Abstract
Prostaglandin E2 (PGE2) increased adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP) formation in tracheal epithelial cells and concomitantly decreased the production/secretion of immunoreactive endothelin (irET).
Naturally occurring prostanoids and selective and non-selective EP receptor agonists showed the following rank order of potency in stimulating cyclic AMP generation by epithelial cells: PGE2 (EP-selective)>16,16-dimethyl PGE2 (EP-selective)>11-deoxy PGE2 (EP-selective)>>>iloprost (IP/EP1/EP3-selective), butaprost (EP2-selective), PGD2 (DP-selective), PGF2α (FP-selective). The lack of responsiveness of the latter prostanoids indicated that the prostanoid receptor present in these cells is not of the DP, FP, IP, EP1, EP2 or EP3 subtype.
Pre-incubating the cells with the selective TP/EP4-receptor antagonists AH23848B and AH22921X antagonized the PGE2-evoked cyclic AMP generation. This suggested that EP4 receptors mediate PGE2 effects. However, in addition to any antagonistic effects at EP4-receptors, both compounds, to a different extent, modified cyclic AMP metabolism. The selective EP1, DP and EP2 receptor antagonist (AH6809) failed to inhibit PGE2-evoked cyclic AMP generation which confirmed that the EP2 receptor subtype did not contribute to the change in cyclic AMP formation in these cells.
The PGE2-induced inhibition of irET production by guinea-pig tracheal epithelial cells was due to cyclic AMP generation and activation of the cyclic AMP-dependent protein kinase since this effect was reverted by the cyclic AMP antagonist Rp-cAMPS.
These results provide the first evidence supporting the existence of a functional prostaglandin E2 receptor that shares the pharmacological features of the EP4-receptor subtype in guinea-pig tracheal epithelial cells. These receptors modulate cyclic AMP formation as well as ET-1 production/secretion in these cells.
Keywords: Prostaglandin E2, cyclic AMP, endothelin-1, EP4-receptor, prostanoid receptors, tracheal epithelial cells
Introduction
Airway epithelial cells have been shown to produce endothelin-1 (ET-1) (Laporte et al., 1996; Pelletier et al., 1998; Yang et al., 1997), which has several effects on the airways such as broncho- and tracheoconstriction (Boichot et al., 1991; Inui et al., 1994; Uchida et al., 1988) and is also an important mediator of pulmonary hypertension (Horgan et al., 1991). In addition to its constrictor effect, ET-1 stimulates epithelial cell growth and the synthesis of various autacoids including prostaglandins (Ninomiya et al., 1992; Murlas et al., 1995; Takimoto et al., 1996). On the other hand, ET-1 synthesis and release are stimulated in response to pro-inflammatory, mitogenic and hypertrophic as well as constrictor mediators (Endo et al., 1992; Yang et al., 1997b; Samransamruajkit et al., 2000). By contrast, basal and stimulated ET-1 production has been shown to be inhibited by vaso- and bronchorelaxing hormones or autacoids, namely adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP)-elevating agents, such as adenosine, β2-adrenoceptor agonists and forskolin, and the cyclic AMP analogue 8-Br-cyclic AMP (Yang et al., 1997a).
Prostaglandin E2 is a potent modulator of the immune system and a major mediator of inflammation (Goodwin & Ceuppens, 1983). PGE2 was shown to relax bronchial smooth muscle and to reduce mucus secretion (Goodwin & Ceuppens, 1983; Marom et al., 1981; Sweatman & Collier, 1968; Walters & Davies, 1982). It was suggested that PGE2 could produce its broncho- and tracheoprotective effect through at least two distinct pathways. First, through relaxation of bronchi or trachea by acting directly on smooth muscle cells (Walters & Davies, 1982) and, secondly, through inhibition of bronchoconstrictor mediator release and/or synthesis by mast cells and other cell populations of the airway (Thien & Walters, 1995). As a consequence of PGE2 stimulation, human airway epithelial cells increase their content of intracellular cyclic AMP (Penn et al., 1994). This observation, combined with the previous one indicating that cyclic AMP elevating agents inhibit ET-1 production by tracheal epithelial cells, suggested that PGE2 could regulate the production/secretion of ET-1 through activation of a Gs-coupled receptor and then contribute to a negative feedback regulation loop on ET-1 synthesis.
PGE2 binds to and activates multiple receptors, namely EP1, EP2, EP3 and EP4 (Coleman et al., 1994a,1994b. These receptors are members of a large family of receptors and have been classified on the basis of their sensitivity to the five naturally occurring prostanoids. These are TP for thromboxane A2 (TxA2), DP for PGD2, EP for PGE2, FP for PGF2α and IP for PGI2 or prostacyclin (Kennedy et al., 1982). The development of selective agonists and antagonists led to the identification of the four isoforms of EP receptors. The EP1-receptor subtype was shown to be a seven transmembrane domain receptor coupled to phosphoinositide turnover and intracellular Ca2+ mobilization through a regulatory Gq protein (Coleman & Kennedy, 1985; Creese & Denborough, 1981). The EP2- and EP4-subtypes are also seven transmembrane domain receptors coupled to a regulatory Gs protein and activate adenylyl cyclase which increases cyclic AMP formation (De Vries et al., 1995; Honda et al., 1993; Jumblatt & Paterson, 1991; Nishigaki et al., 1995; de Brum-Fernandes et al., 1996). The seven transmembrane domain EP3-receptor subtype is expressed as multiple isoforms as a result of alternative splicings (Breyer et al., 1994; Irie et al., 1993; Jon et al., 1997; Negishi et al., 1993). These isoforms, which expressed the same rank order of potency and affinity for the different agonists, were also shown to couple different heterotrimeric G proteins leading to stimulation of various second messenger pathways (Namba et al., 1993).
EP1 receptors were characterized mainly by the use of selective agonists because of the lack of selectivity of receptor antagonists (Coleman et al., 1994a). Similarly, there are numerous EP2-selective agonists, such as butaprost, but no highly selective antagonists are yet available (Gardiner, 1986; Nials et al., 1993). The same applies for the EP3-receptor subtype (Coleman et al., 1994). As far as the EP4-receptor subtype is concerned, there are no selective agonists available but there are selective antagonists such as AH23848B and AH22921X, which allow discrimination between EP receptor subtypes (Coleman et al., 1994a,1994b;). EP-receptor subtypes can be distinguished on the basis of PGE2 action on second messenger pathways (cyclic AMP formation, phosphoinositide turnover and calcium) combined to the rank order of agonist and antagonist potencies. Since the action of PGE2 on prostanoid receptors could be mediated by the activation of any of the prostanoid receptor subtypes, the pharmacological characteristics of the other prostanoid receptor subtypes have to be taken into account. IP and DP receptors were shown to be coupled to a regulatory Gs protein thus activating adenylyl cyclase and increasing cyclic AMP formation (Hashimoto et al., 1990; Ito et al., 1990, 1992; Siegl et al., 1979; Simon et al., 1980). As for the FP and TP receptors, their signalling pathways involve a regulatory Gq protein (Knezevic et al., 1993; Shenker et al., 1991) and the activation of phospholipase C (Brass et al., 1987).
In the present study, the effects of PGE2 on the cyclic AMP generation and on the production/secretion of irET by tracheal epithelial cells were evaluated. A pharmacological characterization of the prostanoid receptor subtype responsible for the PGE2 action was also conducted using selective and non-selective receptor agonists and antagonists on the generation of cyclic AMP.
Methods
Animals
Male Dunkin Hartley guinea-pigs (300–350 g) were obtained from Charles River Laboratory (St Constant, Québec, Canada). The animals were killed by cervical dislocation according to the guidelines of the Canadian Council on Animal Care. The trachea was harvested under sterile conditions and dissected in Krebs–Henseleit physiological solution.
