Abstract
Amantadine can prevent and decrease airway inflammation by inhibiting influenza virus (IV) replication; however, the effect of amantadine on RANTES production by human bronchial epithelial cells (BEC) has not been determined. In the present study, we examined the effect of amantadine on RANTES production and also analysed p38 mitogen-activated protein (MAP) kinase and c-Jun-NH2-terminal kinase (JNK) activation to clarify the mechanism in the effect of amantadine on RANTES production, since we have previously shown that p38 MAP kinase and JNK regulate RANTES production by IV-infected BEC.
BEC that had been preincubated with amantadine were infected with IV and then p38 MAP kinase and JNK activation in the cells and RANTES concentrations in the culture supernatants were determined.
Amantadine-induced inhibition of virus replication resulted in a decrease in p38 MAP kinase and JNK activity and decreased expression of RANTES in IV-infected cells.
Amantadine did not inhibit p38 MAP kinase and JNK activation induced by tumour necrosis factor-α (TNF-α) as a non-viral stimulus.
These results indicate that amantadine inhibits IV infection-induced RANTES production by human BEC and that the inhibition by amantadine of RANTES production might result from an indirect inhibitory effect of amantadine on p38 MAP kinase and JNK activation via the inhibition of virus replication, and we emphasize that amantadine may produce a beneficial effect on controlling bronchial asthma exacerbation caused by IV infection.
Keywords: Amantadine, influenza virus, human bronchial epithelium, MAP kinase, RANTES
Introduction
Influenza virus (IV) infection causes airway inflammation and bronchial asthma exacerbation (Nicholson et al., 1993; Folkerts et al., 1998; Johnston et al., 1998). The pathogenesis of airway inflammation caused by IV is complex, and involves multiple inflammatory cells, cytokines and mediators (Folkerts et al., 1998; Johnston et al., 1998). Airway epithelial cells are the initial site of IV infection, and have the capacity to produce a variety type of biologically active molecules, including cytokines (Polito & Proud 1998). IV-infected airway epithelial cells, at least in part, participate in the production of airway inflammation and asthma exacerbation by expressing various cytokines (Choi et al., 1992; Matsukura et al., 1996; 1998). Consequently, the inhibition of cytokine production by airway epithelial cells is an important strategy for controlling asthma exacerbation.
Amantadine (1-aminoadamantane hydrochloride) can inhibit influenza A virus (IVA) uncoating and subsequent replication by blocking ion channel activity of IVA M2 integral membrane protein (Kato & Eggers 1969; Skehel et al., 1978; Hay, 1992). Thus, amantadine can prevent and decrease the severity of IV infection (Dolin et al., 1982; Jackson, 1986). However, the effect of amantadine on cytokine production by IV-infected human airway epithelial cells has not been determined. In the present study, we therefore examined the effect of amantadine on the production of RANTES that exhibits a chemotactic activity for eosinophils (Baggiolini & Dahinden 1994; Humbert, 1996), by IV-infected human bronchial epithelial cells (BEC). In addition, since we have previously shown that p38 mitogen-activated protein (MAP) kinase and c-Jun-NH2-terminal kinase (JNK) regulate IV infection-induced RANTES production by human BEC (Kujime et al., 2000), we also examined the effect of amantadine on IV infection-induced p38 MAP kinase and JNK activation in the context of its effect on RANTES production.
Methods
Virus stock
Influenza virus strain A/Udon/307/72 (H3N2) was grown in Madian-Darby canine kidney cells (American Type Culture Collection, Rockville, MD, U.S.A.) in DMEM (Nissui Co. Ltd., Tokyo, Japan) and semipurified by two cycles of differential centrifugation from the infected culture supernatants. Virus stock was stored at −80°C.
Cells and reagents
Bronchial epithelial cell lines, NCI-H292, were obtained from American Type Culture Collection. NCI-H292 were grown in culture medium which is RPMI 1640 (Nissui Co. Ltd, Tokyo, Japan) supplemented with 10% heat-inactivated FCS (Mitsubishikasei, Co. Ltd, Japan), streptomycin and penicillin (Meiji Pharmaceutical Co. Ltd, Tokyo, Japan). SB 203580 as the specific inhibitor of p38 MAP kinase activity (Lee et al., 1994) was obtained from Carbiochem-Novabiochem Corporation (La Jolla, CA, U.S.A.). CEP-1347 as the specific inhibitor of JNK activation (Maroney et al., 1998) was kindly provided by Cephalon Incorporated (West Chester, PA, U.S.A.). SB 203580 and CEP-1347 were dissolved in DMSO. Amantadine was obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Human recombinant tumour necrosis factor-α (TNF-α) were kindly provided by Dainippon Pharmaceutical Co. Ltd. (Osaka, Japan).
