Abstract
The effects of the endogenous cannabinoid anandamide were studied on peripheral, polymodal nociceptors recorded from normal and chronically inflamed (Freund's adjuvant) knee joint afferents in rats anaesthetized with pentobarbitone. Anandamide (860 nmol) caused a rapid, short lasting excitation of a sub-population of capsaicin-sensitive nociceptive afferents in normal knee joints (7.2±2.3 impulses s−1; n=15 units from five animals). In arthritic joints there were 9.7±3.0 impulses s−1 (n=11 from six animals), which was not significantly different from normal joints. The excitation was dose dependent (8.6 – 2900 nmol) and mediated by activation of the vanilloid receptor (VR1) as it was abolished by the VR1 antagonist capsazepine (1 mg kg−1). Our results show that anandamide, at high doses, can activate nociceptive afferents innervating the rat knee joints, in contrast with its widely described analgesic actions.
Keywords: Sensory nerves, anandamide, adjuvant arthritis, knee joint, afferent nociceptors, vanilloid receptor
Introduction
Following the discovery of anandamide as an endogenous ligand for the CB (cannabinoid) receptor, there has been intense interest in its physiological function. An important putative role for anandamide is as a modulator of nociception, in part involving activation of the CB1 receptors on sensory neurones and the subsequent inhibition of the release of CGRP (Richardson et al., 1998). Reports have also linked anandamide with the recently cloned vanilloid receptor subtype 1 (VR1) which is sensitive to capsaicin (Caterina et al., 1997). The action of anandamide on the VR1 receptor has been demonstrated using transfected human receptors (Smart et al., 2000), on sensory nerves controlling vasodilatation in isolated arteries (Zygmunt et al., 1999), and the amplitude of electrically-evoked contractions in the mouse isolated vas deferens (Ross et al., 2001). The VR1 agonist capsaicin is a potent activator of C-fibre polymodal nociceptors in the periphery. In view of the reported actions of anandamide, and its structural relationship to capsaicin and other vanilloid agonists, we hypothesized that anandamide can directly activate capsaicin nociceptors and have investigated those innervating the rat knee joint. Using a unilateral adjuvant-induced arthritis model in the rat (Dowd et al., 1998) we have also investigated the effects of anandamide to determine whether the responsiveness of these joint nociceptors was altered during experimental inflammation.
Methods
Experiments were performed on five normal and six arthritic male Wistar rats (body weight range 280 – 470 g; mean±s.e.mean 374±19 g).
Induction of arthritis
Freund's Complete Adjuvant (FCA, 0.15 – 0.20 ml of 1 mg ml−1 heat killed Mycobacterium tuberculosis in paraffin oil, Sigma) was introduced into the left knee (stifle) joint by intra-articular injection under transient halothane anaesthesia (3% in oxygen). The animals were used for electrophysiological recording 14 – 35 days post-injection. Animals displayed mild but persistent unilateral arthritis as characterized by a significant increase in the diameter of the injected joint (injected 10.6±0.1 mm; uninjected 9.8±0.1 mm; n=6, P<0.05, Wilcoxon).
Surgical procedures
Animals were anaesthetized with an intra-peritoneal (i.p.) injection of pentobarbitone (60 mg kg−1) and the trachea was cannulated. Anaesthesia was maintained with an intravenous (i.v.) infusion of pentobarbitone (0.4 – 0.5 mg kg−1 min−1) via a cannula inserted in the right femoral vein and the right carotid artery was cannulated for monitoring blood pressure. An additional catheter was inserted into the right femoral artery with its tip positioned in the lower abdominal aorta to allow for close arterial injection of drugs to the left knee joint. Animals were killed at the end of the experiment by an overdose of anaesthetic.
Electrophysiology
Extracellular recordings were performed on filaments of the medial articular nerve (MAN) innervating the left knee joint using previously described techniques (Dowd et al., 1998). Briefly, the left leg was fixed to a support and an incision was made on the medial aspect of the limb and the skin secured to form a pouch that was filled with heavy liquid paraffin. Three branches of the medial articular nerve (MAN) were exposed at the point where they leave the saphenous nerve. The saphenous nerve was cut centrally to prevent interference from efferent activity. One branch of the MAN was dissected from surrounding tissue and electrical activity was recorded from filaments, typically containing 1 – 4 afferent fibres, using a Pt-Ir bipolar electrode connected to an amplifier. Neural activity was viewed on an oscilloscope, digitally recorded and analysed using a personal computer running Spike 2 software (CED, Cambridge). Drugs were administered by close intra-arterial injection (total volume: 0.1 ml drug+0.2 ml wash), completed within 2 s. The minimum time interval between injections was 20 min to minimize the possibility of desensitization.
