Abstract
M40403 is a low molecular weight, synthetic manganese containing superoxide dismutase mimetic (SODm) that removes superoxide anions (.O2−) without interfering with other reactive species known to be involved in inflammatory responses (e.g. nitric oxide, NO and peroxynitrite, ONOO-).
As such, M40403 represents an important pharmacological tool to dissect the roles of .O2− in acute and chronic inflammation. For this purpose, the pharmacological profile of M40403 was evaluated in carrageenan-induced pleurisy.
Injection of carrageenan into the pleural cavity of rats elicited an acute inflammatory response characterized by: fluid accumulation in the pleural cavity which contained a large number of neutrophils (PMNs) as well as an infiltration of PMNs in lung tissues and subsequent lipid peroxidation, and increased production of nitrite/nitrate (NOx), prostaglandin E2 (PGE2), tumour necrosis factor α, (TNFα), interleukin-1β (IL-1β), interleukin-6 (IL-6) and interleukin-10 (IL-10).
All parameters of inflammation were attenuated by M40403 except for NOx, PGE2 and IL-10 which remained unaltered. Furthermore, carrageenan induced an upregulation of the adhesion molecules ICAM-1 and P-selectin, as well as nitrotyrosine and poly (ADP-ribose) synthetase (PARS) as determined by immunohistochemical analysis of lung tissues.
The degree of staining for the ICAM-1, P-selectin, nitrotyrosine and PARS was reduced by M40403.
These results clearly indicate that .O2− plays a critical role in the development of the inflammatory response by altering key components of the inflammatory cascade. Therefore, synthetic enzymes of SOD such as M40403, offers a novel therapeutic approach for the management of various inflammatory diseases where these radicals have been postulated to play a role.
Keywords: Inflammation, cytokines, adhesion molecules, superoxide anions, superoxide dismutase mimetic
Introduction
Under normal circumstances, formation of superoxide anion (.O2−; the one-electron reduction product of oxygen) is kept under tight control by endogenous superoxide dismutase (SOD) enzymes. These include: the Mn enzyme in mitochondria (SOD2) and Cu/Zn enzyme present in the cytosol (SOD1) or extracellular surfaces (SOD3). The importance of SOD2 is highlighted by the findings that in contrast to SOD1 (Reaume et al., 1996) and SOD3 (Carlsson et al., 1995), SOD2 knockout is lethal to mice (Li et al., 1995; Lebovitz et al., 1996; Melov et al., 1999). In acute and chronic inflammation, the production of .O2− anion is increased at a rate that overwhelms the capacity of the endogenous SOD enzyme defence system to remove them. The result of such imbalance results in .O2− mediated damage. A proposal that .O2− was intimately involved with the inflammatory response was raised as early as the 1970s through the pioneering work of McCord & Fridovich (1969). Some important pro-inflammatory roles for .O2− include: endothelial cell damage and increased microvascular permeability (Droy-Lefaix et al., 1991; Haglind et al., 1994; Xia et al., 1995), formation of chemotactic factors such as leukotriene B4 (Fantone & Ward, 1982; Deitch et al., 1990), recruitment of neutrophils at sites of inflammation (Boughton-Smith et al., 1993; Salvemini et al., 1999Salvemini et al., 1999), lipid peroxidation and oxidation, DNA single-strand damage (Dix et al., 1996) and formation of peroxynitrite (ONOO−), a potent cytotoxic and proinflammatory molecule (Crow & Beckman, 1995; Salvemini et al., 1998a,1998b; 1999a; Beckman et al., 1990; Ischiropoulos et al., 1992; Beckman & Crow, 1993). Most of the knowledge gathered about the roles of superoxide in disease has been collected by the use of the native SOD enzyme and, more recently, by data generated in transgenic animals that overexpress the human enzyme (Huber et al., 1980; Flohe, 1988; Uematsu et al., 1994; Fridovich, 1995). Protective and beneficial roles of SOD have been demonstrated in a broad range of disease, both preclinically and clinically (Halliwell & Gutteridge, 1985; Maxwell, 1995; McCord, 1974). Orgotein® (bovine CuZnSOD) showed promising results as a human therapeutic in acute and chronic conditions including rheumatoid arthritis and osteoarthritis as well as side effects associated with chemotherapy and radiation therapy (Flohe, 1988; Babior, 1982; Niwa et al., 1985). There are drawbacks or problematic issues associated with the use of the native enzymes as therapeutic agents (e.g., solution instability, immunogenicity of non-human enzymes, bell-shaped dose response curves, high susceptibility to proteolytic digestion) and as pharmacological tools (e.g., they do not penetrate cells or cross the blood brain barrier, limiting the dismutation of superoxide only to the extracellular space or compartments).
To overcome the limitations associated with native enzyme therapy we have developed a series of superoxide dismutase mimetics (SODm) that catalytically remove .O2−. M40403 (see chemical structure Figure 1) is a prototypic example of our stable, low molecular weight, manganese-containing, non-peptidic molecules possessing the function and catalytic rate of native SOD enzymes, but with the advantage of being a much smaller molecule (MW 483 vs MW 30,000 for the mimetic and native enzyme, respectively) (Salvemini et al., 1999b). An important property of these SODm is that they catalytically remove superoxide at a high rate without interacting with other reactive species including nitric oxide, peroxynitrite, hydrogen peroxide, oxygen or hydroxyl radicals (Riley et al., 1996; 1997). This property is not shared by other classes of SOD mimetics or scavengers including several metalloporphyrins such as tetrakis-(N-ethyl-2-pyridyl) porphyrin (MnTE-2-PyP) and tetrakis-(benzoic acid) porphyrin (MnTBAP), that interact with other reactive species such as nitric oxide (NO) and ONOO− which clearly play important roles in inflammation (Patel & Day, 1999). Furthermore, SODm are not deactivated by ONOO−, an added advantage over the native MnSOD enzyme that is nitrated and deactivated by ONOO− (Yamakura et al., 1998; Macmillan-Crow & Thompson 1999). We have recently shown that M40403 is anti-inflammatory and protective in ischaemia-reperfusion injury (Salvemini et al., 1999b).
Figure 1.
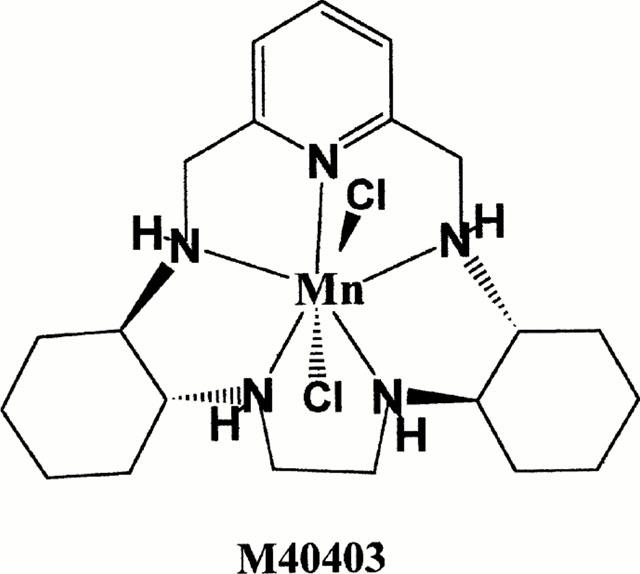
Structure of M40403.
In the current study we have extended our initial observations to a well characterized model of acute pleurisy in rats and show that M40403 inhibits the inflammatory response following the intrapleural injection of carrageenan in rats. Results obtained in this study support the use of SODm as therapeutic agents in diseases associated with overt production of superoxide.
