Abstract
We showed previously that interaction between NO and iron(II), both released following decomposition of sodium nitroprusside (SNP), accounted for the late SNP-induced dopamine (DA) increase in dialysates from the striatum of freely moving rats.
In this study, intrastriatal infusion of the NO-donor S-nitroso-N-acetylpenicillamine (SNAP) (0.2 mM for 180 min) induced a moderate increase in dialysate DA and decreases in ascorbic acid dialysate concentrations; in contrast, SNAP 1 mM infusion induced a long-lasting decrease in both DA and ascorbic acid dialysate concentrations. 3-Methoxy-tyramine (3-MT), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and uric acid levels were unaffected.
Co-infusion of ferrous sulphate [iron(II), 1 mM for 40 min] with SNAP either 1 or 0.2 mM (for 180 min), produced a significant increase in both DA and 3-MT dialysate concentrations, but it did not affect decreases in dialysate ascorbic acid levels. All other dialysate neurochemicals were unaffected.
Co-infusion of ascorbic acid (0.1 mM) with SNAP (1 mM) for 180 min did not modify SNAP-induced decreases in dialysate DA levels. In contrast, co-infusion of uric acid (1 mM) reversed SNAP-induced decreases in dialysate DA; co-infusion of a superoxide dismutase mimetic delayed SNAP-induced DA decreases for a short period, while co-infusion of the antioxidant N-acetylcysteine (NAC, 0.1 mM) significantly increased dialysate DA.
The results of this study show that SNAP induces concentration-related changes in DA dialysate levels. At higher concentrations, SNAP induces non-enzymatic DA oxidation, which is inhibited by uric acid and NAC; ascorbic acid failed to protect dialysate DA from oxidation, probably owing to its promoting effect on SNAP decomposition; exogenous iron(II) may react with NO generated from SNAP decomposition, with a consequent increase in dialysate DA and 3-MT, therefore mimicking SNP effects on striatal DA release.
Keywords: NO-donor SNAP, dopamine, in vivo release, ascorbic acid, iron, microdialysis, rat striatum
Introduction
Nitric oxide (NO) is a widespread and versatile biological messenger molecule. Roles proposed for NO in CNS pathophysiology are increasingly multiform and range from intracellular signaling (Garthwaite & Boulton, 1995; Park et al., 1998), through neurotransmitter release, neural development, synapse plasticity, killing of cells and invading pathogens, apoptosis, to tissue damage or protection (Yun et al., 1996; Murphy, 2000). It is evident that either damaging, protective, or signalling effects of NO depend on both the source of NO (neuronal, glial, extracellular, exogenous) and the timing of NO production (Murphy, 2000). This assumption appears to be also true when dealing with the biological effects of NO-generating drugs. These effects vary according to the chemical structure of NO-generating drugs (Bates et al., 1991; Holm et al., 1998; Menconi et al., 1998) and the composition of the endogenous environment in which NO is generated (Millar, 1995; Reiser et al., 1999; Serra et al., 2000a). Ascorbic acid is a very important component of the endogenous environment, since NO readily reacts with ascorbic acid. Neuronal ascorbic acid concentrations (10 mM) are about 10 times higher than glial and, respectively, 20 – 25 times higher than extracellular concentrations (Miele & Fillenz, 1996; Rice, 2000). Extracellular ascorbic acid levels are also dynamically modulated by glutamate-mediated activity (Rice, 2000), via glutamate-ascorbate heteroexchange (Miele et al., 1994). The close relationship between NO and ascorbic acid has been outlined in several biological systems (Millar, 1995; Lilley & Gibson, 1997). Ascorbic acid may either generate NO from nitrite ions (NO2−) in the extracellular space (Millar, 1995), protect NO from destruction by superoxide anions (Dudgeon et al., 1998; Jackson et al., 1998), or scavenge it (Whiteman & Halliwell, 1996). In this regard, Karanth et al. (2000) claimed that ascorbic acid may even act as an inhibitory transmitter in the hypothalamus by scavenging NO. Neal et al. (1999) showed that dopamine (DA) released from the retina is oxidized by NO and that endogenous ascorbic acid protects DA from oxidation by scavenging NO. Ascorbic acid may trigger decomposition of the NO-donor sodium nitroprusside (SNP) both in biological tissue in vitro (Bates et al., 1991; Reiser et al., 1999) and in the striatal extracellular space in vivo (Serra et al., 2000a). In addition, ascorbic acid potentiates decomposition of S-nitroso-N-acetylpenicillamine (SNAP) in striatal slices in vitro, but not that of 3-morpholinosydnonimine (SIN-1) (Reiser et al., 1999).
In a previous study (Serra et al., 2000a), we showed that intrastriatal infusion of either SNI-1 or SNP produced a NO-mediated increase in DA concentration in dialysates from the striatum of freely moving rats. In addition, we showed that only SNP decreased dialysate ascorbic acid levels. This decrease was related to the ascorbic acid triggering of SNP decomposition (Bates et al., 1991; Reiser et al., 1999) in the striatal extracellular space. The effects of the NO-donor SNAP on DA release in terminal fields of the dopaminergic system in vivo have been often conflicting. West & Galloway (1996; 1997) showed that SNAP increased DA efflux from the striatum of chloral hydrate-anaesthetized rats. In contrast, Guevara-Guzman et al. (1994) showed that SNAP decreased extracellular DA concentration in urethane-anaesthetized rat, as did NO gas, given directly by dissolution in degassed perfusion fluid. Segieth et al. (2000) showed that SNAP promoted DA release from the rat hippocampus in vivo at low concentrations, whilst high concentrations induced long-lasting DA decreases. More recently, Trabace & Kendrick (2000) have shown that short-lasting intrastriatal infusion of SNAP induced increases in dialysate DA at low concentration, and decreases in dialysate DA at high concentrations; these DA changes were attributed to SNAP-induced peroxynitrite formation; at high levels, peroxynitrite would reduce extracellular DA concentration through oxidation, while at low levels it increases DA levels in a cyclic GMP-dependent manner. The study of the role of endogenous ascorbic acid and superoxide anion in SNAP-induced changes in DA release and metabolism in the striatum of freely moving rats was therefore deemed of interest.