Isolation and cell culture
Tracheal epithelial cells were obtained following a 1 h incubation of the trachea at 37°C with a solution of 0.15% protease type XXIV in Krebs–Henseleit buffer, according to a previously described procedure (White et al., 1993). The cells were then mechanically removed from the mucosal surface of the trachea by gentle scraping with a rubber policeman. They were centrifuged and washed twice with 5 ml of culture medium, DMEM-F12, containing 10% (v v−1) foetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (0.08% w v−1) and fungizone (1% w v−1). The cells were resuspended in 10 ml medium, counted and seeded at a concentration of 4–5×105 cells/ml/well in 24-well culture plates. Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2. Cells were cultured for 72 h in the presence and thereafter 48 h in absence of medium before experiments were conducted. All experiments used confluent cells.
ET-1 production/secretion
Before the experiments, the DMEM-F12 medium was aspirated from the 24-well plates and 1ml of fresh medium was added. The cells were incubated with selected pharmacological agents at 37°C in a humidified atmosphere of 5% CO2 for 24 h. At the end of the incubation period, the culture media were collected and stored at −20°C until measurement of immunoreactive-ETs (irETs). Triton X-100 (0.1% v v−1; 0.5 ml) was added to each well and, following an overnight incubation at 4°C, total proteins were assayed using the Bio-Rad protein assay.
Measurement of immunoreactive-ET by radioimmunoassay
The concentrations of irET were measured by radioimmunoassay (RIA) using a commercially available kit (RPA 555; Amersham, Ontario, Canada), as previously described (Laporte et al., 1996). The amount of each aliquot was expressed in picograms per milligram of total proteins.
Cyclic AMP formation
Intracellular cyclic AMP levels were determined by measuring the conversion of [3H]-ATP into [3H]-cyclic AMP as described by Salomon (1979). Briefly, after 5 days of culture in 24-well plates, cells were washed and incubated for 2 h in DMEM-F12 medium with 2 μCi [3H]-adenine/ml. The cells were washed twice with HBSS-glucose and pre-incubated for 15 min in a solution of HBSS-BSA containing 10 μM rolipram (a selective type 4 phosphodiesterase inhibitor) in the presence or absence of selected antagonists and inhibitors. PGE2 and congeners were then added to the incubation medium for various periods of time at 37°C. The reaction was ended by the addition of 100 μl of ice-cold perchloric acid (50% v v−1, final concentration of 5%) and by putting the 24-well plates on ice. Cells were scraped with a rubber policeman and 100 μl of ice-cold solution of ATP and cyclic AMP (5 mM of each) was added to the cellular extract. The samples were vortexed and centrifuged for 15 min at 3000 r.p.m. and 4°C. The supernatants were sequentially chromatographed on Dowex and alumina columns allowing the separation of [3H]-ATP from [3H]-cyclic AMP. Cyclic AMP formation was expressed as per cent conversion of [3H]-ATP to [3H]-cyclic AMP and calculated using the following formula ([3H]-cAMP/([3H]-ATP+[3H]-cAMP))×100. Results are expressed as per cent response to 10 μM PGE2, unless stated otherwise.
Protein assay
Guinea-pig tracheal epithelial cells in each well were disrupted overnight with 0.1% Triton X-100. A 10 μl aliquot was mixed with 200 μl Bio-Rad protein assay reagent and incubated for 15 min at room temperature to evaluate the concentration of cell proteins. The concentration of proteins was determined by measuring the absorbance at 590 nm, using a standard curve of bovine serum albumin (25–400 μg ml−1).
Chemicals and drugs
The following chemicals and drugs were used: culture medium, serum and antibiotics (penicillin, streptomycin and fungizone) (Gibco, New York, U.S.A.); protease type XXIV, ATP, cyclic AMP, bovine serum albumin, imidazol, adenosine and alumina (Sigma Chemical Co., St Louis, MO, U.S.A.); the Bio-Rad protein assay kit, Dowex 1X8 (100–200 mesh) (Bio-Rad, Mississauga, Canada); [3H]-adenine, endothelin radioimmunoassay kits (RPA 555) (Amersham, Oakville, Ontario, Canada); butaprost (Dr Harold Kluender, Bayer Corporation, West Haven, CT, U.S.A); AH22921X ([1α(Z),2β,5α]-(±)-7-[5-[[(1,1′-biphenyl)-4-yl] methoxy] -2- (4 -morpholinyl) -3- oxocyclopentyl] -5-heptenoic acid), AH6809 (6-isopropoxy-9-oxoxanthine-2-carboxylic acid) and AH23848B ([1α(Z),2β,5α]-(±)-7-[5-[[(1,1′-biphenyl)-4-yl] methoxy]-2- (4-morpholinyl) -3-oxocyclopentyl] 4-heptenoic acid) (Dr R.A. Coleman, Glaxo R&D Ltd, Hertfordshire, U.K.); PGE2, D2, F2α and iloprost (Cayman Chemicals, Ann Arbor, MI, U.S.A.); rolipram (RBI, Research Biochemicals International, Natik, CA, U.S.A.); Rp-cAMPS: (Rp)-adenosine cyclic 3′,5′-phosphorothioate (Calbiochem, San Diego, CA, U.S.A.)
Statistical analysis
The results are expressed as the mean±s.e.mean. The degree of significance of differences between experimental groups was performed by ANOVA followed by Dunnett or Bonferroni post-test analysis using Graph Pad Software version 2.01. P values less than 5% were considered significant.
Results
Effect of prostaglandin E2 on cyclic AMP formation by tracheal epithelial cells
PGE2 (1 μM) significantly stimulated (4 fold) the conversion of ATP to cyclic AMP by cultured guinea-pig tracheal epithelial cells during a 5 min incubation period compared to the basal formation. Longer incubation times such as 15, 30 and 60 min in the presence of PGE2 (1 μM) led to 4.3, 5.7 and 11.4 fold increment in conversion of ATP to cyclic AMP (Figure 1A). Stimulation of the cells with increasing concentrations of PGE2 (0.01 to 100 μM) produced a concentration-dependent increase in the conversion of ATP to cyclic AMP and the maximal effect was observed using the concentration of 100 μM, where the values reached 5.03±0.90% (Figure 1B). Subsequent experiments using selected agonists and antagonists were performed at 15 min and expressed as the per cent of the response obtained by the stimulation with 10 μM PGE2 (100%) to minimize the variability between experiments.
Figure 1.
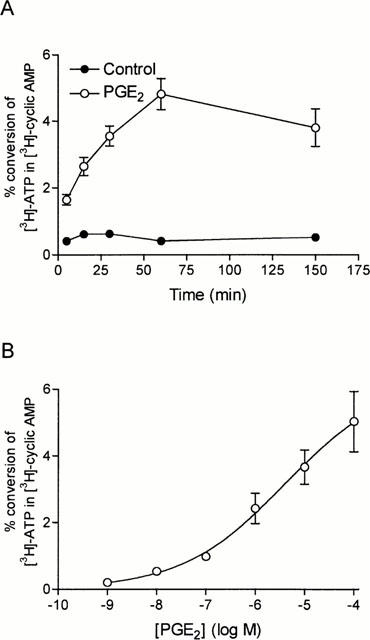
Concentration- and time-dependent effect of PGE2 on cyclic AMP formation in guinea-pig tracheal epithelial cells. (A) Cells were pre-treated for 15 min with rolipram (10 μM) and incubated in the presence (open circles) or the absence (solid circles) of PGE2 (1 μM) for 5, 15, 30, 60 and 150 min. The conversion of [3H]-ATP to [3H]-cyclic AMP was assayed as described in Methods. (B) Cells were pre-treated for 15 min with rolipram (10 μM) and then incubated for 15 min with increasing concentrations of PGE2 (1 nM to 100 μM). The points are the means±s.e.mean of four determinations made with separate cell preparations.
Effect of naturally occurring prostaglandins and iloprost on cyclic AMP formation
In contrast to the results obtained with PGE2, iloprost, PGD2 and PGF2α (0.1–10 μM) did not stimulate any conversion of ATP to cyclic AMP by guinea-pig tracheal epithelial cells (Figure 2).
Figure 2.