Cell cultures
The cells were placed onto 24-well flat bottomed tissue culture plate (Corning, Corning, NY, U.S.A.) for cytokine production and tissue culture plate (Falcon 1007, Oxnard, CA, U.S.A.) for Western blot analysis, and cultured using culture medium at 37°C in humidified 5% CO2 atmosphere. When the cells were grown in subconfluent conditions, the culture medium was replaced with serum free RPMI 1640 and the cells were cultured for 16 h. In order to examine IV infection-induced RANTES production, and p38 MAP kinase and JNK activation; the cells were infected with IV at multiplicity of infection (moi) of 2. In order to examine the effect of amantadine, SB 203580 and CEP-1347 on RANTES production by IV-infected BEC; the cells that had been incubated with amantadine, SB 203580 or CEP-1347 for 1 h were infected with IV and cultured for 24 h. At the end of 24 h, the culture supernatants were harvested and centrifuged, and the supernatants were collected, filtrated with a millipore filter and stored at −80°C until assay. In order to examine the effect of amantadine on IV-infection-induced RANTES production, and p38 MAP kinase and JNK activation and on TNF-α-induced RANTES production, and p38 MAP kinase and JNK activation, the cells that had been incubated with amantadine for 1 h were infected with IV and cultured for the desired times as indicated.
Measurement of RANTES
The concentration of RANTES in the culture supernatants from BEC were measured by commercially available ELISA kits (Amersham International, Aylesbury, U.K.). ELISA was performed according to the manufacturer's instructions. All samples were assayed in duplicate.
Western blot analysis of p38 MAP kinase and JNK
Analysis of threonine and tyrosine phosphorylation of p38 MAP kinase was performed using an anti-phosphorylated threonine and tyrosine of p38 MAP kinase antibody (ab) (anti-phospho-specific p38 MAP kinase ab, New England Biolabs, Inc.) which is specific for active p38 MAP kinase and does not cross react with Erk and JNK. Analysis of threonine and tyrosine phosphorylation of JNK was performed using an anti-phosphorylated threonine and tyrosine of JNK ab (anti-phospho-specific JNK ab, New England Biolabs, Inc.) which is specific for active JNK and does not cross react with p38 MAP kinase and Erk. Analysis of p38 MAP kinase and JNK was performed according to manufacturer's instructions as described previously (Hashimoto et al., 1999). Briefly, after separating proteins from cell lysate by a 15% SDS – PAGE, the cell lysate containing 10 μg of protein was electrophoretically transferred to membrane and the membrane was incubated with specific ab to phosphorylated threonine and tyrosine of p38 MAP kinase (affinity-purified rabbit polyclonal IgG) or specific ab to phosphorylated threonine and tyrosine of JNK (affinity-purified rabbit polyclonal IgG) for analysis of JNK, and then it was incubated with the horseradish peroxidase-conjugated anti-rabbit IgG ab and horseradish peroxidase-conjugated anti-biotin ab to detect biotinylated protein markers. Blots were incubated with ECL (enhanced chemiluminescence) solution for 1 min and exposed on KODAK XAR film. In order to show the amounts of p38 MAP kinase and JNK precipitated, blots were stripped and reprobed using phosphorylation-state independent p38 MAP kinase-specific ab (affinity purified rabbit polyclonal IgG) to determine total p38 MAP kinase levels or phosphorylation-state independent JNK-specific ab (affinity purified rabbit polyclonal IgG) to determine total JNK levels, respectively.
Statistical analysis
Statistical significance was analysed by using analysis of variance (ANOVA). P value less than 0.05 was considered significant. When statistical significance was reached, post hoc tests (Fischer's Protected Least Significant Difference, Scheff's F) were performed.
Results
IV infection induces RANTES production
First, BEC were infected with IV and cultured for 12, 24 and 48 h, and RANTES concentrations of the culture supernatants were determined. As shown in Figure 1, IV infection induced RANTES production was a time-dependent manner.
Figure 1.