Methods
Animals
Male Sprague-Dawley rats (300 – 350 g; Charles River; Milan, Italy) were housed in a controlled environment and provided with standard rodent chow and water. Animal care was in compliance with Italian regulations on protection of animals used for experimental and other scientific purposes (D.M. 116192) as well as with EEC regulations (O.J. of E.C. L 358/1 12/18/1986)
Carrageenan-induced pleurisy
Rats were anaesthetized with isoflurane and submitted to a skin incision at the level of the left sixth intercostal space. The underlying muscle was dissected and saline (0.2 ml) or saline containing 1% w v−1 λ-carrageenan (0.2 ml), injected into the pleural cavity. The skin incision was closed with a suture and the animals allowed to recover. M40403 (5 – 20 mg kg−1), or an equivalent volume (0.3 ml) of vehicle (26 mM sodium bicarbonate buffer, pH 8.1 – 8.3), was injected intraperitoneally (i.p.) 15 min before carrageenan. At 4 h after the injection of carrageenan, the animals were killed by inhalation of CO2. The chest was carefully opened and the pleural cavity rinsed with 2 ml of saline solution containing heparin (5 u ml−1) and indomethacin (10 μg ml−1). The exudate and washing solution were removed by aspiration and the total volume measured. Any exudate, that was contaminated with blood was discarded. The amount of exudate was calculated by subtracting the volume injected (2 ml) from the total volume recovered. The leukocytes in the exudate were suspended in phosphate-buffer saline (PBS composition (in mM): NaCl 137, KCl 2.7, NaH2PO4 1.4, Na2HPO4 4.3, pH 7.4 and counted with an optical microscope in a Burker's chamber after vital Trypan Blue staining. Cytokines (TNFα, IL-1β, IL-6 and I1-10) and PGE2 were measured in the exudates by ELISA's using commercially available kits (Calbiochem-Novabiochem Corporation, U.S.A.). Levels of NOx in the exudates were measured using the Griess reaction as described below.
Measurement of lung-tissue myeloperoxidase activity and malondialdehyde
Myeloperoxidase (MPO) activity, a haemoprotein located in azurophil granules of neutrophils, has been used as a biochemical marker for neutrophil infiltration into tissues (Bradley et al., 1982). In the present study, MPO was measured photometrically by a method similar to that described previously (Laight et al., 1994). At 4 h following the intrapleural injection of carrageenan, lung tissues were obtained and weighed. Each piece of tissue was homogenized in a solution containing 0.5% w v−1 hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 20,000×g at 4°C. An aliquot of the supernatant was then allowed to react with a solution of tetramethylbenzidine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured spectrophotometrically at 650 nm. MPO activity was defined as the quantity of enzyme degrading 1 μmol of peroxide/min−1 at 37°C and was expressed in milliunits per 100 mg weight of wet tissue. Malondialdehyde (MDA) levels in the lung tissue were determined as an indicator of lipid peroxidation (Ohkawa et al., 1979). Lung tissue, collected at the specified time, were homogenized in 1.15% w v−1 KCl solution. An aliquot (100 μl) of the homogenate was added to a reaction mixture containing 200 μl of 8.1% w v−1 v v−1 SDS, 1500 μl of 20% v v−1 acetic acid (pH 3.5), 1500 μl of 0.8% w v−1 thiobarbituric acid and 700 μl distilled water. Samples were then boiled for 1 h at 95°C and centrifuged at 3,000×g for 10 min. The absorbance of the supernatant was measured by spectrophotometry at 650 nm.
Immunofluorescence localization of ICAM-1, P-selectin, nitrotyrosine and PARS
Indirect immunofluorescence staining was performed on 7 μm thick sections of unfixed rat lung. Sections were cut in with a Slee and London cryostat at −30°C, transferred onto clean glass slides and dried overnight at room temperature. Sections were permeabilized with acetone at −20°C for 10 min and rehydrated in PBS (phosphate buffered saline, 150 mM NaCI, 20 mM sodium phosphate pH 7.2) at room temperature (RT) for 45 min. Sections were incubated overnight with (1) rabbit anti-human polyclonal antibody directed towards P-selectin (CD62P) which reacts with rat and with mouse anti-rat antibody directed at ICAM-1 (CD54) (1 : 500 in PBS, v v−1) (DBA, Milan, Italy) or (2) with anti-nitrotyrosine rabbit policlonal antibody (1 : 500 in PBS, v v−1) or with anti-poly (ADP-Ribose) goat policlonal antibody rat (1 : 500 in PBS, v v−1). Sections were washed with PBS, and incubated with secondary antibody (TRITC-conjugated anti-rabbit and with FITC-conjugated anti-mouse (Jackson, West Grove, PA, U.S.A.) or with TRITC-conjugated anti-goat antibody (1 : 80 in PBS, v v−1) for 2 h at RT. Sections were washed as before, mounted with 90% v v−1 glycerol in PBS, and observed with a Nikon RCM8000 confocal microscope equipped with a 40×oil objective.
Histological examination
Lung biopsies were taken 4 h after injection of carrageenan. The biopsies were fixed for 1 week in buffered formaldehyde solution (10% w v−1 in PBS, composition in mM: NaCl 137, KCl 2.7, NaH2PO4 1.4, Na2HPO4 4.3, pH 7.4) at room temperature, dehydrated by graded ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, U.S.A.). Tissue sections (thickness 7 μm) were deparaffinized with xylene, stained with trichromic Van Gieson and studied using light microscopy (Dialux 22 Leitz).
Isolation of pleural macrophages
Resident pleural cell macrophages were collected 4 h after the carrageenan injection from rats treated with or without M40403 (Cuzzocrea et al., 1998a). After exsanguination, the pleural cavity, was opened and the cells in it were collected by repeated washing with 2 ml of medium (11.8 mM Tris-HCL buffer saline, containing (mM): KCl 2.6, MgCl2 1.0, NaH2PO4 0.4, Glucose 5.4, EDTA 1.5 (pH 7.4). Total leukocyte counts in the exudate were measured with a Neubauer cell-count plate after fixation with Turk's solution. Differential counts of the exuded leukocytes were performed after smear preparation and staining with Wright-Giemsa methods as previously described by Ogino et al. (1996). Cells (106/ml), consisting mainly of macrophages (approximately 70%) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with L-glutamine (3.5 mM), penicillin (50 ml−1), streptomycin (50 μg ml−1) and heparin sodium (10 u ml−1) in 12-well plates. Cells were allowed to adhere for 2 h at 37°C in a humidified 5% w v−1 CO2 incubator. Non-adherent cells were removed by rinsing the plates three times with 5% dextrose water. After removing non-adherent cells (approximately 10%), adherent macrophages were scraped off for the measurement of iNOS activity, DNA single strand breaks and cellular levels of NAD+. In another series of experiments, cells were made to adhere (for 2 h) as described above and the levels of nitrite/nitrate (NOx) and peroxynitrite (ONOO−) formation from the cells was measured in the supernatant.
Measurement of nitrite/nitrate (NOx) and peroxynitrite from pleural macrophages
NOx production, an indicator of NO synthesis, was measured in cell supernatants as described previously (Cuzzocrea et al., 1998a). Briefly, the nitrate in the supernatant was first reduced to nitrite by incubation with nitrate reductase (670 mU ml−1) and NADPH (160 μM) at room temperature for 3 h. The nitrite concentration in the samples was then measured by the Griess reaction, by adding 100 μl of Griess reagent (0.1% naphthylethylenediamide dihydrochloride in H2O and 1% sulphanilamide in 5% w v−1 concentrated H2PO4; vol. 1 : 1) to 100 μl samples. The optical density at 550 nm (OD550) was measured using ELISA microplate reader (SLT-Labinstruments, Salzburg, Austria). The formation of peroxynitrite was measured by the peroxynitrite-dependent oxidation of dihydrorhodamine-123 to rhodamine-123 (Ishiropoulos et al., 1999; Wizemann et al., 1994; Kooy et al., 1994). Briefly, cells were rinsed with PBS and the medium was then replaced with PBS containing 5 μM dihydrorhodamine 123. After a 60 min incubation at 37°C, the fluorescence of rhodamine-123 was measured using a fluorimeter at an excitation wavelength of 500 nm, emission wavelength of 536 nm (slit widths 2.5 and 3.0 nm, respectively).
Measurement of mitochondrial respiration in pleural macrophages
Cell respiration was assessed by measuring the mitochondrial-dependent reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan as described previously (Cuzzocrea et al., 1998a; Darley-Usmar et al., 1995). Briefly, cells in 96-well plates were incubated at 37°C with MTT (0.2 mg ml−1) for 1 h. Culture medium was removed by aspiration and the cells were solubilized in DMSO (100 μl). The extent of reduction of MTT to formazan within cells was quantified by the measurement of OD550.