Methods
Animals
Male Wistar rats (Morini, R. Emilia, Italy), weighing between 280 – 330 g were used in all experiments. The rats were maintained under standard animal care conditions (12 : 12 h light/dark cycle, lights coming on at 0700 h; room temperature 21°C), with food and water ad libitum. Prior to the start of any experiment, the health of the rat was assessed according to published guidelines (Morton & Griffiths, 1985). All procedures were specifically licensed under the European Community directive 86/609 included in Decreto No. 116/1992 of the Italian Ministry of Public Health.
Drugs
SNAP, SIN-1, N-acetylcysteine (NAC), ascorbic acid, uric acid, and ferrous sulphate [FeSO4, iron(II)] were purchased from Sigma-Aldrich (Milan, Italy); manganese(III) tetrakis (4-benzoic acid) porphyrin (MnTBP) from Calbiochem (Darmstadt, Germany).
Drug administration
Iron(II), ascorbic acid and SIN-1 concentrations were chosen according to Serra et al. (2000a); SNAP according to Guevara-Guzman et al. (1994) and Trabace & Kendrick (2000); NAC according to Serra et al. (2000b); MnTBP according to Petersen et al. (2000)
Microdialysis probe construction
The striatal probe combined two independent microdialysis probes of concentric design with two separate inlets and a shared outlet, as previously described (Miele et al., 2000; Serra et al., 2000b). The probes were constructed using two section of plastic-coated silica tubing (diameter 0.15 mm; Scientific Glass Engineering, Milton Keynes, U.K.) each placed in the centre of a semi-permeable polyacrylonitrile dialysis fibres (molecular cut-off weight of 12 KD, Filtral 16 Hospal Industrie, France). Each probe had a final diameter of 0.22 mm. The tips of the dialysis fibres were sealed and joined using quick-drying epoxy glue. The two sections of silica tubing served as inlets; the outlet was made also with a section of plastic-coated silica tubing, positioned in the centre of polythene tubing. The semipermeable membrane was coated with epoxy leaving an active length of 4 mm. The diameter of the final probe was approximately 0.50 mm. The striatal probe combining two microdialysis probes of concentric design with two separate inlets and a shared outlet, allowed separate co-infusion of drugs (Serra et al., 2000b).
Surgery
Stereotaxic surgery was performed under chloral hydrate (400 mg kg−1 i.p.) anaesthesia. The microdialysis probes were implanted in the right striatum using the following co-ordinates from the atlas of Paxinos & Watson (1986): A/P +0.5 mm from bregma, +2.5 mm M/L, and −6.0 mm D/V from dura. Body temperature during anaesthesia was maintained at 37°C by means of an isothermal-heating pad (Harvard Apparatus, Kent, U.K.). Following surgery the animals were placed in large plastic bowls (50×55 cm), and maintained in a temperature- and light-controlled environment, with free access to food and water. Experiments were carried out 24 h after probe implantation with the animal in its home bowl. This arrangement allowed the rats free movement.
Microdialysis procedure
The composition of the Ringer solution used was as follows (in mM): NaCl 147, KCl 4, CaCl2 1.2, MgCl2 1 (pH 6.0). A microinfusion pump (CMA/100, Microdialysis, Sweden) pumped Ringer solution at a flow rate of 1.0 μl min−1 using two separate syringes connected to the inlets by a length of polythene tubing; every 20 min, 40 μl dialysate samples were collected manually in 250 μl micro-centrifuge tubes (Alpha Laboratories, U.K.) attached to the outlet. Subsequently, a 20 μl aliquot of collected dialysate was injected into the analytical system. Drugs were added to the Ringer solution and infused via the striatal probe implanted in the striatum.
Chromatographic analysis
DA, 3-methoxytyramine (3-MT), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), ascorbic acid and uric acid were quantified by high performance liquid chromatography with electrochemical detection (HPLC-EC) as previously described (Serra et al., 2000a), using an Alltech 426 HPLC pump equipped with a Rheodyne injector, column 15 cm×4.6 mm i.d. Alltech Adsorbsphere C18 5U, electrochemical detector Antec CU-04-AZ and Varian Star Chromatographic Workstation. The mobile phase was citric acid 0.5 M, Na acetate 1 M, EDTA 12.5 mM, MeOH 10% and sodium octylsulphate 650 mg l−1 (pH=3.0); the flow rate was 1.3 ml min−1. The first sample was collected after 60 min of stabilization (time 0), then dialysates were collected, at 20 min intervals, for 40 min prior to the start of experiments.
Histology
Following the experiments, rats were killed with an overdose of chloral hydrate (800 mg kg−1 i.p.). The location of each microdialysis probe was confirmed by post-mortem histology. Brains were fixed in formal saline and 50 μm coronal sections were made with a cryostat. The slices were stained with cresyl violet and examined under a microscope.
Statistical analysis
The concentrations in the dialysate were expressed in nM (DA, 3-MT) or μM (DOPAC, HVA, ascorbic acid, uric acid) and given as mean±s.e.mean. Drug effects on neurochemicals were statistically evaluated in terms of changes in absolute dialysate concentrations. Statistical significance was assessed using analysis of variance (ANOVA) for difference between groups and over time. Difference within or between groups were determined by paired or unpaired t-tests with Bonferroni multiple comparison adjustment.
