Abstract
The anticonvulsant properties of 2-chloroadenosine (CADO) in the basolateral amygdala rely on the activation of adenosine-specific heptahelical receptors. We have utilized whole-cell voltage-clamp electrophysiology to examine the modulatory effects of CADO and other adenosine receptor agonists on voltage-gated calcium channels in dissociated basolateral amygdala neurons.
CADO, adenosine, and the A1 subtype-selective agonists N6-(L-2-Phenylisopropyl)adenosine (R-PIA) and 2-chloro-N6-cyclopentyladenosine (CCPA) reversibly modulated whole cell Ba2+ currents in a concentration-dependent fashion. CADO inhibition of barium currents was also sensitive to the A1 antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX).
The A2A-selective agonist 4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid (CGS21680) was without effect.
CADO inhibition was predominantly voltage-dependent and sensitive to the sulphydryl-modifying reagent N-ethylmaleimide, implicating a membrane-delimited, Gi/o-coupled signal transduction pathway in the channel regulation.
Using Ca2+ channel subtype-selective antagonists, CADO inhibition appeared to target multiple channel subtypes, with the inhibition of ω-conotoxin GVIA-sensitive calcium channels being more prominent.
Our results indicate that the anti-convulsant effects CADO in the basolateral amygdala may be mediated, in part, by the A1 receptor-dependent inhibition of voltage gated calcium channels.
Keywords: Basolateral amygdala, A1 adenosine receptor, calcium channel, N-ethylmaleimide, nifedipine, ω-conotoxin GVIA, ω-agatoxin IVA
Introduction
As part of the limbic system, the amygdala plays a highly integrative role in the sense/memory-response pathway and is believed to occupy a pivotal position in the regulation of fear and anxiety. Rat models of fear/anxiety have implicated the basolateral complex (BLA), as being centrally important in both the acquisition and expression of fear/apprehension-related behaviours (reviewed in Davis, 1992). Of particular relevance for the studies outlined below, infusion of the non-selective adenosine receptor agonist 2-chloroadenosine (CADO) into the basolateral amygdala suppresses seizure activity following amygdala kindling (Abdul-Ghani et al., 1997; Pourgholami et al., 1997), the long-term decrease in seizure threshold brought about by repeated electrical stimulation. In fact, adenosine receptor activation can even prevent the acquisition of amygdala kindling (Abdul-Ghani et al., 1997). This anticonvulsant activity of CADO is dose-dependent and blocked by caffeine, suggesting that activation of adenosine heptahelical receptors in the basolateral amygdala may regulate neuronal excitability. Previous work has demonstrated that adenosine receptors may act presynaptically in the amygdala to inhibit both excitatory and inhibitory transmission (Heinbockel & Pape, 1999; Nose et al., 1991). However, direct regulation of postsynaptic processes by amygdala adenosine receptors has not been examined.
P1 purinoreceptors are believed to mediate the effects of adenosine in the central nervous system. These receptors belong to the heptahelical family of receptors and are coupled to heterotrimeric G proteins. Several subtypes of P1 receptors can be distinguished from one another by receptor pharmacology or by examination of the signal transduction pathways to which the individual receptors couple. For example, the A1 adenosine receptor subtype is classically associated with the inhibition of cyclic AMP production via pertussis toxin-sensitive, ‘inhibitory' Gi/o heterotrimeric G proteins. A1 receptors also have high affinity for the agonists adenosine and 2-chloro-N6-cyclopentyladenosine (CCPA; Lohse et al., 1988) and the antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; Martinson et al., 1987). Unlike A1 receptors, A2 adenosine receptors appear to couple to cholera toxin-sensitive G proteins and can stimulate cyclic AMP accumulation. Two A2 isoforms, the A2A and A2B receptors, arise from distinct genes and are pharmacologically distinguishable. The A2A has a high affinity for the agonist 2-[p-(2-carbonyl-ethyl)-phenylethylamino]-5′-N-ethylcarboamido-adenosine (CGS 21680) but intermediate/low affinity for the antagonist DPCPX. Conversely, the A2B receptor has a very low affinity for CGS 21680 but a high affinity for DPCPX. The most recently identified P1 receptor, the A3 subtype, binds 2-chloro-N6-(3-iodobenzyl)-5′-(N-methylcarbanoyl)adenosine (Cl-IB-MECA; reviewed in Jacobson, 1998) with high affinity and selectivity but is not believed to be highly or widely expressed in brain (Rivkees et al., 2000; Zhou et al., 1992; but see Dixon et al., 1996). Thus, the pharmacological and signal transduction characteristics can often identify the receptor subtype mediating a particular adenosine-sensitive physiological response.
In the nervous system, adenosine is a potent modulator of neuronal activity, with A1 and A2 receptors often playing contrasting roles. For example, activation of pre-synaptic A1 receptors can depress synaptic transmission in numerous preparations and can alter both long-term (de Medonca & Ribeiro, 1990) and short-term (Lovinger & Choi, 1995) modifications in synaptic efficacy. In contrast, pre-synaptic A2 receptor activation is frequently associated with increased neurotransmitter release and enhanced synaptic function (Cuhna & Ribeiro, 2000; Kessey & Mogul, 1998; Umemiya & Berger, 1994). In addition to these synaptic roles, A1 and A2 receptors often regulate voltage-gated calcium channels in contrasting ways. A1 receptors typically inhibit calcium channel activity (Mynlieff & Beam, 1994; Zhu & Ikeda, 1993). Conversely, A2 receptors can facilitate calcium channel function (Goncalves et al., 1997; Umemiya & Berger, 1994; Mogul et al., 1993). Thus, adenosine receptors appear to modulate neuronal activity via a diverse array of signal transduction pathways.
The inhibition of voltage-gated calcium channels by heterotrimeric G protein-coupled receptors is believed to be an important means of regulating Ca2+ entry and thus has direct consequences for many Ca2+-dependent processes. In this context, dihydropyridine antagonists of somatic voltage-gated calcium channels prevent kindling-related phenomena (Hassan et al., 1999), presumably by attenuating the elevation in intracellular calcium associated with this seizure-like activity (Pal et al., 1999). The inhibition of somatic voltage-gated calcium channels can therefore dramatically influence neuronal excitability and potentially underlies the effects of CADO on amygdala seizure activity. Here we characterize the regulation voltage-gated calcium channels by CADO in acutely isolated basolateral amygdala neurons. The receptor mediating these effects is defined by pharmacological analyses; and its utilization of particular signal transduction pathways is determined. Finally, we examine the discriminate targeting of specific calcium channel subtypes during the modulatory process.
