworthy that in the rat, vascular smooth muscle Na+/K+-ATPase is very insensitive to ouabain and high (mM) concentrations, known to prevent the formation of gap junctions (Watsky et al., 1990), are required to inhibit the enzyme activity. Thus, an additional possible action of ouabain is inhibition of the co-ordinated spread of ATP-induced hyperpolarization between smooth muscle cells. However, carbenoxolone (100 μM, 1 h), a gap junction inhibitor, did not block prolonged relaxations to ATP (unpublished observations), indicating that this mechanism is unlikely to be involved in inhibition by ouabain. There was no effect of charybdotoxin on prolonged relaxation to ATP indicating a lack of involvement of small conductance KCa channels.
P2X receptors are expressed on cell bodies in dorsal root ganglia and on capsaicin-sensitive primary afferent nerves in the periphery (Bland-Ward & Humphrey, 1997; Dowd et al., 1998; Guo et al., 1999; Ueno et al., 1999) including those of rat mesenteric arteries (Kirkup et al., 1999). The present study indicates that P2X receptors do not mediate an efferent function of rat mesenteric sensory nerves, as capsaicin pre-treatment to desensitize and/or deplete the neurotransmitter content of sensory nerves had no effect on vasorelaxation to ATP. ATP and α,β-meATP do, however, evoke excitation of rat mesenteric sensory nerves (Kirkup et al., 1999), which may indicate that P2X receptors expressed on sensory nerves mediate purely an afferent function. In contrast, coexpressed vanilloid receptors mediate both an afferent and efferent function of primary afferent nerves (Maggi & Meli, 1988). High levels of extracellular ATP can be generated when there is damage to tissues (Burnstock & Kennedy, 1996). The present results indicate that endothelial and smooth muscle P2 receptors, and not P2X receptors on sensory nerves, are likely to be the primary local targets mediating defensive vasomotor responses under such conditions (Burnstock, 1987; Ralevic, 1998).
The possibility that P2X receptors on sensory nerves can modulate the efferent function of sensory neurotransmission was additionally investigated. α,β-meATP caused no change in the vasorelaxant response mediated by electrical stimulation of sensory nerves. This indicates that P2X receptors do not modulate neurotransmitter release from the peripheral terminals of primary afferent nerves in the rat mesenteric arterial bed. Others have also shown a lack of effect of α,β-meATP as a modulator of the efferent function of capsaicin-sensitive sensory neurones, in the guinea-pig isolated atria (Rubino et al., 1992). Those authors concluded that the purinoceptors accounting for ATP-modulation of capsaicin-sensitive neurotransmission in guinea-pig atria belong to the P1 subtype (Rubino et al., 1992). P1 receptors (A1 subtype) are also expressed on capsaicin-sensitive sensory nerves in rat mesenteric arteries (Rubino et al., 1993), and similarly may mediate inhibition of neurotransmission by ATP.
Exposure to α,β-meATP, with consequent P2X receptor desensitization, had different effects on the rapid and prolonged phases of relaxation to ATP. Rapid relaxation was uncovered at high doses of ATP that, in the absence of α,β-meATP, elicited primarily coincident contraction. This indicates that under normal conditions there is functional antagonism to the actions of ATP at endothelial P2Y receptors by opposing P2X receptor-mediated smooth muscle contraction. This may involve a depolarizing action of ATP at smooth muscle P2X receptors, which has been shown to counteract hyperpolarization elicited by activation of endothelial P2Y receptors (Malmsjö et al., 1999). Prolonged relaxation to ATP was attenuated in the presence of α,β-meATP. As the data with capsaicin pre-treatment excludes an involvement of sensory nerves, this indicates an involvement of P2X receptors on the smooth muscle. P2X receptors are ligand-gated cation channels that mediate influx of cations and contraction and thus are unlikely to mediate directly prolonged vasorelaxation to ATP. Rebound relaxation following activation of contractile P2X receptors also does not appear to be involved as there was still a significant prolonged relaxation when contraction to ATP was abolished by PPADS. It is possible that the smooth muscle may remain slightly depolarized in the presence of α,β-meATP, and this may counteract a possible hyperpolarizing action involved in prolonged vasorelaxation to ATP. It is noteworthy that the depolarizing action of ATP (100 μM) during perfusion of rat isolated small mesenteric arteries was observed to decline by less than 30% over 5 min, whilst the contractile action of ATP was transient (Juul et al., 1992).
