Abstract
We have examined the involvement of the endocannabinoid system in the cardioprotection triggered by lipopolysaccharide (LPS). Rats were treated with saline or LPS (10 μg Kg−1). 24 h later, hearts were excised, retrogradely perfused, submitted to a low-flow ischaemia (0.6 ml min−1) for 90 min and reperfused for 60 min. Some hearts were perfused with either SR 141716A (a cannabinoid CB1, receptor antagonist 1 μM), SR 144528 (a CB2 receptor anagonist μM), NNLA (3 μM) or sodium nitroprusside (1 μM) 5 min before ischaemia and during the ischaemic period. The cardioprotective effects of LPS treatment, in terms of infarction and functional recovery, were not altered by the perfusion of SR 141716A but abolished by both SR 144528 and NNLA. Finally, SR 144528 abolished the beneficial effects of SNP perfusion. Our results suggest an involvement of endocannabinoids, acting through the CB2 receptors, in the cardioprotection triggered by LPS against myocardial ischaemia. This could be attributed to a relationship between cannabinoids and NO.
Keywords: Isolated rat heart, myocardial ischaemia, lipopolysaccharide, cannabinoids
Introduction
Several events, including treatment with lipopolysaccharides (LPS) (Brown et al., 1989) can trigger endogenous protective mechanisms against ischaemia-reperfusion injury, appearing 24 h following the trigger event, and lasting for several days. Although the mechanisms implicated in the cardioprotection triggered by LPS are not fully understood, it is now thought that the increase in nitric oxide (NO) production may play a role (Yang et al., 1997).
Recently, increased attention has turned to the pharmacology and biochemistry of endogenous cannabinoids, first identified in the central nervous system and now found to be expressed throughout the body. Components of the endocannabinoid system have now been identified. Anandamide and 2-arachidonoylglycerol are the two endocannabinoids isolated so far, acting through interactions with G-protein coupled membrane receptors, namely CB1 and CB2 receptors (for review, Piomelli et al., 2000). Major advances in this area followed the development of potent and highly selective antagonists. SR 141716A, a CB1 receptor antagonist, was found to block the central effects that occur after in vivo exposure to delta9-tetrahydrocannabinol, the main psychoactive component of marijuana (Rinaldi-Carmona et al., 1994). SR 144528 was recently discovered and shown to have a 700 fold higher affinity for the CB2 receptor than for the CB1 receptor (Rinaldi-Carmona et al., 1998).
Many studies have implicated the involvement of endocannabinoids in regulation of the inflammatory response. For example, Smith et al. (2000) reported that cannabinoid agonists can modulate cytokines through CB1 receptor activation in endotoxaemic mice. In addition, in the macrophage line RAW 264.7, delta9-tetrahydrocannabinol inhibits LPS-induced expression of the inducible form of NO synthase, through the inhibition of the NF-κB pathway (Jeon et al., 1996).
The purpose of this study was to examine the involvement of the endocannabinoid system in the cardioprotective effects of LPS.
Methods
All experiments were performed in accordance with the guidelines from the Canadian Council on Animal Care. Male Wistar (280 – 300 g) rats were asphyxiated with CO2 and rapidly decapitated. Hearts were immediately excised and placed in an ice-cold heparin-treated (10 IU ml−1) buffer. They were mounted on a Langendorff set-up and perfused at a constant flow by means of a digital roller pump (Labcor Inc. Anjou, Qc, Canada). The flow rate was adjusted during the stabilisation period to obtain a coronary perfusion pressure of about 75 mmHg and this was held constant, with exception of the ischaemic period during which flow was reduced to 0.6 ml min−1 (low flow ischaemia).
Isovolumetric left ventricular developed pressure (LVDP) and its first derivative dP/dt were measured by a fluid filled latex balloon inserted into the left ventricle and connected to a pressure transducer. Flow rate was measured during the whole protocol with an ultrasonic flow probe (T106, Transonic system Inc., Ithaca, NY, U.S.A.). The perfusion solution consisted of a modified Krebs-Henseleit buffer, containing (in mM): NaCl, 118; KCl, 4; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 24, D-glucose, 5 and pyruvate, 2.0. The perfusate was kept at 37°C and bubbled with 95% O2/5% CO2 (pH 7.4). All drugs were administered through a Y connector in the aortic cannula with syringe pump (model 11, Harvard Apparatus, St Laurent, Qc, Canada) at one hundred of the coronary flow rate.