Methods
Neuron isolation
Neurons were prepared from coronal brain slices of juvenile male rats (∼P17 – P28) as previously described (McCool & Botting, 2000). Briefly, slices were digested with 0.5 – 1 mg ml−1 Pronase (CalBiochem) dissolved in standard artificial CSF (in mM): NaCl 125, KCl 5, NaHCO3 25, NaH2PO4 1.25, MgSO4 1, CaCl2 2.0, and 20 D-glucose, at 37°C for 20 min with constant oxygenation. Following this digestion, slices were removed to ‘isolation buffer' containing (in mM): N-methyl glucamine 130, NaCl 10, MgCl2 1, HEPES 10, D-glucose 10; pH 7.4 with HCl, osmolality 325 mmol kg−1 adjusted with sucrose; and, those regions containing primarily basolateral amygdala were carefully dissected away from the remaining tissue. Individual neurons were isolated from these tissue pieces by mechanical separation using fire-polished Pasteur pipettes. The dispersed tissue was transferred to plastic coverslips (Themonox). Large neurons (15 – 35 pF) with pyramidal or stellate soma were utilized exclusively in these studies and had morphological characteristics that were similar to both isolated BLA neurons (McCool & Botting, 2000; Viana & Hille, 1996) and BLA neurons in situ (McDonald, 1982).
Electrophysiology
All recordings were performed at ambient room temperature with standard patch-clamp techniques (Hamill et al. 1981) using the axopatch-1D amplifier (Axon Instruments, Inc., Foster City CA, U.S.A.) in the voltage clamp mode. Gigaohm seals were formed using patch pipettes made from borosilicate glass (World Precision Instruments, Sarasota FL, U.S.A.). For whole-cell patch clamp recording, patch pipettes typically had input resistances of 0.5 – 2 MΩ. The internal solution in the patch pipette was similar to that reported previously (McCool & Botting, 2000) and contained (in mM): CsCl 120, HEPES 10, EGTA 11, CaCl2 1, Mg-ATP 4, Tris-GTP 0.3, pH 7.2 with cesium hydroxide; adjust to 300 – 310 mmol kg−1 with sucrose. Whole cell capacitance (typically 15 – 25 pF) and series resistance (typically <10 MΩ) were compensated manually after opening the cell. Currents were online leak-subtracted using a ‘p·n−1' protocol and low-pass filtered (three-pole Bessel filter) at 1 kHz with >70% compensation. Depolarizing test pulses were typically given at 0.25 Hz from a holding potential of −80 mV to prevent prolonged channel inactivation.
Cells were continuously bath perfused with an extracellular solution consisting of (in mM): NaCl 150, Dextrose 10, HEPES 10, KCl 2.5, CaCl2 2.5, MgCl2 1.0, pH 7.4 with NaOH; osmolality adjusted to 320 – 340 mmol kg−1 with sucrose. To isolate currents mediated by the calcium channels, cells were locally perfused with the following (in mM): tetraethylammonium chloride 140, HEPES 10, Dextrose 15, BaCl2 5, pH 7.35 with tetraethylammonium hydroxide; osmolarity adjusted to 320 – 330 mmol kg−1 with sucrose.
Data analysis
Data was digitized at up to 10 kHz with a Labmaster DMA (Axon), stored on a computer, and analysed off-line using pClamp software (Axon). Unless otherwise stated, current amplitudes were measured as the difference between current levels immediately prior to and within 10 ms after the initiation of a depolarizing test pulse. For the calcium channel antagonist experiments, per cent contribution by each component following the sequential addition of channel blockers was calculated using current amplitudes during the ‘baseline' of the experiment using the following relationship:
where ‘blocker N' is the nth channel antagonist added during a sequence of blockers and ‘control' refers to current amplitudes prior to the addition of any channel antagonist. A similar relationship was used to calculate the per cent inhibition by adenosine receptor agonists during these occlusion experiments. Numerical analysis was performed using the QuatroPro software package (v 5.00; Borland International Inc., Scotts Valley CA, U.S.A.). Concentration-response curves were generated from fits (GraphPad Prism; GraphPad Software Inc., San Diego CA, U.S.A.) of data to a standard logistic equation of the form:
where Y=response expressed as per cent of Ymax, X=Log ([agonist]), and HillSlope=slope of the concentration response relationship. Because concentration-response data in each neuron were expressed as a fractional response compared to the inhibition by a maximally efficacious concentration of adenosine (10 μM), Ymin=0 and Ymax=1.0.
Statistics
Power calculations (SSD, CECOR Ltd.) to define the minimum sample size for each experiment were performed using standard deviations derived from pilot experiments. For these calculations, α=0.05 and β=0.1. Standard student t-tests compared population means between two treatment groups, with a significant difference being defined as P<0.05 (2-tailed). In those cases where multiple treatment groups were compared, one-way ANOVA analysis using the repeated measure design examined the population means, which were considered significantly different if P<0.05. Bonferroni's multiple comparison test was used in this case to examine various pairs treatment groups. All statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc.).
Drugs
Adenosine (RBI) and N-ethylmaleimide (NEM; Sigma) were prepared as concentrated stock solutions in distilled water fresh each day (adenosine) or every 3 h (NEM). ω-conotoxin GVIA (Alamone Labs), ω-conotoxin MVIIC (RBI), and ω-agatoxin IVA (Alamone Labs), 2-chloroadenosine (CADO; RBI) were similarly prepared but were stored as frozen stock solutions at −20°C. Similarly, nifedipine, 2-chloro-N6-cyclopentyladenosine (CCPA; RBI), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; RBI), R(-)-N6-(2-phenylisopropyl)-adenosine (R-PIA; RBI), and CGS-21680 (RBI) were made as concentrated stocks in dimethylsulphoxide and stored at −20°C. Agonists and antagonists were typically applied for at least 10 s and no longer than 30 s from an array of eight HPLC-grade capillary tubes (150 μm i.d.; Hewlett Packard Analytical Direct, Wilmington DE, U.S.A.) placed within 50 – 100 μm of the cell of interest.
Results
Inhibition of voltage-gated barium currents by P1 purinoreceptors
Because CADO regulation of BLA excitability (Abdul-Ghani et al., 1997; Pourgholami et al., 1997) may involve the regulation of voltage-gated calcium channels (see Magee & Carruth, 1999; Widmer et al., 1997), we tested the effects of CADO and other P1 receptor agonists on barium currents in acutely isolated neurons. Application of CADO (1 – 3 μM) as well as adenosine (3 – 10 μM) caused modest inhibition of whole-cell Ba2+ currents in a substantial number of cells (Figure 1a), with only 10 out of 68 neurons failing to respond to a purinergic agonist. CADO inhibited currents by 21±2% (mean±s.e.mean; n=11) while adenosine attenuated current amplitude in a different set of neurons by 25±2% (n=25). In cells where both were tested simultaneously, the inhibition by a maximally efficacious concentration CADO was not significantly different (paired t-test, P>0.1; Figure 1b) than that found for adenosine (22±2%; n=11). The inhibition by both compounds was characterized by slowing of the macroscopic current activation kinetics, exemplified by the apparent reduction in the amount of inhibition at later times during the depolarizing test pulse. For example in the traces in Figure 1a, inhibition was 28 and 25% for 3 mM adenosine and 1 μM CADO, respectively, when measured 7 ms after the onset of the test pulse; when measured 65 ms after the initiation of the test pulse, inhibition was reduced to 17 and 18% for adenosine and CADO, respectively. Furthermore, inhibition mediated by both compounds was readily reversible and exhibited no apparent desensitization after repeated applications of these maximally effective agonist concentrations.