Determination of DNA single-strand breaks in pleural macrophages
The formation of DNA strand breaks in double-stranded DNA was determined by the alkaline unwinding method (Cuzzocrea et al., 1998a,1998b; Shraufstatter et al., 1986). Cells in 12-well plates were scraped into 0.2 ml of solution A buffer (myoinositol 250 mM, NaH2PO3 10 mM, MgCl2 1 mM, pH 7.2). The cell lysate was then transferred into plastic tubes designated T (maximum fluorescence), P (fluorescence in sample used to estimate extent of DNA unwinding), or B (background fluorescence). To each tube, 0.2 ml of solution B (alkaline lysis solution: NaOH 10 mM, urea 9 M, ethylenediaminetetraacetic acid 2.5 mM, sodium dodecyl sulphate 0.1% w v−1) was added and incubated at 4°C for 10 min to allow cell lysis and chromatin disruption. 0.1 ml each of solutions C (0.45 volume solution B in 0.2 N NaOH) and D (0.4 volume solution B in 0.2 N NaOH) were then added to the P and B tubes. 0.1 ml of solution E (neutralizing solution: glucose 1 M, mercaptoethanol 14 mM) was added to the T tubes before solutions C and D were added. From this point onwards, all incubations were carried out in the dark. A 30 min incubation period at 0°C was then allowed, during which the alkali diffused into the viscous lysate. As the neutralizing solution, solution E, was added to the T tubes before addition of the alkaline solutions C and D, the DNA in the T tubes was never exposed to a denaturing pH. At the end of the 30 min incubation, the contents of the B tubes were sonicated for 30 s to ensure rapid denaturation of DNA in the alkaline solution. All tubes were then incubated at 15°C for 10 min. Denaturation was stopped by chilling to 0°C and adding 0.4 ml of solution E to the P and B tubes. 1.5 ml of solution F (ethidium bromide 6.7 μg ml−1 in 13.3 mM NaOH) was added to all the tubes and fluorescence (excitation: 520 nm, emission: 590 nm) was measured by a fluorimeter. Under the conditions used, in which ethidium bromide binds preferentially to double stranded DNA, the percentage of double stranded DNA (D) may be determined using the equation:
where F(P) is the fluorescence of the sample, F(B) the background fluorescence, i.e. fluorescence due to all cell components other than double stranded DNA, and F(T) the maximum fluorescence.
Measurement of cellular NAD+ levels in pleural macrophages
We measured NAD+ levels as a mean to indirectly evaluate poly(ADP-ribose)synthase (PARS) activation as previously done by others (Carson et al., 1986; Szabò et al., 1997; Hyslop et al., 1988). Cells in 12-well plates were extracted in 0.25 ml of 0.5 N HClO4 scraped, neutralized with 3 M KOH, and centrifuged for 2 min at 10,000×g. The supernatant was assayed for NAD+ using a modification of the colorimetric method (Cuzzocrea et al., 1998a; Zingarelli et al., 1996) in which NADH produced by enzymatic cycling with alcohol dehydrogenase, reduces MTT to formazan through the intermediation of phenazine methasulphate. The rate of MTT reduction is proportional to the concentration of the co-enzyme. The reaction mixture contained 10 μl of a solution of 2.5 mg ml−1 MTT, 20 μl of a solution of 4 mg ml−1 phenazine methosulphate, 10 μl of a solution of 0.6 mg ml−1 alcohol dehydrogenase (300 u mg−1), and 190 μl of 0.065 M glycyl-glycine buffer, pH 7.4, that contained 0.1 M nicotinamide and 0.5 M ethanol. The mixture was warmed to 37°C for 10 min, and the reaction was started by the addition of 20 μl of the sample. The rate of increase in absorbance was read immediately after the addition of NAD+ samples and after 10- and 20-min incubation at 37°C against blank at 560 nm in the ELISA microplate reader (SLT-Labinstruments, Salzburg, Austria).
Determination of nitric oxide synthase activity (pleural macrophages and lung tissue)
The calcium-independent conversion of L-arginine to L-citrulline in the homogenates of either pleural macrophages or lungs (obtained 4 h after carrageenan treatment in the presence or the absence of M40403) served as an indicator of iNOS activity (Szabò et al., 1993). Cells or lung tissue were scraped into a homogenation buffer composed of 50 mM Tris.HCl, 0.1 mM EDTA and 1 mM phenylmethylsulphonyl fluoride (pH 7.4) and homogenized in the buffer on ice using a tissue homogenizer. Conversion of [3H]-L-arginine to [3H]-L-citrulline was measured in the cell/lung homogenates as described previously (Cuzzocrea et al., 1998a). Briefly, homogenates (30 μl) were incubated in the presence of [3H]-L-arginine (10 μM, 5 kBq per tube), NADPH (1 mM), calmodulin (30 nM), tetrahydrobiopterin (5 μM) and EGTA (2 mM) for 20 min at 22°C. Reactions were stopped by dilution with 0.5 ml of ice cold HEPES buffer (pH 5.5) containing EGTA (2 mM) and EDTA (2 mM). Reaction mixtures were applied to Dowex 50 W (Na+ form) columns and the eluted [3H]-L-citrulline activity was measured by a Beckman scintillation counter.
Materials
Cell culture medium, heparin and foetal calf serum were obtained from Sigma (Milan, Italy). Perchloric acid was obtained from Aldrich (Milan, Italy). Primary anti-nitrotyrosine antibody was from Upstate Biotech (DBA, Milan, Italy). All other reagents and compounds used were obtained from Sigma Chemical Company (Sigma, Milan, Italy). The SODm, M40403 was synthesized in house as described previously (Salvemini et al., 1999b).
Data analysis
All values in the figures and text are expressed as mean±standard error of the mean (s.e.m.) for n observations. For the in vitro studies, data represent the number of wells studied (6 – 9 wells from 2 – 3 independent experiments). For the in vivo studies, n represents the number of animals studied. The results were analysed by one-way ANOVA followed by a Bonferroni post-hoc test for multiple comparisons. A P-value less than 0.05 was considered significant. In the experiments involving histology or immunohistochemistry, the figures shown are representative of at least three experiments performed on different experimental days.
Results
Effects of M40403 in carrageenan-induced pleurisy
Histological examination of lung sections revealed significant tissue damage (Figure 2B) when compared with lung sections taken from saline-treated animals (Figure 2A). Histological examination of lung sections from rats treated with carrageenan showed oedema, tissue injury as well as infiltration of the tissue with neutrophils (PMNs) (Figure 2B). M40403 at the highest dose tested (20 mg kg−1, intraperitoneally, i.p.), significantly reduced the degree of injury as well as the infiltration of PMNs (Figure 2C). Furthermore, the injection of carrageenan into the pleural cavity of rats elicited an acute inflammatory response characterized by the accumulation of fluid (oedema) that contained large amounts of PMNs (Figure 3A,B). Neutrophils also infiltrated in the lung tissues (Figure 4A) and this was associated with lipid peroxidation of lung tissues as evidenced by an increase in the levels of malonyldialdehyde (Figure 4B). Oedema, neutrophil infiltration in lung tissue and lipid peroxidation were attenuated in a dose-dependent fashion by the intraperitoneal injection of M40403 (5 – 20 mg kg−1, n=10) (Figures 3 and 4).
Figure 2.
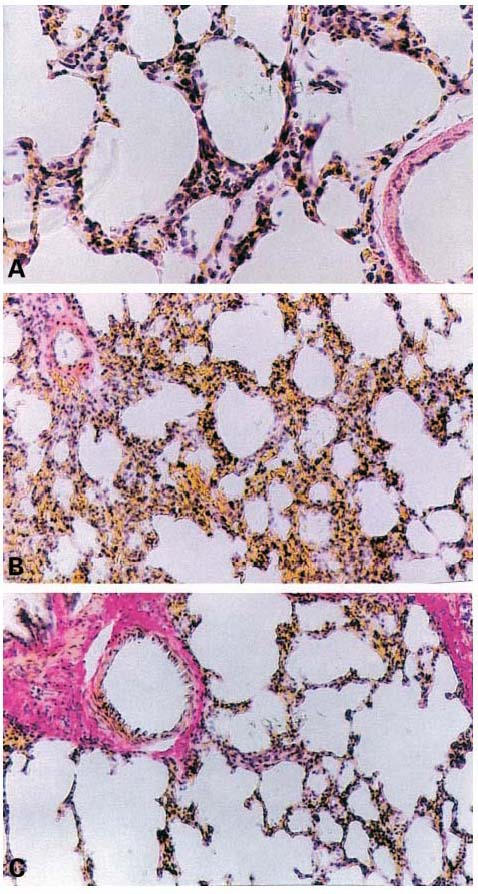
Effect of M40403, on lung injury: When compared to lung sections taken from control animals (A), lung sections from carrageenan-treated rats (B) demonstrates interstitial haemorrhage and polymorphonuclear leukocyte accumulation. Lung sections from a carrageenan-treated rat that had received M40403, (20 mg kg−1, intraperitoneally) (C) exhibit reduced interstitial haemorrhage and a lesser cellular infiltration. Original magnification:×62.5. Figure is representative of at least three experiments performed on different experimental days.