Data analysis
Drug effects were determined by comparing the action potential discharge frequency or the absolute number of events immediately following injection with that in the 15 s period immediately prior to injection. Data are expressed as either the mean change in action potential frequency (impulses s−1) or the change in the absolute number of action potentials evoked during the response (impulses). The maximum possible dose for anandamide was 2900 nmol, the concentration of the stock solution. Differences between means were analysed statistically using the Mann – Whitney test for unpaired data and the Wilcoxon matched-pairs test for paired data. The null-hypothesis was rejected at P<0.05.
Drugs
Anandamide and capsazepine were purchased from Tocris (U.K.) and capsaicin, bradykinin, adenosine 5′-triphosphate (ATP) and α,β-methyleneadenosine 5′-triphosphate (αβmeATP) were purchased from Sigma (U.K.). Capsaicin was dissolved in Tween 80 (10% v v−1), ethanol (10% v v−1) and PBS. Capsazepine stock (10 mg ml−1) was dissolved in 20% cremophor EL (Sigma, U.K.) in distilled water. All drugs were diluted in PBS for injection.
Results
In normal animals (n=5) recordings were made from five afferent fibres consisting of 15 units with receptive fields in the knee joint. These units were identified as either C-fibre polymodal nociceptors (14/15, 93%) or Aδ-mechanonociceptors (1/15, 7%) according to their conduction velocities, mechanosensitivity, and their response to capsaicin (9 nmol, 18.2±5.4 impulses s−1). Basal discharge from these afferents was 0.01±0.01 impulses s−1. Recordings were also made in arthritic animals (n=6) from a total of six mechanosensitive afferents consisting of 11 units, all of which were C-fibre polymodal nociceptors and responded to capsaicin (9 nmol i.a.: 11.6±2.0 impulses s−1). Basal discharge was 0.04±0.02 impulses s−1 which was not significantly different (P>0.05, Mann – Whitney) from the untreated group.
Responses to anandamide in normal and arthritic knee joints
Anandamide (8.6 – 2900 nmol) evoked an increase in afferent discharge in 64% (9/14) of C-fibre polymodal nociceptors but not in the single Aδ-mechanonociceptor recorded from normal knee joints. In arthritic joints anandamide (8.6 – 2900 nmol) evoked a response in 72% (8/11) of C-fibre polymodal nociceptors. Injection of vehicle had no effect in these fibres (soya oil: water (1 : 4), −0.1±0.1 impulses s−1, n=5).
The excitation evoked by anandamide in the sensory fibres was dose dependent (Figure 1) and there was no shift in the dose-response curve when comparing normal with arthritic joints. It was not possible to determine a maximum response, as the amount that could be administered was limited by the solubility of anandamide. Figure 2 shows a typical response to anandamide (860 nmol) recorded from normal and arthritic knee joints. In normal joints the response to this dose was rapid in onset (4.5±0.5 s) and of short duration (4.8±1.0 s) and caused an average change in the discharge frequency of 7.2±2.3 impulses s−1 (n=15 units from five animals). A similar response was obtained from arthritic joints with a response latency of 5.2±0.4 s, response duration of 3.9±1.1 s and a change in the discharge frequency of 9.7±3.0 impulses s−1 (n=11 units from six animals). Figure 3 shows there was no significant difference in the responses when comparing normal and arthritic joints (P>0.05, Mann – Whitney).
Figure 1.
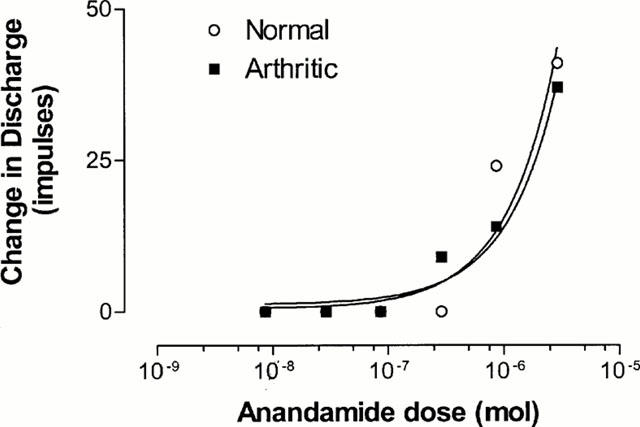
Dose-related increase in absolute discharge (impulses) evoked by close arterial injection of anandamide in a single C-fibre polymodal nociceptor innervating both a normal and an arthritic knee joint.