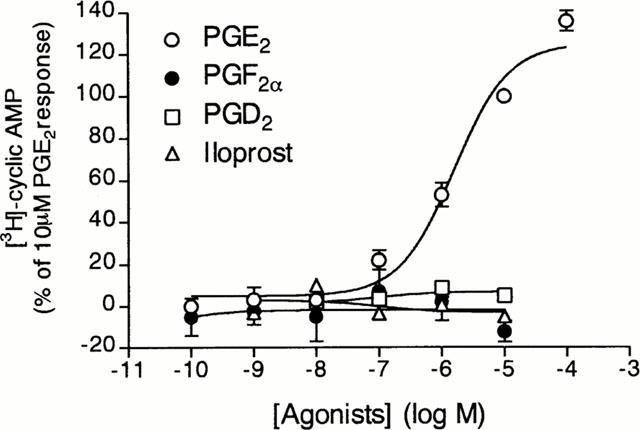
Effects of PGE2, PGD2, PGF2α and iloprost on cyclic AMP formation by guinea-pig tracheal epithelial cells. Cells were pre-incubated for 15 min with rolipram (10 μM) and thereafter incubated with increasing concentrations of PGE2 (open circle), PGD2 (open squares), iloprost (solid circles) or PGF2α(open triangles). The conversion of [3H]-ATP to [3H]-cyclic AMP was assayed as described in Methods. The points are the means±s.e.mean of four determinations made with separate cell preparations.
Effect of selective and non-selective prostanoid-receptor agonists on cyclic AMP generation
To identify the EP receptor subtype that mediates cyclic AMP generation in tracheal epithelial cells, four PGE2 analogues with different affinities for the various EP receptors subtype were tested. PGE2 was the most potent agonist for increasing cyclic AMP in the cells, followed by the non-selective EP receptor agonists 16,16-dimethyl PGE2 and 11-deoxy PGE2, whereas the selective EP2 receptor agonist, butaprost, failed to show a stimulatory effect (Figure 3).
Figure 3.
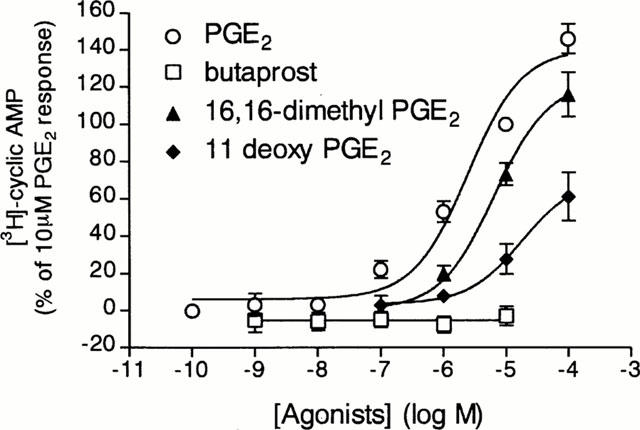
Effects of selective prostaglandin EP receptor agonists on cyclic AMP generation by guinea-pig tracheal epithelial cells. Cells were pre-incubated for 15 min with rolipram (10 μM) and thereafter incubated with increasing concentrations of PGE2 (open circles), 16,16-dimethyl PGE2 (solid triangles), 11-deoxy PGE2 (solid diamonds) or butaprost (open squares). The conversion of [3H]-ATP to [3H]-cyclic AMP was assayed as described in Methods. The points are the mean±s.e.mean of 4–8 determinations made with separate cell preparations.
Effect of selective EP4-receptor antagonists on cyclic AMP generation
The preincubation of tracheal epithelial cells with a selective EP4-receptor subtype antagonist, AH23848B (10 and 100 μM) (Coleman et al., 1994a,1994b), shifted the concentration–response curve elicited by low concentrations of PGE2 to the right (Figure 4A). In contrast, in the presence of high concentrations of PGE2 (10 and 100 μM), AH23848B did not show any antagonist activity. In fact, AH23848B seemed to act synergistically with PGE2 to increase cyclic AMP formation. However, to support the suggestion that PGE2 stimulated the cyclic AMP generation through the activation of the EP4-receptor subtype, the effect of another EP4-receptor antagonist (AH22921X) (Coleman et al., 1994a,1994b) was investigated. In the presence of 10, 50 and 100 μM AH22921X, basal levels of cyclic AMP were not reduced. The concentration–response curves for PGE2-induced cyclic AMP accumulation were shifted to the right by AH22921X in a concentration dependent manner (Figure 4B). Shild plots were not constructed since the Emax values of the concentration–response curves of PGE2 in the absence or in the presence of the antagonist were not reached.
Figure 4.
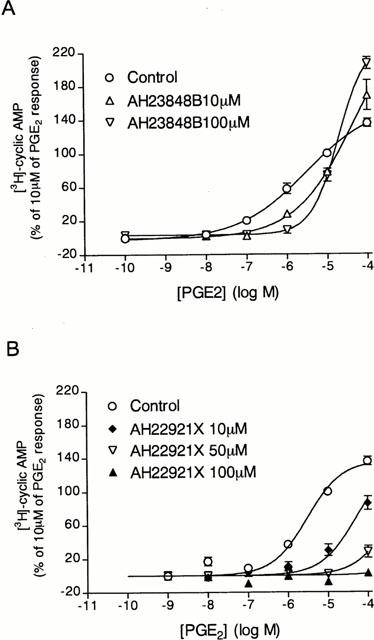
Effect of AH23848B on PGE2-induced cyclic AMP formation by tracheal epithelial cells. Cells were pre-incubated for 15 min with rolipram (10 μM) plus (A) AH23848B (10 μM (open triangles) or 100 μM (solid triangles)) or medium only (open circles), or (B) AH22921X (10 μM (solid diamonds), 50 μM (open triangles) or 100 μM (solid triangles)) or medium only (open circles) and then incubated for a further 15 min with increasing concentrations of PGE2 (A and B). The conversion of [3H]-ATP to [3H]-cyclic AMP was assayed as described in Methods. The points represents the mean±s.e.mean of four determinations made with separate cell preparations.
Specificity of action of AH23848B and AH22921X
In an attempt to define the specificity of action of AH23848B and AH22921X, their effects on forskolin- and isoprenaline-stimulated cyclic AMP formation were evaluated. Neither compound showed any antagonistic activity against forskolin- or isoprenaline-induced cyclic AMP formation (Figure 5A,B). Surprisingly, AH23848B (100 μM) potentiated forskolin-induced (Figure 5A) but not isoprenaline-induced cyclic AMP formation (Figure 5B). AH22912X slightly potentiated forskolin- but not isoprenaline-induced cyclic AMP formation. AH23848B (100 μM) increased by 3 fold the cyclic AMP generation induced by forskolin (100 μM), whereas AH22921X (100 μM) increased it by 1.7 fold (Figure 5B).
Figure 5.
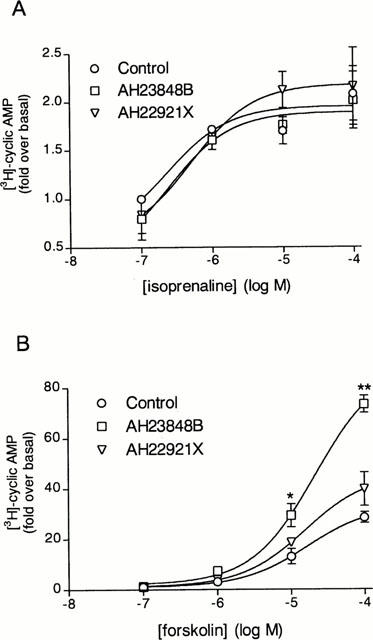
Effects of AH23848B and AH22921X on isoprenaline- and forskolin-induced cyclic AMP generation. Cells were incubated with rolipram (10 μM) in the presence or absence of AH23848B (100 μM) or AH22921X (100 μM) for 15 min and then stimulated for a further 15 min with or without (A) isoprenaline (0.1–100 μM) or (B) forskolin (0.1–100 μM). The conversion of [3H]-ATP to [3H]-cyclic AMP was assayed as described in Methods. The point represents the mean±s.e.mean of four determinations made with separate cell preparations.