Figure 3.
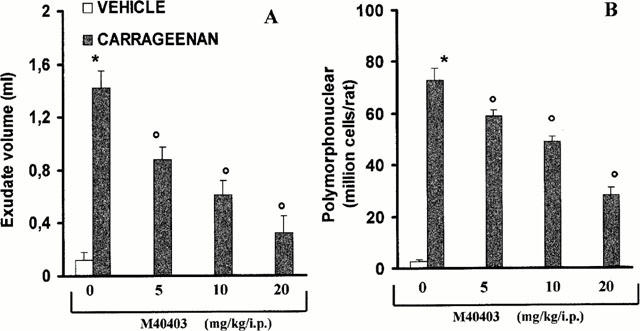
Effect of M40403, on carrageenan-induced inflammation: The increase in volume exudate (A) and accumulation of polymorphonuclear cells (PMNs, B) in pleural cavity at 4 h after carrageenan injection was inhibited in a dose-dependent manner by M40403 (5 – 20 mg kg−1, intraperitoneally). Each value is the mean±s.e.mean for n=10 experiments. *P<0.01 vs sham. oP<0.01 versus carrageenan.
Figure 4.
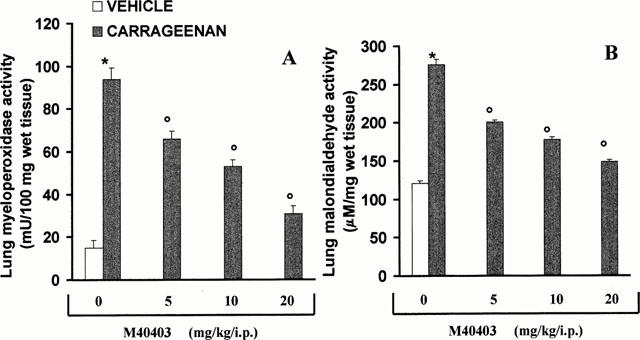
Effect of M40403, on myeloperoxidase (MPO) activity and malondialdehyde (MDA) levels in the lung. Within 4 h, pleural injection of carrageenan led to an increase in neutrophil accumulation in the lung (as measured by MPO activity, A) an effect that was associated with increased lipid peroxidation of lung tissue (as measured by MDA, B). M40403 inhibited in a dose-dependent (5 – 20 mg kg, intraperitoneally) fashion neutrophil infiltration and lipid peroxidation. Each value is the mean±s.e.mean for n=10 experiments. *P<0.01 when compared to control rats and oP<0.01 when compared with rats treated with carrageenan in the absence of M40403.
Effects of M40403 on the expression of adhesion molecules (ICAM-1, P-selectin)
Staining of lung tissue sections obtained from saline-treated rats with anti-ICAM-1 antibody showed a specific staining along bronchial epithelium (arrows), demonstrating that ICAM-1 is constitutively expressed (Figure 5A). No positive staining for P-selectin was found in lung tissue section from saline-treated rats (Figure 5B). At 4 h after carrageenan injection, the staining intensity substantially increased along the bronchial epithelium (Figure 5C; see arrows). Lung tissue sections obtained from carrageenan-treated rats showed positive staining for P-selectin localized in the bronchial epithelium (Figure 5D, see arrows). No positive staining for ICAM-1 or P-selectin was found in the lungs of carrageenan-treated rats that received intraperitoneal injection of M40403 (20 mg kg−1) (Figure 5E,F). To verify the binding specificity for ICAM-1 or P-selectin, some sections were also incubated with only the primary antibody (no secondary) or with only the secondary antibody (no primary). In these situations no positive staining was found in the sections indicating that the immunoreaction was positive in all the experiments carried out.
Figure 5.
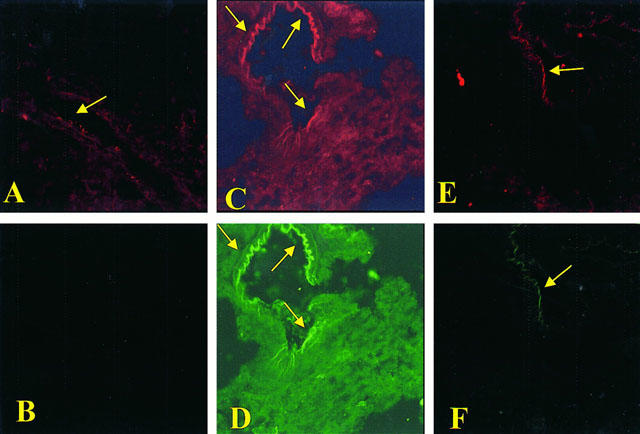
Immunohistochemical localization of ICAM-1 and P-selectin in the lung. Staining of lung tissue sections obtained from sham-operated rats with anti-ICAM-1 antibody showed a specific staining along bronchial epithelium (arrows), demonstrating that ICAM-1 is constitutively expressed (A). Lung section from sham-operated rats revealed no positive staining for P-selectin (B). Section obtained from carrageenan-treated rats showed intense positive staining for ICAM-1 (C) and for P-selectin (D) on bronchial epithelium (arrows). The degree of bronchial epithelium staining for ICAM-1 (E) and for P-selectin (F) was markedly reduced in tissue section obtained from M40403-treated rats (20 mg kg−1, intraperitoneally). Original magnification:×100. Figure is representative of at least three experiments performed on different experimental days.
Effects of M40403 on nitrotyrosine and PARS
At 4 h after carrageenan injection, lung sections were taken in order to determine the immunohistological staining for nitrotyrosine or PARS. Sections of lung from saline-treated rats did not reveal any immunoreactivity for nitrotyrosine (Figure 6A) or for PARS (Figure 6B) within the normal architecture. A positive staining for nitrotyrosine (Figure 6C) and for PARS (Figure 6D) was found primarily localized in the vessels (big arrows) and in the bronchial epithelium (small arrows) of carrageenan treated animals. M40403 (20 mg kg−1, i.p.) reduced the staining for both nitrotyrosine and PARS (Figure 6E,F). In order to confirm that the immunoreaction for the nitrotyrosine was specific some sections were also incubated with the primary antibody (anti-nitrotyrosine) in the presence of excess nitrotyrosine (10 mM) to verify the binding specificity. To verify the binding specificity for PARS, some sections were also incubated with only the primary antibody (no secondary) or with only the secondary antibody (no primary). In these situations, no positive staining was found in the sections indicating that the immunoreaction was positive in all the experiments carried out.
Figure 6.
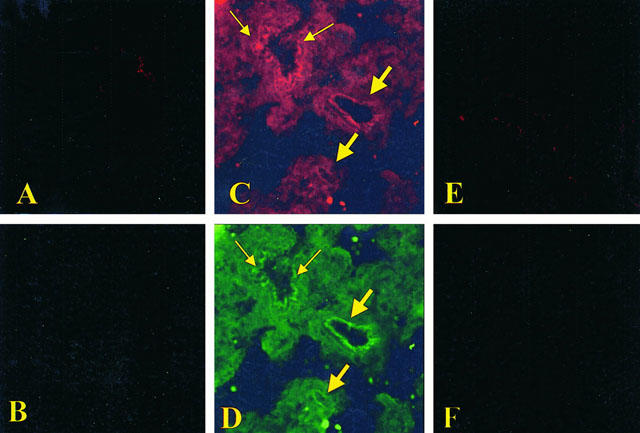
Immunohistochemical localization for nitrotytosine and for PARS in the lung. No positive staining for nitrotyrosine (A) and for PARS (B) was found in the lung section from sham-administered rats. Immunohistochemistry for nitrotyrosine (C) and for PARS (D) show positive staining along the vessels (big arrows) and in the bronchial epithelium (small arrows) from a carrageenan-treated rats. The intensity of the positive staining for nitrotyrosine (E) and for PARS (F) was significantly reduced in the lung from M40403-treated rats (20 mg kg−1, intraperitoneally). Original magnification:×100. Figure is representative of at least three experiments performed on different experimental days.