Figure 1.
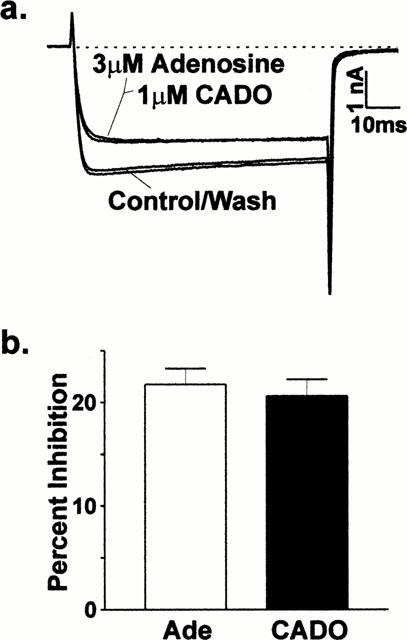
Adenosine receptor modulation of voltage-gated calcium channels in dissociated basolateral amygdala neurons. (a) Both 2-chloroadenosine and adenosine attenuated whole-cell, voltage-gated Ba2+ currents. Unless otherwise stated, the holding potential was −80 mV; and, the test potential was −10 to 0 mV. The inhibition by both adenosine and CADO was characterized by slowing of the macroscopic current activation kinetics, exemplified by the apparent reduction in the amount of inhibition at later times during the depolarizing test pulse. When measured 7 ms after the onset of the test pulse, inhibition was 28 and 25% for adenosine and CADO, respectively; inhibition was reduced to 17 and 18% for adenosine and CADO, respectively, when measured 65 ms after the initiation of the test pulse. Dashed line=zero current level. (b) When maximally efficacious concentrations of both CADO and adenosine were tested in the same neurons (n=11), CADO inhibited currents by 21±2% (mean±s.e.mean) while adenosine attenuated current amplitude by 22±2%. These values were not significantly different (paired t-test, P>0.1).
Inhibition is mediated by the A1 receptor subtype
To further define the receptor subtype responsible for calcium channel inhibition by 2-chloroadenosine, we tested several subtype-selective agonists and an antagonist. In one group of neurons (n=5), R-PIA (500 nM) and CGS21680 (500 nM) were compared to adenosine (Figure 2a). R-PIA inhibited whole-cell barium currents by 26±4%, similar to the level of inhibition seen with adenosine (28±3%; Figure 2a). Conversely, the inhibition by CGS21680 (5±1%) was significantly (P<0.001) less than that for either adenosine or R-PIA, indicating that the A2A receptor does not substantially modulate voltage-gated calcium channels in these neurons. The relative amount of inhibition by R-PIA and CGS21680 was similar when lower concentrations (R-PIA, 100 nM; CGS21680, 100 nM) were used (not shown), indicating that the concentration of agonists used here were sufficient to produce maximal inhibition. To support the hypothesis that the A1 subtype adenosine receptor is responsible for inhibition of calcium channels in isolated BLA neurons, we tested the sensitivity of 2-chloroadenosine inhibition to the selective A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). Co-application of DPCPX (100 nM) with 2-chloroadenosine (0.3 mM) significantly reduced the inhibition from 32±5% to 4±3% (n=4; Figure 2b; P<0.05). Additionally, we examined the concentration-response relationship for several P1 receptor agonists (Figure 3). Adenosine, CADO, and 2-Chloro-N6-cyclopentyladenosine (CCPA) inhibited whole-cell barium currents in a concentration-dependent manner (Figure 3a). The rank order of potency, CCPA (EC50=103 nM, Figure 3b)>adenosine (EC50=225 nM) ≈amp;2-chloroadenosine (EC50=290 nM), was consistent with the A1 subtype being the primary adenosine receptor mediating the barium current inhibition by adenosine and 2-chloroadenosine.
Figure 2.
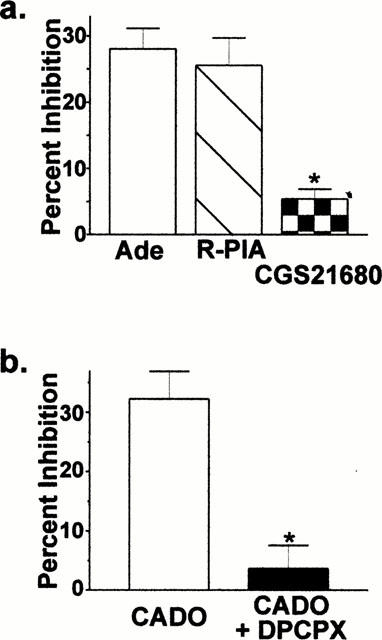
Adenosine receptor modulation of voltage-gated calcium channels in dissociated basolateral amygdala neurons is mediated by the A1 receptor subtype. (a) The adenosine receptor agonists adenosine (Ade), R-PIA, and CGS21680 were tested in one group of neurons (n=5). R-PIA (500 nM) inhibited whole-cell barium currents by 26±4%, similar to the 28±3% inhibition seen with adenosine (P>0.05, repeated measures ANOVA, Bonferronni's post-test). In these same neurons, the inhibition by CGS21680 (500 nM)) was 5±1%, significantly less than that for either adenosine or R-PIA (*P<0.001, repeated measures ANOVA, Bonferroni's post-test). These results indicate that the A2A subtype does not substantially contribute to the CADO modulation of calcium channels. (b) To confirm the contribution of the A1 receptor subtype, the sensitivity of 2-chloroadenosine modulation to the selective A1 receptor antagonist DPCPX was tested. Co-application of DPCPX (100 nM) with 2-chloroadenosine significantly reduced the modulation from 32±5% to 4±3% (n=4; *P<0.05 paired t-test).
Figure 3.