Effect of AH6809 on the generation of cyclic AMP evoked by PGE2
Since PGD2 and iloprost (EP1/EP3/IP receptor agonist) failed to increase cyclic AMP generation in tracheal epithelial cells, AH6809, a DP-, EP1-and EP2-receptor antagonist (Coleman et al., 1987; Keery & Lumley, 1988; Woodward et al., 1995) was used to evaluate the involvement of EP2-receptors in PGE2-evoked cyclic AMP generation. Treatment of tracheal epithelial cells with 10 μM AH6809 (Woodward et al., 1995) changed neither basal nor PGE2-induced cyclic AMP formation (Figure 6). Conversely, AH6809 slightly potentiated the stimulatory effect of PGE2 on the cyclic AMP generation in these cells. However, the leftward shift in the concentration–response curve produced by PGE2 in presence of AH6809 was not significantly different from the concentration–response curve obtained in absence of this compound.
Figure 6.
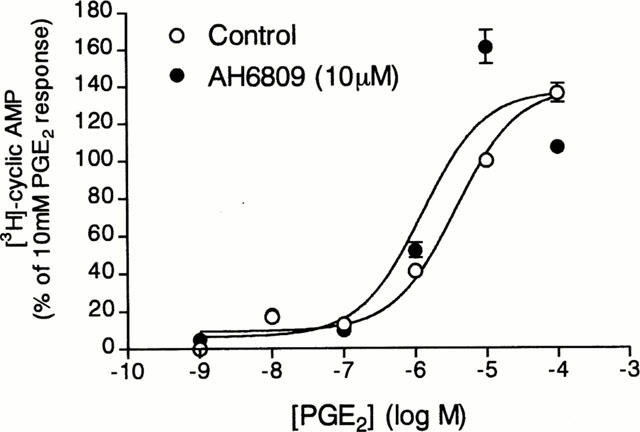
Effect of AH6809, an antagonist at prostanoid EP1, EP2 and DP receptors, on the cyclic AMP generation evoked by PGE2. Cells were pre-incubated for 15 min in the presence of AH6809 (10 μM) and rolipram (10 μM) (solid circles) or in the presence of rolipram alone (10 μM, open circles) and then incubated for a further 15 min with increasing concentrations of PGE2. The conversion of [3H]-ATP to [3H]-cyclic AMP was assayed as described in Methods. The points represents the mean±s.e.mean of eight determinations made in different cell preparations.
Effect of PGE2 on irET production/secretion by tracheal epithelial cells
PGE2 (0.01–100 μM) caused concentration-dependent inhibition of the basal production/secretion of irET from cultured tracheal epithelial cells in a 24 h incubation period (Figure 7). Significant inhibitory effects (35, 44 and 41% from basal levels) were observed at concentrations ranging from 1 to 100 μM.
Figure 7.
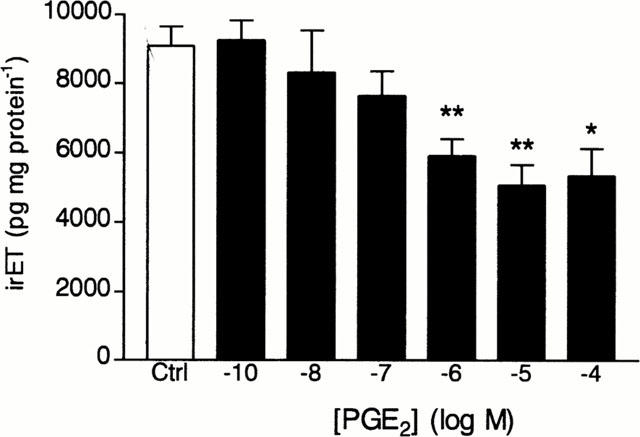
Effect of PGE2 on the production of immunoreactive ET-1 (irET) by tracheal epithelial cells. Cultured cells were incubated for 24 h with PGE2 (10−10 to 10−4 M) or with the medium alone (Control) and irET was measured as described in Methods. Each bar represents the mean±s.e.mean of 4–12 determinations made with separate cell preparations (*P<0.05 and **P<0.01).
Effect of Rp-cAMPS on PGE2- and adenosine-evoked inhibition of irET production/secretion
To confirm that EP4 receptors, which mediate PGE2-evoked cyclic AMP generation, are also responsible for the inhibition of irET production/secretion by PGE2, we evaluated the effects of AH23848B and AH22921X on basal and PGE2-inhibited production/secretion of the peptide. Unfortunately, AH23848B and AH22921X, like PGE2, inhibited basal irET production (data not shown). Another approach was then used to evaluate EP4 receptor involvement in mediating PGE2-inhibition of irET production/secretion. Since the activation of EP4 receptors led to an increase in cyclic AMP generation, the role of this second messenger in mediating inhibition of irET production/secretion by PGE2 was evaluated. Rp-cAMPS, a cyclic AMP antagonist which blocks the activation of the cyclic AMP-dependent protein kinase (Schaap et al., 1993), reversed the inhibitory effect of PGE2 (Figure 8A). The effect of Rp-cAMPS was also tested on the inhibitory effect of adenosine which also activate Gs-coupled receptors in tracheal epithelial cells (Pelletier et al., 2000). As observed with PGE2, Rp-cAMPS reversed the inhibitory effect of adenosine on irET production secretion (Figure 8B).
Figure 8.
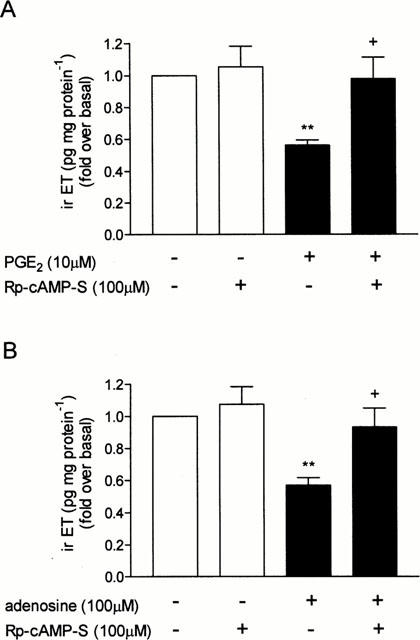
Effect of Rp-cAMPS on the inhibitory effect of Gs-coupled receptor activation on irET production/secretion. Cells were pre-treated for 1 h with Rp-cAMPS (100 μM) or with the medium alone and then stimulated with (A) PGE2 (1 μM) or (B) adenosine, and then incubated for 24 h at 37°C. IrET was measured as described in Methods. Each bar represents the mean±s.e.mean of six determinations made with separate cell preparations (**P<0.01 vs basal level and +P<0.01 vs PGE2 or adenosine).
Discussion
The results presented here show that PGE2 increases cyclic AMP generation in a concentration-dependent manner in guinea-pig tracheal epithelial cells whereas PGI2, PGF2α and PGD2 were ineffective. The rank order of potency of the naturally occurring prostanoids was: PGE2>>>PGI2, PGD2, PGF2α. Based on prostanoid receptor classification (Coleman et al., 1994b), these results suggest the presence of an EP receptor subtype responsible for the increase in cyclic AMP formation by tracheal epithelial cells. To date, two EP receptor subtypes were shown to be coupled to a regulatory Gs protein and to activate adenylyl cyclase. These two subtypes are classified as EP2 and EP4 (Hardcastle et al., 1982; Jumblatt & Paterson, 1991; Nishigaki et al., 1995). In an attempt to characterize the receptor subtype present on guinea-pig airway epithelium, four non-selective and selective agonists of the EP receptor subtypes were tested on cyclic AMP generation. The rank order of PGE2 analogues in stimulating cyclic AMP generation was PGE2>16,16-dimethyl PGE2>11-deoxy PGE1. Butaprost, a selective EP2-receptor agonist, and iloprost, a selective EP1/EP3/IP receptor agonist failed to increase cyclic AMP generation, which suggested that EP1, EP3, IP and EP2 receptors are not present or coupled to cyclic AMP generation in tracheal epithelial cells. When the cells were incubated with the non-selective EP-receptor agonists, 16,16-dimethyl PGE2 and 11-deoxy PGE1, the results showed that 16,16-dimethyl PGE2 was 10 times less potent than PGE2 but appeared to be more potent than 11-deoxy PGE1 in stimulating cyclic AMP generation. These results are different from those observed in a ligand binding assay with the mouse EP4 receptor expressed in CHO cells where 16,16-dimethyl PGE2 had a lower affinity than 11-deoxy PGE1 (Kiriyama et al., 1997). This can be explained by the fact that potency is a function of both affinity and efficacy and there is no direct relationship between affinity and potency. Therefore, the value of comparisons of ligand binding studies and functional assays is limited. In bovine chondrocytes, 11-deoxy PGE1 stimulated the cyclic AMP generation in a concentration–dependent manner and was 10–100 times less potent than PGE2 (de Brum-Fernandes et al., 1996) as observed in our study. However, 16,16-dimethyl PGE2 was not tested on these cell preparations. Species differences could also explain the discrepant results.
