Abstract
The vanilloid receptor of sensory neurons is a polymodal nociceptor sensitive to capsaicin, protons, heat and anandamide. Although it is known that interaction occurs between these different mediators the mechanism by which this occurs is poorly understood. In this study capsaicin elicited currents were recorded from vanilloid receptors found in adult rat isolated dorsal root ganglia (DRG) neurons under conditions of varying pH and the mechanism whereby protons can modulate this capsaicin response investigated.
Under whole-cell voltage clamp, modulating extracellular pH shifted the position of the capsaicin log(concentration)-response curve. Acidification from pH 9.0 to pH 5.5 lowered the EC50 values from 1150±250 nM to 5±2 nM with coincident change in the mean apparent slope factor from 2.3±0.3 to 0.9±0.2 and no change in maximal response.
The magnitude of the potentiation seen on reducing extracellular pH was not significantly affected by changes in extracellular calcium and magnesium concentration.
The response to capsaicin was not potentiated by a reduction in intracellular pH suggesting a site of action more accessible from the extracellular than the intracellular side of the membrane.
Potentiation by low pH was voltage independent indicating a site of action outside the membrane electric field.
At the single channel level, reducing extracellular pH increased channel open probability but had no significant effect on single channel conductance or open time.
These results are consistent with a model in which, on reducing extracellular pH, the vanilloid receptor in rat DRG neurons, changes from a state with low affinity for capsaicin to one with high affinity, coincident with a loss of cooperativity. This effect, presumed to be proton mediated, appears to involve one or more sites with pKa value 7.4 – 7.9, outside the membrane electrical field on an extracellularly exposed region of the receptor protein.
Keywords: Capsaicin, pH, sensory neuron, vanilloid receptor, dorsal root ganglia
Introduction
Capsaicin (8-methyl-N-vanillyl-6-nonenamide), the pungent component of hot chilli peppers excites a subset of primary afferent neurons. This excitation is thought to be mediated by the activation of a ligand-gated non-selective cation channel with subsequent membrane depolarization and action potential generation (reviewed by Bevan & Docherty, 1993). Indeed, such a channel, VR1, has been cloned (Caterina et al., 1997) and found to be expressed in sensory neurons (Caterina et al., 1997; Helliwell et al., 1998; Tominaga et al., 1998). When heterologously expressed in mammalian cells or Xenopus oocytes, VR1 also confers sensitivity to heat (Caterina et al., 1997) and to the endogenous lipid, anandamide (Zygmunt et al., 1999; Smart et al., 2000). The vanilloid receptor is therefore clearly polymodal and interactions between the different mediators are likely to be an important part of its function.
Changes in extracellular pH from the usual value of about 7.4 can be caused by a variety of physiological and pathophysiological conditions including, tissue inflammation and ischaemia (Uchida & Murao, 1975; Jacobus et al., 1977; Steen et al., 1995). Although these changes are usually only fairly small, values below pH 6.0 have been measured (Jacobus et al., 1977). In psychophysical studies intradermal injection or infusion of solution at pH 6.2 is sufficient to cause pain (Lindahl, 1962; Steen & Reeh, 1993). These effects are thought to be mediated via activation and sensitization of nociceptive and some visceral afferent fibres (Steen et al., 1992). In a sub-population of isolated DRG neurons acidification activates at least two different types of cation channel, resulting in a fast rapidly-inactivating current, which may be followed by a slower more slowly-inactivating one (Bevan & Yeats, 1991). Recently, several proton activated channels (e.g. ASIC 1a and 3) with a variety of properties and kinetics have been cloned and their mRNA shown by in situ hybridization to be present in sensory ganglia (Waldmann et al., 1997a,1997b; Linguelis et al., 1997). In addition to these specific proton activated channels there is evidence that some of the effects seen on acidification of DRG neurons are mediated via the capsaicin receptor itself (Bevan et al., 1993; Tominaga et al., 1998). Indeed, studies in transgenic mice lacking the VR1 receptor demonstrate a loss of the more slowly-inactivating, sustained, pH-induced current in DRG neurons, coincident with a loss of capsaicin sensitivity (Caterina et al., 2000; Davies et al., 2000).
In addition to a direct effect on sensory neurons, protons also exert a modulatory role on the capsaicin response whereby low pH substantially potentiates the amplitude of responses to sub-maximal concentrations of capsaicin in DRG and Trigeminal neurons (Petersen & LaMotte, 1993; Martenson et al., 1994; Kress et al., 1996), and in cells expressing cloned VR1 (Caterina et al., 1997; Tominaga et al., 1998; Smart et al., 2000). Studies on the cloned VR1 receptor have demonstrated a shift in the capsaicin log(concentration)-response curve corresponding to a 2 – 6 fold increase in capsaicin affinity on decreasing pH from 7.4 to pH 6.4. A detailed, quantitative analysis of pH modulations of the capsaicin responses of native receptors in DRG neurons is, however, lacking. Protons interact with sites on extracellular regions of the protein (Tominaga et al., 1998; Jung et al., 1999) and site directed mutagenesis studies have identified residues in the region linking the extracellular aspect of the fifth transmembrane domain to the putative pore forming region that are important for VR1 activation by acid solutions and for potentiation of heat responses by protons (Jordt et al., 2000). The mechanisms underlying the potentiation are, however, unclear. It has yet to be resolved whether acid-induced potentiation is identical for the native receptor and for cloned VR1 and whether potentiation is similar for chemical and thermal stimuli.
In this study we have addressed these issues by studying the native vanilloid receptor in DRG neurons isolated from adult rats under whole cell voltage clamp and at the single channel level to look at affects on single channel conductance. The results represent the first comprehensive study of the mechanism of potentiation of the capsaicin response by protons and provide evidence that this effect represents an allosteric modulation affecting the transition between open and closed states of the receptor. A model is proposed based on these observations.
A preliminary account of some of this work has been reported (McLatchie & Bevan, 1998a,1998b).