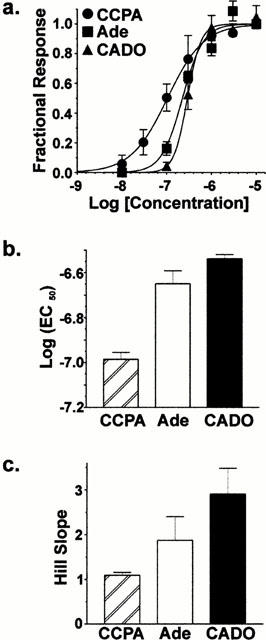
The agonist profile of P1 receptor modulation is consistent with A1 receptor-mediated inhibition. (a) Agonist concentration-response relationships for CCPA (▪ast;), CADO (▪sim;), and adenosine (▪amp;). The rank order of potency, CCPA>adenosine≈amp;CADO, was consistent with the A1 subtype being the primary P1 receptor responsible for the barium current modulation. To reduce the influence of cell-to-cell variability, data in each neuron was normalized to a maximally efficacious concentration of adenosine (10 μM). (b) EC50 values for CCPA, adenosine, and CADO were 103, 225 and 290 nM, respectively. (c) Hill slopes of the concentration-response relationships were 1.1±0.1, 1.9±0.5, and 2.9±0.6 for CCPA, adenosine, and CADO, respectively.
Signal transduction pathway for A1 adenosine receptor inhibition
In order to determine the signal transduction pathway utilized by A1 adenosine receptors, we assessed the voltage-dependence of the inhibition. Using a voltage protocol similar to that in Ikeda (1991), two ‘test' pulses (‘Vt1' and ‘Vt2', Figure 4a) were separated by a large membrane depolarization (+60 mV) and brief recovery period. The voltage-dependence of inhibition is represented in this protocol by a ‘relief' from inhibition in the second test pulse relative to the first test pulse. This ‘relief' is believed to be due to the voltage-dependent association between calcium channel subunits and G protein βγ subunits (Herlitze et al. 1996; Ikeda 1996) that are liberated during receptor activation. The representative traces in Figure 4a generally reflect our findings that inhibition was indeed partially voltage-dependent. In one set of neurons (n=8), the amount of inhibition by adenosine (3 μM) or 2-chloroadenosine (3 μM) in the first test pulse (19±1% inhibition) was significantly (P<0.05, paired t-test) reduced in the second test pulse (12±2% inhibition) by the intervening depolarization (Figure 4b). To further explore this phenomena, membrane potentials were continuously ‘ramped' from −100 to +60 mV to evoke the ‘bell-shaped' current that is characteristic for voltage-gated calcium channels (Figure 4c; see McCool et al., 1996). Application of 2-chloroadenosine (1 μM) during this voltage ramp reduced the amplitude of current response but did not change the general shape of the current, indicating that adenosine receptor activation did not substantially alter voltage sensitivity of the calcium channels. The per cent inhibition by CADO during these voltage ‘ramps' was greatly influenced by the membrane potential (Figure 4c). Specifically, the amount of inhibition decreased as membrane potential increased. Thus, like many other G protein coupled receptors, inhibition of whole-cell barium currents by adenosine or 2-chloroadenosine is mediated via a voltage-dependent signal transduction pathway that is likely to involve a direct interaction between channel and activated G protein.
Figure 4.
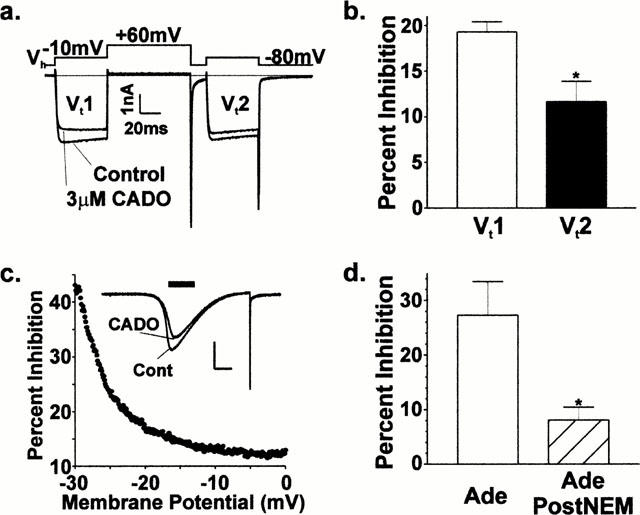
The modulation by CADO is partially voltage-dependent and NEM-sensitive, implicating a membrane-delimited, Gi/o-coupled signaling pathway. (a) Example of the ‘paired-pulse' voltage protocol and resultant whole-cell Ba2+ currents used to examine the voltage dependence of the modulation. Note the large depolarization to +60 mV reduced CADO inhibition during the second test pulse (Vt2; 10% inhibition) relative to that present in the first test pulse (Vt1; 20% inhibition). (b) Pooled data for CADO (3 μM) and adenosine (3 μM; n=6) shows that modulation is partially voltage dependent, with inhibition being significantly reduced from 19±1% to 12±2% (P<0.05, paired t-test). (c) Example of ‘bell-shaped' whole cell currents (inset) evoked by ramping the membrane potential from −100 to +60 mV. Per cent inhibition (▪ast;) by 2-chloroadenosine (CADO) at each sampled interval (500 μs) in this neuron decreased from ∼40 to ∼12% during the increase in membrane potential from −30 to 0 mV (black bar, inset). Inset calibration bars: x=100 ms, y=1 nA. (d) Adenosine modulation is reduced by exposure to the sulfhydryl-modifying reagent, NEM. Neurons responding initially to adenosine were subsequently treated with NEM during the recording; and, the response to adenosine was again measured. NEM treatment (50 mM for 2 min) significantly reduced the amounts of inhibition from 27±6% to 8±2% (n=4; P<0.05).
To further characterize the signal transduction pathway utilized by A1 receptors, we utilized the sulfhydryl-modifying reagent, N-ethylmaleimide (NEM). At the concentrations used here, NEM inactivates pertussis toxin (PTX)-sensitive G protein α subunits, but not the Gq- or Gs-subtypes (McCool et al., 1998), by ethylation of the same cysteine residue that is ADP-ribosylated by PTX (Asano & Ogasawara, 1986; Hoshino et al., 1990). Specifically, neurons responding to adenosine with robust inhibition were subsequently treated with NEM (50 μM for 2 min) during the recording and the response to adenosine again measured. NEM exposure significantly reduced the amount of inhibition by adenosine (Figure 4d) from 27±6 to 8±2% (n=4; paired t-test). These results suggest that inhibition mediated by A1 adenosine receptors primarily utilizes a well characterized, membrane-delimited, Gi/o-dependent signal transduction pathway.