The greater sensitivity of ATP for endothelial P2Y receptors versus smooth muscle P2X receptors indicates that they are selectively activated at low concentrations of ATP, leading to vasorelaxation. At high concentrations, ATP activates simultaneously smooth muscle P2X and endothelial P2Y receptors and their effects are opposite and opposing; thus, P2Y-mediated relaxations are revealed by desensitization of P2X receptors (present study) and P2X-mediated contractions may be augmented by endothelium removal (Ralevic & Burnstock, 1988). At high concentrations of ATP there is additionally a slow and prolonged vasorelaxation. When P2X receptors are activated and at least partly desensitized, both vasocontraction and prolonged vasorelaxation are blocked, and the principal response to ATP is rapid vasorelaxation. ATP can be released from a number of different sources, including sympathetic nerves, platelets and endothelial cells (Burnstock & Kennedy, 1986; Burnstock, 1987). Their relevance to these multiple vascular actions of ATP remains to be determined.
In conclusion, these data indicate that the prolonged phase of vasorelaxation to ATP in the rat isolated mesenteric arterial bed, which may be mediated by P2Y receptors, is endothelium-independent, involves activation of Na+/K+-ATPase and KATP channels, and is inhibited by α,β-meATP. Neither prolonged nor rapid vasorelaxation to ATP involves capsaicin-sensitive sensory nerves, adenosine P1 receptors, prostanoids or KCa channels.
Acknowledgments
I am grateful to the Royal Society for financial support. I would also like to thank Dr M.D. Randall for comments on the manuscript.
Abbreviations
- α,β-meATP
α,β-methylene ATP
- CGRP
calcitonin gene-related peptide
- EFS
electrical field stimulation
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid
- TEA
tetraethylammonium
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- BLAND-WARD P.A., HUMPHREY P.P.A. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br. J. Pharmacol. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOARDER M.P., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BRAYDEN J.E. Hyperpolarization and relaxation of resistance arteries in responses to adenosine diphosphate. Circ. Res. 1991;69:1415–1420. doi: 10.1161/01.res.69.5.1415. [DOI] [PubMed] [Google Scholar]
- BRIZZOLARA A., BURNSTOCK G. Endothelium-dependent and endothelium-independent vasodilatation of the hepatic artery of the rabbit. Br. J. Pharmacol. 1991;103:1206–1212. doi: 10.1111/j.1476-5381.1991.tb12325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. Local control of blood pressure by purines. Blood Vessels. 1987;24:156–160. doi: 10.1159/000158691. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KENNEDY C. A dual function for adenosine 5′-triphosphate in the regulation of vascular tone. Circ. Res. 1986;58:319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., WOOD J.N. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr. Op. Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK S.P., VULCHANOVA L., HARGREAVES K.M., ELD R., MCCLESKEY E.W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- DOWD E., MCQUEEN D.S., CHESSELL I.P., HUMPHREY P.P.A. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br. J. Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLINGE D., HOU M., WEBB T.E., BARNARD E., MOLLER S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT–PCR. Biochem. Biophys. Res. Commun. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN K.T., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- GUO A., VULCHANOVA L., WANG J., LI X., ELDE R. Immunocytochemical localization of the vanilloid receptor 1 (VR1), relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neuroscience. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- JUUL B., PLESNER L., AALKJAER C. Effects of ATP and UTP on [Ca2+]i, membrane potential and force in isolated rat small mesenteric arteries. J. Vasc. Res. 1992;29:385–395. doi: 10.1159/000158955. [DOI] [PubMed] [Google Scholar]
