Abstract
Effects of NS-1619, an opener of large conductance Ca2+-activated K+ (BK) channel, on intracellular Ca2+ concentration ([Ca2+]i) and membrane potential were examined in single myocytes freshly isolated from porcine coronary artery.
Under current clamp mode, the application of 1 – 30 μM NS-1619 hyperpolarized the membrane in concentration-dependent manner. The NS-1619-induced hyperpolarization was abolished by the presence of 100 nM iberiotoxin.
Application of 1 – 10 μM NS-1619 hyperpolarized the membrane by approximately 6 mV or less but did not change significantly the [Ca2+]i. When membrane hyperpolarization of 12 mV or so was caused by 30 μM NS-1619, [Ca2+]i was unexpectedly increased by approximately 200 nM. This increase in [Ca2+]i and the concomitant outward current activation were also observed under voltage-clamp at holding potential of −40 mV.
The increase in [Ca2+]i by 30 μM NS-1619 occurred mainly in peripheral regions than in the centre of the myocytes. The removal of extracellular Ca2+ affected neither the membrane hyperpolarization nor the increase in [Ca2+]i.
In the presence of 10 mM caffeine and 10 μM ryanodine, the increase in [Ca2+]i by 30 μM NS-1619 was not observed and the membrane hyperpolarization was reduced to approximately 67% of the control.
These results indicate that the opening of BK channels by NS-1619 at 30 μM, which is the most frequently used concentration of this agent, is partly due to Ca2+ release from caffeine/ryanodine-sensitive intracellular storage sites but is mainly due to the direct activation of the channels.
Keywords: NS-1619, coronary artery, smooth muscle, Ca2+ -activated K+ channel, K+ channel opener, hyperpolarization, Ca2+ release
Introduction
Large conductance Ca2+-activated K+ (BK) channels are highly expressed in smooth muscle cells of various organs, including blood vessels (Kuriyama et al., 1998). In some smooth muscles, the blockade of BK channels results in the membrane depolarization by several millivolts and the rise of muscle tone, so that the channels may contribute to the regulation of the resting membrane potential and the muscle tone (Leblanc et al., 1994; Nelson & Quayle, 1995; Carl et al., 1996). Several compounds have been reported to be BK channel openers; dehydrosoyasaponin-I (McManus et al., 1993; Giangiacomo et al., 1998), maxikdiol (Singh et al., 1994), niflumic acid (Ottolia & Toro, 1994), NS-004 (Sargent et al., 1993; McKay et al., 1994; Xu et al., 1994), NS-1619 (Olesen et al., 1994; Edwards et al., 1994; Holland et al., 1996), Evans blue (Hollywood et al., 1998; Wu et al., 1999) and nordihydroguaiaretic acid (Nagano et al., 1996; Yamamura, et al., 1999). A possibility has been suggested that BK channel openers may have a substantial potency for the treatment of angina, hypertension, bronchial asthma, hypersensitive urinary bladder and some other diseases characterized by the increased tonus of smooth muscles (Edwards & Weston, 1995). These are expected to be a new category of K+ channel openers following those acting on ATP-sensitive K+ (KATP) channel. The major mechanism underlying the vasodilating effect of KATP channel openers is supposed to be the depression of Ca2+ influx through the voltage dependent Ca2+ channel via the membrane hyperpolarization and the subsequent decrease in intracellular Ca2+ concentration ([Ca2+]i) in vascular myocytes (Nelson & Quayle, 1995). The modulation of Ca2+ mobilization by BK channel openers remains, however, unclear.
The present study was undertaken to examine the possibility that the regulation of intracellular Ca2+ mobilization could be involved in the mechanisms underlying of the activation of BK channel by the most popular synthetic BK channel opener, NS-1619, in porcine coronary arterial smooth muscle cells. Based on the input resistance of single smooth muscle cells as high as approximately 2 – 4 GΩ, the measurement of membrane hyperpolarization under current clamp mode is often more sensitive to detect BK channel opening than the direct measurement of whole cell BK channel current under voltage clamp. In the present study, the confocal Ca2+ images and either membrane potentials or currents were simultaneously recorded from single coronary arterial smooth muscle cells in the absence or presence of NS-1619.
Methods
Cell isolation
Single smooth muscle cells of porcine coronary artery were prepared as described previously (Yamamura et al., 1999). In brief, whole hearts from young pigs (6 months old) were obtained at a local slaughterhouse and transported to the laboratory in ice-cold normal Krebs' solution. A small piece of left circumflex coronary artery was dissected, cleaned of blood and surrounding tissues and immersed for 40 min in Ca2+-free Krebs' solution containing 1% albumin, 0.2% collagenase, 0.1% papain and 0.2% trypsin inhibitor at 37°C. After the incubation, the solution was replaced with Ca2+- and collagenase-free Krebs' solution. Myocytes were isolated by gentle agitation with a glass pipette and stored at 4°C until use. A few drops of cell suspension were placed in a recording chamber, which was mounted on the stage of a phase contrast microscope (Nikon TMD, Tokyo, Japan). After these cells were settled, the bath was continuously perfused with the HEPES-buffered solution at a flow rate of 5 ml min−1.