Methods
Preparation of cells
Dorsal root ganglion (DRG) neurons were isolated from adult male Sprague-Dawley rats by methods similar to those described previously (Bevan & Winter, 1995). Briefly, ganglia from all levels of the spinal cord were dissected from rats of ∼180 g killed by over exposure to CO2 (according to a Home Office approved procedure). Ganglia were placed in Ham's Nutrient mix F14 (GIBCO) to which was added Ultraser-G artificial serum (4%), L-glutamine (1 mM), penicillin (50 IU ml−1) and streptomycin (50 μg ml−1). They were digested in collagenase (Worthington type IV 0.125%) for 2 – 3 h before mechanical dissociation, filtering (90 μm pore) and spinning through 15% BSA to remove debris and myelin. Neurons were then resuspended in F14 media as above, with addition of nerve growth factor (NGF, 50 ng ml−1), and plated on polyornithine coated culture dishes (35 mm). They were incubated at 37°C in a humidified incubator with 3% CO2 in air, for 16 h – 4 days before use, usually being replated 1 – 8 h before recording, to remove neurites, which may compromise the space clamp of the neurons.
Electrophysiological recording
Whole cell recordings were made from small to medium sized neurons (capacitance 42±1 pF, diameter 29.6±0.8 μm (n=217)) without obvious neurites. Borosilicate glass patch pipettes (2 – 6 MΩ) were filled with (mM): KCl 140, CaCl2 1, EGTA/2 10, MgATP 1 (or MgCl2/10 1,2-bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)), HEPES 10, pH 7.4 (KOH). One hundred mM CsCl and 30 mM N-methyl glucamine replaced KCl in experiments in which responses were studied at positive membrane potentials (shown in Figures 2, 3, 6, 7, 8). For the experiments in Figure 5 where internal pH was modified, N-methyl glucamine was substituted with buffer to give a final buffer concentration of 40 mM whilst maintaining osmolarity. For these experiments neurons were left for at least 5 min after reaching the whole-cell configuration before recordings were made to allow time for exchange of internal solution and neurons were only used if the series resistance was 8 MΩ or under. The culture dishes were continually perfused (3 ml min−1) with either standard bath solution (mM): NaCl 140, KCl 5, glucose 10, buffer 10 (MES (2-[N-Morpholino]ethanesulphonic acid); pH 5.5 – 6.6, HEPES, pH 7.0 – 7.8, AMPSO (3-[(1,1-Dimethyl-2-hydroxyethyl0amino]-2-hydroxypropanesulphonic acid); pH 8.2 – 9.0), CaCl2 2, MgCl2 1, pH 7.4 (NaOH)), or for experiments studying the current-voltage relation (Figure 6), with a modified solution to minimize potassium currents (mM): NaCl 130, glucose 10, HEPES 10, CsCl 1, CdCl2 200 μM, TEA 10 mM, MgCl2 3, pH 7.4 (NaOH). Capsaicin (Sigma) was applied rapidly by means of a U-tube (Fenwick et al., 1982). CaCl2 and MgCl2 were replaced by NaCl in some experiments. Experiments were carried out at room temperature (17 – 25°C). To avoid the introduction of any temperature related effects, pH comparisons were either made on individual neurons (Figures 1, 3b,c, 6, 7, 8), between comparable numbers of neurons recorded on the same day (Figures 4, 5) or, using a randomized pH order and repeated measurements (Figures 2, 3a). Only one neuron was used from each dish to minimize desensitization. In experiments in which a neuron was exposed to more than one pH value experiments were bracketed (i.e. 1st pH, 2nd pH, 1st pH again) and/or the order of pH presentation was reversed between experiments.
Figure 2.
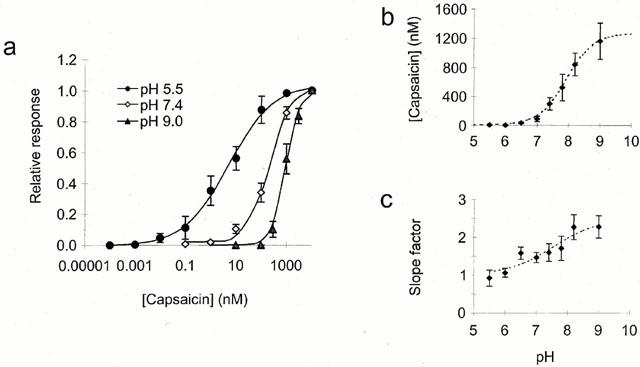
Effect of extracellular pH on the capsaicin log(concentration)-response curve. (a) Log(concentration)-response curves for capsaicin recorded at +60 mV with extracellular pH of 5.5 (circles), 7.4 (open diamonds) and 9.0 (triangles). Curve fitted of form [Imin−Imax/(1+(x/x0)p]+Imax (where; Imin is the minimum current, Imax the maximum, x0 the x value for half maximal response and p the apparent slope factor). Current was normalized to that elicited by 10 μM capsaicin for each neuron. (b) Concentration of capsaicin for half maximal response at each of these pH values with additional such curves as a function of the extracellular pH. Curve fitted based on model described in text constrained to a lower limit of 4.5±1.7 nM calculated from the mean data at pH 5.5 and pH 6.0 (n=18). Upper limit of 1270±160 nM and pKa value of 7.9±0.2 determined from best fit (n=75). (c) Slope factors from the curves in (a) and additional such curves as a function of extracellular pH. Curve of same form as in (b) with limits of 1.1±0.2 and 2.3±0.2 and pKa value of 7.4±0.4, determined from best fit (n=75).
Figure 3.
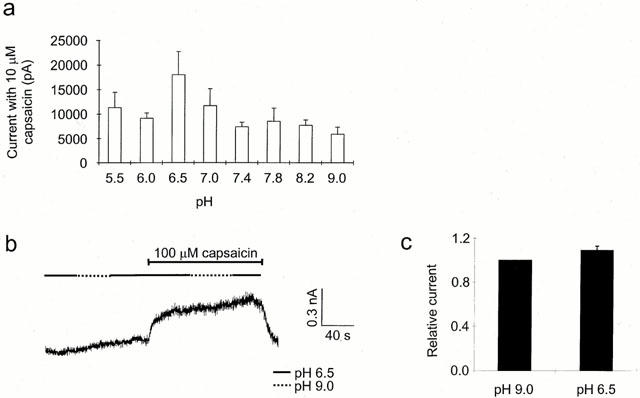
Effect of extracellular pH on the maximal capsaicin current. (a) Mean peak currents elicited by 10 μM capsaicin from the data in Figure 2 as a function of pH. (b) Effect of extracellular pH on the current elicited by 100 μM capsaicin in a single neuron at +40 mV. One hundred μM capsaicin was applied at pH 6.5 then in the maintained presence of capsaicin extracellular pH increased to pH 9, then returned to pH 6.5 as shown. (pH order was reversed in half the neurons tested). Only one set of recordings was used from each neuron corresponding to the first application of capsaicin to that dish and only one neuron was used from each dish. Decreasing the extracellular pH had very little effect on the capsaicin elicited current. (c) Mean data from experiments such as that in (b) with any current elicited by the pH change alone subtracted, showing the relative size of the current at pH 6.5 if that at pH 9.0 is 1 (n=8).