Analysis of the agonist profile suggested that the receptor expressed in tracheal epithelial cells was the EP4-receptor subtype. The antagonist profile suggested a similar conclusion. Both selective EP4-receptor antagonists used, AH23848B and AH22921X, inhibited in a concentration–dependent (AH22921X) or independent manner (AH23848B, see below) the cyclic AMP generation evoked by PGE2 in tracheal epithelial cells. Since the maximal effect was not observed in the absence and in the presence of AH22921X, the Schild slope was not calculated and the nature of its antagonistic effect was not defined. Coleman et al. (1994a) showed that these two antagonists (AH23848B and AH22921X) have similar pA2 values at EP4 receptors. However, in our study, pA2 values were very different: AH22921X was much more potent than AH23848B and showed a clear concentration-related inhibition of PGE2-induced cyclic AMP formation whereas AH23848B did not. This discrepancy could have been explained by the agonist activity of AH23848B observed in many EP4-receptor preparations (Lydford et al., 1996; Meja et al., 1997; Wise, 1998). However, in tracheal epithelial cells, AH23848B (10–100 μM) did not show any agonist activity on cyclic AMP accumulation. It acted in synergy with PGE2 only when high concentrations of PGE2 (10–100 μM) were used (Figure 4A) or possibly when a marked accumulation of cyclic AMP was observed. This synergistic effect was also observed in forskolin-induced cyclic AMP formation and only when high concentrations (10 and 100 μM) of forskolin were used (Figure 5B). Interestingly, this synergistic effect was not observed in isoprenaline-induced cyclic AMP formation (Figure 5A). These observations suggested that, at concentrations used in these experiments, AH23848B could act as an antagonist at EP-receptor (EP4) and was probably specific since isoprenaline-induced cyclic AMP formation was not affected in its presence. These effects of AH22921X on forskolin-induced cyclic AMP were less marked than those observed with AH23848B. At the present time, there is no obvious explanation for the difference in potency, but it is clear that these compounds need further studies before being used as selective antagonists of EP4 receptors.
To better characterize the EP receptor present in this cell preparation, another EP antagonist was used. AH6809, which is selective for EP1 and DP receptor subtypes (Coleman et al., 1987; Keery & Lumley, 1988) was also shown to block PGE2-evoked cyclic AMP accumulation in COS-7 cells expressing recombinant human EP2 receptors (Woodward et al., 1995). In our study, treatment of tracheal epithelial cells with AH6809 did not affect the generation of cyclic AMP induced by PGE2. These results suggested that the EP1-, DP- and EP2-receptors were not responsible for mediating the action of PGE2 on tracheal epithelial cells. This is consistent with the lack of efficacy of butaprost on this cell preparation. The small potentiation of PGE2-evoked cyclic AMP generation by the AH6809 is still unexplained. However, this compound was found to inhibit phosphodiesterase (Keery & Lumley, 1988), which could explain its small potentiating effect on cyclic AMP generation induced by PGE2 in our model.
Comparisons with other EP4 receptors or EP receptors positively coupled to adenylyl cyclase
Among the various EP receptor agonists, PGE2 was shown to exhibit the highest affinity and to be the most potent for stimulating the EP4 receptor subtype in both ligand binding assay and functional assay (Coleman et al., 1994a; de Brum-Fernandes et al., 1996; Kiriyama et al., 1997; Marshall et al., 1997; Milne et al., 1994). In all EP4 receptor systems studied, 11-deoxy PGE1 appeared to be less potent than PGE2 but more potent than 16,16-dimethyl PGE2 (de Brum-Fernandes et al., 1996; Kiriyama et al., 1997), except in our study and in human monocytes (Milne et al., 1994), where they had the same potency. The selective EP2 receptor agonist, butaprost, was the least potent agonist in all studies reported on the EP4 receptor-subtype (Coleman et al., 1994a) and was not active at concentrations up to 10 μM in bovine chondrocytes (de Brum-Fernandes et al., 1996). Furthermore, it had a very low affinity in binding studies on human EP4-receptor subtype expressed in Xenopus oocytes (Marshall et al., 1997) and on the mouse EP4-receptor expressed in CHO cells (Kiriyama et al., 1997). Iloprost showed very little activity in many studies including ours. In other studies, selective-EP1 receptor agonists also showed very poor affinity on the EP4-receptor subtype (Coleman et al., 1994a; de Brum-Fernandes et al., 1996; Marshall et al., 1997).
AH23848B and AH22921X were shown to be of low affinity but selective for EP4 receptors (Coleman et al., 1994a). To our knowledge, AH22921X has only been tested on piglet saphenous vein, where the pA2 value was 5.3 (Coleman et al., 1994a). In contrast, AH23848B was more widely used to discriminate the effect produced by the EP2 or EP4-receptor subtypes (Coleman et al., 1994a; de Brum-Fernandes et al., 1996; De Vries et al., 1995; Feoktistov & Biaggioni, 1997; Kiriyama et al., 1997; Lydford et al., 1996; Marshall et al., 1997; Milne et al., 1995; Wise, 1998). In all these experiments, a high concentration (10 μM) of this compound was used to inhibit the effect of PGE2. Other studies reported the presence of a receptor positively coupled to adenylyl cyclase that responded to PGE2 but not to butaprost in bovine chondrocytes and in Jurkat T-cell line (de Brum-Fernandes et al., 1996; De Vries et al., 1995). In these last two studies, AH23848B antagonized the effect of PGE2 in a concentration-dependent manner but the nature of the antagonism was unclear. The first report describing the effect of AH23848B presented this compound as a competitive antagonist on PGE2-induced relaxation of piglet saphenous vein (Coleman et al., 1994a). In contrast, De Vries et al. (1995) described compound AH23848B as a non-competitive antagonist. Based on these data, they postulated that a new EP-receptor subtype was present in Jurkat cells. De Brum-Fernandes et al. (1996) reported that the EP receptor expressed in bovine chondrocytes possessed some characteristic features of the EP4-subtype. AH23848B was also used to determine the receptor subtype responsible for the inhibition of LPS-induced TNFα generation in blood monocytes (Meja et al., 1997).
Prostaglandin E2 and endothelin-1
The results presented here show that, in addition to increasing cyclic AMP generation, PGE2 inhibits irET production/secretion by tracheal epithelial cells. To confirm that activation of EP4 receptor which increases cyclic AMP generation was the mechanism by which inhibition of irET production/secretion occurred, the effects of AH22921X and AH23848B on irET production/secretion were also studied. Unfortunately, both compounds inhibited basal irET-production/secretion, in common with PGE2. It was, therefore, very difficult to evaluate the role of the EP4 receptor subtype in mediating the inhibition of irET production/secretion using these tools. This inhibitory effect could be due to their activity on cyclic AMP metabolism. Therefore, another approach was used to answer this question. The ability of a cyclic AMP antagonist (Rp-cAMPS) to inhibit the effect of PGE2 on irET production/secretion was tested. At the concentration of 100 μM, Rp-cAMPS clearly reversed the inhibitory effect of PGE2 on irET production. Furthermore, the ability of this compound to reverse the inhibitory effect of adenosine (acting through the A2b-adenosine receptor which is also coupled to a Gs-protein; Pelletier et al., 2000), was evaluated on irET production. The results showed that Rp-cAMPS (100 μM) almost completely reversed the effect of adenosine. These results suggested that PGE2 and adenosine are able to inhibit basal production of irET by activating the cyclic AMP-adenylyl cyclase system. The results also suggested that the EP4 receptor subtype mediated PGE2 effects since it is were the only one that increased cyclic AMP generation in guinea-pig tracheal epithelial cells. In addition, a strong correlation between the cyclic AMP generation and the inhibitory effect of PGE2 on the production/secretion of irET was observed. Linear regression analysis of the responses observed on cyclic AMP generation and inhibition of irET production/secretion gave a slope of 0.91 and a correlation coefficient (r) of 0.95 (data not shown).