Solutions
The normal Krebs' solution had an ionic composition of (in mM): NaCl 112, KCl 4.7, CaCl2 2.2, MgCl2 1.2, NaHCO3 25, KH2PO4 1.2 and glucose 14. The pH was adjusted to 7.4 by gassing with a mixture of 95% O2 and 5% CO2. The Ca2+-free Krebs' solution was prepared by the removal of 2.2 mM CaCl2 from the normal Krebs' solution. The HEPES-buffered solution for electrophysiological recording had an ionic composition of (in mM): NaCl 137, KCl 5.9, CaCl2 2.2, MgCl2 1.2, glucose 14 and HEPES 10. The pH of the solution was adjusted to 7.4 with NaOH. The pipette solution contained (in mM): KCl 140, MgCl2 1, HEPES 10 and Na2ATP 2. The pH was adjusted to 7.2 with KOH.
Electrophysiological experiments
The whole cell patch clamp technique was applied to single cells by means of the techniques originally introduced by Hamill et al. (1981) using CEZ-2400 amplifier (Nihon Kohden, Tokyo, Japan). The procedures of electrophysiological recordings and data analyses were performed as described previously by use of programs, Data-Acquisition and Cell-Soft, which were developed in University of Calgary (Imaizumi et al., 1989). All electrophysiological recordings were carried out at 30±1°C.
[Ca2+]i measurements
Two dimensional Ca2+ images were obtained by a fast scanning confocal fluorescent microscope (Nikon RCM-8000; Nikon, Tokyo, Japan) equipped with objective lens (Fluor 40×1.15 NA, water immersion, Nikon) and Ratio3 software (Nikon). Recordings were started at least 3 min after rupturing the patch membrane to make indo-1 diffuse into the cell from the pipette, which was filled with the solution containing 100 μM indo-1. Excitation wavelength from an argon ion laser was 351 nm and the emission wavelengths were 405 and 485 nm. The resolution of the microscope is approximately 0.33×0.27 μm (1 pixel) and approximately 1.5 μm to Z-axis direction. The Ca2+ image was scanned over a full frame (512×512 pixels) every 20 or 30 s. The calibration of indo-1 signal was performed by the method reported by Kawanishi et al. (1994). The data analyses were performed as described previously (Imaizumi et al., 1998).
Statistics
Pooled data were shown as mean±s.e.mean. Statistical significance between two and multi groups was determined by Student's t-test and Scheffé's test after one-way ANOVA, respectively. Significant difference was expressed in figures as ** or ##P<0.01.
Drugs
Pharmacological reagents were obtained from Sigma (St. Louis, U.S.A.) except for NS-1619 (1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl) phenyl]-5-(tr ifluoromethyl)-2H-be nzimidazol-2-one; Research Biochemicals International, Natick, U.S.A.), iberiotoxin (IbTx; Peptide Institute, Osaka, Japan), caffeine (Wako, Osaka, Japan), collagenase (Amano, Nagoya, Japan), HEPES, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), indo-1, fluo-3 (Dojin, Kumamoto, Japan) and fura-2 (Molecular Probes, Eugene, U.S.A.). NS-1619 was dissolved in dimethyl sulphoxide (DMSO) at the concentration of 10 mM as a stock solution. The external solutions always contained 0.3% DMSO throughout the experiments, regardless of the presence/absence of NS-1619. It was confirmed that 0.3% DMSO did not affect the membrane potential, current and [Ca2+]i.
Results
Membrane hyperpolarization by NS-1619
When the membrane potential of single coronary smooth muscle cells was recorded under current clamp mode, the averaged resting membrane potential was −39.3±1.1 mV (n=10). The application of 30 μM NS-1619 hyperpolarized the membrane by 11.7±1.1 mV (n=10, P<0.01 vs control; Figure 1) in all myocytes examined. The membrane potential was mostly recovered by washout of NS-1619 (−40.2±1.1 mV, n=10). The hyperpolarization induced by 30 μM NS-1619 was not affected significantly by either 10 μM glibenclamide, a KATP channel blocker, or 100 nM apamin, a small conductance Ca2+-activated K+ channel blocker (P>0.05, n=5 for each). In contrast, the addition of 100 nM IbTx, a selective BK channel inhibitor, abolished the NS-1619-induced hyperpolarization and, moreover, depolarized the myocytes by approximately 5 mV (5.0±0.6 mV depolarization from the initial resting potential, n=3; Figure 1). Application of 100 nM IbTx in the absence of NS-1619 depolarized the membrane from −38.8±1.2 to −34.3±1.2 mV (n=3, P<0.05) and the subsequent addition of 30 μM NS-1619 did not induce further change in the membrane potential (−33.8±0.4 mV, P>0.05 vs only IbTx).
Figure 1.