Figure 6.
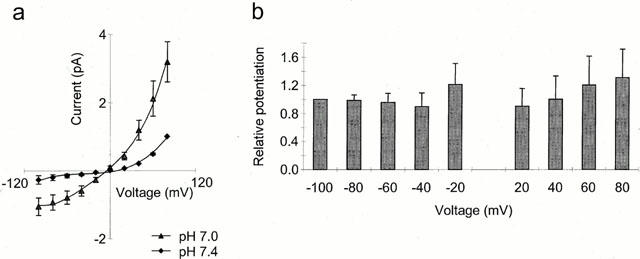
Voltage dependency of the potentiation of the capsaicin response by extracellular protons. (a) Current-voltage relationships at extracellular pH 7.4 (diamonds) and 7.0 (triangles) with subthreshold concentration of capsaicin, either 50 or 100 nM for each neuron. Current normalized to that at +80 mV and pH 7.4 for each neuron. Responses to application of pH in the absence of capsaicin subtracted (n=7). (b) Relative potentiation (current pH 7.0/current pH 7.4) as a function of voltage, normalized to that at −100 mV for each neuron (n=7).
Figure 7.
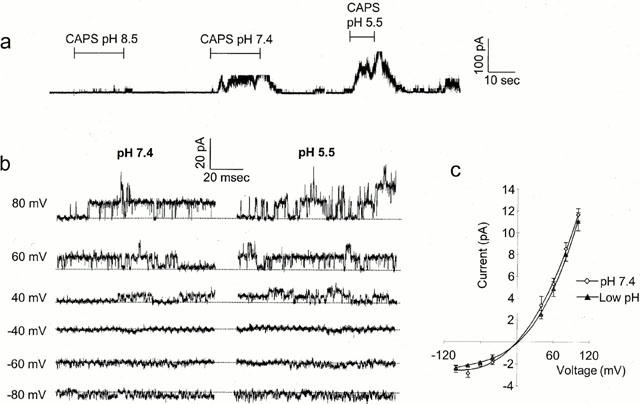
Effects of pH on the response to capsaicin at the single channel level. (a) Current elicited by outside-out patch at +80 mV on application of 10 μM capsaicin at pH 8.5, pH 7.4 and pH 5.5 as indicated. Filtered at 2 kHz. (b) Same patch as in (a) showing single channel activity at a range of potentials. No activity was seen in this patch on application of the pH solutions alone. (c) Mean single channel currrent-voltage relationship, combined data from five patches at pH 7.4 (open diamonds) and reduced pH (filled triangles).
Figure 8.
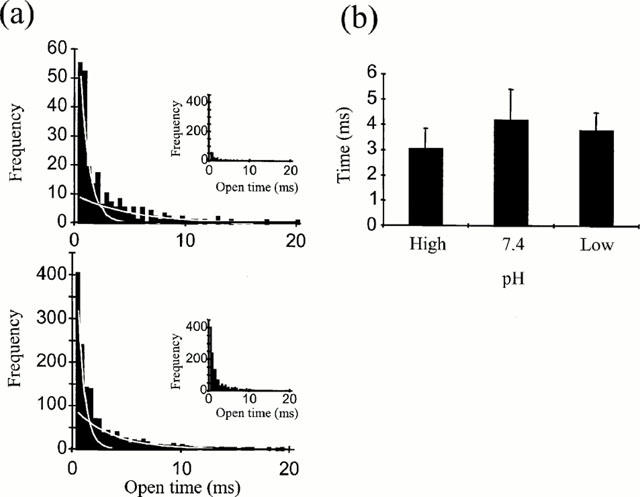
The effect of extracellular pH on mean channel open times. (a) Mean open time distributions of single channel activity in an outside-out patch at +80 mV with extracellular pH 8.5 (upper) and pH 7.4 (lower) each fitted with two exponentials. Inserts show data on the same scale to allow easier comparison of the relative frequencies. (b) Mean values for slower of these exponentials from 6 – 9 patches as a function of pH.
Figure 5.
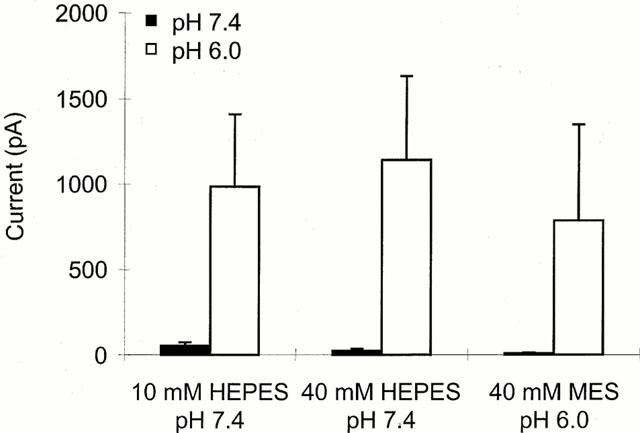
Effect of intracellular pH on the response to capsaicin. Current elicited by neurons held at −60 mV with internal pH 7.4 (buffered with either 10 or 40 mM HEPES) or pH 6.0 (buffered with 40 mM MES) to external application of 10 nM capsaicin at pH 7.4 (filled bars) or pH 6.0 (open bars) (n=8,9,9). Series resistance ⩽8 MΩ, ⩾5 min equilibration period after reaching whole-cell configuration before recording.
Figure 1.