Experimental protocols
The rats were treated with saline or LPS (serotype 011b4, 10 μg kg−1, i.v) and left to recover for 24 h. They were then assigned randomly to either one of the 16 groups. In all ischaemic groups, hearts were submitted to a 20-min stabilization period, followed by 90 min of low flow ischaemia (flow rate 0.6 ml min−1) before a 60-min reperfusion period. In a sham group, hearts were perfused during 170 min without any change in the perfusion rate. Perfusion with either SR 141716A (1 μM) SR 144528 (1 μM) NNLA (3 μM) or sodium nitroprusside (1 μM) was initiated 5 min before ischaemia and maintained during the entire ischaemic period.
Measurement of the infarct size
At the end of the protocol, atria were removed and the heart was frozen at −80°C for 10 min. It was then cut into 0.6 – 0.8 mm transverse sections from apex to base (6 – 7 slices/heart). Once thawed, the slices were incubated at 37°C with 1% triphenyltetrazolium chloride in phosphate buffer (pH 7.4) for 10 min and fixed in 10% formaldehyde solution to distinguish the clearly stained viable tissue from unstained necrotic tissue. Infarct size (I) was determined using a computerized planimetric technique (Scion image for Windows) and expressed as the percentage of the ventricular area (V).
Drugs
SR 141716A (N-(piperidin-1-yl)-5-(4-(chlorophenyl)-1- (2,4-dichlorophenyl) -4-methyl-1H- pyrazole-3- carboxamide hydrochloride), a CB1 receptor antagonist and SR 144528 (N-( [1s] -endo-1,3,3-trimethylbicyclo [2, 2, 1] heptan-2-yl) -5-(4- chloro-3-methylphenyl) -1-(4-methylbenzyl) -pyrazole-3-carbo-xamide), a CB2 receptor antagonist, were kindly provided by Sanofi Research. All other drugs were obtained from Sigma (Oakville, ON, Canada). Stock solutions of Nω-nitro-L-arginine (NNLA), a NO synthase inhibitor, and sodium nitroprusside, a NO donor, were prepared in distilled water and further diluted in Krebs-Henseleit buffer. Stock solutions of SR 141716A and SR 144528 were prepared in 100% dimethyl suphoxide (DMSO) and further diluted in Krebs-Henseleit buffer in order to obtain the desired final concentration. DMSO at the concentration obtained in the final dilution (0.01%) had no effect either on the infarct size, or on any of the haemodynamic variables studied.
Statistical analysis
Data are presented as mean±s.e.mean. Infarct size values were compared using a one-way ANOVA. Haemodynamic data were analysed using a two-way ANOVA, with post-hoc multiple comparison Tukey test.
Results
Myocardial function
Pre-ischemic haemodynamic data are described in Table 1. SNP perfusion significantly decreased the coronary resistance without affecting LVDP and dP/dT.
Table 1.
Pre-ischemic haemodynamic values in the different experimental groups
Myocardial infarction
Figure 1A, B show myocardial infarct size, expressed as the percentage of the ventricular area, in untreated or endotoxic hearts.
Figure 1.
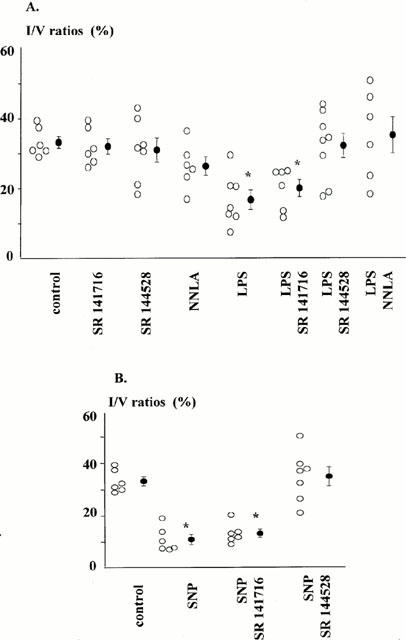
Infarct size expressed as the percentage of ventricular area assessed following low-flow ischaemia (0.6 ml min−1, 90 min)-reperfusion (60 min) sequence, in: (A) control or endotoxic hearts (LPS 10 μg kg−1, i.v. 24 h earlier), perfused or not with either SR 141716A (1 μM), SR 144528 (1 μM), or NNLA (30 μM). (B) control hearts perfused or not with SNP, in absence or in presence of either SR 141716A (1 μM), or SR 144528 (1 μM). Drug perfusions are initiated 5 min before ischaemia and maintained during the entire ischaemic period. Open circles, individual values, and closed circles, mean±s.e.mean. *P<0.05 vs control group.