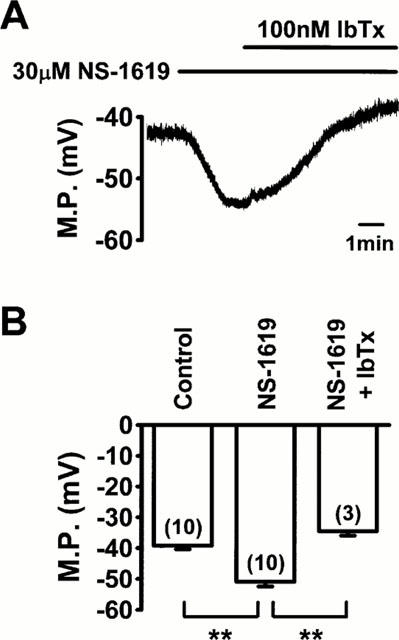
Effects of NS-1619 on membrane potential (M.P.) in single smooth muscle cells of porcine coronary artery under current clamp mode. (A) Application of 30 μM NS-1619 hyperpolarized the myocyte by 12 mV. The NS-1619-induced hyperpolarization was abolished by 100 nM iberiotoxin (IbTx). (B) Summarized data obtained from experiments typically shown in (A). The number of myocytes used is given in parentheses. The statistical significance of the difference between three groups is indicated by **P<0.01.
Effects of NS-1619 on [Ca2+]i and membrane potential or current
The simultaneous measurements of [Ca2+]i and membrane potential were performed using a confocal microscope and 100 μM indo-1 under current clamp mode. The averaged resting membrane potential and [Ca2+]i as the average from whole cell area were −40.0±1.0 mV and 114±6 nM, respectively (n=21). As shown in Figure 2, the application of 30 μM NS-1619 caused the membrane hyperpolarization by 12.5±1.0 mV (n=11, P<0.01 vs control). Unexpectedly, a small but significant increase in [Ca2+]i to 308±20 nM (n=11, P<0.01 vs control) was observed during the membrane hyperpolarization (Figure 2). These effects of 30 μM NS-1619 were removed by washout and reproduced at least three times in each myocyte. Of importance is that the membrane hyperpolarization was caused by NS-1619 in a concentration-dependent manner in the range of 1 and 30 μM, but the significant increase in [Ca2+]i was elicited only by 30 μM NS-1619 in all or none manner. Effects of 100 μM NS-1619 were similar to those of 30 μM but had faster onset and were removed only partly by washout (not shown). Therefore, most experiments were performed using NS-1619 in the range of 1 and 30 μM. The addition of 100 nM IbTx abolished the hyperpolarization induced by 30 μM NS-1619, but did not affect significantly the [Ca2+]i increase (299±21 nM, n=4, P>0.05 vs 308±20 nM). A change in cell shape was not detected during the response to 30 μM NS-1619 in any myocytes examined (n=∼50).
Figure 2.

Effects of NS-1619 on membrane potential (M.P.) and [Ca2+]i. M.P. and [Ca2+]i were recorded simultaneously using a fast scanning confocal fluorescent microscope with a Ca2+ fluorescent indicator 100 μM indo-1 under whole cell current clamp mode. (A) The changes in M.P. and [Ca2+]i as the average from whole cell area are shown against time. Application of 30 μM NS-1619 produced a membrane hyperpolarization and, simultaneously, the [Ca2+]i increase, which were removed by washout. (B) and (C) The summarized data of the relationships between concentrations of NS-1619 and either the membrane hyperpolarizations (B) or the changes in [Ca2+]i (C). The number of myocytes used is given in parentheses. The statistical significance of the difference vs control is indicated by **P<0.01.
Figure 3 shows effects of 30 μM NS-1619 on [Ca2+]i and membrane current measured under voltage clamp at holding potential of −40 mV. Application of NS-1619 first increased the frequency of spontaneous transient outward current with a small increase in [Ca2+]i and then induced a sustained outward current with a larger increase in [Ca2+]i. The averaged sustained component of the outward current and the concomitantly measured [Ca2+]i were 21.7±6.4 pA and 295±32 nM (n=7, P<0.01 vs control), respectively, indicating that the [Ca2+]i increase under voltage-clamp at −40 mV was comparable to that measured under current-clamp conditions (308±20 nM, P>0.05).
Figure 3.