Results
Effect of intrastriatal infusion of t SNAP on DA, 3-MT, DOPAC, HVA, ascorbic acid and uric acid dialysate levels
Intrastriatal infusion of SNAP (1 mM for 180 min, n=4) induced a long-lasting decrease in DA and ascorbic acid dialysate concentrations (Figure 1A,C), whilst 3-MT (Figure 1B), DOPAC, HVA and uric acid (Figure 2) were unaffected.
Figure 1.
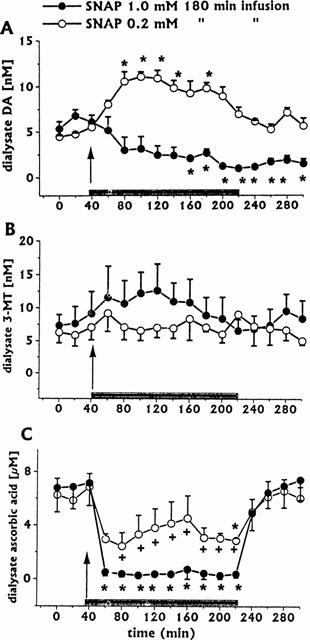
Effect of intrastriatal infusion of SNAP 1 mM (n=4) or 0.2 mM (n=3) on DA (A), 3-MT (B), and ascorbic acid (C) dialysate concentrations. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e. mean. *P<0.05 compared with baseline values.
Figure 2.
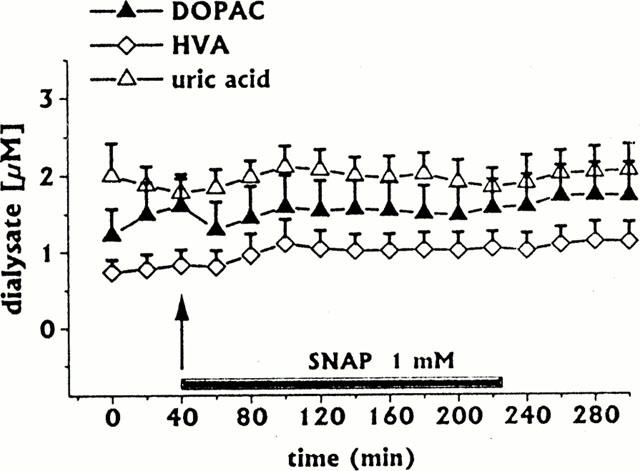
Effect of intrastriatal infusion of SNAP 1 mM (n=4) on DOPAC, HVA and uric acid dialysate concentrations. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean.
Intrastriatal infusion of SNAP (0.2 mM for 180 min, n=3) induced increases in dialysate DA (Figure 1A) and a decrease in dialysate ascorbic acid (ANOVA P<0.02) lower than that induced by the higher SNAP concentration (Figure 1C). 3-MT levels were unaffected (Figure 1B), as were other neurochemicals (data not shown).
Effect of intrastriatal co-infusion of ascorbic acid on SNAP-induced changes in dialysate neurochemical concentrations
We showed previously (Serra et al., 2000a) that the peroxynitrite generator SIN-1 increased dialysate DA without changes in dialysate ascorbic acid concentration; in addition, we showed that intrastriatal ascorbic acid co-infusion inhibited SIN-1-induced increases in dialysate DA. The inhibition was related to NO scavenging by ascorbic acid. In the present study, SNAP induced a concentration-related decrease in dialysate ascorbic acid. The study of ascorbic acid co-infusion on SNAP-induced decreases in DA dialysate concentrations was therefore deemed of interest.
Co-infusion of ascorbic acid (0.1 mM for 180 min, n=3) did not affect SNAP (1 mM)-induced decreases in dialysate DA (Figure 3A). In addition, ascorbic acid co-infusion affected neither 3-MT (Figure 3B), uric acid (Figure 3D), nor DOPAC and HVA dialysate levels (data not shown).
Figure 3.
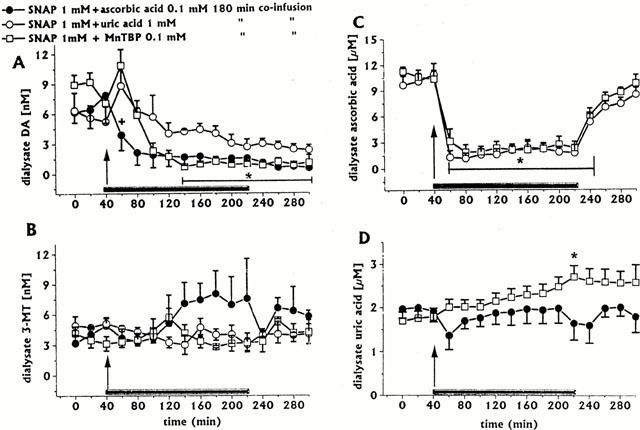
Effect of intrastriatal co-infusion of ascorbic acid 0.1 mM (n=3), uric acid 1 mM (n=3), or MnTBP 0.1 mM (n=4) on SNAP-induced changes in DA (A), 3-MT (B), ascorbic acid (C), and uric acid (D) dialysate concentrations. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. *P<0.05 compared with baseline values. Thin horizontal black bar in (A) indicates significant decreases for both SNAP/ascorbic acid SNAP/MnTBP groups; in (C), it indicates significant decreases for both SNAP/uric acid and SNAP/MnTBP groups. +P<0.05 compared with SNAP/MnTBP group.
Co-infusion of ascorbic acid (0.1 mM for 180 min, n=3) inhibited SNAP (0.2 mM)-induced increases in dialysate DA; during co-infusion, dialysate levels of DA were always in the range of baseline values. All other neurochemicals were unaffected (data not shown).
