Abstract
There is controversy as to whether somatostatin sst4 receptors internalize. In this study, CHO-K1 cells expressing human sst4 receptor (CHOsst4 cells) cells internalized [125I]-[11Tyr]-SRIF in a time-dependent manner, reaching a steady state at 60 min (1.4±0.1×104 molecules internalized per cell). Internalization was blocked by hypertonic sucrose (0.5 M), ATP depletion or by decreasing the temperature to 4°C.
Internalization of [125I]-[11Tyr]-SRIF was also inhibited (pIC50 values) by increasing concentrations of SRIF (7.74), L-362855 (6.27) and NNC-296100 (6.50) with pIC50 values approximately 10 fold lower than those obtained for inhibition of [125I]-[11Tyr]-SRIF binding to membrane homogenates.
Internalized ligand recycled rapidly to the extracellular media (t1/2 3.9±0.7 min) with only 6.8±0.6% of internalized radioactivity remaining in the cell after 45 min.
Confocal microscopy of permeabilized, HA-epitope tagged CHOsst4 cells labelled with a Cy-3 conjugated antibody revealed little internal immunostaining after SRIF (1 μM) treatment, consistent with the small proportion of receptors (3.5%) estimated to be internalized by radioimmunoassay.
In summary, CHOsst4 cells internalized [125I]-[11Tyr]-SRIF in a clathrin- and ATP-dependent manner with subsequent rapid recycling to the extracellular medium. Rapid receptor recycling and the consequent low proportion of receptors internalized at any one time may explain the inability to visualize internalized receptors by confocal microscopy. It seems unlikely therefore that the marked receptor desensitization observed in CHOsst4 cells following SRIF treatment can be accounted for by a decrease in cell surface receptor expression.
Keywords: Somatostatin (SRIF), sst2 receptors, sst4 receptors, internalization, receptor endocytosis
Introduction
The binding of agonist to cell surface G-protein coupled receptors initiates receptor mediated endocytosis. Once endocytosed, receptors are channelled through multiple intracellular pathways leading to lysosomal degradation or recycling to the cell surface (for review see Böhm et al., 1997; Koenig & Edwardson, 1997). The physiological importance of receptor endocytosis is subject to some controversy, but has been postulated to be involved in receptor resensitization (Yu et al., 1993; Pippig et al., 1995), intracellular signalling (Vieira et al., 1996; Daaka et al., 1998; Chow et al., 1998; Ignatova et al., 1999), receptor down regulation (Moore et al., 1995), removal of circulating hormone (Wu-Wong et al., 1995) and nutrient uptake (van Gelder et al., 1998). Many G-protein coupled receptors, including, bradykinin (Faussner et al., 1998), 5-HT1A (Della Rocca et al., 1999), P2Y2 (Sromek & Harden, 1998), muscarinic (Koenig & Edwardson, 1996), β2 adrenoceptors (January et al., 1997), and μ opiate receptors (Arden et al., 1995) are internalized as a consequence of agonist treatment.
The 14-amino acid neuropeptide hormone somatostatin (somatotrophin release inhibitory factor, SRIF), and its N-terminally extended form, SRIF-28, are native ligands of a family of five G-protein coupled somatostatin receptors (see Hoyer et al., 1995). The five somatostatin receptors (sst1–sst5) are distributed widely through the CNS and periphery, and mediate a wide range of effects (for reviews see Hoyer et al., 1994; Schindler et al., 1996). Clinical studies have shown that some tumours, particularly those of neuroendocrine origin, express high levels of somatostatin receptors (Lamberts et al., 1991; Reubi & Laissue, 1995). With radiolabelled somatostatin analogues being routinely used in pre-operative tumour scintigraphy, there is clinical relevance of somatostatin receptor internalization (Lamberts et al., 1993; Hurst & Modlin, 1993).
Initial reports of somatostatin ligand internalization were performed on native cell lines expressing heterogeneous receptor populations, which often provided conflicting results (Morel et al., 1986; Sullivan & Schonbrunn, 1986; Viguerie et al., 1987; Presky & Schonbrunn, 1988). More recent work has focused upon recombinant receptor expression systems, allowing individual receptor subtypes to be studied in isolation. There is evidence from some studies that the choice of expression system may also exert an influence on the internalization characteristics of a variety of G-protein coupled receptors (Koenig & Edwardson, 1996). Thus it has been demonstrated that all human sst receptors, apart from human sst1, internalize [125I]-LTT SRIF-28 when expressed in CHO-K1 (Chinese hamster ovary) cells (Hukovic et al., 1996). Whereas in another study, COS-7 cells expressing the human sst1 receptor were shown to internalize both [125I]-SRIF and a fluorescein-labelled SRIF analogue (Nouel et al., 1997). Studies using human embryonic kidney cells (HEK) or transfected rat insulinoma cells, expressing rat rsst4 receptor, did not demonstrate SRIF induced receptor internalization (Roth et al., 1997; Roosterman et al., 1997). However, ligand internalization experiments on CHO-K1 cells expressing the human sst4 receptor clearly showed the internalization of [125I]-LTT SRIF-28 (Hukovic et al., 1996).
In a recent study using human sst4 receptor expressing CHO-K1 cells, we demonstrated that SRIF-induced increases extracellular acidification rate (EAR) are highly susceptible to a marked desensitization after pre-treatment with SRIF, but not the SRIF analogue L-362855 (Smalley et al., 1998). As very little is known about the operational characteristics of the human sst4 receptor, we were interested to determine whether receptor internalization and any subsequent recycling were responsible for the observed desensitization. It was of particular interest to investigate the internalization characteristics of this receptor in light of work which suggested that rat sst4 receptors do not internalize (Roth et al., 1997). In the present study, we have examined both the internalization and recycling characteristics of [125I]-[Tyr11]-SRIF. In addition, we have looked at the internalization of the haemoagglutin (HA) epitope-tagged human sst4 receptor using confocal microscopy in order to reconcile internalization of ligand with changes in receptor trafficking.