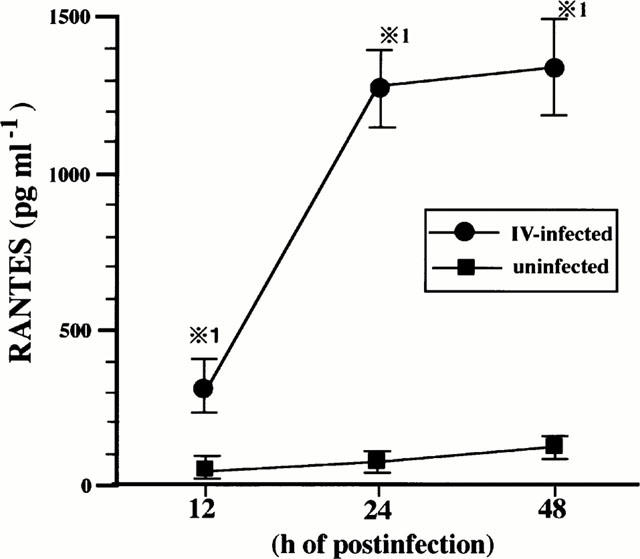
IV infection induces RANTES production by BEC. BEC were cultured with medium or infected with IV and the concentrations of RANTES in the culture supernatants were determined at 12, 24 and 48 h after cultivation. The results are expressed as mean±s.d. in the five different experiments. *P<0.01 compared with RANTES concentrations in BEC cultured with medium.
p38 MAP kinase and JNK regulate IV infection-induced RANTES production
We have previously shown that p38 MAP kinase and JNK, at least, regulate IV-induced RANTES production (Kujime et al., 2000). In order to confirm the previous results and verify the experimental condition in this study, we examined the threonine and tyrosine phosphorylation of p38 MAP kinase and JNK in IV-infected cells, and the effect of SB 203580 and CEP-1347 on IV induced RANTES production. Activation of p38 MAP kinase and JNK is mediated by dual phosphorylation of the threonine and tyrosine residues of p38 MAP kinase and JNK (Derijard et al., 1994; Raingeaud et al., 1995). Increases in the threonine and tyrosine phosphorylation of p38 MAP kinase and JNK reflect activation state of p38 MAP kinase and JNK. We therefore examined the threonine and tyrosine phosphorylation of p38 MAP kinase kinase and JNK in IV-infected cells. Our previous study with the time-course of p38 MAP kinase and JNK activation in IV-infected BEC showed that p38 MAP kinase and JNK activation in IV-infected BEC were maximal at 6 h. Therefore, in the present study we performed the brief experiment to examine p38 MAP kinase and JNK activation in IV-infected cells. To this end, the cells were lysed for analysis of p38 MAP kinase and JNK activation at 1, 4, 6 and 12 h after IV infection. As shown in Figure 2, IV infection activated p38 MAP kinase and JNK and their maximal responses occurred at 6 h. Lower panel of Figure 2a showed that equal amounts of p38 MAP kinase protein were immunoblotted with phosphorylation-independent p38 MAP kinase-specific ab regardless of time of culture periods, indicating that IV infection-induced p38 MAP kinase activation occurred in the absence of changes in p38 MAP kinase protein levels. Similarly, IV infection-induced JNK activation occurred in the absence of changes in JNK protein levels (lower panel of Figure 2b). SB 203580 and CEP1347 inhibited RANTES production by IV-infected cells (Figure 2c). The present results verified the role of p38 MAP kinase and JNK in IV infection-induced RANTES production by BEC. Addition of DMSO vehicle alone did not attenuate IV infection-induced increases in p38 MAP kinase and JNK activation (data not shown).
Figure 2.