Effects of M40403 on the release of cytokine, nitrate/nitrite (NOx) and PGE2
A significant increase in the levels of TNFα, IL-1β, IL-6 and IL-10 was observed in pleural exudates from carrageenan-treated (Figure 7A,B). At the highest dose tested, M40403 (20 mg kg−1) attenuated the release of TNFα, IL-1β and IL-6 (Figure 7A,B), but had no effect on IL-10 (if anything a slight increase was observed) (Figure 7A). Furthermore, a significant increase in NOx and PGE2 concentration was found in pleural exudates (from 5±1 to 15±3 nmol NOx ml−1 exudate (n=10), and from 0 to 250±10 pg PGE2 ml−1 exudate (n=10). M40403 had no effect on NOx or PGE2 release (from 15±3 to 20±2 nmol NOx ml−1 pleural exudate and from 250±10 to 200±20 pg PGE2 ml−1 pleural exudate in rats treated with carrageenan alone and rats treated with carrageenan in the presence of M40403 respectively, (n=10).
Figure 7.
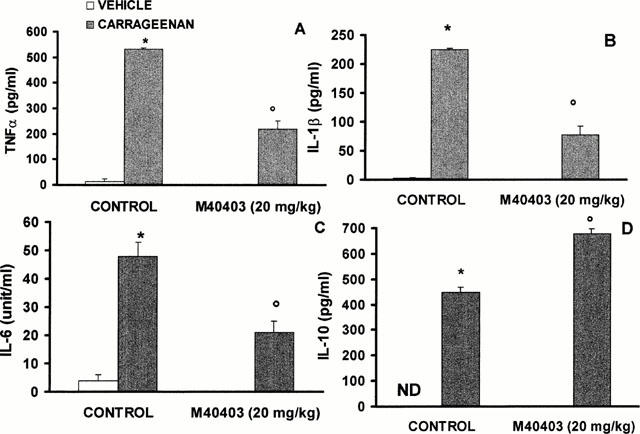
Pleural injection of carrageenan caused by 4 h an increase in the release of the cytokines, tumour necrosis factor alpha (TNFα, A), interleukin-1β (IL-1β, B), interleukin-6 (IL-6, C) and interleukin-10 (IL-10, D). When tested at the highest dose, M40403 (20 mg kg−1, intraperitoneally) inhibited TNFα, IL-1β and IL-6 (A – C) but had no effect on IL-10 (D). Each value is the mean±s.e.mean for n=10 experiments. *P<0.01 vs control and oP<0.01 vs carrageenan treated in the absence of M40403.
Effects of M40403 on nitrate/nitrite (NOx) and peroxynitrite formation from pleural macrophages
Carrageenan induced the expression of inducible NOS in macrophages isolated from the pleural cavity (from 5±1 to 29±1 fmol mg−1 min−1 for macrophages taken from non-injected rats or injected with carrageenan respectively n=10, P<0.01). This induction was, in turn, associated with the release of NOx into the pleural cavity (Figure 8A). At the highest dose tested (20 mg kg−1), intraperitoneal injection of M40403 (5 – 20 mg kg−1) had no effect on the release of NOx (Figure 8A) nor did it inhibit the activity of iNOS (from 29±1 to 31±1 fmol mg−1 min−1 for macrophages taken from rats injected with carrageenan alone or carrageenan with M40403 (n=10), P<0.5). When compared to the supernatant of macrophages collected from the pleural cavity of sham-operated animals, the supernatant of macrophages obtained from carrageenan-treated rats showed a significant increase in the concentration of peroxynitrite (Figure 8B). As expected, M40403 (5 – 20 mg kg−1, i.p.) attenuated peroxynitrite formation from pleural macrophages in a dose-dependent manner (Figure 8B).
Figure 8.
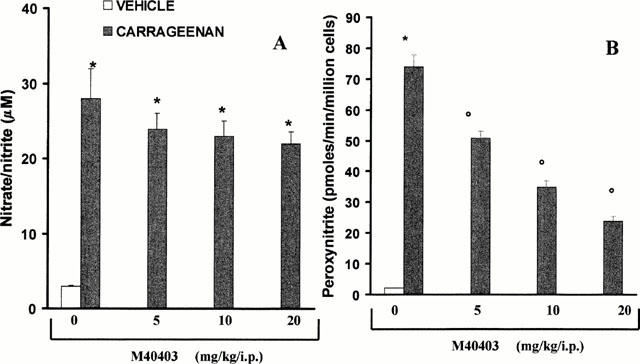
Effect of M40403, on nitrite/nitrate (NOx) (A) and peroxynitrite (B) formation by macrophage ex vivo. Production of NOx release was unaffected by pre-treatment of rats with M40403, (5 – 20 mg kg−1, intraperitoneally) (A). On the other hand, M40403 (5 – 20 mg kg−1) caused a dose-dependent inhibition of peroxynitrite production (B). Each value is the mean±s.e.mean for n=10 experiments. *P<0.01 when compared to control macrophages and oP<0.01 when compared to macrophages taken from M40404-treated rats injected with carrageenan in the presence of M40403.
Effects of M40403 on DNA damage and injury of pleural macrophages
When compared to normal rats, a significant increase in the occurrence of single-strand breaks in DNA was seen in pleural macrophages taken from rats that received an injection of carrageenan (Figure 9A). Furthermore, a reduction in mitochondrial respiration (Figure 9B) as well as a significant fall in the intracellular levels of NAD+ (Figure 9C) was observed in these cells. M40403 had significant protective effects on all parameters of damage (Figure 9A – C).
Figure 9.
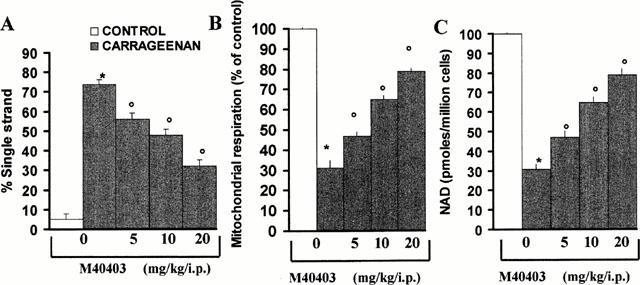
Effect of M40403, on DNA damage and cellular dysfunction in macrophages taken from carrageenan-challenged rats. When evaluated at 4 h post carrageenan injection, a significant damage of DNA (as measured by DNA single strand breakage) was observed in pleural macrophages when compared to control macrophages (A). In addition to this damage, a reduction of mitochondrial respiration and cellular levels of NAD+ was also seen. M40403 (5 – 20 mg kg−1, intraperitoneally) attenuated DNA damage (A) and restored cellular respiration (B) and intra-cellular levels of NAD+ (C). *P<0.01 when compared to control macrophages and oP<0.01 when compared to macrophages taken from M40403-treated rats injected with carrageenan in the presence of M40403.
Discussion
Reactive oxygen and nitrogen species including nitric oxide, superoxide and the product of their reaction, peroxynitrite, are involved in acute and chronic inflammation. The relative contribution of each of these species is becoming increasingly substantiated through the development of selective agents that either inhibit their formation or remove them. The use of native SOD enzymes both preclinically and clinically shed light on the importance of .O2− in disease and, thus, the therapeutic potential of exogenous SOD enzymes (Huber et al., 1980; Flohe, 1988; Uematsu et al., 1994). Because of the limitations associated with the native enzymes, strategies to develop low molecular weight molecules that selectively remove .O2− for therapeutic application has been sought.
While a number of synthetic metal complexes such as the MnIII or FeIII Porphyrins or the MnIII(Salen) complexes have been claimed to possess SOD activity, they also react with all of the other pertinent biological oxidants such as H2O2, OONO−, NO. (Patel & Day, 1999). Such compounds have been tested in several animal models of disease (Patel & Day, 1999). However, it is difficult from those studies to decipher the role of superoxide using such non-selective redox catalysts – they lack specificity. This is in contrast to the selective MnII mimics of the biscyclohexylpyridine class such as M40403 whose unique selectivity resides in the nature of the manganese(II) centre in the complex. The resting oxidation state of the complex is the reduced state, Mn(II); as a consequence, the complex has no reactivity with reducing agents until it is oxidized to Mn(III) by protonated superoxide, whereupon, the complex is rapidly reduced back to the Mn(II) state by the superoxide anion at diffusion-controlled rates. Since the complex is so difficult to oxidize (+0.78 v (SHE)) many one-electron oxidants cannot oxidize (including nitric oxide and oxygen) this and its related complexes. Further, since M40403 operates via a facile one-electron oxidation pathway other two-electron non-radical, but nevertheless, potent oxidants are not kinetically competent to oxidize the Mn(II) complex; e.g., OONO−, H2O2, OCl−.