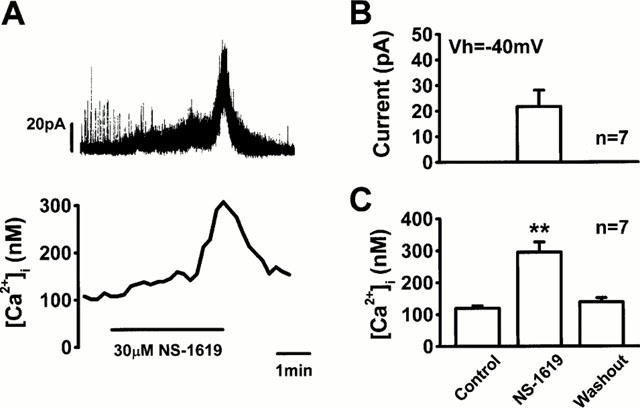
Effects of NS-1619 on membrane current and [Ca2+]i. (A) Membrane current was measured under voltage-clamp at a holding potential of −40 mV. [Ca2+]i was simultaneously measured in the same manner as in Figure 2. Application of 30 μM NS-1619 induced an outward current and increased [Ca2+]i. (B) The amplitude of the sustained component of outward current induced by 30 μM NS-1619 at holding potential of −40 mV was measured in seven myocytes. (C) The [Ca2+]i before and during the application and after washout of 30 μM NS-1619 under voltage-clamp at −40 mV were summarized from the results obtained in the same cells shown in (B). The statistical significance of the difference vs the control group is indicated by **P<0.01.
Localized increase in [Ca2+]i by NS-1619
The confocal Ca2+ images were obtained in the absence (Figure 4A,a) and presence of 30 μM NS-1619 (A,b) and after the removal of NS-1619 (A,c), respectively. The profiles of [Ca2+]i along a cross-section of short axis of the myocyte (3 μm in thickness) as indicated by the bars in Figure 4A were measured and plotted against the section in Figure 4B. It is clear from the analysis that the increase in [Ca2+]i occurred mainly in the peripheral regions within 1 μm from both edges of the myocyte. To examine quantitatively the localized [Ca2+]i increase by NS-1619, the averaged [Ca2+]i in square areas (3.0×1.0 μm) along the long axis of a myocyte was measured in peripheral and central regions (five for each). The initial [Ca2+]i in central and peripheral regions was 102±9 and 109±14 nM, respectively (n=11, P>0.05; Figure 4C). Approximately 3 min after the application of 30 μM NS-1619, the [Ca2+]i in central and peripheral regions was 181±9 and 438±52 nM, respectively (n=11, P<0.01; Figure 4C). These effects of NS-1619 were removed by washout.
Figure 4.
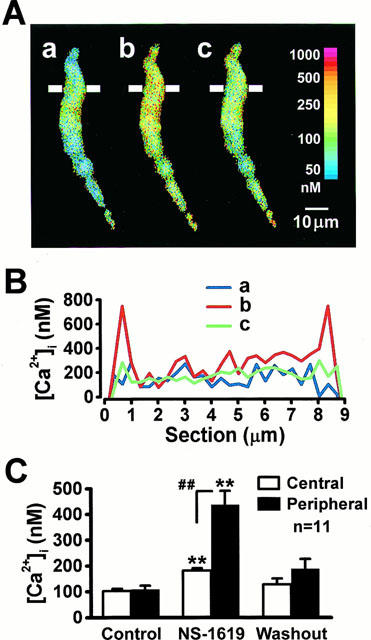
Analyses of confocal Ca2+ images. (A) Three images show the distribution of intracellular Ca2+ in the absence (a), in the presence of 30 μM NS-1619 for 3 min (b) and after washout (c). (B) The profile of [Ca2+]i along the cross-section indicated by the line in (A) (3 μm in thickness), are plotted along the section. The colour lines indicated by a, b and c were [Ca2+]i profiles obtained in the corresponding images in (A). (C) [Ca2+]i in squares (3.0×1.0 μm) along the long axis of the cells was measured in the central and peripheral regions. The average from five squares for each region was obtained in a myocyte and the results from 11 myocytes were summarized. The statistical significance of the difference vs control and vs central region is indicated by ** and ##P<0.01, respectively.
Source of Ca2+ increased by NS-1619
To determine the source of Ca2+ increased by NS-1619, the effects of NS-1619 were examined after the removal of extracellular Ca2+. When 2.2 mM Ca2+ in the external solution was replaced by 0.5 mM Cd2+, the application of 30 μM NS-1619 significantly increased [Ca2+]i to 333±10 nM (n=4, P>0.05 vs under the control conditions) during the membrane hyperpolarization of 11.5±1.0 mV (n=4, P>0.05; Figure 5).
Figure 5.

Effects of the removal of extracellular Ca2+ on NS-1619-induced responses of membrane potential (M.P.) and [Ca2+]i. The 2.2 mM Ca2+ in the external solution was replaced by 0.5 mM Cd2+. (A) The change in M.P. and that in [Ca2+]i as the average from whole cell area induced by application of 30 μM NS-1619 in the absence of external Ca2+. (B) and (C) Summarized results of the changes in M.P. (B) and [Ca2+]i (C) by 30 μM NS-1619 under normal and Ca2+-free conditions are illustrated. The number of experiments is given in parentheses. The statistical significance of the difference vs control is indicated by **P<0.01.