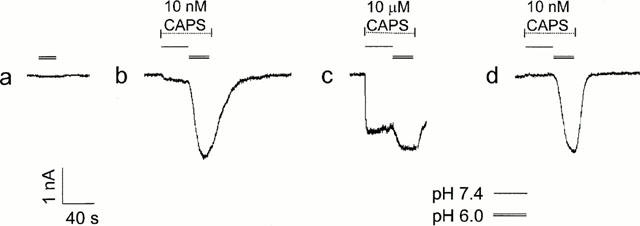
Potentiation of the response to capsaicin by low pH. DRG neuron was voltage clamped at −60 mV and capsaicin applied at pH 7.4 (single line) or pH 6.0 (double line) at either 10 nM (b, d) or 10 μM (c) as indicated. (a) shows response to pH 6.0 solution without capsaicin. Inward current downwards, responses to low pH alone not subtracted.
Figure 4.
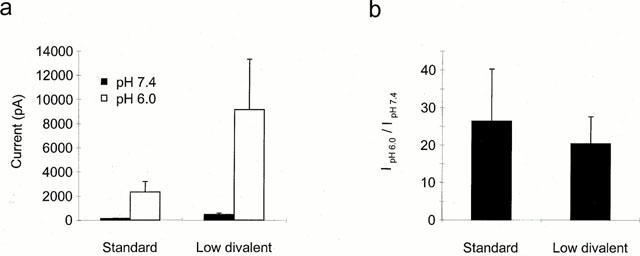
Effect of divalent cations on the potentiation of the capsaicin response. (a) Mean current elicited at −60 mV on application of 10 nM capsaicin at pH 7.4 (filled bars) or pH 6.0 (open bars) in standard or divalent solution as indicated. Currents elicited by pH alone were subtracted. (b) Current at pH 6.0/current at pH 7.4 for each neuron in the two solutions (n=11 and 8 respectively).
With the exception of Figure 1 where the response to low pH alone is shown, the current elicited by pH change alone was either subtracted or in the case of capsaicin log(concentration)-response curves at a constant pH the neuron was equilibrated at that pH value prior to capsaicin exposure. This means that all currents described are capsaicin rather than pH activated. Responses to pH alone required for subtraction were always obtained prior to any capsaicin exposure to avoid problems associated with persistence of capsaicin in the membrane. Capsaicin log(concentration)-response curves responses were obtained by increasing rather than decreasing capsaicin concentration for the same reason. All solutions contained 0.1% dimethyl sulphoxide (DMSO) as a solvent for capsaicin; 10 and 30 μM capsaicin solutions contained 1 and 3% DMSO respectively.
Data analysis
Recordings were made with an Axoclamp 200A amplifier, filtered at 5 kHz (unless otherwise stated) and analysed using pClamp6 software (Axon instruments). Statistical tests: ANOVA with post hoc Bonferroni and paired t-tests were used. All errors are standard errors of the mean.
Results
Figure 1 shows the effect of reducing the extracellular pH on the capsaicin-elicited current recorded from a DRG neuron held at −60 mV. Low pH solution was initially applied in the absence of capsaicin to measure the small response to low pH alone (Figure 1a). The neuron was then exposed to either a low (10 nM) or high (10 μM) concentration of capsaicin, as indicated, first at physiological pH 7.4 and then at pH 6.0. In the presence of the lower capsaicin concentration (Figure 1b), there was a large increase in current on reducing the pH, this increase being much greater than the current elicited by the low pH alone, indicating a potentiation of the capsaicin evoked current (Petersen & Lamotte, 1993; Martensen et al., 1994; Kress et al., 1996; Caterina et al., 1997). A smaller degree of potentiation was seen at the higher concentration of capsaicin (Figure 1c). This difference was not simply the result of some reduction in potentiation with time as a larger degree of potentiation was again seen on reapplication of the lower capsaicin concentration (Figure 1d).
To examine this phenomenon of potentiation further, full log(concentration)-response curves to capsaicin were constructed. A potential of +60 mV was used to minimize the effects of desensitization (Yeats et al., 1992; Bevan & Docherty, 1993; Liu & Simon, 1996) and each neuron was equilibrated for several minutes at the test extracellular pH before application of capsaicin at that pH. The results for three pH values are shown in Figure 2a. Data from individual neurons were normalized to the current elicited by 10 μM capsaicin, at that pH, to allow for comparison of the curves. A comparison of response amplitude was made later (see below). The effect of reducing pH was to shift the position of the log(concentration)-response curve to the left corresponding to an increase in the apparent affinity for capsaicin. The pH dependence of the concentration of capsaicin required for a half maximal effect is plotted in Figure 2b. The curve fitted, which gives a good description of these data, assumes a shift on reducing pH, from a low affinity state (EC50 value 1270±160 nM) to a high affinity state of the receptor (EC50 4.5±1.7 nM) with a pKa value of 7.9±0.2 determining the relative ratios of the two forms. In addition, as seen in Figure 2a, reducing extracellular pH caused a reduction in the mean apparent slope factors of the individual log(concentration)-response curves to capsaicin from 2.3±0.3 (at pH 9.0) to 0.9±0.2 (at pH 5.5). The apparent slope factors, were plotted as a function of pH, in Figure 2c. These pooled data were fitted with a curve assuming the same type of model as that in Figure 2b yielding maximum and minimum values of 2.3±0.2 and 1.1±0.2 respectively and pKa value of 7.4±0.4. The reduction in slope factor from a value close to 2 at high pH to one close to 1 at lower extracellular pH suggests that the capsaicin interaction with its receptor shifts from being cooperative (whereby binding of the first capsaicin molecule facilitates the binding of subsequent molecules) to non-cooperative (independent binding) on reducing extracellular pH. These effects of pH are unlikely to be due to changes in the degree of protonation of the capsaicin molecule itself, because with a pKa value of 10.10±0.05 (Sirius PCA101 pKa-log P analyser) the change in the ratio of charged to uncharged capsaicin will be small over the pH range used here.