Calcium channel subtypes modulated by A1 adenosine receptors
Two separate experiments were performed to determine the relative contribution of each calcium channel subtype to the whole cell barium currents recorded from basolateral amygdala neurons. In one set of neurons (n=6), sequential application of the L-type channel antagonist nifedipine (5 μM), nifedipine plus the N-type calcium channel antagonist ω-conotoxin GVIA (1 – 2 μM), and then nifedipine plus the P/Q-type calcium channel antagonist ω-agatoxin IVA (0.1 μM) inhibited total whole-cell barium currents by 28±3, 27±4 and 18±2%, respectively (Figure 5b). Thus, in this experiment, 28±6% of the total current that is ‘resistant' to antagonist exposure. ω-Conotoxin GVIA inhibition of N-type channels is not reversible in these neurons under our recording conditions (data not shown). In a second experiment (n=4), sequential application of nifedipine, nifedipine plus ω-conotoxin GVIA, and then nifedipine plus the mixed N-type and P/Q-type antagonist ω-conotoxin MVIIC (3 μM) inhibited whole cell currents by 18±4, 41±5 and 10±4%, respectively, leaving 30±8% ‘resistant' current. Assuming ω-agatoxin IVA and ω-conotoxin MVIIC inhibit a similar population of channels following treatment with ω-conotoxin GVIA, our results are consistent, with following contributions to whole-cell current: 20 – 30% L-type, 30 – 40% N-type, 10 – 20% P/Q-type, and 30% ‘resistant' channel subtype.
Figure 5.
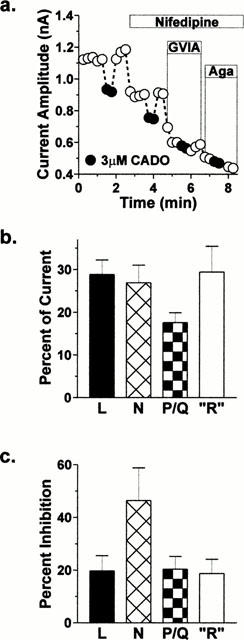
A1 adenosine receptors modulate different calcium channel subtypes in dissociated basolateral amygdala neurons. (a) Example of calcium channel antagonist effects of the modulation by CADO. For this neuron, CADO (▪ast;) inhibition was 19% in the absence of any calcium channel antagonist. The inhibition was reduced to 13% in the presence of nifedipine (5 μM) and to 3% in the presence of both nifedipine and ω-conotoxin GVIA (1 μM). Boxes indicate the duration of channel antagonist application. ‘GVIA'=ω-conotoxin GVIA. ‘Aga'=ω-agatoxin IVA. (b) Calcium channel subtype-selective antagonists indicate that dihydropyridine-sensitive, ω-conotoxin GVIA-sensitive, ω-agatoxin IVA, and ‘resistant' channels contribute 28±3, 27±4, 18±2 and 29±6% to the whole cell current, respectively, in isolated basolateral amygdala neurons (n=6). (c) Comparison of the amount of inhibition present during co-application with channel antagonists with the relative contribution of each channel subtype allowed us to examine A1 receptor modulation of specific classes of voltage-gated calcium channels. CADO inhibits 20±6% of the nifedipine-sensitive current (L-type), 46±12% of the ω-conotoxin GVIA-sensitive current (N-type), 20±5% of the ω-agatoxin IVA-sensitive current (P/Q-type), and 19±5% of the current resistant to all the channel antagonists (R-type).
To determine whether A1 adenosine receptors modulate specific calcium channel subtypes, the inhibition mediated by 2-chloroadenosine (3 μM) was measured in the presence of nifedipine (5 μM), nifedipine plus ω-conotoxin GVIA (1 μM), and then nifedipine plus ω-Agatoxin IVA (0.1 μM). A representative experiment in a single neuron is shown Figure 5a. In a population of neurons (n=6), inhibition by CADO was 24±3% without channel antagonists. During sequential application of channel antagonists, this inhibition was reduced to 18±3% during co-application of nifedipine, to 9±2% during nifedipine+ω-conotoxin GVIA, and to 5±2% in the presence of nifedipine+ω-agatoxin IVA. Comparing these values with the relative contribution of each channel subtype, CADO inhibited 46±12% of the N-type current, 20±6% of the L-type current, 20±5% of the P/Q-type current, and 19±5% of the current ‘resistant' to all channel antagonists (Figure 5c). However, the relative amounts of inhibition across different channel subtypes only approached statistical significance, suggesting that, while A1 receptors may preferentially target the N-type channels, these receptors can inhibit a variety of calcium channel subtypes in acutely dissociated basolateral amygdala neurons.
Discussion
Based on agonist pharmacology and sensitivity to the antagonist DPCPX, we propose that 2-chloroadenosine inhibition of calcium channels in basolateral amygdala neurons is mediated by the adenosine A1 adenosine receptor. This is consistent with the distribution of adenosine receptor subtypes in the central nervous system. A1 receptor mRNA is widely expressed in the forebrain (Reppert et al., 1991); and, A1-specific radioligand binding is present in the lateral/basolateral amygdala (Fastbom et al., 1987). The lack of effect by CGS21680 is also consistent with the predominant expression of A2A receptors in the striatum, nucleus accumbens, and olfactory tubercle (Schiffman et al., 1990; Wan et al., 1990). However, A2A mRNA and binding are also present elsewhere in the forebrain (Cuhna et al., 1994; Johansson et al., 1993); and, the whole-cell recording conditions used here would tend to minimize any contribution by diffusable second messengers that might be produced by activation of this adenosine receptor subtype (e.g. cyclic AMP). Conventional radioligand or mRNA analyses have failed to demonstrate significant A2B or A3 receptor expression in the forebrain (Rivkees et al., 2000; Dixon et al., 1996); however, polymerase chain reaction-based methodologies suggest that both subtypes may be expressed at low levels within the amygdala (Dixon et al., 1996). While our pharmacologic analyses strongly suggest that A1 receptors represent the predominant influence on voltage-gated calcium channels in dissociated basolateral amygdala neurons, we can not rule out possible contributions by other subtypes under some circumstances. It is also possible that other adenosine receptor subtypes are expressed in a population of neurons that is distinct from those examined here.
A1 receptors have been classically associated with the inhibition of cyclic AMP production. However, it is also clear that these adenosine receptors can modulate numerous signal transduction pathways. In basolateral amygdala neurons, A1 receptors appear to utilize primarily voltage-dependent, NEM-sensitive signal transduction pathways to inhibit voltage-gated calcium channels. These characteristics are very similar to Gi/o-mediated inhibition in many other systems. NEM treatment does not alter antagonist binding to A1 receptors (Ukena et al., 1984), suggesting the NEM-sensitive inhibition described here is most likely related to the uncoupling of A1 receptors from PTX-sensitive G proteins. However, A1 receptors may utilize both PTX-sensitive and PTX-resistant pathways to modulate calcium channels in basolateral amygdala neurons since their inhibition is only partially NEM-sensitive. In support of this, A1 receptors couple to PTX/NEM-resistant αZ-containing G proteins to modulate cyclic AMP levels in heterologous systems (Ho & Wong, 1997; Wong et al., 1992) and voltage-gated calcium channels in isolated hypothalamic neurons (Noguchi & Yamashita, 2000). Additional studies focusing on the potential interaction between A1 receptors and different G protein subtypes in these particular neurons may be warranted.