For further experiments to determine the source of Ca2+ increased by NS-1619, the effects of NS-1619 were examined after intracellular Ca2+ storage sites were depleted by the treatment with caffeine and ryanodine. The simultaneous application of 10 mM caffeine and 10 μM ryanodine caused a transient rise of [Ca2+]i to 401±50 nM and, concomitantly, a transient membrane hyperpolarization of 8.2±2.0 mV at the peak (n=10; Figure 6A). Slight shortening of myocytes in the longitudinal direction was occasionally observed when caffeine and ryanodine were applied. In the presence of caffeine and ryanodine for 5 min, the membrane potential and [Ca2+]i were changed to a stable value of approximately −38 mV (−38.4±1.8 mV, n=10, P<0.05 vs −41.5±1.1 mV before the treatment with caffeine and ryanodine) and 223±33 nM (n=10, P<0.05 vs 121±10 nM before caffeine and ryanodine), respectively (Figure 6A). Under these conditions, the addition of 30 μM NS-1619 induced significant membrane hyperpolarization of 8.3±0.6 mV (n=10, P<0.01 before application of NS-1619). The hyperpolarization was a slightly but significantly smaller than that in the absence of caffeine and ryanodine (P<0.01 vs value in the absence of caffeine and ryanodine of 12.5±1.0 mV; Figure 6B). On the other hand, the increase in [Ca2+]i by NS-1619 was almost abolished in the presence of caffeine and ryanodine (P<0.01 vs in the absence; Figure 6C).
Figure 6.

Effects of NS-1619 in the co-presence of 10 mM caffeine and 10 μM ryanodine on membrane potential (M.P.) and [Ca2+]i. (A) The changes in M.P. and [Ca2+]i as the average from whole cell area in the presence of caffeine and ryanodine, are shown against time. (B) and (C) The data about the extent of membrane hyperpolarization (B) and [Ca2+]i increase (C) by 30 μM NS-1619 in the absence and presence of caffeine/ryanodine are summarized. The number of experiments is given in parentheses. The statistical significance of the difference vs the corresponding control is indicated by **P<0.01.
Discussion
The present study clearly shows that 30 μM NS-1619 releases Ca2+ from caffeine/ryanodine-sensitive Ca2+ storage sites and that the Ca2+ release may partially contribute to the membrane hyperpolarization via the activation of BK channels in porcine coronary artery smooth muscle cells.
The membrane hyperpolarization induced by NS-1619 was completely inhibited by IbTx, indicating that it was due to the activation of BK channels. The major mechanism underlying vasodilation induced by the enhancement of BK channel activity has been suggested to be similar to that via the opening of KATP channel, which may be due to the decrease in Ca2+ influx through voltage dependent Ca2+ channels by the membrane hyperpolarization in smooth muscle cells (Nelson & Quayle, 1995). A decrease in [Ca2+]i was, therefore, expected to be elicited by the application of NS-1619 in porcine coronary artery smooth muscle cells. On the contrary, the results showed the increase in [Ca2+]i during the membrane hyperpolarization induced by 30 μM NS-1619. Since the [Ca2+]i increase was also observed under voltage-clamp at holding potential of −40 mV, it can be neglected that NS-1619-evoked Ca2+ release is triggered by the membrane hyperpolarization. The possibility that the increase in [Ca2+]i by NS-1619 is attributable to optical artifacts can be also ruled out for the following reasons: (1) The [Ca2+]i increase by NS-1619 was minimized after the treatment with caffeine and ryanodine (Figure 6) or in the presence of 10 mM BAPTA in the pipette solution (data not shown). (2) The fluorescence intensity of indo-1 at emission wavelengths of 405 and 485 nm was increased and decreased, respectively, by the application of NS-1619. (3) The NS-1619-induced [Ca2+]i increase was also observed when fluo-3 (excitation wavelength of 488 nm; emission wavelength of 515 nm) was used as a fluorescence indicator in the same equipment. (4) It was also confirmed using fura-2 (excitation wavelengths of 340 and 380 nm; emission wavelength of ⩾520 nm) in a different system for the Ca2+ image measurements (Argus/HiSCA, Hamamatsu Photonics, Hamamatsu, Japan).
The membrane hyperpolarization induced by 30 μM NS-1619 was reduced to approximately 67%, when the increase in [Ca2+]i was mostly abolished by the pretreatment with caffeine and ryanodine. This result indicates that the activation of BK channel by NS-1619 is partly attributable to the increase in [Ca2+]i, in addition to the direct action on the channel itself or associated sites, which has been reported by use of single channel recording (Sellers & Ashford, 1994; Holland et al., 1996). Similar possibility has been speculated for one of the mechanisms underlying the activation of BK channel current by nordihydroguaiaretic acid in porcine coronary artery myocytes (Yamamura et al., 1999). The increase in [Ca2+]i elicited by NS-1619 occurred mainly in peripheral regions of the myocytes based on the analyses of confocal Ca2+ images. Although this result might fit with an idea that Ca2+ influx was enhanced by NS-1619, the fact that the [Ca2+]i increase was not affected by the removal of external Ca2+ strongly suggests the Ca2+ release from intracellular storage sites. The reason why the Ca2+ release occurred mainly in peripheral storage sites and did not diffuse over whole cell area was not clear in the present study. It is likely that NS-1619 was highly soluble to lipids and, therefore, may preferentially distribute to the plasmalemma and subplasmalemma membranes including superficial storage sites.