Here we have shown that superoxide anions plays a major role in the development of inflammation and that their removal by the synthetic SOD enzyme M40403 is anti-inflammatory. The anti-inflammatory profile of such agents warrants their development as clinical candidates for inflammatory diseases.
A mechanism by which M40403 attenuates inflammation is by reducing ONOO− formation by simply removing .O2− before it reacts with NO. This is important since the pro-inflammatory and cytotoxic effects of ONOO− are numerous (Squadrito & Pryor 1995; Salvemini et al., 1998a). Removal of ONOO− by agents such as FeTMPS, a porphyrin-containing molecule which increases the rate of isomerization of ONOO− to nitrate is cytoprotective and anti-inflammatory (Salvemini et al., 1996a,1996b; 1998a,1998b; Stern et al., 1996; Misko et al., 1998). Peroxynitrite also nitrosates tyrosine residues in proteins and nitrotyrosine formation along with its detection by immunofluorescence has been used as a marker for the detection of the endogenous formation of peroxynitrite (Beckman, 1996). Using nitrotyrosine as a marker for the presence of ONOO− has been challenged by the demonstration that other reactions can also induce tyrosine nitration; e.g., the reaction of nitrite with hypochlorous acid and the reaction of myeloperoxidase with hydrogen peroxide can lead to the formation of nitrotyrosine (Eiserich et al., 1998). Thus, increased nitrotyrosine staining is considered, as an indicator of ‘increased nitrosative stress' rather than a specific marker of the generation of peroxynitrite (Eiserich et al., 1998). We have found that nitrotyrosine is indeed present in lung sections taken after carrageenan injection and that M40403 reduced the staining in these tissues. Based on these findings, we conclude that carrageenan evoked, in part, a superoxide-driven ONOO− formation that was, in turn, responsible for the formation of nitrotyrosine. The fact that there is residual nitration can be explained by the presence of neutrophils in the lung. A similar pattern of immunoreactivity for nitrotyrosine is observed in a lung model of ischaemia (Ischiropoulos et al., 1995). Macrophages harvested from pleural exudates generated substantial amounts of ONOO− and this was attenuated by some 60% with M40403. .O2− and peroxynitrite can also cause DNA single-strand damage which is the obligatory trigger for PARS activation (Inoue & Kawanishi, 1995; Salgo et al., 1998) resulting in the depletion of its substrate NAD+ in vitro and a reduction in the rate of glycolysis. Since NAD+ functions as a cofactor in glycolysis and the tricarboxylic acid cycle, NAD+ depletion leads to a rapid fall in intracellular ATP and, ultimately, cell injury (Szabò & Dawson, 1998). Furthermore, substantial evidence exists to support the fact that PARS activation is important in inflammation (Szabò, 1999). PARS inhibitors such as nicotinamide and 3-aminobenzamide attenuate both acute and chronic inflammatory processes (Cuzzocrea et al., 1998b; Szabò et al 1997; Szabò & Dawson, 1998; Szabò, 1999; Eiserich et al., 1998). As shown in Figures 5 and 8, M40403 reduced PARS immunofluorescence and attenuated the reduction of NAD+. The overall effect of M40403 was a significant protection of cellular viability (Figure 8). In light of the role of PARS in inflammation, it is possible that PARS inhibition by M40403 accounts for its anti-inflammatory response.
Besides attenuating ONOO− production and PARS activation, M40403 also reduced the development of oedema, neutrophil accumulation and lipid peroxidation and had an overall protective effect on the degree of lung injury as assessed by histological examination. In previous studies, superoxide, has been found to increase both neutrophil infiltration and adhesion (Hirschelmann & Bekemser, 1981; Scraufstatter et al., 1987; Warren et al., 1990). A possible mechanism by which M40403 attenuates PMNs infiltration is by down-regulating adhesion molecules ICAM-1 and P-selectin. Other adhesion molecules may also be affected by superoxide. For instance, SOD enzyme attenuates monocyte infiltration in glomeruli post endotoxin; an effect associated with an attenuation of the expression of various adhesion molecules including glomerular ICAM-1 and VCAM-1 and leukocyte LFA-1 and VLA-4 (Faas et al., 1998).
.O2− plays a role in the regulation of cytokine release since M40403 inhibited the release of the pro-inflammatory cytokines, TNFα and IL-1β in acute inflammation and post ischaemia-reperfusion injury (Salvemini et al., 1999b, this study). Furthermore, M40403 attenuated IL-6 release an important cytokine that plays a role in the inflammatory response associated with pleurisy. Thus, IL-6 knockout mice are resistant to the acute inflammation of the lung caused by carrageenan (Cuzzocrea et al., 1999). Our data is substantiated by a recent report which demonstrates that superoxide anions (generated from xanthine-xanthine oxidase) increase TNFα release from macrophages (Volk et al., 1999). Besides pro-inflammatory cytokines, inflammation is associated with the release of anti-inflammatory cytokines in an attempt to regulate the extent and severity of the response. Release of IL-10, a major anti-inflammatory cytokine, was not affected by M40403.
Injection of carrageenan in the pleural cavity of rats induces the expression of iNOS and COX-2 that in turn release large amounts of pro-inflammatory NOx and PGs (Tomlinson et al., 1994; Hatanaka et al., 1999). Consistant with our previous findings (Salvemini et al., 1999b), M40403 had no effect on the activity of iNOS nor on the release of NOx or PGE2, indicating that the pro-inflammatory effects of superoxide do not involve NO or PGE2. The fact that M40403 did not affect PGE2 release furthers our understanding of the role of reactive species in the activation of COX enzymes. NO activates both COX-1 and COX-2 (Salvemini et al., 1993) leading to an exaggerated production of pro-inflammatory PGE2 and dual inhibition of both NO and NO-driven COX-2 activation accounts for the anti-inflammatory effect of NOS inhibitors (Salvemini et al., 1995; 1996a,1996b). Since the discovery of the ability of NO to activate COX enzymes several hypotheses have been put forward to explain the increased production of PGs by NO (Salvemini, 1997). The possible role of ONOO− in the activation of COX (Landino et al., 1997) cannot be supported by the present study. Indeed, if ONOO− did activate COX, M40403 (by removing .O2 and reducing ONOO−) should have reduced PGE2 but this was not observed. The relative roles of NO and ONOO− in COX activation remains to be fully elucidated.
Data generated from the present study indicate that selective removal of superoxide is anti-inflammatory. These findings support: (1) the concept that superoxide is a crucial mediator of inflammation and, (2) the potential use of SODm as therapeutic agents in inflammation. To date, it is unclear by which mechanism(s) .O2− modulates these events. An attractive possibility would be the activation of transcription factors such as NFkB or AP-1 which in turn regulate a variety of genes that encode for pro-inflammatory cytokines, chemokines, inflammatory enzymes, adhesion molecules and receptors and as such play pivotal roles in inflammation (Bauerle & Henkel, 1994; Barnes & Karin, 1997). At present, we cannot assess the relative contribution of ONOO− in the overall pro-inflammatory effect of .O2−. This area will be expanded as novel and selective inhibitors/catalysts for ONOO− become available. Nevertheless in light of the known pro-inflammatory and cytotoxic activity of ONOO−, its attenuation by M40403 is clearly an added benefit to the overall property of the molecule.
The challenge in the future will be to understand the signal transduction mechanisms used by superoxide to modify key components of the inflammatory response as this will undoubtedly elucidate important molecular targets for future pharmacological intervention.
Acknowledgments
This study was supported by grant from Consiglio Nazionale delle ricerche. The authors would like to thank Giovanni Pergolizzi and Carmelo La Spada for their excellent technical assistance during this study, Mrs Caterina Cutrona and Deborah A. Schaller for secretarial assistance and Miss Valentina Malvagni for editorial assistance with the manuscript. We would also like to thank Drs H. Ischiropoulos (Stokes Research Institute, Philadelphia, U.S.A.), T.P. Misko and J.A. Sikorski (G.D. Searle, St. Louis MO, U.S.A.) for useful discussions and critical evaluation of this manuscript.