Interestingly, Prins et al. (1994) showed that PGE2 and PGI2 decreased ET-1 production/secretion by endothelial cells in a concentration and time-dependent manner. In their study, PGE2 and PGI2 increased cyclic GMP formation and activated cyclic GMP-dependent protein kinase leading to a decrease of ET-1 gene expression, thus resulting in a diminution of the ET-1 production/secretion. The data also showed that the signalling pathway was independent of the activity of a cyclic AMP-dependent protein kinase which suggested that cyclic AMP was not involved in the regulation of ET-1 production/secretion. However, this group did not clearly demonstrate that cyclic AMP was not involved in the inhibition of endothelin secretion.
The involvement of cyclic AMP in the regulation of the expression and/or the production/secretion of ET-1 has already been reported in rat mesangial cells where the production of ET-1 was reduced by stimulation with a β-adrenoceptor agonist (isoprenaline) and by forskolin, a direct activator of adenylyl cyclase (Sakamoto et al., 1992). These authors reported that a cyclic AMP-dependent protein kinase appeared to be involved in the signalling pathway. In the two previous studies reported here (Prins et al., 1994; Sakamoto et al., 1992), the implication of cyclic AMP-dependent protein kinase was investigated on the inhibition of ET-1 secretion. In the first study, Prins et al. (1994) tested the effect of a cyclic AMP-dependent protein kinase inhibitor, Rp-cAMPS, and a selective cyclic GMP-dependent protein kinase inhibitor, KT5823, and found that the inhibitory effect of PGE2 and PGI2 on ET-1 production/secretion was unchanged when cells were incubated in the presence of the Rp-cAMPS whereas the PG-induced inhibition of ET-1 production/secretion was reversed in the presence of the KT5823. In the second study, H-8, a non-selective cyclic AMP-dependent and cyclic GMP-dependent protein kinase inhibitor, was used and blocked forskolin- and isoprenaline-induced inhibition of ET-1 production/secretion by rat mesengial cells. Thus, Sakamoto et al. (1992) suggested that a cyclic AMP-dependent protein kinase was involved in the inhibition of ET-1 production/secretion whereas Prins et al. (1994) suggested a cyclic GMP-dependent protein kinase mechanism. These two studies suggested different intracellular pathways involved in the regulation of the production of ET-1 by these two cell types. In fact, a different interpretation of the results obtained by Sakamoto et al. (1992) could suggest that, considering that the H-8 compound was recognized as a non-selective cyclic AMP- and cyclic GMP-dependent protein kinase inhibitor (Hagiwara et al., 1987; Hidaka et al., 1984), the inhibitory effect of forskolin and isoprenaline on ET-1 production secretion was mediated through a cyclic GMP-dependent protein kinase. Studies from our laboratory have already demonstrated that compounds able to bind Gs-coupled receptors in tracheal epithelial cells reduced the production/secretion of ET-1 (Yang et al., 1997a). Furthermore, inhibition of ET-1 secretion by activation of Gs-coupled receptors was observed by Durieu-Trautman et al. (1993) in brain microvessel endothelial cells, where isoprenaline inhibited ET-1 secretion. Biphasic effects of cyclic AMP analogues were also observed on ET-1 secretion by brain microvessel endothelial cells. Micromolar concentrations of 8-Br-cyclic AMP reduced ET-1 production/secretion by cultured cells whereas high concentrations stimulated the production and secretion of the peptide (Durieu-Trautman et al., 1993). These and our findings suggested that the activation of a Gs-coupled receptor was able to decrease ET-1 production/secretion but the mechanism involved needs further elucidation. More recently, we reported that adenosine can also inhibit ET-1 production by tracheal epithelial cells by activating an A2b adenosine receptor which is coupled to a regulatory Gs-protein (Pelletier et al., 2000). This study also suggested an important role for cyclic AMP in regulating ET-1 production.
In conclusion, the results of the present study suggest that guinea-pig tracheal epithelial cells express an EP4 receptor positively coupled to adenylyl cyclase that inhibits the production/secretion of irET. This receptor subtype may play a major protective role by controlling the release of ET-1, a potent bronchoconstrictor agent which may have a deleterious effect in asthma and other lung diseases.
Acknowledgments
The authors would like to thank the Medical Research Council of Canada and Pierre Pelletier for their financial support. The authors would also thank Ms Solange Cloutier for excellent technical assistance, willing help and enriching discussions.
Abbreviations
- Cyclic AMP
adenosine 3′ : 5′ cyclic monophosphate
- ET-1
endothelin-1
- G protein
guanine nucleotide-binding regulatory protein
- irET
immunoreactive endothelin
- Rp-cAMPS
(Rp)-adenosine cyclic 3′,5′-phosphorothioate
References
- BOICHOT E., CARRE C., LAGENTE V., PONS F., MENCIA-HUERTA J.M., BRAQUET P. Endothelin-1 and bronchial hyperresponsiveness in the guinea pig. J. Cardiovasc. Pharmacol. 1991;17 Suppl.:S329–S331. doi: 10.1097/00005344-199100177-00094. [DOI] [PubMed] [Google Scholar]
- BRASS L.F., SHALLER C.C., BELMONTE E. Inositol 1,4,5-triphosphate-induced granule secretion in platelets: evidence that the activation of phospholipase C mediated by platelet thromboxane receptors involves a guanine nucleotide binding protein-dependent mechanism distinct from that of thrombin. J. Clin. Invest. 1987;79:1269–1275. doi: 10.1172/JCI112947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREYER R.M., EMESON R.B., TARNG J.-L., BREYER M.D., DAVIS L.S., ABROMSON R.M., FERRENBACH S.M. Alternative splicing generates multiple isoforms of a rabbit prostaglandin E2 receptor. J. Biol. Chem. 1994;269:6163–6169. [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I. Characterisation of the prostanoid receptors mediating contraction of isolated guinea pig trachea. Prostaglandins. 1985;29:363–375. doi: 10.1016/0090-6980(85)90096-6. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., GRIX S.P., HEAD S.A., LOUTTIT J.B., MALLET A., SHELDRICK R.L.G. A novel inhibitory prostanoid receptor on piglet saphenous vein. Prostaglandins. 1994a;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I., SHELDRICK R.L.G. New evidence with selective agonists and antagonists for the subclassification of PGE2-sensitive (EP) receptors. Adv. Prostaglandin Thromboxane Leukot. Res. 1987;17:467–470. [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. Classification of prostanoids receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994b;46:205–229. [PubMed] [Google Scholar]
- CREESE B.R., DENBOROUGH M.A. The effects of prostaglandin E2 on contractility and cyclic AMP levels of guinea-pig tracheal smooth muscle. Clin. Exp. Pharmacol. Physiol. 1981;8:616–617. doi: 10.1111/j.1440-1681.1982.tb00791.x. [DOI] [PubMed] [Google Scholar]