It has been suggested that there are, at least, two spatially and functionally distinctive types of Ca2+ storage sites in smooth muscle cells with respect to intracellular Ca2+ mobilization coupled with the cellular functions. One type of storage sites is localized in the superficial areas just beneath the cell membrane and does not directly contribute to the activation of contractile system; ‘non-contractile compartment' (van Breemen et al., 1995; Karaki et al., 1997). The other type is located in relatively centre of the cell and surrounded with contractile elements; ‘contractile compartment'. Spontaneous Ca2+ release from local storage sites in the superficial areas through ryanodine receptor Ca2+ releasing channels has been detected as a Ca2+ spark in smooth muscle cells (Nelson et al., 1995). It has been clarified that Ca2+ sparks in superficial areas elicit spontaneous transient outward currents via BK channel activation (Zhuge et al., 1999; Pérez et al., 1999) but are not related to cell contraction (Bolton & Imaizumi, 1996; Imaizumi et al., 1999; Jaggar et al., 2000). The reason why NS-1619-induced [Ca2+]i increase activated BK channels but did not induce cell shortening may probably be due to the localized increase in [Ca2+]i in peripheral regions. Actually, 30 μM NS-1619 induces relaxation rather than contraction in smooth muscle tissues (Edwards et al., 1994; Holland et al., 1996).
The potentiation of BK channels by NS-1619 has been demonstrated in smooth muscles of vascular tissues (Olesen et al., 1994; Edwards et al., 1994; Holland et al., 1996), trachea (Macmillan et al., 1995), vas deferens (Huang et al., 1997), urinary bladder (Sheldon et al., 1997), and also neurons (Sellers & Ashford, 1994; Lee et al., 1995). It has been reported that the BK channel activation by NS-1619 appears to be independent from ligand receptors, G-proteins or channel phosphorylation (Olesen et al., 1994). NS-1619 has been found to be a highly effective relaxant with an EC50 of about 10 – 30 μM in several smooth muscles of blood vessels (Edwards et al., 1994; Holland et al., 1996; Cadorette et al., 2000) and other tissues (Huang et al., 1997; Sheldon et al., 1997). NS-1619, however, also inhibits the voltage dependent Ca2+ channel (Edwards et al., 1994; Holland et al., 1996; Sheldon et al., 1997), the voltage dependent K+ channel (Edwards et al., 1994; Holland et al., 1996), KATP channel (Edwards et al., 1994), and even the cholinergic neurotransmission (Patel et al., 1998). In addition to these, it was found in the present study that NS-1619 at the concentration of 30 μM or higher releases Ca2+ from peripheral storage sites.
It has been reported that the application of carbonyl cyanide p-trifluoromethoxyphenylhydrazone, a mitochondrial uncoupler, to single smooth muscle cells of the rat pulmonary artery induces Ca2+ release probably from mitochondria and results in the activation of BK channels (Yuan et al., 1996). In the present study, the depletion of Ca2+ stored in sarcoplasmic reticulum (SR) by the treatment with caffeine and ryanodine almost abolished NS-1619-induced [Ca2+]i increase. This strongly suggests that the Ca2+ release was mainly from SR rather than mitochondria. The mechanisms underlying the Ca2+ release by NS-1619 from SR remain to be determined.
In conclusion, the application of 30 μM NS-1619 to porcine coronary arterial smooth muscle cells releases Ca2+ from peripheral storage sites, which were sensitive to caffeine/ryanodine and, therefore, selectively activated BK channels. The Ca2+ release by NS-1619 is partly responsible for the activation of BK channels.
Acknowledgments
We thank Dr Wayne Giles (University of Calgary, Calgary, Canada) for providing data acquisition and analysis programs. This work was supported by Grant-in-Aid for Scientific Research by Japan Society for the Promotion of Sciences and also by Research Grant for Cardiovascular Diseases (11C-1) from the ministry of Health and Welfare (to Y. Imaizumi). Y. Imaizumi was also supported by Daiko Foundation for Scientific Research.