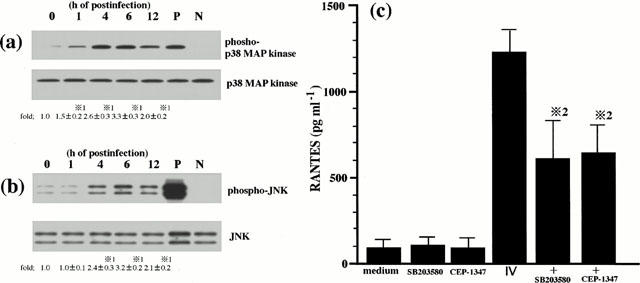
p38 MAP kinase and JNK regulate IV infection-induced RANTES production. BEC were stimulated with IV for the desired times as indicated. The lysates from BEC were separated by a 15% SDS – PAGE, transferred to membranes, and blotted either with a specific antibody (ab) to phosphorylated threonine and tyrosine of p38 MAP kinase (phospho-p38 MAP kinase; upper panel of (a)) or a specific ab to phosphorylated threonine and tyrosine of JNK (phospho-JNK; upper panel of (b)). Blots shown in the upper panel of (a) were stripped and reprobed using a phosphorylation state-independent p38 MAP kinase specific ab to show the amounts of p38 MAP kinase blotted (p38 MAP kinase; lower panel of (a)). Blots shown in the upper panel of (b) were stripped and reprobed using a phosphorylation state-independent JNK specific antibody to show the amounts of JNK blotted (JNK; lower panel of (b)). Lane P of (a) and (b) represent phosphorylated p38 MAP kinase and JNK control protein for positive control, respectively (New England Biolabs, Inc.). Lane N of (a) and (b) represent nonphosphorylated p38 MAP kinase and JNK control protein for negative control, respectively (New England Biolabs, Inc.). The amounts of phosphorylated p38 MAP kinase and JNK proteins were quantified by NIH image analyzer and are presented as the amounts of phosphorylated p38 MAP kinase and JNK proteins relative to control cells treated without IV (1.0). Three identical experiments independently performed gave similar results. Fold increase in amounts of phosphorylated p38 MAP kinase and JNK proteins as indicated below are expressed as the mean±s.d. in three different experiments. *1=P<0.01 compared with amounts of phosphorylated p38 MAP kinase or JNK proteins in IV-uninfected BEC. BEC that had been preincubated either with medium, SB 203580 (10 μM) or CEP-1347 (1 μM) for 1 h were cultured with medium or infected with IV and the concentrations of RANTES in the culture supernatants were determined at 24 h after IV infection (c). The results are expressed as the mean±s.d. in six different experiments. *2=P<0.01 compared with RANTES concentrations in BEC cultured without inhibitor.
Amantadine inhibits IV infection-induced RANTES production
In order to examine the effects of amantadine on IV infection-induced RANTES production, the cells that had been preincubated with various doses of amantadine for 1 h were infected with IV, and cultured for 24 h. RANTES concentrations in the supernatants were determined. As shown in Figure 3, amantadine inhibited RANTES production in a dose-dependent manner.
Figure 3.
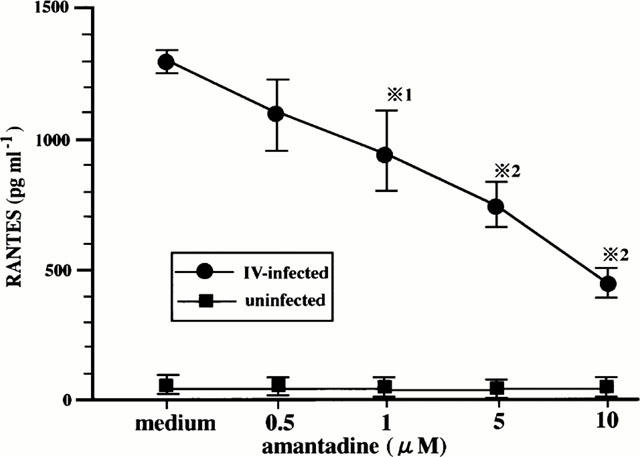
Amantadine inhibits IV infection-induced RANTES production. BEC that had been preincubated either with medium or various concentrations of amantadine for 1 h were cultured with medium or infected with IV. The concentrations of RANTES in the culture supernatants were determined at 24 h after infection. The results are expressed as the mean±s.d. in six different experiments. *1=P<0.05 compared with RANTES concentrations in BEC cultured without amantadine. *2=P<0.01 compared with RANTES concentrations in BEC cultured without amantadine.