To determine whether modulation of extracellular pH had any effect on maximal response, the currents elicited by 10 μM capsaicin, from the cumulative log(concentration)-response curves in Figure 2 were compared (Figure 3a). Although these data showed no significant overall trend in maximal current magnitude as a function of pH there was some suggestion of larger responses at the lower pH values, such as pH 6.5, than at very high pH values. This possible trend could represent a real difference, but masked by variable desensitization between neurons or, simply sampling error when studying a population with very variable sensitivities to capsaicin (see Bevan & Winter, 1995). To differentiate between these possibilities a comparison was made between the potentiation seen at pH 6.5 and 9.0, with a very high concentration of capsaicin (100 μM) in individual neurons not previously exposed to capsaicin. Figure 3b shows a sample trace illustrating the type of protocol used, in which a neuron held at +60 mV in solution at pH 7.4 was exposed to solutions of pH 6.5 and 9.0, first in the absence of capsaicin, and then in the maintained presence of 100 μM capsaicin. In this neuron, selected for its small current and small degree of desensitization (see below), there was virtually no effect of this change in pH in the absence of capsaicin and only a very small decrease in current on increasing the pH in the presence of capsaicin. Figure 3c shows the mean data from eight experiments of this type with variable order of pH presentation, where the average ratio of the current at pH 6.5 as compared to that at pH 9.0 was 1.09±0.04. This value is not significantly different from 1 (paired t-test, P=0.2 (n=8)), indicating that the maximal response was not modified by changes in extracellular pH but that instead, the potentiation seen could be explained entirely by a shift in the position of the log(concentration)-response curve. Interestingly, following desensitization seen with subsequent applications of capsaicin to the same neuron, responses were more susceptible to the effects of pH change even at high capsaicin concentrations. This suggests that in this aspect at least, desensitization was equivalent to reducing the effective concentration of capsaicin.
In the next series of experiments the mechanism whereby the position of the capsaicin log(concentration)-response curves may be shifted by modifications in extracellular pH was addressed. To establish whether any changes in divalent cation permeability were involved, the magnitude of potentiation was compared in standard extracellular solution and in solution without added calcium or magnesium. As shown in Figure 4a there was an increase in the magnitude of the currents evoked by 10 nM capsaicin at both pH 7.4 and pH 6.0 on removing divalent cations (Docherty et al., 1996), but no significant effect on the actual degree of potentiation of the response (Figure 4b). This finding indicates that no part of the potentiation described above can be explained by a change in divalent cation permeability or by displacement of fixed charge contributed by divalent cations.
To investigate more closely the site of action of protons the effects of modifying internal pH were compared with those seen with changes in extracellular pH. Figure 5 shows the current obtained on extracellular application of a low concentration (10 nM) of capsaicin at pH 7.4 (dark bars) or pH 6.0 (open bars), to neurons with one of three different internal solutions, standard internal solution at pH 7.4 buffered with 10 mM HEPES (left), solution at pH 7.4 with 40 mM HEPES (middle) or reduced pH solution, pH 6.0 with 40 mM MES (right). Although there was a small decrease in the current elicited with extracellular pH 7.4 on decreasing intracellular pH, the large potentiated current seen at extracellular pH 6.0 was not significantly different between the three groups (P>0.9, ANOVA). If the site responsible for potentiation were intracellular or close to the intracellular face of the membrane, then changing intracellular pH would be expected to have a greater effect than changing the extracellular pH. In contrast, these results suggest that the site responsible for capsaicin potentiation is more accessible from the extracellular than the intracellular side of the membrane.
To investigate whether the site could be within the membrane electrical field the dependence of potentiation on voltage was examined. Figure 6a shows the outwardly rectifying current voltage relationships elicited at pH 7.4 and pH 7.0 by a concentration of capsaicin chosen to be sub maximal at both pH values for each neuron, (either 50 or 100 nM in each case). Current was potentiated at all voltages. In Figure 6b the relative potentiation, i.e. the current evoked at pH 7.0 as a function of that at pH 7.4 normalized to the ratio at −100 mV for each neuron is plotted as a function of voltage. There was no significant difference in this ratio as a function of voltage indicating that potentiation was not voltage dependent. This implies that the site of action is not within the membrane electrical field and therefore more likely to be on some extracellular region of the protein. This finding also implies that this site is likely to be remote from the capsaicin binding site which has recently been reported to be intracellular (Jung et al., 1999).
The absence of effect of reducing extracellular pH on the maximal response to capsaicin (Figure 3) suggests that the reduction in pH may affect open channel probability rather than to increase single channel conductance. This question was addressed directly by making recordings at the single channel level. In excised outside-out membrane patches, application of capsaicin to the extracellular side of the membrane elicited channel activity (Forbes & Bevan, 1987; Oh et al., 1996). At a constant concentration of capsaicin, reducing pH from 8.5 to 5.5 led to a dramatic increase in the channel activity elicited and the number of open channels (Figure 7a), corresponding to an increase in channel open probability, assuming a constant total channel population in the patch, of over 20 times. No channel activity was elicited by the pH change alone in this experiment (data not shown) and recordings where ‘background' channel activity was apparent at the pH value under study were not included in the analysis. The increase in channel activity was particularly marked (15 fold) with the decrease in pH from 8.5 to 7.4 which corresponds to the region of maximum pH dependence of the EC50 and apparent slope factors seen with whole-cell recordings (Figure 2). Figure 7b shows current recordings with capsaicin elicited channel activity at a range of membrane potentials at extracellular pH 7.4 and pH 5.5 respectively. It is apparent that this figure and the single channel current-voltage relationship shown in Figure 7c containing data from five patches that the single channel conductance was not significantly modified by changes in extracellular pH. Although there was no significant difference in single channel conductance which was, 91±11 pS and 35±2 pS at +60 mV and −60 mV respectively at physiological pH and 81±11 pS and 32±1 pS at reduced pH, there was a trend towards reduced conductance at low pH as recently reported by Baumann & Martenson (2000).
Open time distributions were constructed from excised outside-out patches held at +60 to +80 mV with an external pH of either 8.5, 7.4 or low pH (pH 5.5 – 6.5). In cases where multi-channel patches were used data were only included from periods of openings from baseline to the single open channel level. Figure 8a shows typical open time distributions for pH 8.5 and 7.4, which were best fitted by two exponentials with a faster and a slower time constant respectively. Double exponential fits to open time distributions have been reported previously for the vanilloid receptor in rat DRG neurons (Oh et al., 1996) although a single exponential was found to best fit the open time distribution for the cloned VR1 receptor (Tominaga et al., 1998). In some of the single channel experiments data were filtered at 2 KHz which limited the resolution of the brief events. For this reason analysis of measurements has been restricted to the second, slower component of the open time distributions. Mean values for the constant as a function of pH (Figure 8b) revealed no significant change with pH (ANOVA, P>0.2), with values of 3.1±0.8 ms (n=6, pH 8.5), 4.2±1.2 ms (n=9, pH 7.4) and 3.8±0.7 ms (pH 5.5 – 6.5).