Dialysate ascorbic acid concentrations attained during ascorbic acid/SNAP (1 mM) co-infusion were compared with those attained with ascorbic acid/SIN-1 (1 mM) co-infusion (n=3). As shown in Figure 4, dialysate ascorbic acid concentrations attained during 180 min co-infusion with SNAP (1 mM) were significantly lower (by about 28 – 30%) than those attained during 180 min co-infusion with SIN-1. Dialysate ascorbic acid concentrations attained during co-infusion with SNAP 0.2 mM did not statistically differ from those attained during 180 min co-infusion with SIN-1 (data not shown).
Figure 4.
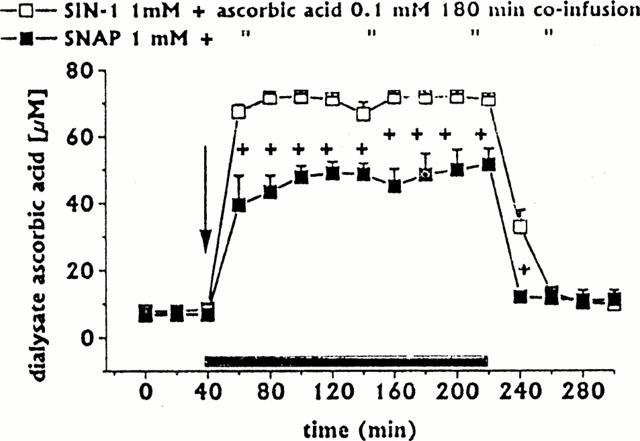
Dialysate ascorbic acid concentrations following intrastriatal co-infusion of ascorbic acid 0.1 mM with SIN-1 1 mM (n=3) or SNAP 1 mM (n=3). Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. +P<0.05 compared with ascorbic acid/SIN-1 group.
Effect of uric acid co-infusion on SNAP-induced changes in DA and ascorbic acid dialysate concentrations
In their very recent paper, Trabace & Kendrick (2000) suggest that SNAP induces peroxynitrite formation which results, according to the peroxynitrite level attained, either in extracellular DA oxidation, or in increases in DA release from the striatum of freely moving rats. Uric acid is a natural strong scavenger of peroxynitrite (Hooper et al., 1998). In addition, one of the scavenging activity of uric acid is to maintain ascorbic acid in its reduced form in biological fluids (Sevanian et al., 1991). Although in the present study SNAP infusion failed to modify dialysate uric acid concentrations (Figure 2), the study of uric acid co-infusion on SNAP-induced changes in DA and ascorbic acid dialysate concentrations was deemed of interest.
In preliminary experiments, it was found that uric acid dialysate concentrations ranging from 80 to 100 μM could be attained with the infusion of uric acid 1 mM. Co-infusion of uric acid 1 mM with SNAP 1 mM for 180 min (n=3) reverted SNAP-induced decreases in dialysate DA levels (Figure 3A), whilst 3-MT levels were unaffected (Figure 3B); in addition, uric acid co-infusion attenuated SNAP-induced decreases in dialysate ascorbic acid (Figure 3C); DOPAC and HVA levels were unaffected (data not shown).
Effect of superoxide dismutase (SOD) mimetic MnTBP co-infusion on SNAP-induced changes in DA and ascorbic acid dialysate concentrations
Peroxynitrite is formed by reaction of NO with superoxide anions. Ascorbic acid, at high physiological concentrations, is a natural inhibitor of this reaction (Jackson et al., 1998); however, in this study, co-infusion of ascorbic acid failed to protect dialysate DA from SNAP-induced oxidation. SOD is too large a molecule to cross the dialysis membrane used; therefore, we used the cell-permeant SOD mimetic MnTBP (Patel & Day, 1999), in order to assess the role of peroxynitre in SNAP-induced oxidation of dialysate DA.
Co-infusion of MnTBP (0.1 mM for 180 min, n=3) shortly delayed SNAP (1 mM)-induced decreases in dialysate DA (Figure 3A) and attenuated ascorbic acid decreases (Figure 3C). MnTBP co-infusion affected neither 3-MT (Figure 3B), nor DOPAC and HVA dialysate levels (data not shown); in contrast, MnTBP significantly increased uric acid levels (up to 60% of baseline at end of co-infusion) (Figure 3D).
Effect of NAc-cysteine co-infusion on SNAP-induced changes in DA and ascorbic acid dialysate concentrations
We showed previously (Serra et al., 2000b) that intrastriatal infusion of NAC protected DA and L-DOPA, which are both catechol-containing compounds, from autoxidation. It is well known that O-methylation of either DA or L-DOPA protects these compounds from non-enzymatic oxidation (Miller et al., 1996). The fact that SNAP 1 mM induced decreases in dialysate DA concentration leaving unaffected that of 3-MT, the DA O-methylated derivative, prompted us to evaluate the effect of NAC co-infusion on the SNAP-induced dialysate DA decrease.
Co-infusion of NAC 0.1 mM with SNAP 1 mM for 180 min (n=4) resulted in a significant increase in dialysate DA levels (Figure 5A), whilst 3-MT levels were unaffected (Figure 5B); in addition, NAC co-infusion attenuated the decrease in dialysate ascorbic acid (Figure 5C), whilst DOPAC, HVA and uric acid levels were unaffected (data not shown).
Figure 5.
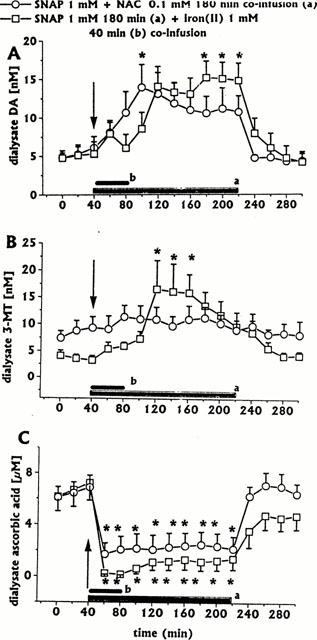
Effect of intrastriatal co-infusion of NAC (0.1 mM for 180 min, n=4) or iron(II) (1 mM for 40 min, n=4), on SNAP 1 mM-induced changes in DA (A), 3-MT (B), and ascorbic acid (C) dialysate concentrations Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean (n=3). *P<0.05 compared with baseline values.