Amantadine inhibits IV-induced p38 MAP kinase and JNK activation
IV infection-induced RANTES production is, at least, regulated by p38 MAP kinase and JNK (Kujime et al., 2000). In the next, we examined the effect of amantadine on IV-induced p38 MAP kinase and JNK activation in order to clarify the mechanism of amantadine-mediated inhibition of RANTES production. To this end, we examined the effect of amantadine on IV infection-induced p38 MAP kinase and JNK activation at 6 h after IV infection. Amounts of phosphorylated threonine and tyrosine of p38 MAP kinase and JNK were lower in IV-infected cells that had been preincubated with amantadine than those that had been preincubated without amantadine, showing that amantadine inhibited IV infection-induced p38 MAP kinase and JNK activation (Figure 4). Lower panel of Figure 4a showed that equal amounts of p38 MAP kinase protein were immunoblotted with phosphorylation-independent p38 MAP kinase-specific ab regardless of culture conditions, indicating that the inhibition of IV infection-induced p38 MAP kinase activation by amantadine occurred in the absence of changes in p38 MAP kinase protein levels. Similarly, the inhibition of IV infection-induced JNK activation by amantadine occurred in the absence of changes in JNK protein levels (lower panel of Figure 4b).
Figure 4.
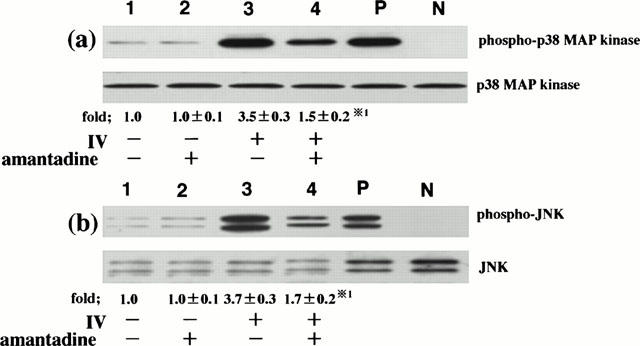
Amantadine inhibits IV infection-induced p38 MAP kinase and JNK activation. BEC that had been preincubated either with medium or amantadine (10 μM) for 1 h were infected with IV. p38 MAP kinase and JNK activation was analysed at 6 h after IV infection. The cells were cultured with medium (lane 1), amantadine (lane 2), IV (lane 3) and IV and amantadine (lane 4). Three identical experiments independently performed gave similar results. Fold increase in amounts of phosphorylated p38 MAP kinase and JNK proteins as indicated below are expressed as the mean±s.d. in three different experiments. *1=P<0.01 compared with amounts of phosphorylated p38 MAP kinase or JNK proteins in IV-infected BEC cultured without amantadine.
Amantadine does not inhibit TNF-α-induced p38 MAP kinase and JNK activation
Amantadine inhibited IV-induced p38 MAP kinase and JNK activation and consequent RANTES production. Since this drug is well known to inhibit virus replication (Kato et al., 1969; Skehel et al., 1978; Hay, 1992), the inhibition by amantadine of IV infection-induced p38 MAP kinase and JNK activation might result from the inhibition of virus replication. However, a direct inhibitory effect of amantadine on p38 MAP kinase and JNK activation has not been determined. To test this possibility, since it has been shown that TNF-α activates p38 MAP kinase to produce RANTES by BEC (Hashimoto et al., 2000), we employed TNF-α as non-viral stimulus to activate p38 MAP kinase and JNK and examined the effect of amantadine on TNF-α-induced p38 MAP kinase and JNK activation and RANTES production. As shown in Figure 5a and 5b, amantadine did not inhibit TNF-α-induced p38 MAP kinase and JNK activation, indicating that amantadine does not have a direct inhibitory effect on p38 MAP kinase and JNK activation. We also measured the concentrations of RANTES in the culture supernatants from TNF-α-infected BEC in the presence or absence of amantadine. Amantadine did not inhibit TNF-α-induced RANTES production (Figure 5c). The total number of the cells and cell viability at the end of the culture period of each experiment, determined by trypan blue exclusion dye, did not differ with culture conditions, suggesting that IV infection-induced RANTES production and the inhibition by amantadine and MAP kinase inhibitors of RANTES production did not result from cell cytotoxicity.
Figure 5.