Abbreviations
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- BK channel
large conductance Ca2+-activated K+ channel
- [Ca2+]i
intracellular Ca2+ concentration
- DMSO
dimethyl sulphoxide
- IbTx
iberiotoxin
- KATP channel
ATP-sensitive K+ channel
- SR
sarcoplasmic reticulum
References
- BOLTON T.B., IMAIZUMI Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996;20:141–152. doi: 10.1016/s0143-4160(96)90103-7. [DOI] [PubMed] [Google Scholar]
- CADORETTE C., SICOTTE B., BROCHU M., ST-LOUIS J. Effects of potassium channel modulators on myotropic responses of aortic rings of pregnant rats. Am. J. Physiol. 2000;278:H567–H576. doi: 10.1152/ajpheart.2000.278.2.H567. [DOI] [PubMed] [Google Scholar]
- CARL A., LEE H.K., SANDERS K.M. Regulation of ion channels in smooth muscles by calcium. Am. J. Physiol. 1996;271:C9–C34. doi: 10.1152/ajpcell.1996.271.1.C9. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., NIEDERSTE-HOLLENBERG A., SCHNEIDER J., NOACK T.H., WESTON A.H. Ion channel modulation by NS 1619, the putative BKCa channel opener, in vascular smooth muscle. Br. J. Pharmacol. 1994;113:1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. Pharmacology of the potassium channel openers. Cardiovasc. Drugs Ther. 1995;9:185–193. doi: 10.1007/BF00878465. [DOI] [PubMed] [Google Scholar]
- GIANGIACOMO K.M., KAMASSAH A., HARRIS G., MCMANUS O.B. Mechanism of maxi-K channel activation by dehydrosoyasaponin-I. J. Gen. Physiol. 1998;112:485–501. doi: 10.1085/jgp.112.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HOLLAND M., LANGTON P.D., STANDEN N.B., BOYLE J.P. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br. J. Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLYWOOD M.A., COTTON K.D., MCHALE N.G., THORNBURY K.D. Enhancement of Ca2+-dependent outward current in sheep bladder myocytes by Evans blue dye. Pflügers Arch. 1998;435:631–636. doi: 10.1007/s004240050563. [DOI] [PubMed] [Google Scholar]
- HUANG Y., LAU C.W., HO I.H.M. NS 1619 activates Ca2+-activated K+ currents in rat vas deferens. Eur. J. Pharmacol. 1997;325:21–27. doi: 10.1016/s0014-2999(97)00102-7. [DOI] [PubMed] [Google Scholar]
- IMAIZUMI Y., MURAKI K., WATANABE M. Ionic currents in single smooth muscle cells from the ureter of the guinea-pig. J. Physiol. 1989;411:131–159. doi: 10.1113/jphysiol.1989.sp017565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMAIZUMI Y., OHI Y., YAMAMURA H., OHYA S., MURAKI K., WATANABE M. Ca2+ spark as a regulator of ion channel activity. Jpn. J. Pharmacol. 1999;80:1–8. doi: 10.1254/jjp.80.1. [DOI] [PubMed] [Google Scholar]
- IMAIZUMI Y., TORII Y., OHI Y., NAGANO N., ATSUKI K., YAMAMURA H., MURAKI K., WATANABE M., BOLTON T.B. Ca2+ images and K+ current during depolarization in smooth muscles cells of the guinea-pig vas deferens and urinary bladder. J. Physiol. 1998;510:705–719. doi: 10.1111/j.1469-7793.1998.705bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGAR J.H., PORTER V.A., LEDERER W.J., NELSON M.T. Calcium sparks in smooth muscle. Am. J. Physiol. 2000;278:C235–C256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MATSUI-SAITO M., AMANO K., HARADA K., MIYAMOTO S., NAKAZAWA H., WON K.J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KAWANISHI T., ASOU H., KATO T., UNEYAMA C., TOYODA K., OHATA H., MOMOSE K., TAKAHASHI M. Ratio-imaging of calcium waves in cultured hepatocytes using rapid scanning confocal microscope and indo-1. Bioimages. 1994;2:7–14. [Google Scholar]
- KURIYAMA H., KITAMURA K., ITOH T., INOUE R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- LEBLANC N., WAN X., LEUNG P.M. Physiological role of Ca2+-activated and voltage-dependent K+ currents in rabbit coronary myocytes. Am. J. Physiol. 1994;266:C1523–C1537. doi: 10.1152/ajpcell.1994.266.6.C1523. [DOI] [PubMed] [Google Scholar]
- LEE K., ROWE I.C., ASHFORD M.L. NS 1619 activates BKCa channel activity in rat cortical neurones. Eur. J. Pharmacol. 1995;280:215–219. doi: 10.1016/0014-2999(95)00251-f. [DOI] [PubMed] [Google Scholar]
- MACMILLAN S., SHERIDAN R.D., CHILVERS E.R., PATMORE L. A comparison of the effects of SCA40, NS 004 and NS 1619 on large conductance Ca2+-activated K+ channels in bovine tracheal smooth muscle cells in culture. Br. J. Pharmacol. 1995;116:1656–1660. doi: 10.1111/j.1476-5381.1995.tb16387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKAY M.C., DWORETZKY S.I., MEANWELL N.A., OLESEN S.P., REINHART P.H., LEVITAN I.B., ADELMAN J.P., GRIBKOFF V.K. Opening of large-conductance calcium-activated potassium channels by the substituted benzimidazolone NS 004. J. Neurophysiol. 1994;71:1873–1882. doi: 10.1152/jn.1994.71.5.1873. [DOI] [PubMed] [Google Scholar]