Effect of iron(II) intrastriatal co-infusion on SNAP-induced changes in dialysate neurochemicals concentrations
We showed previously (Serra et al., 2000a) that interaction between NO and iron(II), both released following decomposition of SNP, accounted for the late but quite substantial SNP-induced dopamine (DA) increase in dialysates from the striatum of freely moving rats. In addition, we showed (unpublished observation) that the late SNP-induced increase in dialysate DA was mimicked by co-infusing the NO-donor SIN-1 with either iron(II), or potassium ferrocyanide, the iron-carrier of SNP, therefore confirming that an exogenous NO/exogenous iron interaction was responsible for the late great SNP-induced increase in dialysate DA. The evaluation of iron(II) co-infusion on SNAP-induced changes in DA dialysate concentrations was therefore deemed of interest.
Intrastriatal infusion of iron(II) (1 mM for 40 min, n=3) induced a late moderate increase in dialysate DA concentration, whilst all other neurochemicals (3-MT, DOPAC, HVA, ascorbic acid, and uric acid) dialysate concentrations were unaffected (data not shown).
When SNAP (1 mM for 180 min) was co-infused with iron(II) (1 mM for 40 min, n=4), dialysate DA greatly increased progressively to a peak (316% of baseline) at the end of 180 min SNAP infusion (Figure 5A). Similarly, iron(II) co-infusion significantly increased 3-MT dialysate levels, but in this case the peak (407% of baseline) occurred early (Figure 5B). Iron(II) co-infusion did not affect SNAP-induced dialysate ascorbic acid decreases (Figure 5C). All other neurochemicals (DOPAC, HVA and uric acid) were unaffected (data not shown).
When SNAP (0.2 mM for 180 min) was co-infused with iron(II) (1 mM for 40 min, n=3), the early SNAP-induced increase in dialysate DA concentration was inhibited during 40 min iron(II) co-infusion. Following discontinuation of iron(II) co-infusion, dialysate DA significantly increased to a peak (340% of baseline) at the end of 180 min SNAP infusion (Figure 6A). Similarly, iron(II) co-infusion significantly increased 3-MT dialysate levels, but in this case the peak (265% of baseline) occurred early (Figure 6B). Dialysate ascorbic acid concentration significantly decreased during 40 min of iron(II) co-infusion. Following discontinuation of iron(II) co-infusion, dialysate ascorbic acid showed a trend to recovery (Figure 6C). All other neurochemicals (DOPAC, HVA, uric acid) were unaffected (data not shown).
Figure 6.
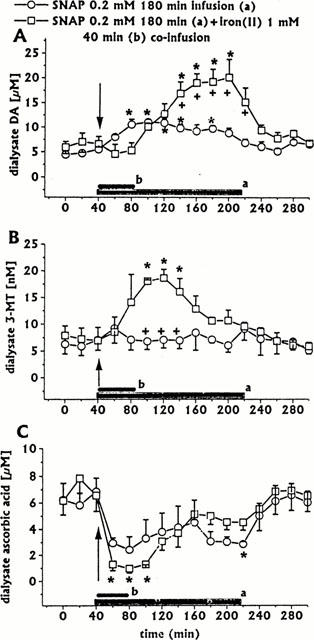
Effect of intrastriatal co-infusion of iron(II) (1 mM for 40 min, n=3) on SNAP 0.2 mM-induced changes in DA (A), 3-MT (B), and ascorbic acid (C) dialysate concentrations. SNAP 0.2 mM, same group as in Figure 1. Dialysates were collected, at 20 min intervals, for 180 min during drug infusion (horizontal black bar) and for 80 min after discontinuation of drug infusion. Values are given as mean±s.e.mean. *P<0.05 compared with baseline values; +P<0.05 compared with SNAP group.
Discussion
The results of the present study confirm the results of a previous study (Serra et al., 2000a) that both ascorbic acid and iron(II) play a key role in DA changes induced by NO-donors in dialysates from the striatum of freely moving rats.