Figure 2.
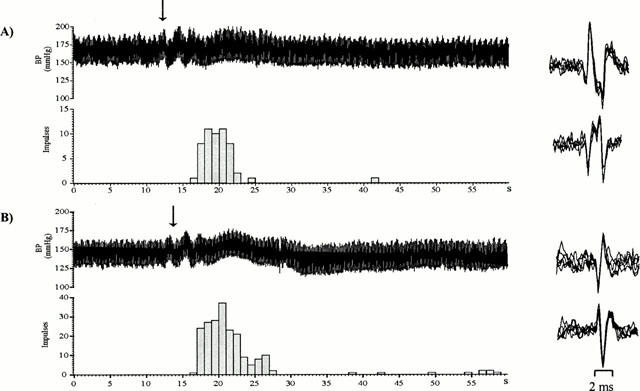
Spike 2 traces showing the evoked discharge from an afferent fibre of the medial articular nerve innervating the (A) normal and (B) arthritic rat knee joint evoked by close arterial injection of anandamide (860 nmol; arrow). The upper panel shows the blood pressure and the lower panel is a bar graph representing the pooled discharge of the individual units recorded from the afferent fibre. Pooled data are shown because the responses evoked are similar in all units. Discharge is displayed in 1 s bins. The panels on the right show an overlay of the individual units (2) present in the pooled discharge.
Figure 3.
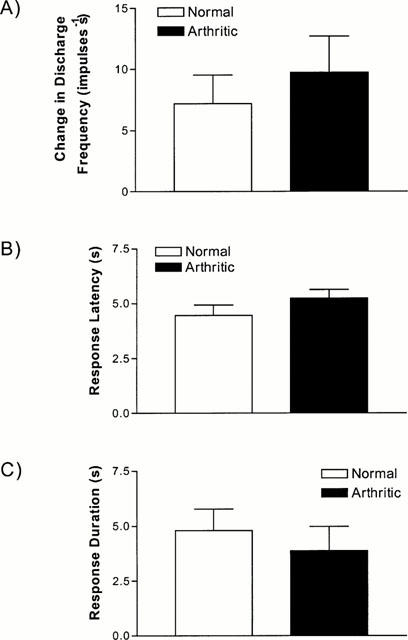
Comparison of the (A) increase in nociceptive discharge, (B) latency to onset, (C) duration of the response following a close arterial injection of anandamide (860 nmol). Data shown is for normal (15 units from five animals) and chronically arthritic (n=11 units from six animals) joints. There is no significant difference in any facet of the response between normal and arthritic joints (P>0.05, Mann – Whitney).
Other algogens that are capable of activating polymodal nociceptors were also examined to ascertain whether or not they were affected by the administration of anandamide (860 – 2900 nmol). In normal joints the response to bradykinin (9 nmol) was 1.7±0.7 impulses s−1 and after anandamide it was not significantly different (P>0.05, Mann – Whitney) with a response of 1.3±0.6 impulses s−1 (n=8 units from two animals). The response to ATP (2000 nmol) was unchanged after the administration of anandamide (P>0.05, Mann – Whitney) with a response of 14.8±6.0 impulses s−1 prior to anandamide and 11.2±4.7 impulses s−1 following (n=5 units from two animals). In arthritic joints after anandamide, there was no significant difference (P>0.05, Mann – Whitney) in the response to bradykinin (9 nmol) or ATP (2000 nmol) with responses of 2.2±0.6 impulses s−1 and 1.3±0.5 impulses s−1 (n=6 units from three animals) before and after bradykinin respectively and 2.3±1.5 impulses s−1 and 6.5±2.5 impulses s−1 (n=4 units from three animals) before and after ATP respectively.
The response to capsaicin (9 nmol) was also examined before and after anandamide. In normal joints, the afferent discharge was 18.2±5.4 impulses s−1 before and 8.1±2.8 impulses s−1 after anandamide and this difference was not found to be significant (P>0.05, Wilcoxon, n=12 from four animals). Similarly, no difference was seen in the arthritic joints (P>0.05, Wilcoxon) with responses of 11.6±2.0 impulses s−1 before and 8.1±2.2 impulses s−1 after anandamide (n=11 from six animals).