The utilization of multiple signal transduction pathways may also be reflected by the apparent inhibition of multiple channel subtypes in isolated amygdala neurons. A1 receptors appear to preferentially target ω-conotoxin GVIA-sensitive channels in these neurons. The inhibition of this channel subtype by G protein-coupled receptors is voltage-dependent in most systems, suggesting a common signal transduction pathway regardless of the tissue. However, the inhibition of dihydropyridine-sensitive channels and antagonist-resistant channels by G protein-coupled receptors is a novel finding for basolateral amygdala neurons. For example, somatostatin receptors appear to modulate primarily ω-conotoxin GVIA- and ω-agatoxin IVA-sensitive channels (Viana & Hille, 1996). It is therefore likely that different G protein-coupled receptors expressed by basolateral amygdala neurons may inhibit specific populations of calcium channel subtypes. The inhibition of overlapping, yet distinct, populations of calcium channels by muscarinic and adenosine receptors in striatal cholinergic interneurons (Song et al., 2000; Yan & Surmeier, 1996) appears to support this idea. Regardless, it remains to be determined if the different calcium channel subtypes are modulated by A1 receptors via identical signal transduction pathways.
Using central amygdala neurons from young rats (<P19), Yu & Shinnick-Gallagher (1997) find a distribution of channel subtypes that is similar to the basolateral neurons used here, with whole cell currents being 30 – 31% ω-conotoxin GVIA-sensitive, ∼28% ‘resistant' to antagonists, 22 – 27% dihydropyridine-sensitive, and 18% agatoxin IVA-sensitive. Furthermore, the relative contributions of each channel to whole-cell current is consistent with the expression of their mRNAs, with prominent expression of CaV α12.2 (N-type or α1B; see Ertel et al., 2000 for nomenclature) and CaV α12.3 mRNA (R-type or α1E; Ludwig et al., 1997; Williams et al., 1994; Fujita et al., 1993) and lower levels of CaV α12.1 (α1A), α11.2 (α1C), and α11.3 (α1D) mRNA expression (Ludwig et al., 1997) in the amygdala. Compared to our juvenile animals, whole cell calcium currents from adult basolateral amygdala neurons (Foehring & Scroggs, 1994) possess larger contributions by dihydropyridine-sensitive channels (30 – 42% of whole cell current amplitude) and ω-agatoxin IVA (31 – 33%) channels, with a subsequent reduction in the contribution by channel antagonist ‘resistant' currents (to ∼15%). These data may indicate that expression of different calcium channel subtypes in the amygdala is developmentally regulated well into adulthood.
The implications associated with A1 receptor inhibition of voltage-gated calcium channels will certainly depend upon the circumstances responsible for the release of adenosine. In the hippocampus for example, adenosine release during hypoxia/hypoglycemia depresses synaptic transmission (Coelho et al., 2000; Fowler, 1993). In the amygdala, increases in extracellular adenosine can arise from either the degradation of synaptically-released adenine nucleotides via ‘ecto-nucleotidases' or by direct release of adenosine from the intracellular space, probably via reversal of nucleoside transporters (reviewed by Brundege & Dunwiddie, 1997) since the amygdala possesses among the highest levels of adenosine-like immunoreactivity (Braas et al., 1986) and ATPase activity (Mohanakumar & Sood, 1985) in the forebrain. Furthermore, both spontaneous release of adenosine, probably via degradation of ‘extracellular' nucleotide (MacDonald & White, 1985), and depolarization-evoked adenosine release (White & MacDonald, 1990) are present in synaptosomes prepared from amygdala. Regardless, A1 receptor inhibition of voltage-gated calcium channels is likely to influence both neuronal excitability and local synaptic transmission within the amygdala. This may be especially relevant during times of heightened neuronal activity when increases in extracellular adenosine are probable.
Acknowledgments
We would like to thank Dr Jerry Trzeciakowski for his review of this manuscript and helpful comments. This work is supported in part by a Pharmaceutical Research and Manufacturers of America Foundation Starter Grant (B.A. McCool).
Abbreviations
- BLA
basolateral amygdaloid complex
- CADO
2-chloroadenosine
- CCPA
2-chloro-N6-cyclopentyladenosine
- CGS21680
4-[2-[[6-Amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- NEM
N-ethylmaleimide
- PTX
pertussis toxin
- R-PIA
N6-(L-2-Phenylisopropyl)adenosine
References
- ABDUL-GHANI A.-S., ATTWELL P.J.E., BRADFORD H.F. The protective effect of 2-chloroadenosine against the development of amygdala kindling and on amygdala-kindled seizures. Eur. J. Pharmacol. 1997;326:7–14. doi: 10.1016/s0014-2999(97)00139-8. [DOI] [PubMed] [Google Scholar]
- ASANO T., OGASAWARA N. Uncoupling of gamma-aminobutyric acid B receptors from GTP- binding proteins by N-ethylmaleimide: effect of N-ethylmaleimide on purified GTP-binding proteins. Mol. Pharmacol. 1986;29:244–249. [PubMed] [Google Scholar]
- BRAAS K.M., NEWBY A.C., WILSON V.S., SNYDER S.H. Adenosine-containing neurons in the brain localized by immunocytochemistry. J. Neurosci. 1986;6:1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNDEGE J.M., DUNWIDDIE T.V. Role of adenosine as a modulator of synaptic activity in the central nervous system. Adv. Pharmacol. 1997;39:353–391. doi: 10.1016/s1054-3589(08)60076-9. [DOI] [PubMed] [Google Scholar]
- COELHO J.E., DE MENDONCA A., RIBEIRO J.A. Presynaptic inhibitory receptors mediate the depression of synaptic transmission upon hypoxia in rat hippocampal slices. Brain Res. 2000;869:158–165. doi: 10.1016/s0006-8993(00)02381-7. [DOI] [PubMed] [Google Scholar]
- CUHNA R.A., RIBEIRO J.A. Adenosine A2A receptor facilitation of synaptic transmission in the CA1 area of the rat hippocampus requires protein kinase C but not protein kinase A activation. Neurosci. Lett. 2000;289:127–130. doi: 10.1016/s0304-3940(00)01295-7. [DOI] [PubMed] [Google Scholar]