Infarct size were smaller in hearts from rats treated with LPS 24 h earlier, compared with that in hearts from saline-treated animals. Blockade of cannabinoid receptors or NO synthase inhibition had no effect on infarct size in hearts from saline-treated rats.
The CB1 receptor antagonist SR 141716A did not alter the ability of LPS treatment to reduce infarct size. In contrast, both the CB2 receptor antagonist, SR 144528 and the NO synthase inhibitor, NNLA antagonized the infarct size limitation due to LPS treatment. The possible contribution of cannabinoids in the cardioprotective effect of NO was investigated. As shown in Figure 1B, SNP perfusion significantly decreased the infarct size. Although this cardioprotective effect was still observed in hearts perfused with SR 141716A, it was inhibited in hearts perfused with SR 144528.
Functional recovery
As shown in Figure 2A, B, a 90-min low-flow ischemia was accompanied by a marked reduction in dP/dt in all hearts. Most hearts from saline-treated rats did not recover on reperfusion. In contrast, functional ventricular recovery was greatly improved in hearts from LPS-treated rats. The improved recovery in LPS-treated hearts was not altered by SR 141716A perfusion, and was abolished by SR 144528 as well as NNLA perfusion. A concordance between infarct size and functional recovery was also observed with SNP : Perfusion of SR 144528, but not SR 141716A abolished the beneficial effect of the NO donor.
Figure 2.
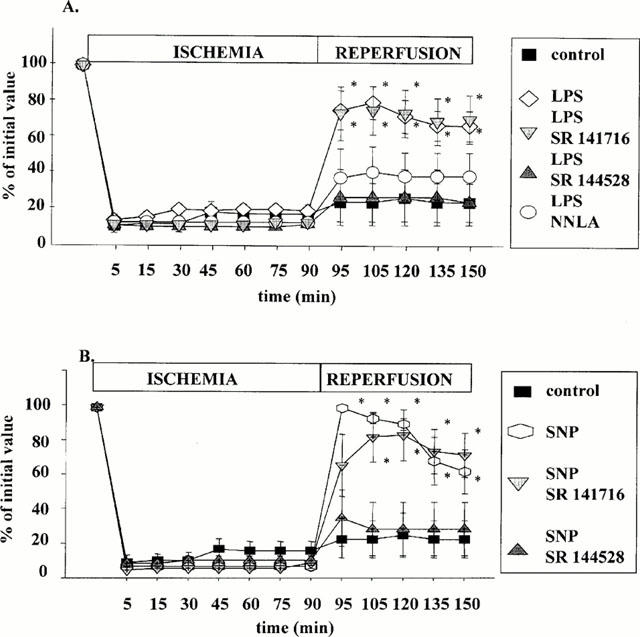
Functional recovery assessed by the percentage of maximum dP/dt from its pre-ischeamic value, during low-flow ischaemia (0.6 ml min−1, 90 min)-reperfusion (60 min) sequence. (A) Control or endotoxic hearts (LPS 10 μg kg−1, i.v, 24 h earlier) are perfused or not with either SR 141716A (1 μM), SR 144528 (1 μM), or NNLA (30 μM). (B) Control hearts are perfused or not with SNP, in absence or in presence of either SR 141716A (1 μM), or SR 144528 (1 μM). Drug perfusions are initiated 5 min before ischaemia and maintained during the entire ischaemic period. Values are mean±s.e.mean. *P<0.05 vs control group.