- DE BRUM-FERNANDES A.J., MORISSET S., BKAILY G., PATRY C. Characterization of the PGE2 receptor subtype in bovine chondrocytes in culture. Br. J. Pharmacol. 1996;118:1597–1604. doi: 10.1111/j.1476-5381.1996.tb15580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE VRIES G.W., GUARINO P., MCLAUGHLIN A., CHEN J., ANDREWS S., WOODWARD D.F. An EP receptor with a novel pharmacological profile in the T-cell line Jurkat. Br. J. Pharmacol. 1995;115:1231–1234. doi: 10.1111/j.1476-5381.1995.tb15030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURIEU-TRAUTMAN O., FÉDÉRICI C., CRÉMINON C., FOIGNANT-CHAVEROT N., ROUX F., CLAIRE M., STROSBERG A.D., COURAUD P.O. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J. Cell. Physiol. 1993;155:104–111. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- ENDO T., UCHIDA Y., MATSUMOTO H., SUZUKI N., NOMURA A., HIRATA F., HASEGAWA S. Regulation of endothelin-1 synthesis in cultured guinea-pig airway epithelial cells by various cytokines. Biochem. Biophys. Res. Commun. 1992;186:1594–1599. doi: 10.1016/s0006-291x(05)81590-6. [DOI] [PubMed] [Google Scholar]
- FEOKTISTOV I., BREYER R.M., BIOGGIONI I. Prostanoid receptor with a novel pharmacological profile in human erythroleukemia cells. Biochem. Pharmacol. 1997;54:917–926. doi: 10.1016/s0006-2952(97)00288-8. [DOI] [PubMed] [Google Scholar]
- GARDINER P.J. Characterisation of prostanoid relaxant/inhibitory receptors (psi) using a highly selective agonist, TR4979. Br. J. Pharmacol. 1986;87:45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN J.S., CEUPPENS J. Regulation of the immune response by prostaglandins. J. Clin. Invest. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- HAGIWARA M., INAGAKI M., HIDAKA H. Specific binding of a novel compound, N-[2-methylamino)ethyl]-5-isoquinolinesulfonamine (H-8) to the active site of cAMP-dependent protein kinase. Mol. Pharmacol. 1987;31:523–528. [PubMed] [Google Scholar]
- HARDCASTLE J., HARDCASTLE P., REDFERN J.Morphine has no direct effect on PGE2-stimulated cyclic AMP production by rat isolated enterocytes J. Pharm. Pharmacol. 19823468[Abstract] [DOI] [PubMed] [Google Scholar]
- HASHIMOTO H., NEGISHI M., ICHIKAWA A. Identification of a prostacyclin receptor coupled to the adenylate cyclase system via a stimulatory GTP-binding protein in mouse mastocytoma P-815 cells. Prostaglandins. 1990;40:491–505. doi: 10.1016/0090-6980(90)90111-8. [DOI] [PubMed] [Google Scholar]
- HIDAKA H., INAGAKI M., KAWAMOTO S., SASAKI Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984;23:5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- HONDA A., SUGIMOTO Y., NAMBA T., WATABE A., IRIE A., NEGISHI M., NARUMIYA S., ISHIKAWA A. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. J. Biol. Chem. 1993;268:7759–7762. [PubMed] [Google Scholar]
- HORGAN M.J., PINHEIRO J.M.B., MALIK A.B. Mechanism of endothelin-1-induced pulmonary vasoconstriction. Circ. Res. 1991;69:157–164. doi: 10.1161/01.res.69.1.157. [DOI] [PubMed] [Google Scholar]
- INUI T., JAMES A.F., FUJITANI Y., TAKIMOTO M., OKADA T., YAMAMURA T., URADE Y. ETA and ETB receptors on single smooth muscle cells cooperate in mediating guinea pig tracheal contraction. Am. J. Physiol. 1994;266:L113–L124. doi: 10.1152/ajplung.1994.266.2.L113. [DOI] [PubMed] [Google Scholar]
- IRIE A., SUGIMOTO Y., NAMBA T., HARAZONO A., HONDA A., WATABE A., NEGISHI M., NARUMIYA S., ICHIKAWA A. Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur. J. Pharmacol. 1993;217:313–318. doi: 10.1111/j.1432-1033.1993.tb18248.x. [DOI] [PubMed] [Google Scholar]
- ITO S., HASHIMOTO H., NEGISHI M., SUZUKI M., KOYANO H., NOYORI R., ICHIKAWA A. Identification of the prostacyclin receptor by use of [15-3H1]19-(3-azidophenyl)-20-norisocarbaprostacyclin, an irreversible specific photoaffinity probe. J. Biol. Chem. 1992;267:20326–20330. [PubMed] [Google Scholar]
- ITO S., OKUDA E., SUGAMA K., NEGISHI M., HAYAISHI O. Evalation of ZK110841 and AH6809, an agonist and an antagonist of prostaglandin DP receptor on human platelets, with PGD2-responsive cell line from bovine embryonic trachea. Br. J. Pharmacol. 1990;99:13–14. doi: 10.1111/j.1476-5381.1990.tb14645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JON J., MAO G.F., ASHBY B. Constitutive activity of human prostaglandin E receptor EP3 isoforms. Br. J. Pharmacol. 1997;121:317–323. doi: 10.1038/sj.bjp.0701121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUMBLATT M.M., PATERSON C.A. Prostaglandin E2 effects on corneal endothelial cyclic adenosine monophosphate synthesis and cell shape are mediated by a receptor of the EP2 subtype. Invest. Ophth. Vis. Sci. 1991;32:360–365. [PubMed] [Google Scholar]
- KEERY R.J., LUMLEY P. AH 6809, a prostaglandin DP receptor blocking drug on human platelets. Br. J. Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY I., COLEMAN R.A., HUMPHREY P.P.A., LEVY G.P., LUMLEY P. Studies on the characterisation of the prostanoid receptors: a proposed classification. Prostaglandins. 1982;24:667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in chinese hamster ovary cells. Br. J. Pharmacol. 1997;121:1673–1678. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNEZEVIC I., BORG C., LE BRETON G.C. Identification of Gq as one of the G-proteins which copurify with human platelet thromboxane A2/prostaglandin H2 receptors. J. Biol. Chem. 1993;268:26011–26017. [PubMed] [Google Scholar]
- LAPORTE J., D'ORLÉANS-JUSTE P., SIROIS P. Guinea-pig Clara cells secrete endothelin-1 through a phosphoramidon-sensitive pathway. Am. J. Resp. Cell Mol. Biol. 1996;14:356–362. doi: 10.1165/ajrcmb.14.4.8600940. [DOI] [PubMed] [Google Scholar]
- LYDFORD S.J., MCKECHNIE K.C.W., DOUGALL I.G. Pharmacological studies on prostanoid receptors in rabbit iolated saphenous vein: a comparison with the rabbit ear artery. Br. J. Pharmacol. 1996;117:13–20. doi: 10.1111/j.1476-5381.1996.tb15148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAROM Z., SHELHAMER J.H., KALINER M. Effect of arachidonic acid, monohydroxyeicosatetraenoicacid and prostaglandins on the release of mucous glycoproteins from human airways in vitro. J. Clin. Invest. 1981;67:1695–1702. doi: 10.1172/JCI110207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL F.H., PATEL K., LUNDSTROM K., CAMACHO J., FOORD S.M., LEE M.G. Characterization of [3H]-prostaglandin E2 binding to prostaglandin EP4 receptors expressed with Semliki Forest Virus. Br. J. Pharmacol. 1997;121:1673–1678. doi: 10.1038/sj.bjp.0701332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEJA K.K., BARNES P.J., GIEMBYCZ M.A. Characterization of the prostanoid receptor(s) on human blood monocytes at which prostaglandin E2 inhibits lipopolysaccharide-induced tumor necrosis factor-α generation. Br. J. Pharmacol. 1997;122:149–157. doi: 10.1038/sj.bjp.0701360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNE S.A., ARMSTRONG R.A., WOODWARD D.F. Comparison of the EP receptor subtypes mediating relaxation of the rabbit jugular and pig saphenous veins. Prostaglandins. 1995;49:225–237. doi: 10.1016/0090-6980(95)00018-6. [DOI] [PubMed] [Google Scholar]