Ascorbic acid promotes degradation of both SNAP (Reiser et al., 1999) and SNP (Bates et al., 1991; Reiser et al., 1999), but not that of SIN-1 (Reiser et al., 1999). In this study, SNAP induced a concentration-dependent decrease in dialysate ascorbic acid levels; we showed previously (Serra et al., 2000a) that SNP infusion induced time-dependent decreases in dialysate ascorbic acid levels, whilst SIN-1 did not affect dialysate ascorbic acid. These finding are consistent with the role of endogenous ascorbic acid as an active promoter of both SNAP and SNP degradation in vivo. Following SNAP degradation, and the ensuing NO release, DA dialysate levels showed concentration-dependent changes: long-lasting decreases at higher SNAP concentration (1 mM), and short lasting increases at lower concentration (0.2 m). These findings are in agreement with those of Trabace & Kendrick (2000). Decrease in DA dialysate induced by the higher concentration of SNAP is undoubtedly due to DA non-enzymatic oxidation (Trabace & Kendrick, 2000), since dialysate levels of 3-MT, the extracellular O-methylated DA metabolite which is resistant to oxidation (Miller et al., 1996), were unaffected by SNAP. The question arises as to whether SNAP-induced oxidation of DA might be mediated by excess of NO generated from SNAP degradation, by peroxynitrite formed following endogenous superoxide anion reaction with NO generated from SNAP degradation, or both. Intrastrial infusion of either SIN-1, a well-known peroxynitrite generator (Menconi et al., 1998), or SNP, which also induces peroxynitrite formation in vitro (Keller et al., 1998), resulted in a long-lasting increase in dialysate DA (Serra et al., 2000a). These findings seem to exclude a peroxynitrite role in DA oxidation. However, both the chemical structure of NO-generating drugs and the composition of the endogenous environment in which NO is generated must be taken in consideration. The NO generated in vitro by SNAP is far greater than that by SIN-1 (Holm et al., 1998). In addition, NO generation from SNAP degradation, in the present study in vivo, is likely to be increased by the potentiating effect of endogenous ascorbic acid on SNAP degradation (Reiser et al., 1999). NO released in excess by the higher SNAP concentration might act both as a free radical to promote extracellular DA oxidation, and as a promoter of peroxynitrite formation by reacting with endogenous superoxide anion. The SOD mimetic MnTBP delayed SNAP-induced decreases in dialysate DA for a short period, attenuated decreases in dialysate ascorbic acid, and increased dialysate uric acid levels. These convergent findings suggest an initial formation of peroxynitrite, which might initially participate to extracellular DA oxidation. Thereafter, NO continuously generated from SNAP degradation would take the place of peroxynitrite in the extracellular DA oxidation. This hypothesis is supported by the fact that: (1) Co-infusion with uric acid protected DA from SNAP-induced oxidation. Uric acid is a natural strong scavenger of peroxynitrite, but not of NO (Hooper et al., 1998). Uric acid is an active component of the neuronal antioxidant pool (Becker, 1993). It is capable of inhibiting SNP-and free radical-initiated lipid peroxidation and DNA damage (Cohen et al., 1984; Keller et al., 1998); in addition, it forms strong complexes with iron ions, particularly Fe3+ (Cohen et al., 1984), and inhibits DA autoxidation (Church & Ward, 1994); (2) Co-infusion of the antioxidant NAC with SNAP completely protected DA from oxidation with a consequent significant increase in dialysate DA levels. Wang et al. (1998) showed that NAC is an active antioxidant also in presence of NO.
Co-infusion of SNAP with iron(II), which is known to react readily with NO (Stamler et al., 1992; Le Brun et al., 1997), significantly increased dialysate DA and 3-MT levels. The latter finding further highlights the role of iron in the NO-donor drug-induced increase in dialysate DA. In a previous study (Serra et al., 2000a), we showed that NO released following SIN-1 decomposition increased dialysate DA. In contrast to SNAP (Holm et al., 1998), SIN-1 also generates the superoxide anion; thus, as a potential peroxynitrite generating drug (Menconi et al., 1998), SIN-1 should promote DA oxidation (to 6-hydroxyindole-5-one, according to Kerry & Rice-Evans, 1999), as SNAP does. Evidently, this peroxynitrite-induced DA oxidation does not occur in striatal dialysates following SIN-1 infusion. In a submitted study, in order to explain the fact that the iron chelator deferoxamine inhibited the SIN-1-induced increase in dialysate DA (unpublished observations), we suggested that the superoxide anion generated by SIN-1 decomposition would preferentially release iron from storage proteins and enzymic [4Fe-4S] clusters, rather than react with NO, with a consequent elevation in free iron levels (Keyer & Imlay, 1996) The endogenous iron would then react with NO released by SIN-1 decomposition, to form NO-iron complexes (Stamler et al., 1992; Le Brun et al., 1997), therefore mimicking the effect of SNP (which notoriously releases both NO and iron) on striatal DA release (Serra et al., 2000a).
Endogenous ascorbic, acting as a NO scavenger (Whiteman & Halliwell, 1996), may either protect DA from oxidation (Neal et al., 1999) or even act as an inhibitory transmitter in the hypothalamus by scavenging NO (Karanth et al., 2000). In a previous study (Serra et al., 2000a) we hypothesized that exogenous ascorbic acid inhibited SIN-1-induced increases in dialysate DA by scavenging NO. The results of this study confirm this hypothesis, since the DA releasing effect of the lower SNAP concentration (0.2 mM) was completely blocked by ascorbic acid co-infusion. It is likely that exogenous ascorbic acid, besides promoting further the degradation of SNAP (Reiser et al., 1999), had scavenged the ensuing NO. Exogenous ascorbic acid exerts antidopaminergic action on striatal function in vivo (Desole et al., 1987; Rebec & Pierce, 1994; Gulley & Rebec, 1999). The NO scavenging activity of ascorbic acid might be one of the mechanisms of this antidopaminergic action.
The results of this study raise the question as to whether NO-generating drugs might be useful tools for the in vivo study of the role of endogenous NO in striatal dopaminergic transmission. The composition of the endogenous environment in which NO is generated may play a key role. When NO is generated intraneuronally, it has to face an ascorbic acid concentration of about 10 mM (Rice, 2000), which is 20 – 25 times higher than that found in vivo in the striatal extracellular space (Miele & Fillenz, 1996). Therefore, the neuronal ascorbic acid concentration far exceeds the extracellular one at which exogenous ascorbic acid acts as a scavenger of exogenous NO.
In conclusion, NO generated from low SNAP concentrations increases DA dialysate levels; endogenous extracellular ascorbic acid may promote SNAP decomposition, with a consequent generation of an excess of NO; an excess of NO may induce both peroxynitrite formation and DA dialysate non-enzymatic oxidation; these effects are both inhibited by exogenous uric acid and the antioxidant NAC; exogenous iron(II) may preferentially react with NO generated from SNAP decomposition; this interaction results in increases in dialysate DA, and therefore mimics the SNP effect on striatal DA release.
Acknowledgments
The research was supported by University of Sassari (ex 60% fund).