Discussion
In the present study, we have evaluated whether antagonism of cannabinoid receptors could modulate the beneficial effects induced by pre-treatment with LPS, a well-known trigger of a cardioprotective response. We have also evaluated the effects of exogenous NO perfusion. We observed that the cardioprotection induced by pre-treatment with LPS was abolished by a CB2 receptor antagonist but not by a CB1 receptor antagonist. Similarly, we observed that the CB2-receptor antagonist abolished the cardioprotective effect observed with sodium nitroprusside, a NO donor, whereas the CB1 receptor antagonist had no effect.
It now appears that a functional endocannabinoid system exists in the myocardium. Firstly, both CB1 and CB2 transcripts were found to be expressed in the heart and in other peripheral tissues (Galliegues et al., 1995). Secondly, a new sensitive technique allowed the detection of the two endocannabinoids isolated so far, namely anandamide and 2-arachidonoylglycerol in the heart (Schmid et al., 2000). Finally, these compounds exert a variety of regulatory functions, including cardiovascular ones. For example, many studies reported, in different models that exogenous cannabinoids inhibit noradrenaline release, via the interaction with pre-synaptic CB1 receptors (Molderings et al., 1999; Niederhoffer > Szabo 2000). Numerous studies also revealed non-neuronal locations, such as vascular smooth muscle (Gebremedhin et al., 1999) or endothelial (Liu et al., 2000) cells.
In addition to all of these observations, the pronounced hypotension in response to cannabinoid injection (Lake et al., 1997), suggests an involvement of endocannabinoids in pathological situations associated with extreme hypotension, such as endotoxic shock, a potentially lethal failure of multiple organs that is initiated by LPS.
It was demonstrated in the rat that in response to LPS treatment, platelets and macrophages generate different endogenous cannabinoids, that may be paracrine mediators of endotoxin-induced hypotension via the activation of vascular CB1 receptors (Varga et al., 1998).
Our results suggest that the LPS-induced effect against myocardial ischaemia involves endocannabinoids that may contribute to the cardioprotection via the activation of CB2 receptors.
Our observations may seen to contradict the ability of endocannabinoids to modulate the inflammatory processes by favouring anti-inflammatory cytokines to the detriment of pro-inflammatory ones. A recent study reported that in the tumour-bearing mouse, treatment with delta9-tetrahydrocannabinol down-regulated INFγ and increased anti-inflammatory IL-10 and TGFβ activity. This modulatory effect of exogenous cannabinoids was blocked by SR 144528, indicating a role for CB2 receptors (Zhu et al., 2000). In a previous study (Berdyshev et al., 1998) it was reported that delta9-tetrahydrocannabinol decreased the levels of TNFα in a murine model of endotoxin-induced pulmonary inflammation. Finally, several studies have demonstrated the involvement of CB1 receptor in the modulation of the cytokine network by cannabinoid agonists (Schmid et al., 2000).
However, results obtained in hearts perfused with sodium nitroprusside suggest a functional relationship between endocannabinoids and NO, already reported in several studies and are thought to be dependent on either CB1 (Waksman et al., 1999) or CB2 receptor-stimulation. For example, it was recently reported that in endotoxin- or cytokine stimulated brain mesanglial cells, release of NO, another pro-inflammatory mediator, is inhibited by CB1 receptor agonists (Waksman et al., 1999). It was also shown that the cannabinomimetic WIN55212 inhibits NO production in macrophages stimulated by LPS through an interaction with CB2 receptors (Ross et al., 2000).
NO has often been described as a biological ‘double-edged sword' with established roles in cellular protection and also, when excessively released, in cytotoxicity. This double status could be reflected in our results. We could argue that LPS pre-treatment or perfusion with a NO donor leads to an amount of NO associated with beneficial effects leading to myocardial cytoprotection. In hearts treated with SR 144528, a detrimental and cytotoxic higher amount of NO could be produced, resulting from the blockade of the anti-inflammatory action of cannabinoids.
In conclusion, our data suggest, that endocannabinoid, acting through CB2 receptors, are involved in the cardioprotective effects of LPS against ischaemia in the rat heart. A relationship between cannabinoids and NO does exist, although the precise mechanisms of such a relation remain to be clarified.