- MILNE S.A., LEE J., ARMSTRONG R.A., WOODWARD D.F.Human monocytes and cultured CHO cells both express EP4 receptors positively coupled to adenylyl cyclase Br. J. Pharmacol. 19941138P[Abstract] [Google Scholar]
- MURLAS C.G., GULATI A., SINGH G., NAJMABADI F. Endothelin-1 stimulates proliferation of normal airway epithelial cells. Biochem. Biophys. Res. Commun. 1995;212:953–959. doi: 10.1006/bbrc.1995.2062. [DOI] [PubMed] [Google Scholar]
- NAMBA T., SUGIMOTO Y., NEGISHI M., IRIE A., USHIKUBI F., KAKIZUKA A., ITO S., ICHIKAWA A., NARUMIYA S. Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature. 1993;364:166–170. doi: 10.1038/365166a0. [DOI] [PubMed] [Google Scholar]
- NEGISHI M., SUGIMOTO Y., IRIE A., NARUMIYA S., ICHIKAWA A. Two isoforms of prostaglandin E receptor EP3 subtype: different COOH-terminal domains determine sensitivity to agonist-induced desensitization. J. Biol. Chem. 1993;268:9517–9521. [PubMed] [Google Scholar]
- NIALS A.T., VARDEY C.J., DENYER L.H., THOMAS M., SPARROW S., SHEPHERD G., COLEMAN R.A. AH13205, a selective prostanoid EP2 receptor agonist. Cardiovasc. Drug. Rev. 1993;11:165–179. [Google Scholar]
- NINOMIYA H., YU X.Y., HASEGAWA S., SPANNHAKE E.W. Endothelin-1 induces stimulation of prostaglandin synthesis in cells obtained from canane airways by bronchoalveolar lavage. Prostaglandins. 1992;43:401–411. doi: 10.1016/0090-6980(92)90124-c. [DOI] [PubMed] [Google Scholar]
- NISHIGAKI N., NEGISHI M., HONDA A., SUGIMOTO Y., NAMBA T., NARUMIYA S., ICHIKAWA A. Identification of a prostaglandin E receptor ‘EP2' cloned from mastocytoma cells as EP4 subtype. FEBS Lett. 1995;364:339–341. doi: 10.1016/0014-5793(95)00421-5. [DOI] [PubMed] [Google Scholar]
- PELLETIER S., BATTISTINI B., JENG A.Y., SIROIS P. Inhibitor effects of dual endothelin-converting enzyme/neutral endopeptidase inhibitors, CGS26303 and CGS26393, on lipopolysaccharide or interleukin-1β-stimulated release of endothelin from guinea pig tracheal epithelial cells. J. Cardiovasc. Pharm. 1998;31 Suppl.:S10–S12. doi: 10.1097/00005344-199800001-00005. [DOI] [PubMed] [Google Scholar]
- PELLETIER S., DUBÉ J., VILLENEUVE A., GOBEIL F.J., BERNIER S.J., BATTISTINI B., GUILLEMETTE G., SIROIS P. Adenosine induces cyclic AMP formation and inhibits endothelin-1 production/secretion in guinea-pig tracheal epithelial cells through A2B adenosine receptors. Br. J. Pharmacol. 2000;129:243–250. doi: 10.1038/sj.bjp.0702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENN R.B., KELSEN S.G., BENOVIC J.L. Regulation of β-agonist- and prostaglandin E2-mediated adenylyl cyclase activity in human airway epithelial cells. Am. J. Resp. Cell Mol. Biol. 1994;11:496–505. doi: 10.1165/ajrcmb.11.4.7917318. [DOI] [PubMed] [Google Scholar]
- PRINS B.A., HU R.-M., NAZARIO B., PEDRAM A., FRANK H.J.L., WEBER M.A., LEVIN E.R. Prostaglandin E2 and prostacyclin inhibit the production and secretion of endothelin from cultured endothelial cells. J. Biol. Chem. 1994;269:11938–11944. [PubMed] [Google Scholar]
- SAKAMOTO H., SASAKI S., NAKAMURA Y., FUSHIMI K., MARUMO F. Regulation of endothelin-1 production in cultured rat mesangial cells. Kidney Int. 1992;41:350–355. doi: 10.1038/ki.1992.48. [DOI] [PubMed] [Google Scholar]
- SALOMON Y. Adenylate cyclase assay. Adv. Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- SAMRANSAMRUAJKIT R., GOLLAPUDI S., KIM C.H., GUPTA S., NASSBAUM E. Modulation of endothelin-1 expression in pulmonary epithelial cell line (A459) after exposure to RSV. Int. J. Mol. Med. 2000;6:101–105. doi: 10.3892/ijmm.6.1.101. [DOI] [PubMed] [Google Scholar]
- SCHAAP P., VAN MENTS-COHEN M., SOEDE R.D.M., BRANDT R., FIRTEL R.A., DOSTMAN W., GENIESER H.-G., JASTORFF B., VAN HAASTERT P.J.M. Cell-permeable non-hydrolyzable cAMP derivatives as tools for analysis of signaling pathways controlling gene regulation in Dictyostelium. J. Biol. Chem. 1993;268:6323–6331. [PubMed] [Google Scholar]
- SHENKER A., GOLDSMITH P., UNSON C., SPIEGEL A. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J. Biol. Chem. 1991;257:13570–13575. [PubMed] [Google Scholar]
- SIEGL A.M., SMITH J.B., SILVER M., NICOLAOU K., AHERN D. Selective binding site for [3H]-prostacyclin on platelets. J. Clin. Invest. 1979;63:215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON B., KATHER H., KOMMERELL B. Activation of human colonic mucosal adenylate cyclase by prostaglandins. Adv. Thromboxane Res. 1980;8:1617–1620. [PubMed] [Google Scholar]
- SWEATMAN W.J., COLLIER H.O. Effect of prostaglandins on bronchial muscle. Nature. 1968;217:69. doi: 10.1038/217069a0. [DOI] [PubMed] [Google Scholar]
- TAKIMOTO M., ODA K., SASAKI Y., OKADA T. Endothelin-A receptor-mediated prostanoid secretion via autocrine and deoxyribonucleic acid synthesis via paracrine signaling in human bronchial epithelial cells. Endocrinology. 1996;137:4542–4550. doi: 10.1210/endo.137.11.8895315. [DOI] [PubMed] [Google Scholar]
- THIN F.C.K., WALTERS E.H. Eicosanoids and asthma: an update. Prostaglandins Leukot. Essent. Fatty Acids. 1995;52:271–288. doi: 10.1016/0952-3278(95)90027-6. [DOI] [PubMed] [Google Scholar]
- UCHIDA Y., NINOMIYA H., SAOTOME M. Endothelin, a novel vasoconstrictor peptide, a potent bronchoconstrictor. Eur. J. Pharmacol. 1988;154:227–238. doi: 10.1016/0014-2999(88)90106-9. [DOI] [PubMed] [Google Scholar]
- WALTERS E.H., DAVIES B.H. Dual effect of prostaglandin E2 on normal airway smooth muscles in vitro. Thorax. 1982;37:918–922. doi: 10.1136/thx.37.12.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE S.R., SIGRIST K.S., SPAETHE S.M. Prostaglandin secretion by guinea-pig tracheal epithelial cells caused by eosinophil major basic protein. Am. J. Physiol. 1993;265:L234–L242. doi: 10.1152/ajplung.1993.265.3.L234. [DOI] [PubMed] [Google Scholar]
- WISE H. Activation of the prostaglandin EP4-receptor subtype is highly coupled to the inibition of N-formyl-methionyl-leucyl-phenylalanine-stimulated rat neutrophil aggregation. Prostaglandins Leukot. Essent. Fatty Acids. 1998;58:77–84. doi: 10.1016/s0952-3278(98)90133-8. [DOI] [PubMed] [Google Scholar]
- WOODWARD D.F., PEPPERL D.J., BURKEY T.H., REGAN J.W. 6-isopropoxy-9-oxoxanthine-2-carboxylic acid (AH 6809), a human EP2-receptor antagonist. Biochem. Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- YANG Q., BATTISTINI B., SIROIS P. Inhibition of the release of endothelins by cAMP stimulation in guinea-pig cultured tracheal epithelial cells. Pharmacologist. 1997a;39:93. [Google Scholar]
- YANG Q., LAPORTE J., BATTISTINI B., SIROIS P. Effect of dexamethasone on the basal and cytokine-stimulated release of endothelin-1 from guinea-pig cultured tracheal epithelial cells. Can. J. Physiol. Pharmacol. 1997b;75:576–581. doi: 10.1139/cjpp-75-6-576. [DOI] [PubMed] [Google Scholar]