- CUHNA R.A., JOHANSSON B., VAN DER PLOEG I., SEBASTIAO A.M., RIBEIRO J.A., FREDHOLM B.B. Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res. 1994;649:208–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- DAVIS M.The role of the amygdala in conditioned fear The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction 1992New York:Wiley-Liss; 255–306.ed. Aggleton, J.P. pp [Google Scholar]
- DE MENDONCA A., RIBEIRO J.A. 2-Chloroadenosine decreases long-term potentiation in the hippocampal CA1 area of the rat. Neurosci. Lett. 1990;118:107–111. doi: 10.1016/0304-3940(90)90260-g. [DOI] [PubMed] [Google Scholar]
- DIXON A.K., GUBITZ A.K., SIRINATHSINGHJI D.J.S., RICHARDSON P.J., FREEMAN T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERTEL E.A., CAMPBELL K.P., HARPOLD M.M., HOFMANN F., MORI Y., PEREZ-REYES E., SCHWARTZ A., SNUTCH T.P., TANABE T., BIRNBAUMER L., TSIEN R.W., CATTERALL W.A. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- FASTBOM J., PAZOS A., PALACIOS J.M. The distribution of adenosine A1 receptors and 5′-nucleotidase in the brain of some commonly used experimental animals. Neuroscience. 1987;22:813–826. doi: 10.1016/0306-4522(87)92961-7. [DOI] [PubMed] [Google Scholar]
- FOEHRING R.C., SCROGGS R.S. Multiple high-threshold calcium currents in acutely isolated rat amygdaloid pyramidal cells. J. Neurophysiol. 1994;71:433–436. doi: 10.1152/jn.1994.71.1.433. [DOI] [PubMed] [Google Scholar]
- FOWLER J.C. Purine release and inhibition of synaptic transmission during hypoxia and hypoglycemia in rat hippocampal slices. Neurosci. Lett. 1993;157:83–86. doi: 10.1016/0304-3940(93)90648-5. [DOI] [PubMed] [Google Scholar]
- FUJITA Y., MYNLIEFF M., DIRKSEN R.T., KIM M.S., NIIDOME T., NAKAI J., FRIEDRICH T., IWABE N., MIYATA T., FURUICHI T., FURUTAMA D., MIKOSHIBA K., MORI Y., BEAM K.G. Primary structure and functional expression of the omega-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10:585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- GONCALVES M.L., CUNHA R.A., RIBEIRO J.A. Adenosine A2A receptors facilitate 45Ca2+ uptake through class A calcium channels in rat hippocampal CA3 but not CA1 synaptosomes. Neurosci. Lett. 1997;238:73–77. doi: 10.1016/s0304-3940(97)00803-3. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HASSAN H., GRECKSCH G., RUTHRICH H., KRUG M. Effects of nicardipine, an antagonist of L-type voltage-dependent calcium channels, on kindling development, kindling-induced learning deficits and hippocampal potentiation phenomena. Neuropharmacol. 1999;38:1841–1850. doi: 10.1016/s0028-3908(99)00067-2. [DOI] [PubMed] [Google Scholar]
- HEINBOCKEL T., PAPE H.C. Modulatory effects of adenosine on inhibitory postsynaptic potentials in the lateral amygdala of the rat. Br. J. Pharmacol. 1999;128:190–196. doi: 10.1038/sj.bjp.0702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERLITZE S., GARCIA D.E., MACKIE K., HILLE B., SCHEUER T., CATTERALL W.A. Modulation of Ca2+ channels by Gprotein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- HO M.K., WONG Y.H. Functional role of aminoterminal serine16 and serine27 of G alphaZ in receptor and effector coupling. J. Neurochem. 1997;68:2514–2522. doi: 10.1046/j.1471-4159.1997.68062514.x. [DOI] [PubMed] [Google Scholar]
- HOSHINO S., KIKKAWA S., TAKAHASHI K., ITOH H., KAZIRO Y., KAWASAKI H., SUZUKI K., KATADA T., UI M. Identification of sites for alkylation by N-ethylmaleimide and pertussis toxin-catalyzed ADP-ribosylation on GTP-binding proteins. FEBS Lett. 1990;276:227–231. doi: 10.1016/0014-5793(90)80548-w. [DOI] [PubMed] [Google Scholar]
- IKEDA S.R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J. Physiol. (Lond.) 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKEDA S.R. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol. Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHANSSON B., GEORGIEV V., PARKINSON F.E., FREDHOLM B.B. The binding of the adenosine A2 receptor selective agonist [3H]CGS 21680 to rat cortex differs from its binding to rat striatum. Eur. J. Pharmacol. 1993;247:103–110. doi: 10.1016/0922-4106(93)90066-i. [DOI] [PubMed] [Google Scholar]
- KESSEY K., MOGUL D.J. Adenosine A2 receptors modulate hippocampal synaptic transmission via a cyclic-AMP-dependent pathway. Neurosci. 1998;84:59–69. doi: 10.1016/s0306-4522(97)00504-6. [DOI] [PubMed] [Google Scholar]
- LOHSE M.J., KLOTZ K.N., SCHWABE U., CRISTALLI G., VITTORI S., GRIGANTINI M. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., CHOI S. Activation of adenosine A1 receptors initiates short-term synaptic depression in rat striatum. Neurosci. Lett. 1995;199:9–12. doi: 10.1016/0304-3940(95)12024-x. [DOI] [PubMed] [Google Scholar]
- LUDWIG A., FLOCKERZI V., HOFMANN F. Regional expression and cellular localization of the alpha(1) and beta subunit of high voltage-activated calcium channels in rat brain. J. Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD W.F., WHITE T.D. Nature of extrasynaptosomal accumulation of endogenous adenosine evoked by K+ and veratidine. J. Neurochem. 1985;45:791–797. doi: 10.1111/j.1471-4159.1985.tb04062.x. [DOI] [PubMed] [Google Scholar]
- MAGEE J.C., CARRUTH M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J. Neurophysiol. 1999;82:1895–1901. doi: 10.1152/jn.1999.82.4.1895. [DOI] [PubMed] [Google Scholar]
- MARTINSON E.A., JOHNSON R.A., WELLS J.N. Potent adenosine receptor antagonists that are selective for the A1 receptor subtype. Mol. Pharmacol. 1987;31:247–252. [PubMed] [Google Scholar]
- MCCOOL B.A., BOTTING S.K. Characterization of strychnine-sensitive glycine receptors in acutely isolated neurons from adult rat basolateral amygdala. Brain Res. 2000;859:341–351. doi: 10.1016/s0006-8993(00)02026-6. [DOI] [PubMed] [Google Scholar]