- KAWASAKI H., TAKASAKI K., SAITO A., GOTO K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:165–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- KIRKUP A.J., BOOTH C.E., CHESSELL I.P., HUMPHREY P.P.A., GRUNDY D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J. Physiol. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. The sensory-efferent function of capsaicin-sensitive nerves. Gen. Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- MALMSJÖ M., ERLINGE D., HÖGESTÄTT E.D., ZYGMUNT P.M. Endothelial P2Y receptors induce hyperpolarisation of vascular smooth muscle by release of endothelium-derived hyperpolarising factor. Eur. J. Pharmacol. 1999;364:169–173. doi: 10.1016/s0014-2999(98)00848-6. [DOI] [PubMed] [Google Scholar]
- NORI S., FUMAGALLI L., BO X., BOGDANOV Y., BURNSTOCK G. Coexpression of mRNAs for P2X1, P2X2 and P2X4 receptors in rat vascular smooth muscle: an in situ hybridization and RT–PCR study. J. Vasc. Res. 1997;35:179–185. doi: 10.1159/000025582. [DOI] [PubMed] [Google Scholar]
- OLSSON R.A., PEARSON J.D. Cardiovascular purinoceptors. Physiol. Rev. 1990;70:761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- RALEVIC V.P2 Receptors in blood vessels Cardiovascular Biology of Purines 1988Kluwer Academic Publishers, Massachusetts; 206–224.G. Burnstock, J.G. Dobson, B.T. Liang and J. Linden (eds) [Google Scholar]
- RALEVIC V. Prolonged phase of vasodilatation to ATP in rat mesenteric bed is attenuated by P2X receptor desensitization but does not involve capsaicin-sensitive sensory nerves. Br. J. Pharmacol. 2000;129:258P. [Google Scholar]
- RALEVIC V., BURNSTOCK G. Actions mediated by P2-purinoceptors in the isolated perfused mesenteric bed of the rat. Br. J. Pharmacol. 1998;95:637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Discrimination by PPADS between endothelial P2Y- and P2U-purinoceptors in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1996;118:428–434. doi: 10.1111/j.1476-5381.1996.tb15420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., RANDALL M.D., ZYGMUNT P.M., MOVAHED P., HÖGESTÄTT E.D. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br. J. Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., RUBINO A., BURNSTOCK G. Augmented sensory-motor vasodilation of the rat mesenteric arterial bed after chronic infusion of the P1-purinoceptor antagonist, DPSPX. Br. J. Pharmacol. 1996;118:1675–1680. doi: 10.1111/j.1476-5381.1996.tb15591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINO A., AMERINI S., LEDDA F., MANTELLI L. ATP modulates the efferent function of capsaicin-sensitive neurones in guinea-pig isolated atria. Br. J. Pharmacol. 1992;105:516–520. doi: 10.1111/j.1476-5381.1992.tb09011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBINO A., RALEVIC V., BURNSTOCK G. The P1-purinoceptors that mediate the prejunctional inhibitory effect of adenosine on capsaicin-sensitive nonadrenergic noncholinergic neurotransmission in the rat mesenteric arterial bed are of the A1 subtype. J. Pharmacol. Exp. Ther. 1993;267:1100–1104. [PubMed] [Google Scholar]
- STANFORD S.J., MITCHELL J.A. ATP-induced vasodilatation in the rat isolated mesenteric bed exhibits two apparent phases. Br. J. Pharmacol. 1998;125:94P. [Google Scholar]
- UENO S., TSUDA M., IWANAGA T., INOUE K. Cell type-specific ATP-activated responses in rat dorsal root ganglion cells. Br. J. Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON KÜGELGEN I., STARKE K. Evidence for two separate vasoconstriction-mediating nucleotide receptors, both distinct from the P2X-receptor, in rabbit basilar artery: a receptor for pyrimidine nucleotides and a receptor for purine nucleotides. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;341:538–546. doi: 10.1007/BF00171734. [DOI] [PubMed] [Google Scholar]
- WATSKY M.A., MCCARTNEY M.D., MCLAUGHLIN B.J., EDELHAUSER H.F. Corneal endothelial junctions and the effect of ouabain. Invest. Opthalmol. Vis. Sci. 1990;31:933–941. [PubMed] [Google Scholar]