Abbreviations
- ANOVA
analysis of variance
- DA
dopamine
- DOPAC
dihydroxyphenylacetic acid
- HVA
homovanillic acid
- MnTBP
manganese(III) tetrakis (4-benzoic acid) porphyrin
- 3-MT
3-methoxytyramine
- NAC
n-acetylcysteine
- NO
nitric oxide
- SIN-1
3-morpholinosydnonimine
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
References
- BATES J.N., BAKER M.T., GUERRA R., JR, HARRISON D.G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem. Pharmacol. 1991;42:S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- BECKER B.B. Towards the physiological function of uric acid. Free Rad. Biol. Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- COHEN A.M., ABERDROTH R.E., HOCHSTEIN P. Inhibition of free radical-induced DNA damage by uric acid. FEBS Lett. 1984;174:147–150. doi: 10.1016/0014-5793(84)81094-7. [DOI] [PubMed] [Google Scholar]
- CHURCH W.H., WARD V.L. Uric acid is reduced in the substantia nigra in Parkinson's disease: effect on dopamine autoxidation. Brain Res. Bull. 1994;33:419–425. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- DESOLE M.S., ANANIA V., ESPOSITO G., CARBONI F., SENINI A., MIELE E. Neurochemical and behavioural changes induced by d-amphetamine and ascorbic acid in th rat. Pharmacol. Res. Comm. 1987;19:441–450. doi: 10.1016/0031-6989(87)90083-x. [DOI] [PubMed] [Google Scholar]
- DUDGEON S., BENSON D.P., MACKENZIE A., PAISLEY-ZYSZKIEWICZ K., MARTIN W. Recovery by ascorbate of impaired nitric-oxide dependent relaxation resulting from oxidant stress in rat aorta. Br. J. Pharmacol. 1998;125:782–786. doi: 10.1038/sj.bjp.0702120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTHWAITE J., BOULTON C.L. Nitric oxide signaling in the central nervous system. Ann. Rev. Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- GUEVARA-GUZMAN R., EMSON P.C., KENDRICK K.M. Modulation of in vivo striatal transmitter release by nitric oxide and cyclic GMP. J. Neurochem. 1994;62:807–810. doi: 10.1046/j.1471-4159.1994.62020807.x. [DOI] [PubMed] [Google Scholar]
- GULLEY J.M., REBEC G.V. Modulatory effects of ascorbate, alone or with haloperidol, on a lever-release conditioned avoidance response task. Pharmacol. Biochem. Behav. 1999;63:125–129. doi: 10.1016/s0091-3057(98)00249-4. [DOI] [PubMed] [Google Scholar]
- HOLM P., KANKAANRANTA H., METSA-KETELA T., MOILANEN E. Radical releasing properties of nitric oxide donors GEA 3162, SIN-1 and S-nitroso-N-acetylpenicillamine. Eur. J. Pharmacol. 1998;346:97–102. doi: 10.1016/s0014-2999(98)00009-0. [DOI] [PubMed] [Google Scholar]
- HOOPER D.C., SPITSIN S., KEAN R.B., CHAMPION J.M., DICKSON G.M., CHAUDHRY L., KOPROWSKY H. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON T.S., XU A., VITA A.J., KEANEY J.F. , JR Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- KARANTH S., YU W.H., WALCZEWSKA A., MASTRONARDI C., MCCANN S.M. Ascorbic acid acts as inhibitory transmitter in the hypothalamus to inhibit stimulated luteinizing hormone-releasing hormone release by scavenging nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 2000;15:1891–1896. doi: 10.1073/pnas.97.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLER J.N., KINDY M.S., HOLTSBERG F.W., STCLAIR D.K., HSIU-CHAN Y., GERMEYER A., STEINER S.M., BRUCE-KELLER A.J., HUTCHINS J.B. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury; suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERRY N., RICE-EVANS C. Inhibition of peroxynitrite-mediated oxidation of dopamine by flavonoid and phenolic antioxidants and their structural relationships. J. Neurochem. 1999;73:247–253. doi: 10.1046/j.1471-4159.1999.0730247.x. [DOI] [PubMed] [Google Scholar]
- KEYER K., IMLAY J.A. Superoxide accelerates DNA damage by elevating free iron levels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE BRUN N.E., ANDREWS S.C., MOORE G.R., THOMSON A.J. Interaction of nitric oxide with non-haem iron sites of escherichia coli bacterioferritin: reduction of nitric oxide to nitrous oxide and oxidation of iron(II) to iron(III) Biochem. J. 1997;326:173–179. doi: 10.1042/bj3260173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENCONI M.J., UNNO N., SMITH M., AGUIRRE D.E., FINK M.P. Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochim. Biophys. Acta. 1998;1425:189–203. doi: 10.1016/s0304-4165(98)00072-5. [DOI] [PubMed] [Google Scholar]
- MIELE M., BOUTELLE M.G., FILLENZ M. The physiologically-induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience. 1994;62:87–91. doi: 10.1016/0306-4522(94)90316-6. [DOI] [PubMed] [Google Scholar]
- MIELE M., FILLENZ M. In vivo determination of extracellular brain ascorbate. J. Neurosci. Meth. 1996;69:21–24. doi: 10.1016/S0165-0270(96)00094-5. [DOI] [PubMed] [Google Scholar]
- MIELE M., MURA M.A., ENRICO P., ESPOSITO G., SERRA P.A., MIGHELI R., ZANGANI D., MIELE E., DESOLE M.S. On the mechanism of d-amphetamine-induced changes in glutamate, ascorbic acid and uric acid release in the striatum of freely moving rats. Br. J. Pharmacol. 2000;129:582–588. doi: 10.1038/sj.bjp.0703066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLAR J. The nitric oxide/ascorbate cycle: how neurones may control their own oxygen supply. Med. Hypoth. 1995;45:21–26. doi: 10.1016/0306-9877(95)90194-9. [DOI] [PubMed] [Google Scholar]