Abbreviations
- LPS
lipopolysaccharide
- NNLA
Nω-nitro-L-arginine
- SR 141716
(N-(piperidin-1-yl)-5-(4-(chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- SR 144528
(N-([1s]-endo-1,3,3-trimethylbicyclo[2,2,1]heptan-2-yl)-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
References
- BERDYSHEV E., BOICHOT E., CORBEL M., GERMAIN N., LAGENTE V. Effects of cannabinoid receptor ligands on LPS-induced pulmonary inflammation in mice. Life Sci. 1998;63:PL125–129. doi: 10.1016/s0024-3205(98)00324-5. [DOI] [PubMed] [Google Scholar]
- BROWN J.M., GROSSO M.A., TERADA L.S., WHITMAN G.J., BANERJEE A., WHITE C.W., HARKEN A.H., REPINE J.E. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2516–2520. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., CAMPBELL W.B., HILLARD C.J., HARDER D.R. Cannabinoid CB1 receptor of cat cerebral arteriol muscle funtions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- GALIEGUE S., MARY S., MARCHAND J., DUSSOSSOY D., CARRIERE D., CARAYON P., BOUABOULA M., SHIRE D., LE FUR G., CASELLAS P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- JEON Y.J., YANG K.H., PULASKI J.T., KAMINSKI N.E. Attenuation of inducible nitric oxide synthase gene expression by delta 9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor-kappa B/Rel activation. Mol. Pharmacol. 1996;50:334–341. [PubMed] [Google Scholar]
- LAKE K.D., COMPTON D.R., VARGA K., MARTIN B.R., KUNOS G. Cannabinoid-induced hypotension and bradycardia in rats mediated by CB1-like cannabinoid receptors. J. Pharmacol. Exp. Ther. 1997;281:1030–1037. [PubMed] [Google Scholar]
- LIU J., GAO B., MIRSHAHI F., SANYAL A.J., KHANOLKAR A.D., MAKRIYANNIS A., KUNOS G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000;15:835–840. [PMC free article] [PubMed] [Google Scholar]
- MOLDERINGS G.J., LIKUNGU J., GOTHERT M. Presynaptic cannabinoid and imidazoline receptors in the human heart and their potential relationship. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360:157–164. doi: 10.1007/s002109900043. [DOI] [PubMed] [Google Scholar]
- NIEDERHOFFER N., SZABO B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- PIOMELLI D., GIUFFRIDA A., CALIGNANO A., RODRÍGUEZ D.E., FONSECA F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol. Sc. 2000;21:218–223. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;22:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.C., LE FUR G.L. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., PERTWEE R.G. Inhibition of nitric oxide production in RAW264.7 macrophages by cannabinoid and palmitoyl-ethanolamide. Eur. J. Pharmacol. 2000;401:121–130. doi: 10.1016/s0014-2999(00)00437-4. [DOI] [PubMed] [Google Scholar]
- SCHMID P.C., SCHWARTZ K.D., SMITH C.N., KREBSBACH R.J., BERDYSHEV E.V., SCHMID H.H. A sensitive endocannabinoid assay. The simultaneous analysis of N-acylethanolamines and 2-monoacylglycerols. Chem. Phys. Lipids. 2000;104:185–191. doi: 10.1016/s0009-3084(99)00124-3. [DOI] [PubMed] [Google Scholar]
- SMITH S.R., TERMINELLI C., DENHARDT G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharmacol. Exp. Ther. 2000;293:136–150. [PubMed] [Google Scholar]
- VARGA K., WAGNER J.A., BRIDGEN D.T., KUNOS G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- WAKSMAN Y., OLSON J.M., CARLISLE S.J., CABRAL G.A. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J. Pharmacol. Exp. Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- YANG B.C., CHEN L.Y., SALDEEN T.G., MEHTA J.L. Reperfusion injury in the endotoxin-treated rat heart: reevaluation of the role of nitric oxide. Br. J. Pharmacol. 1997;120:305–311. doi: 10.1038/sj.bjp.0700891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU L.X., SHARMA S., STOLINA M., GARDNER B., ROTH M.D., TASHKIN D.P., DUBINETT S.M. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. 2000;165:373–380. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]