- MCCOOL B.A., PIN J.-P., BRUST P.F., HARPOLD M.M., LOVINGER D.M. Functional coupling of rat group II metabotropic glutamate receptors to an ω-conotoxin GVIA-sensitive calcium channel in human embryonic kidney 293 cells. Mol. Pharmacol. 1996;50:912–922. [PubMed] [Google Scholar]
- MCCOOL B.A., PIN J.-P., HARPOLD M.M., BRUST P.F., STAUDERMAN K.A., LOVINGER D.M. Rat group I metabotropic glutamate receptors inhibit neuronal Ca2+ channels via multiple signal transduction pathways in HEK 293 cells. J. Neurophysiol. 1998;79:379–391. doi: 10.1152/jn.1998.79.1.379. [DOI] [PubMed] [Google Scholar]
- MCDONALD A.J. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J. Comp. Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- MOGUL D.J., ADAMS M.E., FOX A.P. Differential activation of adenosine receptors decreases N-type but potentiates P-type Ca2+ current in hippocampal CA3 neurons. Neuron. 1993;10:327–334. doi: 10.1016/0896-6273(93)90322-i. [DOI] [PubMed] [Google Scholar]
- MOHANAKUMAR K.P., SOOD P.P. Inhibitory action of morphine on adenosine triphosphatase content in the whole and individual nuclei of mouse brain during tolerance-dependence development and its reversal by naloxone. J. fur Hirnforsh. 1985;26:695–708. [PubMed] [Google Scholar]
- MYNLIEFF M., BEAM K.G. Adenosine acting at an A1 receptor decreases N-type calcium current in mouse motoneurons. J. Neurosci. 1994;14:3628–3634. doi: 10.1523/JNEUROSCI.14-06-03628.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOGUCHI J., YAMASHITA H. Adenosine inhibits voltage-dependent Ca2+ currents in rat dissociated supraoptic neurones via A1 receptors. J. Physiol. 2000;526:313–326. doi: 10.1111/j.1469-7793.2000.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSE I., HIGASHI H., INOKUCHI H., NISHI S. Synaptic responses of guinea pig and rat central amygdala neurons in vitro. J. Neurophysiol. 1991;65:1227–1241. doi: 10.1152/jn.1991.65.5.1227. [DOI] [PubMed] [Google Scholar]
- PAL S., SOBATI S., LIMBRICK D.D., DELORENZO R.J. In vitro status epilepticus casuses sustained elevation of intracellular calcium levels in hippocampal neurons. Brain Res. 1999;851:20–31. doi: 10.1016/s0006-8993(99)02035-1. [DOI] [PubMed] [Google Scholar]
- POURGHOLAMI M.H., ROSTAMPOUR M., MIRNAJAFI-ZADEH J., PALIZVAN M.R. Intra-amygdala infusion of 2-chloroadenosine suppresses amygdala-kindled seizures. Brain Res. 1997;775:37–42. doi: 10.1016/s0006-8993(97)00769-5. [DOI] [PubMed] [Google Scholar]
- REPPERT S.M., WEAVER D.R., STEHLE J.H., RIVKEES S.A. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol. Endocrinol. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- RIVKEES S.A., THEVANANTHER S., HAO H. Are A3 adenosine receptors expressed in the brain. Neuroreport. 2000;11:1025–1030. doi: 10.1097/00001756-200004070-00026. [DOI] [PubMed] [Google Scholar]
- SCHIFFMANN S., LIBERT F., VASSART G., DUMONT J.E., VANDERHAEGHEN J.-J. A cloned G protein-coupled protein with a distribution restricted to striatal medium-size neurons. Possible relationship with D1 dopamine receptor. Brain Res. 1990;519:333–337. doi: 10.1016/0006-8993(90)90097-u. [DOI] [PubMed] [Google Scholar]
- SONG W.-J., TKATCH T., SURMEIER D.J. Adenosine receptor expression and modulation of Ca2+ channels in rat striatal cholinergic interneurons. J. Neurophysiol. 2000;83:322–332. doi: 10.1152/jn.2000.83.1.322. [DOI] [PubMed] [Google Scholar]
- UKENA D., POESCHLA E., HUTTEMANN E., SCHWABE U. Effects of N-ethylmaleimide on adenosine receptors of rat fat cells and human platelets. Naunyn Schmiedebergs Arch. Pharmacol. 1984;327:247–253. doi: 10.1007/BF00502457. [DOI] [PubMed] [Google Scholar]
- UMEMIYA M., BERGER A.J. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994;13:1439–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- VIANA F., HILLE B. Modulation of high voltage-activated calcium channels by somatostatin in acutely isolated rat amygdaloid neurons. J. Neurosci. 1996;16:6000–6011. doi: 10.1523/JNEUROSCI.16-19-06000.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAN W., SUTHERLAND G.R., GEIGER J.D. Binding of the adenosine A2 receptor ligand [3H]CGS 21680 to human and rat brain: Evidence for multiple affinity sites. J. Neurochem. 1990;55:1763–1771. doi: 10.1111/j.1471-4159.1990.tb04967.x. [DOI] [PubMed] [Google Scholar]
- WHITE T.D., MACDONALD W.F. Neural release of ATP and adenosine. Ann. N.Y. Acad. Sci. 1990;603:287–298. doi: 10.1111/j.1749-6632.1990.tb37680.x. [DOI] [PubMed] [Google Scholar]
- WIDMER H., AMERDEIL H., FONTANAUD P., DESARMENIEN M.G. Postnatal maturation of rat hypothalamoneurohypophysial neurons: evidence for a developmental decrease in calcium entry during action potentials. J. Neurophysiol. 1997;77:260–271. doi: 10.1152/jn.1997.77.1.260. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M.E., MARUBIO L.M., DEAL C.R., HANS M., BRUST P.F., PHILIPSON L.H., MILLER R.J., JOHNSON E.C., HARPOLD M.M., ELLIS S.B. Structure and functional characterization of neuronal alpha 1E calcium channel subtypes. J. Biol. Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- WONG Y.H., CONKLIN B.R., BOURNE H.R. Gz-mediated hormonal inhibition of cyclic AMP accumulation. Science. 1992;255:339–342. doi: 10.1126/science.1347957. [DOI] [PubMed] [Google Scholar]
- YAN Z., SURMEIER D.J. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J. Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU B.J., SHINNICK-GALLAGHER P. Dihydropyridine- and neurotoxin-sensitive and -insensitive calcium currents in acutely dissociated neurons of the rat central amygdala. J. Neurophysiol. 1997;77:690–701. doi: 10.1152/jn.1997.77.2.690. [DOI] [PubMed] [Google Scholar]
- ZHOU Q.-Y., LI C., OLAH M.E., JOHNSON R.A., STILES G.L., CIVELLI O. Molecular cloning and characterization of an adenosine receptor: The A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU Y., IKEDA S.R. Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J. Neurophysiol. 1993;70:610–620. doi: 10.1152/jn.1993.70.2.610. [DOI] [PubMed] [Google Scholar]