Discussion
Mechanism of action
The data presented here demonstrate a modulatory role for protons in setting the affinity of the native vanilloid receptor in adult rat DRG neurons for capsaicin without changing the maximal response or single channel conductance. This is clearly an important mechanism from the scale of the shifts seen, 200 – 300 fold over the full pH range studied here (pH 5.5 – pH 9) and some 8 – 9 fold over the more physiological range of pH 7.4 to pH 6.5. This latter shift compares with values of 2 – 6 fold seen for the cloned rat and human VR1 receptors over this pH range (Tominaga et al., 1998; Smart et al., 2000). Our analysis of the dependence of EC50 values and slope factors on pH indicates that the relationships can be fitted by a sigmoidal curve with a mid-point at pH 7.4 – 7.9. These values are similar to the mid-point of ∼pH 7 recently reported for the low pH-induced potentiation of noxious heat responses of the rat cloned wild-type VR1 (Jordt et al., 2000). In contrast low pH solutions have been reported to have no effect on the response of rat cloned VR1 to another activator, anandamide (Smart et al., 2000). This finding suggests that pH modulation of VR1 activity may depend on the type of agonist.
Jordt et al. (2000) identified a glutamate residue (E600) thought to be located in the region that links the extracellular domain of the fifth transmembrane spanning region to the putative pore forming loop. Site directed mutagenesis showed that this residue is particularly important for potentiation of the heat responses. Replacement of glutamate with neutral or positively charged residues potentiated the current responses to heat and resulted in a reduction or loss of pH modulation of the evoked heat responses. Such amino acid changes also shifted the log(concentration)-response curves for capsaicin to lower concentrations, although the authors did not report whether the shifts were associated with any change in pH modulation of the capsaicin-evoked responses.
In the current study potentiation of the capsaicin response at low pH was not associated with any increase in the amplitude of the maximum response. This finding contrasts with a recent report that acidification from pH 8.0 to pH 6.0 is associated with a greater than 3 fold increase in the maximal capsaicin-evoked current response of human cloned VR1 expressed in Xenopus oocytes (Hayes et al., 2000). The reasons for this difference are unclear although they may be associated with either a species difference or the cellular environments of the native and cloned receptors.
A shift in the log(concentration)-response curve with constant maximal response could be described by assuming the conversion of individual receptors from a low affinity, unprotonated state at high pH values to a higher affinity, protonated state, on reducing pH. The population response at any given pH value would then reflect the ratio of the numbers of receptors in the two states. The pH dependence of the shift in the apparent half maximal effect and slope factors are both consistent with this interpretation if the proton(s) interact(s) with a site, or sites, with pKa value of 7.4 – 7.9. This simple model is shown in scheme 1 with two pathways representing the protonated and non-protonated states of the receptor. It is unknown how many subunits make up each capsaicin receptor or how many capsaicin molecules are required to bind for activation. Hill coefficients (slope factors) of 1.7 – 2.2 (Vlachova & Vyklicky, 1993; Koplas et al., 1997 and Figure 2a) for capsaicin interaction at physiological pH, suggest at least two capsaicin molecules are involved. This finding suggests the involvement of at least two subunits but from comparison with potassium channels or nicotinic receptors for example, (e.g. Popot & Changeux, 1984; Mackinnon, 1991), an overall composition of four or more subunits may occur. The model in Scheme 1 considers the simplest case and shows the sequential binding of two capsaicin molecules whilst acknowledging that this may be an over simplification and that additional steps could be added. Activation of the receptor by protons alone has been omitted from the model for clarity.
Scheme 1.
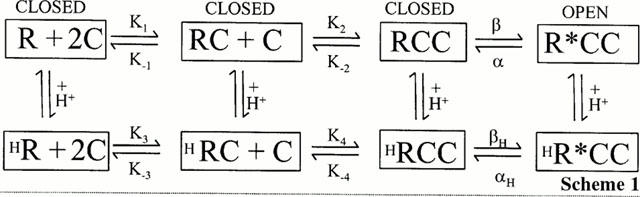
K1 to K4 are rate constants for capsaicin binding and K−1 to K−4 for unbinding. β and α (or βH and αH) are rate constants for the conversion of the liganded receptor between the open and closed states. The reduction in cooperativity seen on reducing extracellular pH could imply the loss of one of the steps indicated in the lower pathway, or simply an increase in the rate constant such that that step is no longer limiting. Since it is not possible to determine from these data which of these two situations is occurring an equivalent number of steps have been included in both pathways.
The single channel results showing that conductance is unchanged but that open probability is increased on reducing extracellular pH are consistent with this model in that they favour a change in the conversion rate between states rather than the development of a new state. From the analysis of the single channel open times the values α and αH, the rate constants for transition from the open to closed state of the channel, appear to be equal. This implies that changes in the binding rate constants or β values must mediate the increase in open channel probability.
Whilst this model is consistent with the majority of the data presented here it may be an oversimplification. For example protons may interact with more than one site on the receptor. Although we have fitted the relationships between pH and either EC50 values or slope factors with single sigmoidal curves, close inspection of the slope factor data suggests that the pH dependence of the slope factors (Figure 2c) could be fitted by a double sigmoidal function with pKa values of about pH 6 and 8. Support for the contribution of a single site is provided by the identification of the importance of the glutamate residue (E600) for potentiation of noxious heat responses in site directed mutagenesis studies (Jordt et al., 2000). Nevertheless we cannot rule out at present that other residues may have a role in proton-induced potentiation.
It is possible that pH modulation of VR1 involves changes in both chemical sensitivity and thermal thresholds and that our experiments have monitored the net agonism of VR1 induced by capsaicin at a lowered temperature threshold. In the present study temperature in individual experiments was steady, despite some day to day variations, and low pH alone often had no significant agonist effect at the experimental temperatures used (see e.g. Figure 1). We cannot rule out the possibility that capsaicin also modulates temperature threshold. It would be interesting to extend the present findings to study the effects of temperature on pH modulation of chemical sensitivity, but such a study is beyond the scope of the present investigation. It is hoped that this initial ‘simple' model will serve as a valuable starting point from which to study interactions at this receptor further and for comparison of the native receptor with the cloned VR1.