Response to anandamide is abolished by VR1 antagonist capsazepine
In order to confirm the algogenic effects of anandamide were attributable to activation of VR1 receptors the effects of both capsaicin (9 nmol) and anandamide (2900 nmol) were examined in the presence of the VR1 antagonist capsazepine (1 mg kg−1). In a normal rat knee joint, capsazepine completely abolished the afferent response to both capsaicin and anandamide (Figure 4). The effect was short lasting and the response to both drugs recovered after 20 min. Furthermore the effect was specific to capsaicin and anandamide, as responses to both bradykinin (9 nmol) and αβmeATP (60 nmol) were not altered by capsazepine (data not shown).
Figure 4.
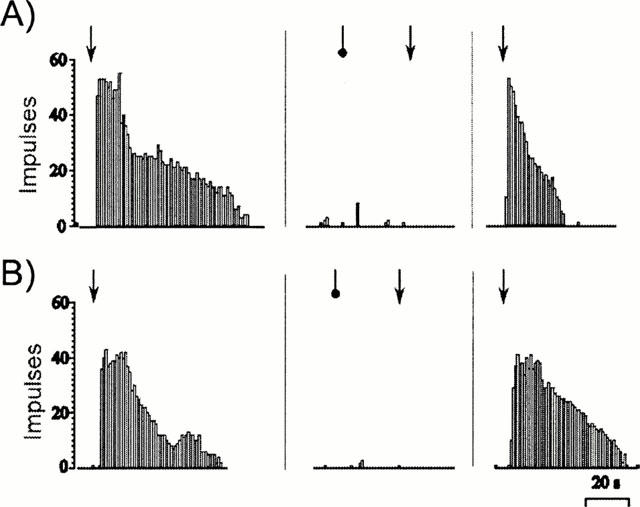
Afferent discharge from a single C-fibre evoked by (A) capsaicin (9 nmol) on (B) anandamide (2900 nmol) innervating a normal knee joint before and after the bolus injection of capsazepine 1 mg kg−1. Capsaicin is administered at the arrows and capsazepine at the circle. The time interval between panels is 20 min.
Discussion
The primary finding from this in vivo study is that anandamide causes a rapid short-lasting excitation in a sub-population of peripheral, polymodal nociceptors in normal and arthritic rat knee joints in a dose dependent manner, producing a rapid, short-lasting excitation.
Anandamide is an agonist at CB1 and CB2 receptors; both these receptors are present in the periphery, with CB1 receptors localized to the sensory nerves and the CB2 receptors expressed on immune cells such as B-cells and natural killer cells. The ability of anandamide to activate nociceptors seems inconsistent with previously reported anti-nociceptive actions of the CB1 receptor. Cannabinoids act on the CB1 receptor both centrally, in the brain and spinal cord, and peripherally in the dorsal root ganglion to produce their analgesia (reviewed in Walker et al., 1999 and Fuentes et al., 1999). However, our studies focus in particular on the role of anandamide acting directly on peripheral nerve terminals located in an articular joint and its ability to initiate a nociceptive response. Analgesic effects of the CB1 receptor appear to result from relatively low doses of cannabinoids, and the high doses of anandamide required to activate peripheral nociceptors in our study are likely to result from actions on a different pharmacological receptor.
The rapid onset of the response to anandamide eliminates a number of potential indirect mechanisms, such as activation of the CB2 receptor on immune cells triggering the release of algogenic mediators. Furthermore, even allowing for the rapid metabolism of anandamide to arachadonic acid and subsequent eicosanoids, the latency of onset of the response is too short to be the result of a metabolite. The rapid response is not characteristic of G-protein coupled receptor activation, rather it is more representative of the response of a ligand gated ion-channel such as the VR1 receptor channel (Szallasi & Blumberg, 1999).
Anandamide is a full agonist at the human VR1 receptor when expressed in HEK293 cells (Smart et al., 2000), and the vasodilatory action of anandamide is a result of VR1 mediated release of CGRP (Zygmunt et al., 1999). The afferents activated by anandamide were all sensitive to capsaicin, however the corollary was untrue with 64 and 72% of capsaicin sensitive afferents being activated by anandamide in normal arthritic joints respectively. The VR1 receptor is expressed on approximately 88% of small to medium neurones in the dorsal root ganglion (Michael & Priestley, 1999). These are the cell bodies for thinly myelinated (Aδ) or unmyelinated (C) fibres running from the periphery. Although the VR1 receptor is not present on all sensory afferents our study did not demonstrate any afferents that were sensitive to anandamide without showing sensitivity to capsaicin. Furthermore, we have shown that direct activation of capsaicin sensitive sensory nerves in vivo by anandamide can be abolished by the VR1 antagonist capsazepine (1 mg kg−1). Therefore, the activation of peripheral nociceptors by high doses of anandamide appears to involve a VR1-dependent mechanism. In addition, it can be concluded that if cannabinoid receptors are present on peripheral terminals they are not excitatory.