- MCMANUS O.B., HARRIS G.H., GIANGIACOMO K.M., FEIGENBAUM P., REUBEN J.P., ADDY M.E., BURKA J.F., KACZOROWSKI G.J., GARCIA M.L. An activator of calcium-dependent potassium channels isolated from a medicinal herb. Biochemistry. 1993;32:6128–6133. doi: 10.1021/bi00075a002. [DOI] [PubMed] [Google Scholar]
- NAGANO N., IMAIZUMI Y., HIRANO M., WATANABE M. Opening of Ca2+-dependent K+ channels by nordihydroguaiaretic acid in porcine coronary arterial smooth muscle cells. Jpn. J. Pharmacol. 1996;70:281–284. doi: 10.1254/jjp.70.281. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., CHENG H., RUBART M., SANTANA L.F., BONEV A.D., KNOT H.J., LEDERER W.J. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- OLESEN S.P., MUNCH E., MOLDT P., DREJER J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur. J. Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- OTTOLIA M., TORO L. Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys. J. 1994;67:2272–2279. doi: 10.1016/S0006-3495(94)80712-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL H.J., GIEMBYCZ M.A., KEELING J.E., BARNES P.J., BELVISI M.G. Inhibition of cholinergic neurotransmission in guinea pig trachea by NS 1619, a putative activator of large-conductance, calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 1998;286:952–958. [PubMed] [Google Scholar]
- PÉREZ G.J., BONEV A.D., PATLAK J.B., NELSON M.T. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J. Gen. Physiol. 1999;113:229–237. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARGENT C.A., GROVER G.J., ANTONACCIO M.J., MCCULLOUGH J.R. The cardioprotective, vasorelaxant and electrophysiological profile of the large conductance calcium-activated potassium channel opener NS-004. J. Pharmacol. Exp. Ther. 1993;266:1422–1429. [PubMed] [Google Scholar]
- SELLERS A.J., ASHFORD M.L. Activation of BKCa channels in acutely dissociated neurones from the rat ventromedial hypothalamus by NS 1619. Br. J. Pharmacol. 1994;113:659–661. doi: 10.1111/j.1476-5381.1994.tb17041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELDON J.H., NORTON N.W., ARGENTIERI T.M. Inhibition of guinea pig detrusor contraction by NS-1619 is associated with activation of BKCa and inhibition of calcium currents. J. Pharmacol. Exp. Ther. 1997;283:1193–1200. [PubMed] [Google Scholar]
- SINGH S.B., GOETZ M.A., ZINK D.L., DOMBROWSKI A.W., POLISHOOK J.D., GARCIA M.L., SCHMALHOFER W., MCMANUS O.B., KACZOROWSKI G.J. Maxikdiol: A novel dihydroxyisoprimane as an agonist of maxi-K channels. J. Chem. Soc. (Perkin) 1994;1:3349–3352. [Google Scholar]
- VAN BREEMEN C., CHEN Q., LAHER I. Superficial buffer barrier function of smooth muscle sarcoplasmic reticulum. Trends. Pharmacol. Sci. 1995;16:98–105. doi: 10.1016/s0165-6147(00)88990-7. [DOI] [PubMed] [Google Scholar]
- WU S.N., JAN C.R., LI H.F., CHEN S.A. Stimulation of large-conductance Ca2+-activated K+ channels by Evans blue in cultured endothelial cells of human umbilical veins. Biochem. Biophys. Res. Commun. 1999;254:666–674. doi: 10.1006/bbrc.1998.0120. [DOI] [PubMed] [Google Scholar]
- XU X., TSAI T.D., WANG J., LEE E.W., LEE K.S. Modulation of three types of K+ currents in canine coronary artery smooth muscle cells by NS-004, or 1-(2′-hydroxy-5′-chlorophenyl)-5-trifluoromethyl-2(3H) benzimidazolone. J. Pharmacol. Exp. Ther. 1994;271:362–369. [PubMed] [Google Scholar]
- YAMAMURA H., NAGANO N., HIRANO M., MURAKI K., WATANABE M., IMAIZUMI Y. Activation of Ca2+-dependent K+ current by nordihydroguaiaretic acid in porcine coronary arterial smooth muscle cells. J. Pharmacol. Exp. Ther. 1999;291:140–146. [PubMed] [Google Scholar]
- YUAN X.J., SUGIYAMA T., GOLDMAN W.F., RUBIN L.J., BLAUSTEIN M.P. A mitochondrial uncoupler increases KCa currents but decreases KV currents in pulmonary artery myocytes. Am. J. Physiol. 1996;270:C321–C331. doi: 10.1152/ajpcell.1996.270.1.C321. [DOI] [PubMed] [Google Scholar]
- ZHUGE R., TUFT R.A., FOGARTY K.E., BELLVE K., FAY F.S., WALSH J.V., JR The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J. Gen. Physiol. 1999;113:215–228. doi: 10.1085/jgp.113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]