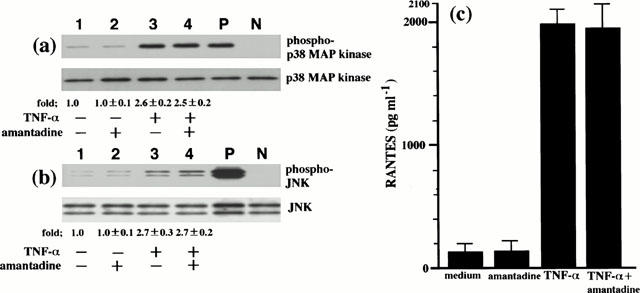
Amantadine does not inhibit TNF-α-induced p38 MAP kinase and JNK activation, and RANTES production. BEC that had been preincubated either with medium or amantadine (10 μM) for 1 h were stimulated with TNF-α (10 ng ml−1). p38 MAP kinase and JNK activation was analysed at 10 min after stimulation with TNF-α (a) and (b). The cells were cultured with medium (lane 1), amantadine (lane 2), TNF-α (lane 3) and TNF-α and amantadine (lane 4). The fold increases in amounts of phosphorylated p38 MAP kinase and JNK proteins are indicated below. Three identical experiments independently performed gave similar results. Fold increase in amounts of phosphorylated p38 MAP kinase and JNK proteins as indicated below are expressed as the mean±s.d. in three different experiments. The concentrations of RANTES in the culture supernatants were determined at 24 h after TNF-α stimulation (c). The results are expressed as the mean±s.d. in three different experiments.
Discussion
In the present study, we examined the effect of amantadine on IV infection-induced RANTES production by human BEC. The results showed that (1) amantadine-induced inhibition of virus replication resulted in a decrease in p38 MAP kinase and JNK activity and decreased expression of RANTES in IV-infected cells, and (2) amantadine did not inhibit p38 MAP kinase and JNK activation, and RANTES production induced by TNF-α as a non-viral stimulus. These results indicate that amantadine inhibits IV infection-induced RANTES production by human BEC and that the inhibition by amantadine of RANTES production might result from an indirect inhibitory effect of amantadine on p38 MAP kinase and JNK activation via the inhibition of virus replication, but not a direct inhibitory effect of p38 MAP kinase and JNK activation.
We firstly examined the effect of amantadine on IV infection-induced RANTES production by human BEC. The results showed that amantadine inhibited RANTES production in a dose-dependent manner. 10 μM of amantadine partially inhibited RANTES production. Next, we examined the effect of amantadine on IV infection-induced p38 MAP kinase and JNK activation to clarify the mechanism in amantadine-mediated inhibition of RANTES production, since our previous results (Kujime et al., 2000) and the present study showed that p38 MAP kinase and JNK, at least, regulated IV infection-induced RANTES production by human BEC. The results showed that 10 μM of amantadine partially inhibited IV infection-induced p38 MAP kinase and JNK activation. This correlated with a partial inhibition of RANTES production by amantadine. These results indicated that amantadine-mediated inhibition of RANTES production resulted from amantadine-mediated inhibition of p38 MAP kinase and JNK activation.
The relationship between the IV growth and cellular functions has been determined, including cytokine production and apoptosis (Pahl & Baeuerle, 1995; Takizawa et al., 1996; Schultz-Cheery & Hinshaw, 1996; Bussfeld et al., 1998; Morris et al., 1999). Therefore, it is of interest to determine a relationship between IV growth and the induction of p38 MAP kinase and JNK activation and subsequent cytokine production. The present study showed that the treatment of cells with amantadine resulted in a decrease in p38 MAP kinase and JNK activity, and decreased expression of RANTES in IV-infected cells. Since amantadine is a well-known inhibitor of IV uncoating, thus inhibiting subsequent IV replication (Kato et al., 1969; Skehel et al., 1978; Hay, 1992), virus replication is required for p38 MAP kinase and JNK activation and RANTES production. Recently, double-strand RNA (dsRNA) analogue poly(IC) has been demonstrated to activate p38 MAP kinase and JNK (Jordanov et al., 2000). We are currently investigating the IV-specific molecule involved, including dsRNA for p38 MAP kinase and JNK activation to clarify this point.
IV infection results in acute inflammation and increased cytokine secretion at areas of viral replication (Nicholson et al., 1993; Folkerts et al., 1998; Johnston et al., 1998; Polito et al., 1998; Choi et al., 1992; Matsukura et al., 1996; 1998). Amantadine can prevent and decrease airway inflammation by inhibiting IV replication (Kato et al., 1969; Skehel et al., 1978; Hay, 1992). In addition, our results indicated that the inhibition of IV replication by amantadine inhibits p38 MAP kinase and JNK activation, resulting in the decreased RANTES production. RANTES plays an important role in the production of airway inflammation in asthmatics (Baggiolini et al., 1994; Humbert, 1996; Pizzichini et al., 1999). Therefore, the inhibition of RANTES production can be important in the attenuation of airway inflammation in asthmatics. Consequently, an inhibitory effect of amantadine on RANTES production by IV-infected BEC may have a beneficial effect on controlling bronchial asthma exacerbation.