- MILLER J.W., SELHUB J., JOSEPH J.A. Oxidative damage caused by free radicals produced during catecholamine autoxidation: protective effects of O-methylation and melatonin. Free Rad. Biol. Med. 1996;21:241–249. doi: 10.1016/0891-5849(96)00033-0. [DOI] [PubMed] [Google Scholar]
- MORTON D.B., GRIFFITHS P.H.M. Guidelines on the recognition of pain, distress and discomfort in experimental animals and a hypothesis for assessment. Vet. Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- MURPHY S. Production of nitric oxide by glial cells: regulation and potential roles in CNS. Glia. 2000;29:1–13. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- NEAL M.J., CUNNINGHAM J.M., MATTHEWS K.L. Release of endogenous ascorbic acid preserves extracellular dopamine in the mammalian retina. Invest. Ophtalmol. Vis. Sci. 1999;40:2893–2897. [PubMed] [Google Scholar]
- PARK J.-H., STRAUB V.A., O'SHEA M. Anterograde signaling by nitric oxide: characterization and in vitro reconstitution of an identified nitrergic synapse. J. Neurosci. 1998;18:5463–5479. doi: 10.1523/JNEUROSCI.18-14-05463.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL M., DAY B.J. Metalloporphyin class of therapeutic catalytic antioxidants. Trends Pharmacol. Sci. 1999;20:359–364. doi: 10.1016/s0165-6147(99)01336-x. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego; 1986. [Google Scholar]
- PETERSEN A., CASTILHO R.F., HANSSON O., WIELOCH T., BRUNDIN P. Oxidative stress, mitochondrial permeability transition and activation of caspases in calcium ionophore A23187-induced death of cultured striatal neurones. Brain Res. 2000;857:20–29. doi: 10.1016/s0006-8993(99)02320-3. [DOI] [PubMed] [Google Scholar]
- REBEC G.V., PIERCE C.P. A vitamin as neuro-modulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Progr. Neurobiol. 1994;43:537–685. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- REISER M., SCHILD L., KEILHOFF G., WOLF G. Interaction of nitric oxide donors and ascorbic acid on D-[3H] aspartate efflux from rat striatal slices. Neurochem. Res. 1999;24:61–67. doi: 10.1023/a:1020980013915. [DOI] [PubMed] [Google Scholar]
- RICE M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- SEGIETH J., FOWLER L., WHITON P., PEARCE B. Nitric oxide-mediated regulation of dopamine release in the hippocampus in vivo. Neuropharmacology. 2000;39:571–577. doi: 10.1016/s0028-3908(99)00178-1. [DOI] [PubMed] [Google Scholar]
- SERRA P.A., ESPOSITO G., DELOGU M.R., MIGHELI R., ROCCHITTA G., GRELLA G., MIELE E., MIELE M., DESOLE M.S. Analysis of 3-morpholinosydnonimine and sodium nitroprusside effects on dopamine release in the striatum of freely moving rats: role of nitric oxide, iron and ascorbic acid. Br. J. Pharmacol. 2000a;131:832–842. doi: 10.1038/sj.bjp.0703635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERRA P.A., ESPOSITO G., ENRICO P., MURA M.A., MIGHELI R., DELOGU M.R., MIELE M., DESOLE M.S., GRELLA G., MIELE E. Manganese increases L-DOPA autoxidation in the striatum of freely moving rats: potential implications to long-term L-DOPA therapy of Parkinson's disease. Br. J. Pharmacol. 2000b;130:937–945. doi: 10.1038/sj.bjp.0703379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVANIAN A., DAVIES K.J.A., HOCHSTEIN P. Serum urate as an antioxidant for ascorbic acid. Amer. J. Clin. Nutr. 1991;54:129S–134S. doi: 10.1093/ajcn/54.6.1129s. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., SINGEL D.J., LOSCALZO J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- TRABACE L., KENDRICK K.M. Nitric oxide can differentially modulate neurotransmitter concentrations via soluble guanylate cyclase and peroxynitrite formation. J. Neurochem. 2000;75:1664–1674. doi: 10.1046/j.1471-4159.2000.0751664.x. [DOI] [PubMed] [Google Scholar]
- WANG D., YU X., BRECHER P. Nitric oxide and N-acetylcysteine inhibit activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J. Biol. Chem. 1998;273:33027–33034. doi: 10.1074/jbc.273.49.33027. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Intrastriatal infusion of (+/−)-S-nitroso-N-acetylpenicillamine release vesicular dopamine via an ionotropic glutamate receptor-mediated mechanism: an in vivo microdialysis study in chloral hydrate-anesthetized rats. J. Neurochem. 1996;66:1971–1980. doi: 10.1046/j.1471-4159.1996.66051971.x. [DOI] [PubMed] [Google Scholar]
- WEST A.R., GALLOWAY M.P. Endogenous nitric oxide facilitates striatal dopamine and glutamate efflux in vivo: role of ionotropic glutamate receptor-dependent mechanisms. Neuropharmacology. 1997;36:1571–1581. doi: 10.1016/s0028-3908(97)00148-2. [DOI] [PubMed] [Google Scholar]
- WHITEMAN M., HALLIWELL B. Protection against peroxynitrite-dependent tyrosine nitration and α1-antiproteinase inactivation by ascorbic acid. A comparison with other biological antioxidants. Free Radic. Res. 1996;25:275–283. doi: 10.3109/10715769609149052. [DOI] [PubMed] [Google Scholar]
- YUN N.Y., DAWSON V.L., DAWSON T.M. Neurobiology of nitric oxide. Crit. Rev. Neurobiol. 1996;10:291–316. doi: 10.1615/critrevneurobiol.v10.i3-4.20. [DOI] [PubMed] [Google Scholar]