Site of proton action
All of the data presented here argue against the site or sites of proton action mediating potentiation being within the channel pore or membrane electrical field. If the proton(s) acted within the membrane electrical field a voltage dependence would be expected which was not seen. Similarly, the absence of any interaction with divalent cations or effect on the single channel conductance and hence maximal response support this view. The larger effect of extracellular than intracellular pH change suggest that the site(s) is(are) on an extracellular region of the protein. These characteristics are consistent with the identification of a glutamate residue (E600), which is extracellular and not part of the putative pore region, as an important modulatory site for protons. This modulatory site appears to differ from the extracellular site responsible for proton activation of the VR1 (Jung et al., 1999; Tominaga et al., 1998), which has been identified, at least in part, as another glutamate residue (E648). Both sites appear to be remote from the proposed intracellular capsaicin site (Jung et al., 1999) and imply that the potentiating effects of pH are likely to be mediated via an allosteric change in the receptor rather than a direct interaction of a proton with the capsaicin binding site. The identification of glutamate residues as important mediators of proton activation and potentiation of VR1 highlights the differences between the pKa values for the side chain of free glutamic acid (pH 4.28) and the observed pH for half maximal potentiation of capsaicin or noxious heat responses (pH 7 – 8) and activation by low pH (pH 5.3 – 5.8; Bevan & Yeats, 1991; Jordt et al., 2000). Such differences in the titration of the side chains of amino acids in solution and when in the complex chemical microenvironment of proteins are well known and have been reported for other ion channels including L-type calcium channels (see Chen & Tsien, 1997) and cyclic nucleotide gated channels (Morrill & MacKinnon, 1999).
Physiological implications
The vanilloid receptor has also been shown to be sensitive not only to capsaicin and protons but also to both heat (Tominaga et al., 1998) and the endogenous cannabinoid, anandamide (Zygmunt et al., 1999; Smart et al., 2000). Reduction in pH has been demonstrated to potentiate the response of VR1 to heat (Tominaga et al., 1998) although not to anandamide (Smart et al., 2000). It is unclear why this should be the case and whether the same is true of the native receptor.
The potentiation of the response to heat could be very important physiologically, for, as suggested by Vyklicky et al. (1999) protons may function to decrease the threshold for the heat response such that a previously ineffective temperature becomes stimulatory and hence painful.
A reduction in pH has also been shown to potentiate the response of P2X2 containing receptors to ATP (King et al., 1996; Li et al., 1996; 1997; Stoop et al., 1997). This potentiation shows similarities to that seen here for capsaicin except that cooperativity is not affected by changes in pH. Like the capsaicin response this potentiation of the P2X2 containing receptor is voltage independent, involves a site with pKa value of 7.6 (Li et al., 1996) and the interaction of a single effector and could involve a similar mechanism.
It is clear from the polymodal nature of the vanilloid receptor that interaction between its different activators is an important part of its function. Here, by studying one of these pairs of interactions we have developed a model in which on reducing extracellular pH the vanilloid receptor in rat DRG neurons undergoes an allosteric change, shifting it from a low to high affinity state coincident with a loss of cooperativity. This effect, presumed to involve proton interaction is voltage independent indicating a site or sites of action outside the membrane electrical field. The absence of potentiation seen on reducing intracellular pH points to an extracellular site. The increase in channel open probability and the reduction in cooperativity suggest a facilitation in the transition between the open and closed states and possibly a reduction in the number of capsaicin molecules required for activation. It is hoped that this model should provide a useful starting point from which to explore additional interactions at this receptor.
Acknowledgments
We wish to thank Hendrikus Eggelte and David Bentley for measuring the pKa of capsaicin, Prof Humphrey Rang for helpful comments and discussion on the manuscript and Dr Alison Reeve for some of the single channel recordings.
Abbreviations
- AMPSO
3-[(1,1-Dimethyl-2-hydroxyethyl0amino]-2-hydroxypropanesulphonic acid
- ASIC
Acid sensing Ionic channel
- BAPTA
1,2-bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- DMSO
Dimethyl Sulphoxide
- DRG
Dorsal Root Ganglia
- MES
2-[N-Morpholino]ethanesulphonic acid
- NGF
Nerve Growth Factor
- VR1
Vanilla receptor 1
References
- BAUMANN T.K., MARTENSON E. Extracellular protons both increase the activity and reduce the conductance of capsaicin-gated channels. J. Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-11-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEVAN S., DOCHERTY R.J.Cellular mechanisms of the action of capsaicin Capsaicin in the study of pain 1993Academic Press; 27–44.ed. Wood, J. Chapter 2, pp [Google Scholar]
- BEVAN S., FORBES C.A., WINTER J. Protons and capsaicin activate the same ion channels in rat isolated dorsal root ganglion cells. J. Physiol. 1993;459:401P. [Google Scholar]
- BEVAN S., WINTER J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J. Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEVAN S., YEATS J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurons. J. Physiol. 1991;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSON-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHEN X.H., TSIEN R.W. Aspartate substitutions establish the concerted action of P-region glutamates in repeats I and III in forming the protonation site of L-type Ca2+ channels. J. Biol. Chem. 1997;272:30002–30008. doi: 10.1074/jbc.272.48.30002. [DOI] [PubMed] [Google Scholar]
- DAVIS J.B., GRAY J., GUNTHORPE M.J., HATCHER J.P., DAVEY P.T., OVEREND P., HARRIES M.H., LATCHMAN J., CLAMPHAM C., ATKINSON K., HUGHES S.A., RANCE K., GRAU E., HARPER A.J., PUGH P.L., ROGERS D.C., BINGHAM S., RANDALL A., SHEARDOWN S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., BEVAN S., BODDEKE H.W.G.M. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurons from adult rats. Pflugers Archiv. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- FENWICK E.M., MARTY A., NEHER E. Sodium and calcium channels in bovine chromaffin cells. J. Physiol. 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES C.A., BEVAN S. Single channels activated by capsaicin in patches of membrane from adult rat sensory neurons in culture. Neurosci. Lett. Suppl. 1987;32:S3. [Google Scholar]