From the data presented here, we conclude that amantadine inhibits RANTES production by IV-infected BEC. Therefore, the inhibition of RANTES production by amantadine is an important strategy for controlling bronchial asthma exacerbation caused by IV infection.
Acknowledgments
This work was supported by grant-in-aid for General Scientific Research from the Ministry of Education of Japan (11670596). We gratefully acknowledge Dr Yuzuru Matsuda (Kyowa-Hakko Kogyo Co., Ltd) and Dr Jeffery Vaught and Dr Matthew Miller (Cephalon Incorporated) for the generous gift of CEP-1347.
Abbreviations
- ab
antibody
- BEC
bronchial epithelial cells
- FCS
foetal calf serum
- IV
influenza virus
- IVA
influenza A virus
- JNK
c-Jun-NH2-terminal kinase
- MAP
mitogen-activated protein kinase
- moi
multiplicity of infection
- TNF-α
tumour necrosis factor-α
References
- BAGGIOLINI M., DAHINDEN C.A. CC chemokines in allergic inflammation. Immunol. Today. 1994;15:127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- BUSSFELD D., KAUFMANN A., MEYER R.G., GEMSA D., SPRENGER H. Differential mononuclear leukocyte attracting chemokine production after stimulation with active and inactive influenza A virus. Cell. Immunol. 1998;186:1–7. doi: 10.1006/cimm.1998.1295. [DOI] [PubMed] [Google Scholar]
- CHOI A.K., JACOBY D.B. Influenza virus A induces interleukin-8 gene expression in human airway epithelial cells. FEBS Lett. 1992;309:327–329. doi: 10.1016/0014-5793(92)80799-m. [DOI] [PubMed] [Google Scholar]
- DERIJARD D., HIBI M., WU I.-H., BARRETT T., SU B., DENG T., KARIN M., DAVIS R.J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- DOLIN R., REICHMAN R.C., MADORE H.P.H., MAYNARD R., LINTON P.N., WEBBER-JONES J.A. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. New Engl. J. Med. 1982;307:580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- FOLKERTS G., BUSSE W.W., NIJKAMP F.P., SORKNESS R., GERN J.E. Virus-induced airway hyperresponsiveness and asthma. Am. J. Respir. Crit. Care Med. 1998;157:1708–1720. doi: 10.1164/ajrccm.157.6.9707163. [DOI] [PubMed] [Google Scholar]
- HAY J. The action of amantanamines against influenza A viruses: inhibition of the M2 ion channel protein. Semin. Virol. 1992;3:21–30. [Google Scholar]
- HASHIMOTO S., MATSUMOTO K., GON Y., NAKAYAMA T., TAKESHITA I., HOTIE T. Hyperosmolarity-induced IL-8 expression in human bronchial epithelial cells through p38 MAP kinase. Am. J. Respir. Crit. Care Med. 1999;159:634–640. doi: 10.1164/ajrccm.159.2.9712090. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO S., MATSUMOTO K., GON Y., MARUOKA S., KUJIME K., HAYASHI S., TAKEHSITA I., HORIE T. p38 mitogen-activated protein kinase regulate TNF-α-1, IL-1α- and PAF-induced RANTES GM-CSF production by human bronchial epithelial cells. Clin. Exp. Allergy. 2000;30:48–55. doi: 10.1046/j.1365-2222.2000.00641.x. [DOI] [PubMed] [Google Scholar]
- HUMBERT M. Pro-eosinophilic cytokines in asthma. Clin. Exp. Allergy. 1996;26:123–127. doi: 10.1111/j.1365-2222.1996.tb00069.x. [DOI] [PubMed] [Google Scholar]
- IORDANOV M.S., PARANJAPE J.M., ZHOU A., WONG J., WILLIAMS B.G., MEURS E.F., SILVERMAN R.H., MAGUN B.E. Activation of p38 mitogen-activated protein kinase and c-jun-NH2 terminal kinase by double strand RNA and encephalomyocarditis virus: involvement of Rnase L, protein kinase R, and alternative pathway. Mol. Cell Biol. 2000;20:617–627. doi: 10.1128/mcb.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON G.G.The development and clinical evaluation of amantadine for the prophylaxis and treatment of influenza A Opinion for the control of influenza 1986New York: Alan R. Liss; 293–315.eds. A.P. Kendal & P.A. Patriarca. pp [Google Scholar]