Abbreviations
- iNOS
inducible nitric oxide synthase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-10
interleukin-10
- MPO
myeloperoxidase
- NO
nitric oxide
- PMNs
polymorphonuclear cells
- PGE2
prostaglandin E2
- .O2−
superoxide anions
- SODm
superoxide dismutase mimetic
- TNFα
tumour necrosis factor α
References
- BABIOR B.M. Superoxide: a two-edged sword. Braz. J. Med. Biol. Res. 1982;30:141–155. doi: 10.1590/s0100-879x1997000200001. [DOI] [PubMed] [Google Scholar]
- BAEUERLE P.A., HENKEL T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-kappa B: a pivotal transcription factor in chronic inflammatory diseases. New Engl. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S. Oxidative damage and tyrosine nitration from peroxynitrite. Chem. Res. Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., CROW L.P. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans. 1993;21:330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., BECKMAN T.W., CHEN J., MARSHALLAND P.A., FREEMAN B.A. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., EVANS S.M., LASZLO F., WHITTLE B.J., MONCADA S. The induction of nitric oxide synthase and intestinal vascular permeability by endotoxin in the rat. Br. J. Pharmacol. 1993;110:1189–1195. doi: 10.1111/j.1476-5381.1993.tb13940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY P.P., PRIEBAT D.A., CHRISTENSEN R.D., ROTHSTEIN G. Measurement of cutaneous inflammation. Estimation of neutrophil content with an enzyme marker. J. Clin. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- CARLSSON L.M., JONSSON J., EDLUNDAND T., MARKLUND S.L. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSON D.A., SETO S., WASSON B., CARRERA C.J. DNA strand breaks, NAD metabolism, and programmed cell death. Exp. Cell. Res. 1986;164:273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- CROW J.P., BECKMAN J.S. The role of peroxynitrite in nitric oxide-mediated toxicity. Current Top Microbiol. Immunol. 1995;196:57–73. doi: 10.1007/978-3-642-79130-7_7. [DOI] [PubMed] [Google Scholar]
- CUZZOCREA S., CAPUTI A.P., ZINGARELLI B. Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology. 1998a;93:96–101. doi: 10.1046/j.1365-2567.1998.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUZZOCREA S., SAUTEBIN L., DE SARRO G., COSTANTINO G., ROMBOLA L., MAZZON E., IALENTI A., DE SARRO A., DI ROSA M., CAPUTI A.P., THIEMERMANN C. Role of IL-6 in the pleurisy and lung injury caused by carrageenan. J. Immunol. 1999;163:5094–5104. [PubMed] [Google Scholar]
- CUZZOCREA S., ZINGARELLI B., GILARD E., HAKE P., SALZMAN A.L., SZABÓ C. Protective effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthase in carrageenan-induced models of local inflammation. Eur. J. Pharmacol. 1998b;342:67–76. doi: 10.1016/s0014-2999(97)01417-9. [DOI] [PubMed] [Google Scholar]
- DARLEY-USMAR V., WISEMAN H., HALLIWELL B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–136. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- DEITCH E.A., BRIDGES W., BERG R., SPECIAN R.D., GRANGER N. Hemorrhagic Shock-induced Bacterial Translocation: The role of neutrophils and hydroxyl radicals. J. Trauma. 1990;30:942–951. doi: 10.1097/00005373-199008000-00002. [DOI] [PubMed] [Google Scholar]
- DIX T.A., HESS K.M., MEDINA M.A., SULLIVAN R.W., TILLY S.L., WEBB T.L.L. Mechanism of site-selective DNA nicking by the hydrodioxyl (perhydroxyl) radical. Biochemistry. 1996;35:4578–4583. doi: 10.1021/bi952010w. [DOI] [PubMed] [Google Scholar]
- DROY-LEFAIX M.T., DROUET Y., GERAUD G., HOSFOD D., BRAQUET P. Superoxide dimutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia-reperfusion. Free Rad. Res. Commun. 1991;12–13:725–735. doi: 10.3109/10715769109145852. [DOI] [PubMed] [Google Scholar]
- EISERICH J.P., HRISTOVA M., CROSS C.E., JONES A.D., FREEMAN B.A., HALLIWELL B., VAN DER VLIET A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- FAAS M.M., SCHUILING G.A., VALKHOF N., BALLER J.W., BAKKER W.W. Superoxide-mediated glomerulopathy in the endotoxin-treated pregnant rat. Kidney Blood Press Res. 1998;21:432–437. doi: 10.1159/000025896. [DOI] [PubMed] [Google Scholar]
- FANTONE J.C., WARD P.A. A review: role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am. J. Pathol. 1982;107:395–418. [PMC free article] [PubMed] [Google Scholar]
- FLOHE L. Superoxide dismutase for therapeutic use: clinical experience, dead ends and hopes. Mol. Cell. Biochem. 1988;84:123–131. doi: 10.1007/BF00421046. [DOI] [PubMed] [Google Scholar]
- FRIDOVICH I. Superoxide radical and superoxide dismutases. Annual Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- HAGLIND E., XIA G., RYLANDER R. Effects of antioxidants and PAF receptor antagonist in intestinal shock in the rat. Circ. Shock. 1994;42:83–91. [PubMed] [Google Scholar]
- HALLIWELL B., GUTTERIDGE J.M.C. Free Radicals in Biology and Medicine 1985Oxford University Press; 89–193.ed. Baum H., Gergely J. & Fanburg B.L. pp [Google Scholar]
- HATANAKA K., KAWAMURA M., OGINO K., MATSUO S., HARADA Y. Expression and function of cyclooxygenase-2 in mesothelial cells during late phase of rat carrageenin-induced pleurisy. Life Sci. 1999;65:161–166. doi: 10.1016/s0024-3205(99)00384-7. [DOI] [PubMed] [Google Scholar]
- HIRSCHELMANN R., BEKEMSER H. Effects of catalase, peroxidase, superoxide dismutase and 10 scavengers of oxygen radicals in carrageenan edema and in adjuvant arthritis of rats. Experientia. 1981;37:1313–1314. doi: 10.1007/BF01948381. [DOI] [PubMed] [Google Scholar]
- HUBER W., MENANDER-HUBER K.B., SAIFER M.G.P., WILLIAMS L.D. Bioavailability of superoxide dismutase: implications for the anti-inflammatory action mechanism of orgotein. A.A.S. 1980;7:185–195. [PubMed] [Google Scholar]
- HYSLOP P.A., HINSHAW D.B., HALSEY W.A., SCHRAUFSTATTER I.U., SAUERHEBER R.D., SPRAGG R.G., JACKSON J.H., COCHRANE C.G. Mechanisms of oxidant-mediated cell injury: The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J. Biol. Chem. 1988;263:1665–1675. [PubMed] [Google Scholar]
- INOUE S., KAWANISHI S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., AL-MEHDI A.B., FISHER A.B. Reactive species in ischemic rat lung injury: contribution of peroxynitrite. Am. J. Physiol. 1995;269:L158–L164. doi: 10.1152/ajplung.1995.269.2.L158. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., GOW A., THOM S.R., KOOY N.W., ROYALL J.A., CROW J.P. Detection of recative nitrogen species using 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123. Met. Enzymol. 1999;301:367–373. doi: 10.1016/s0076-6879(99)01100-3. [DOI] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., BECKMAN I.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- KOOY N.W., ROYALL J.A., ISCHIROPOULOS H., BECKMAN J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Rad. Biol. Med. 1994;16:149–156. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- LAIGHT D.W., LAD N., WOODWARD B., WATERFALL J.F. Assessment of myeloperoxidase activity in renal tissue after ischemia/reperfusion. Eur. J. Pharmacol. 1994;292:81–88. doi: 10.1016/0926-6917(94)90029-9. [DOI] [PubMed] [Google Scholar]