- HAYES P., MEADOWS H.J., GUNTHORPE M.J., HARIES M.H., DUCKWORTH D.M., CAIRNS W., HARRISON D.C., CLARKE C.E., ELLINGTON K., PRINJHA R.K., BARTON A.J.L., MEDHURST A.D., SMITH G.D., TOPP S., MURDOCK P., SANGER G.J., TERRETT J., JENKINS O., BENHAM C.D., RANDALL A.D., GLOGER I.S., DAVIS J.B. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- HELLIWELL R.J.A., MCLATCHIE L.M., CLARKE M., WINTER J., BEVAN S., MCINTYRE P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- JACOBUS W.E., TAYLOR G.J., HOLLIS D.P., NUNNALLY R.L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977;265:756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- JORDT S.-V., TOMINAGA M., JULIUS D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.-L., KANG C.-J., KIM W.B., DONGHEE K., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B., ZIGANSHINA L.E., PINTOR J., BURNSTOCK G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br. J. Pharmacol. 1996;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPLAS P.A., ROSENBERG R.L., OXFORD G.S. The Role of Calcium in the desensitization of Capsaicin Responses in Rat Dorsal Root Ganglion Neurons. J. Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRESS M., FETZER S., REEH P.W., VYKLICKY L. Low pH facilitates capsaicin responses in isolated sensory neurons of the rat. Neurosci. Lett. 1996;211:5–8. doi: 10.1016/0304-3940(96)12691-4. [DOI] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.W. Proton potentiation of ATP-gated ion channel responses to ATP and Zn2+ in rat nodose ganglion neurons. J. Neurophysiol. 1996;76:3048–3058. doi: 10.1152/jn.1996.76.5.3048. [DOI] [PubMed] [Google Scholar]
- LI C., PEOPLES R.W., WEIGHT F.F. Enhancement of ATP-activated current by protons in dorsal root ganglion neurons. Pflugers Archiv. 1997;433:446–454. doi: 10.1007/s004240050299. [DOI] [PubMed] [Google Scholar]
- LINDAHL O. Pain: a chemical explanation. Acta Rheumatologica Scandinavica. 1962;8:161–169. doi: 10.3109/rhe1.1962.8.issue-1-4.17. [DOI] [PubMed] [Google Scholar]
- LINGUEGLIS E., DE WEILLE J.R., BASSILANA F., HEURTEAUX C., SAKAI H., WALDMAN R., LAZDUNSKI M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Capsaicin-induced currents with distinct desensitisation and Ca2+ dependence in rat trigeminal ganglion cells. J. Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- MACKINNON R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- MARTENSON M.E., INGRAM S.L., BAUMANN T.K. Potentiation of rabbit trigeminal responses to capsaicin in a low pH environment. Brain Research. 1994;651:143–147. doi: 10.1016/0006-8993(94)90690-4. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L.M., BEVAN S. Protons affect the affinity and apparent cooperativity of the interaction between capsaicin and the vanilloid receptor in adult rat isolated DRG neurons. J. Physiol. 1998a;513:129P. [Google Scholar]
- MCLATCHIE L.M., BEVAN S. Effects of protons on the affinity and cooperativity of capsaicin interaction with the vanilloid receptor in rat DRG neurons. Soc. Neurosci. Abstr. 1998b. p. 721.6.
- MORRILL J.A., MACKINNON R. Isolation of a single carboxyl-carboxylate proton binding site in the pore of a cyclic nucleotide-gated channel. J. General Physiol. 1999;114:71–83. doi: 10.1085/jgp.114.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OH U., HWANG S.W., KIM D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J. Neurosci. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSEN M., LAMOTTE R.H. Effect of protons on the inward current evoked by capsaicin in isolated dorsal root ganglion cells. Pain. 1993;54:37–42. doi: 10.1016/0304-3959(93)90097-9. [DOI] [PubMed] [Google Scholar]
- POPOT J.-L., CHANGEUX J.-P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiological Reviews. 1984;64:1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., KJERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anadamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEEN K.H., REEH P.W. Sustained graded pain and hyperalgesia from harmless experiments tissue acidosis in human skin. Neurosci. Lett. 1993;154:113–116. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- STEEN K.H., STEEN A.E., REEH P.W. A dominant role of acid pH in inflammatory excitation and sensitization of nociception in rat skin in vitro. J. Neurosci. 1995;15:3982–3989. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEEN K.H., REEH P.W., ANTON F., HANDWERKER H.O. Protons selectively induce lasting excitation to mechanical stimulation of nociceptors in rat skin, in vitro. J. Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOOP R., SUPRENANT A., NORTH R.A. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J. Neurophysiol. 1997;78:1837–1840. doi: 10.1152/jn.1997.78.4.1837. [DOI] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- UCHIDA Y., MURAO S. Acid-induced excitation of afferent cardiac sympathetic nerve fibres. Am. J. Physiol. 1975;228:27–33. doi: 10.1152/ajplegacy.1975.228.1.27. [DOI] [PubMed] [Google Scholar]
- VYKLICKY L., VLACHOVA V., VITASKOVA Z., DITTERT I., KABAT M., ORKAND R.K. Temperature coefficient of membrane currents induced by noxious heat in sensory neurons of the rat. J. Physiol. 1999;517:181–192. doi: 10.1111/j.1469-7793.1999.0181z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VLACHOVA V., VYKLICKY L. Capsaicin-induced membrane currents in cultured sensory neurons of the rat. Physiological Research. 1993;42:301–311. [PubMed] [Google Scholar]
- WALDMANN R., CHAMPIGNY G., BASSILANA F., HEURTEAUX C., LAZDUNSKI M. A proton-gated cation channel involved in acid-sensing. Nature. 1997a;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- WALDMANN R., BASSILANA F., WEILLE J.-D., CHAMPIGNY G., HEURTEAUX C., LAZDUNSKI M. Molecular cloning of a non-inactivating proton-gated Na2+ channel specific for sensory neurons. J. Biol. Chem. 1997b;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- YEATS J.C., DOCHERTY R.J., BEVAN S. Calcium-dependent and independent desensitization of capsaicin-evoked responses in voltage-clamped adult dorsal root ganglion (DRG) neurons in culture. J. Physiol. 1992;446:390P. [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.-H., SORGARD M., MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