- JOHNSTON S.L. Viruses and asthma. Allergy. 1998;53:922–932. doi: 10.1111/j.1398-9995.1998.tb03792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATO N., EGGERS H.J. Inhibition of uncoating of fowl plague virus by 1-adamantanamine hydrochloride. Virology. 1969;37:632–641. doi: 10.1016/0042-6822(69)90281-5. [DOI] [PubMed] [Google Scholar]
- KUJIME K., HASHIMOTO S., GON T., SHIMIZU K., HORIE T. p38 MAP kinase and c-Jun-NH2-terminal kinase regulates RANTES production by influenza virus-infected human bronchial epithelial cells. J. Immunol. 2000;164:3222–3228. doi: 10.4049/jimmunol.164.6.3222. [DOI] [PubMed] [Google Scholar]
- LEE J.C., LAYDON J.T., MCDONNELL P.C., GALLAGHER T.F., KUMMER S., GREEN D., MCNULTY D., BLUMENTHAL M.J., HEYS J.R., LANDVATTER S.W., STRICKLER J.E., MCLAIGHLIN M.M., SIEMENS I.R., FISHER S.M., LIVI G.P., WHITE J.R., ADAMS J.L., YOUNG P. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- MARONEY A.C., GLICKMAN M.A., BASMAN A.N., WALTON K.M., KNIGHT E., Jr, MURPHY C.A., BARLETT B.A., FINN J.P., ANGEKES T., MATSUDA Y., NEFF N.T., DIONNE C.A. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J. Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUKURA S., KOKUBU F., NODA H., TOKNAGA H., ADACHI M. Expression of IL-6, IL-8, and RANTES on human bronchial epithelial cells, NCI-H292, induced by influenza virus A. J. Allergy Clin. Immunol. 1996;98:1090–1087. doi: 10.1016/s0091-6749(96)80195-3. [DOI] [PubMed] [Google Scholar]
- MATSUKURA S., KOKUBU F., KUBO F., TOMITA T., TOKUNAGA H., KADOKURA M., YAMAMOTO T., KUROIWA Y., OHNO T., SUZAKI H., ADACHI M. Expression of RANTES by normal airway epithelial cells after influenza virus infection. Am. J. Respir. Cell. Mol. Biol. 1998;18:255–264. doi: 10.1165/ajrcmb.18.2.2822. [DOI] [PubMed] [Google Scholar]
- MORRIS S.J., PRICE G.E., BARNETT J.M., HISCOX S.A., SMITH H., SWEET C. Role of neuramidase in influenza virus-induced apoptosis. J. Gen. Virol. 1999;80:137–146. doi: 10.1099/0022-1317-80-1-137. [DOI] [PubMed] [Google Scholar]
- NICHOLSON R.G., KENT J., IRELAND D.C. Respiratory viruses and exacerbations of asthma in adults. British Med. J. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLITO A.J., PROUD D. Epithelial cells as regulator of airway inflammation. J. Allergy Clin. Immunol. 1998;102:714–718. doi: 10.1016/s0091-6749(98)70008-9. [DOI] [PubMed] [Google Scholar]
- RAINGEAUD J., GUPTA S., ROGERS J.S., DICKENS M., HAN J., ULEVITCH R., DAVIS R.J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- PAHL H.L., BAEUERLE P.A. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J. Viol. 1995;169:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZZICHINI M., PIZZICHINI M.E., EFTHIMIADIS A., CHAUHAN A.J., JOHNSTON S.L., HUSSACK P., MAHONY J., DOLOVICH J., HARGREAVE F.E. Asthma and natural colds, inflammatory indices in induced sputum: a feasibility study. Am. J. Respir. Crit. Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.4.9712082. [DOI] [PubMed] [Google Scholar]
- SCHULTZ-CHERRY S., HINSHAW V.S. Influenza virus neuramidase activates latent transforming growth factor β. J. Viol. 1996;70:8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKEHEL J., HAY A.J., ARMSTRONG J.A. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J. Gen. Virol. 1978;38:97–110. doi: 10.1099/0022-1317-38-1-97. [DOI] [PubMed] [Google Scholar]
- TAKIZAWA T., OHASHI K., KANANISHI Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J. Viol. 1996;70:8128–8132. doi: 10.1128/jvi.70.11.8128-8132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