Capsaicin, even in small doses, causes desensitization of the VR1 receptor in our preparation. However, anandamide at the doses tested did not produce desensitization either to capsaicin or itself. Further experiments are required to examine the afferent response to anandamide after a desensitizing dose of capsaicin (>86 nmol).
As stated earlier, low doses of cannabinoids can produce anti-hyperalgesia and anti-nociception when acting both centrally and peripherally. We examined the effect of the highest dose of anandamide on responses evoked by other algogens commonly used to characterize primary nociceptive afferents. Responses to three algogens (capsaicin, bradykinin, and ATP) were not significantly different after anandamide (860, 2900 nmol). Therefore anandamide did not reduce the chemosensitivity of primary sensory afferents. More detailed experiments are required to determine whether the high threshold mechanosensitivity or thermosensitivity of these afferents is affected by high doses of anandamide.
There has been recent debate in the literature that the concentrations of anandamide required to activate VR1 are not physiologically relevant (Smart & Jerman, 2000; Szolcsanyi, 2000a,2000b; Zygmunt et al., 2000). It is still not clear at what concentration anandamide might be present at peripheral nerve terminals, and it is possible that levels of locally produced ligand are sufficient for activation of nearby sensory nerve terminals. Similarly, it may be that there is an unknown endogenous compound similar to anandamide in structure with a higher affinity for the VR1 receptor. Regardless of whether the ability of anandamide to activate the vanilloid receptor has any physiological significance, it does have pharmacological relevance with regard both to the pharmacological profile of anandamide and to the development of novel vanilloid receptor ligands.
Abbreviations
- αβmeATP
α,β-methyleneadenosine 5′-triphosphate
- anandamide
arachidonylethanolamide
- ATP
adenosine triphosphate
- capsaicin
8-methyl-N-vanillyl-6-nonenamide
- CGRP
calcitonin-gene-related peptide
- PBS
phosphate buffered saline
- VR1
vanilloid receptor
References
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- DOWD E., MCQUEEN D.S., CHESSEL I.P., HUMPHREY P.P. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br. J. Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUENTES J.A., RUIZ-GAYO M., MANZANARES J., VELA G., RECHE I., CORCHERO J. Cannabinoids as potential new analgesics. Life Sci. 1999;65:675–685. doi: 10.1016/s0024-3205(99)00290-8. [DOI] [PubMed] [Google Scholar]
- MICHAEL G.J., PRIESTLEY J.V. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J. Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON J.D., KILO S., HARGREAVES K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S., DI MARZO V., PERTWEE R.G.Structure-activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens Br. J. Pharmacol. 2001(In press) [DOI] [PMC free article] [PubMed]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C. Anandamide: an endogenous activator of the vanilloid receptor [letter] Trends Pharmacol. Sci. 2000;21:134. doi: 10.1016/s0165-6147(00)01459-0. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- SZOLCSANYI J. Anandamide and the question of its functional role for activation of capsaicin receptors [letter] Trends Pharmacol. Sci. 2000a;21:203–204. doi: 10.1016/s0165-6147(00)01484-x. [DOI] [PubMed] [Google Scholar]
- SZOLCSANYI J. Are cannabinoids endogenous ligands for the VR1 capsaicin receptor. Trends Pharmacol. Sci. 2000b;21:41–42. doi: 10.1016/s0165-6147(99)01436-4. [DOI] [PubMed] [Google Scholar]
- WALKER J.M., HOHMANN A.G., MARTIN W.J., STRANGMAN N.M., HUANG S.M., TSOU K. The neurobiology of cannabinoid analgesia. Life Sci. 1999;65:665–673. doi: 10.1016/s0024-3205(99)00289-1. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., JULIUS I., DI MARZO I., HOGESTATT E.D. Anandamide – the other side of the coin. Trends Pharmacol. Sci. 2000;21:43–44. doi: 10.1016/s0165-6147(99)01430-3. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