- LANDINO L.M., CREWS B.C., TIMMONS M.D., MORROW J.D., MARNETT L.J. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 1997;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBOVITZ R.M., ZHANG H., VOGEL H., CARTWRIGHT J., JR, DIONNE L., LU N., HUANG S., MATZUK M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y., HUANG T.T., CARLSON E.J., MELOV S., URSELL P.C., OLSON J.L., NOBLE L.J., YOSHIMURA M.P., BERGER C., CHAN P.H. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- MACMILLAN-CROW L.A., THOMPSON J.A. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch. Biochem. Biophys. 1999;66:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- MAXWELL S.R.J. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- MCCORD J.M., FRIDOVICH I. Superoxide dismutase: an enzymatic function for erythrocuprein. J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- MCCORD J.M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- MELOV S., COSKUN P., PATEL M., TUINSTRA R., COTTRELL B., JUN A.S., ZASTAWNY T.H., DIZDAROGLU M., GOODMAN S.I., HUANG T.T., MIZIORKO H., EPSTEIN C.J., WALLACE D.C. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. U.S.A. 1999;96:846–851. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISKO T.P., HIGHKIN M.K., VEENHUIZEN A.W., MANNING P.T., STERN M.K., CURRIE M.G., SALVEMINI D. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J. Biol. Chem. 1998;273:15646–15653. doi: 10.1074/jbc.273.25.15646. [DOI] [PubMed] [Google Scholar]
- NIWA Y., SOMIYA K., MICHELSO A.M., PUGET K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free Rad. Res. Comms. 1985;1:137–153. doi: 10.3109/10715768509056547. [DOI] [PubMed] [Google Scholar]
- OGINO M., MAJIMA M., KAWAMURA M., HATANAKA K., SAITO M., HARADA Y., KATORI M. Increased migration of neutrophils to granulocyte-colony stimulating factor in rat carrageenin-induced pleurisy: roles of complement, bradykinin, and inducible cyclooxygenase-2. Inflamm. Res. 1996;45:335–346. doi: 10.1007/BF02252946. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI H., YAGI K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Analytical Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- PATEL M., DAY B.J. Metalloporphyrin class of therapeutic catalyic antioxidants. TiPS. 1999;20:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- REAUME A.G., ELLIOTT J.T., HOFFMAN E.K., KOWALL N.W., FERRANTE R.J., SIWEK D.F., WILCOX H.M., FLOOD D.G., BEAL M.F., BROWN R.H., JR, SCOTT R.W., SNIDER W.D. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- RILEY D.P., HENKE S.L., LENNON P.J., WEISS R.H., NEUMANN W.L., RIVERS W.J., ASTON K.W., SAMPLE K.R., LING C.S., SHIEH J.J., BUSCH D.H., SZULBINSKI W. Synthesis, characterization and stability of manganese (II) C-substituted 1,4,7,10,13-Pentaazacyclopentadecane complexes exhibiting superoxide dismutase activity. Inorg. Chem. 1996;35:5213–5231. [Google Scholar]
- RILEY D.P., LENNON P.J., NEUMANN W.L., WEISS R.H. Toward the rational design of superoxide dismutase mimics: Mechanistic studies for the elucidation of substituent effects on the catalysis activity of macrocyclic manganese (II) complexes. J. Am. Chem. Soc. 1997;119:6522. [Google Scholar]
- SALGO M.G., BERMUDEZ E., SQUADRITO G., PRYOR W. DNA damage and oxidation of thiols peroxynitrite causes in rat thymocytes. Arch. Biochem. Biophys. 1998;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- SALVEMINI D. Regulation of cyclooxygenase enzymes by nitric oxide. Cell Mol. Life Sci. 1997;53:576–582. doi: 10.1007/s000180050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., JENSEN M.P., RILEY D.P., MISKO T.P. Therapeutic manipulations of peroxynitrite. Drug News and Perspectives. 1998a;11:204–214. [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MARINO M.H., SEIBERT K.Activation of the cyclooxygenase pathway by nitric oxide: new concepts of inflammation and therapy Drug News and Perspectives 1996aScience Publisher; 204–219.ed. Prous, J.R. [Google Scholar]
- SALVEMINI D., MANNING P.T., ZWEIFEL B.S., SEIBERT K.. Dual inhibition of nitric oxide and prostaglandin production contributes to the anti-inflammatory properties of nitric oxide synthase inhibitors. J. Clin. Invest. 1995;96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., RILEY D.P., LENNON P.J., WANG Z.Q., CURRIE M.G., MACARTHUR H., MISKO T.P. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br. J. Pharmacol. 1999a;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., STERN M.K., CURRIE M.G., MISKO T.P. Peroxynitrite decomposition catalysts: novel therapeutics for peroxynitrite-mediated pathology. Proc. Natl. Acad. Sci. U.S.A. 1998b;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., WYATT P., BOURDON D.M., MARINO M.H., MANNING P.T., CURRIE M.G. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br. J. Pharmacol. 1996b;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., WANG Z.Q., ZWEIER J.L., SAMOUILOV A., MACARTHUR H., MISKO T.P., CURRIE M.G., CUZZOCREA S., RILEY D. Synzymes: potent non-peptidic agents against superoxide-driven tissue injury. Science. 1999b;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., HINSHAW D.B., HYSLOP P.A., SPRAGG R.G., COCHRANE C.G. Oxidant injury of cells: DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J. Clin. Invest. 1986;77:1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHRAUFSTATTER I.U., HYSLOP P.A., JACKSON J., COCHRANE C.C. Oxidant injury of cells. Int. J. Tissue React. 1987;9:317–324. [PubMed] [Google Scholar]
- SQUADRITO G.L., PRYOR W.A. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chemico-Biological Interactions. 1995;96:203–206. doi: 10.1016/0009-2797(94)03591-u. [DOI] [PubMed] [Google Scholar]
- STERN M.K., JENSEN M.P., KRAMER K. Peroxynitrite decomposition catalysts. J. Am. Chem. Soc. 1996;118:8735–8736. [Google Scholar]
- SZABÓ C., LIM L.H.K., CUZZOCREA S., GETTING S.J., ZINGARELLI B., FLOWER R.J., SALZMAN A.L., PERRETTI M. Inhibition of poly (ADP-ribose) synthetase exerts anti-inflammatory effects and inhibits neutrophil recruitment. J. Exp. Med. 1997;186:1041–1049. doi: 10.1084/jem.186.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., DAWSON V.L. Role of poly (ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol. Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- SZABÓ C.Nitric oxide, peroxynitrite and poly (ADP-ribose) synthetase: biochemistry and pathophysiological implications Pathophysiology and clinical applications of nitric oxide 199969–98.ed. Rubanyi G.M. pp
- TOMLINSON A., APPLETON I., MOOREGILROY A.R., WILLIS D., MITCHELL J.A., WILLOUGHBY A. Cyclo-oxygenase and nitric oxide isoforms in rat carrageenin-induced pleurisy. Br. J. Pharmacol. 1994;113:693–698. doi: 10.1111/j.1476-5381.1994.tb17048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEMATSU T., NAGASHIMA S., UMEMURA K., KANAMARU M., NAKASHIMA M. Pharmacokinetics and safety of intravenous recombinant human superoxide dismutase (NK341) in healthy subjects. Int. J. Clin. Pharmacol. Therapeut. 1994;32:638–641. [PubMed] [Google Scholar]
- VOLK T., GERST J., FAUST-BELBE G., STROEHMANN A., KOX W.J. Monocyte stimulation by reactive oxygen species: role of superoxide and intracellular Ca2+ Inflamm. Res. 1999;48:544–549. doi: 10.1007/s000110050501. [DOI] [PubMed] [Google Scholar]
- WARREN J.S., YABROFF K.R., MANDEL D.M., JOHNSON K.J., WARD P.A. Role of O2− in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Rad. Biol. Med. 1990;8:163–172. doi: 10.1016/0891-5849(90)90089-2. [DOI] [PubMed] [Google Scholar]
- WIZEMANN T.M., GARDNER C.R., LASKIN J.D., QUINONES S., DURHAM S.K., GOLLER N.L., OHNISHI S.T., LASKIN D.L. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J. Leukoc. Biol. 1994;56:759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- XIA Z.F., HOLLYOAK M., BARROW R.E., HE F., MULLER M.J., HERNDON D.N. Superoxide dismutase and leupeptin prevent delayed reperfusion injury in the rat small intestine during burn shock. J. Burn Care and Rehabilitation. 1995;16:111–117. doi: 10.1097/00004630-199503000-00004. [DOI] [PubMed] [Google Scholar]
- YAMAKURA F., TAKA H., FUJIMURA T., MURAYAMA K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- ZINGARELLI B., O'CONNOR M., WONG H., SALZMAN A.L., SZABÓ C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipolysaccharide. J. Immunol. 1996;156:350–358. [PubMed] [Google Scholar]