Some of the preliminary findings of this study have been presented to the British Pharmacological Society (Smalley et al., 1999).
Methods
Cell culture
The cDNA encoding the human sst4 receptor (Glaxo Wellcome, Stevenage, U.K.) was subcloned into CHO-K1 cells using the mammalian expression vector pCIN4 harbouring a neomycin resistant gene as a selection marker. Transfection was achieved using 10 μg of sst4-pCIN4/ 0.5×106 cells using a cationic liposome formulation-mediated transfer (LipofectAMINE™, Life Technologies, U.K.). Stable cell lines expressing the cDNA were prepared by single-cell cloning and receptor expression assessed by binding of [125I]-[Tyr11]- SRIF (see below). Cells were grown in monolayer culture in Dulbecco's modified Eagle's medium/Hams F-12 (1 : 1) mix supplemented with Glutamax, 10% Foetal calf serum and G418 (0.5 mg ml−1). Cultures were maintained at 37°C in a 5% CO2/humidified air atmosphere. Cells used for microphysiometer experiments were typically between passage 10–40.
Some experiments used cells transfected with either the human sst2 or human sst4 receptors labelled with an N-terminal haemoagglutin (HA) tag (Affymax, USA) (CHOsst2 and CHOsst4-tag cells).
Cell membrane preparation
CHO-K1 cells expressing either the human sst4 or sst4-tag receptor were homogenized in assay buffer (mM): N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulphonic acid] HEPES 10 (pH 7.4), MgCl2 11, EDTA 1, 10 μg ml−1 leupeptin, 1 μg ml−1 soyabean trypsin inhibitor, and 0.2 mg ml−1 bacitracin, in a Dounce glass homogenizer (50 strokes, 4°C). The homogenate was centrifuged at 500×g for 10 min at 4°C and the supernatant spun at 20,000 g for 30 min at 4°C. The resultant pellet was resuspended in cold assay buffer and stored in 200 μl aliquots at −70°C.
Radioligand binding assays
Cell membranes were incubated with 0.03 nM [125I]-[Tyr11]-SRIF and increasing concentrations of competing ligand for 60 min at 37°C unless otherwise stated. In assays performed at pH 5.0 the assay buffer additionally contained 10 mM 2-[N-morpholino] ethane sulphonic acid (MES). Non-specific binding was defined with 1 μM cold SRIF. The assay was terminated by rapid filtration through Whatman GF/C glass fibre filters soaked in 0.5% polyethylenimine (PEI), followed by 7×3 ml washes of 50 mM Tris-HCl. Membrane radioactivity was determined using a Canberra Packard Cobra II auto-γ counter.
For dissociation experiments, membranes were incubated with 0.03 nM [125I]-[Tyr11]-SRIF for 60 min at 37°C, dissociation was initiated at 15°C by the addition of excess cold SRIF (1 μM). In these experiments the dissociation buffer contained guanosine 5′ triphosphate (GTP, 100 μM) in a buffered balanced salt solution (mM): HEPES 10, MES 10, NaCl 140, KCl 5, MgCl2 0.5, CaCl2 1.3, pH 5.0.
To calculate KD and Bmax values from competition studies with SRIF, the following equations were used: KD=IC50-[A], where KD is the equilibrium dissociation constant of the radio-ligand, IC50 is the half-maximal inhibitory concentration of SRIF and [A] is the concentration of [125I]-[11Tyr]-SRIF present: Bmax=([B].IC50/[A]), where Bmax is the receptor density and [B] is the concentration of specific [125I]-[11Tyr]-SRIF bound in the absence of competing ligand.
Internalization of [125I]-[Tyr11]-SRIF
CHO-K1 cells were seeded out into 24-well plates at a density of 1×105 cells per well (giving a final cell count at time of experimentation of 1.97±0.23×105 cells per well) and were incubated overnight. Plates were washed twice with assay buffer (serum-free DMEM/HEPES, pH 7.3 with 0.2 mg ml−1 bacitracin) before being left to equilibrate for 10 min in 0.2 ml buffer at 37°C, after which 0.05 ml of 0.5 nM [125I]-[Tyr11]-SRIF was added to each well and left for increasing periods of time. Non-specific internalization was determined by inclusion of 1 μM SRIF. In some studies cells were incubated for 10 min in DMEM/HEPES containing 0.5 M sucrose before the SRIF was added. In another series of studies the whole internalization experiment was carried out at 4°C.
The amount of ligand internalized was determined by treating cells with 2 ml of ice-cold acid-wash buffer (DMEM/HEPES, 10 mM MES, pH 5.0) for 20 min to remove surface bound ligand. Plates were then washed with a further 2×2 ml of acid-wash buffer. To determine internalized radioactivity 0.25 ml 1% v v−1 Triton X-100 was added to each well and left for 60 min before being transferred to vials and counted in a γ counter. Internalized ligand data was fitted to a one-phase exponential association curve using the equation Y=Ymax (1−e−kx) and t1/2=0.69/k (where Y= ligand internalized, Ymax=radioligand internalization at equilibrium and k=internalization rate constant) using Graph Pad Prism software.
To determine the effect of ATP depletion upon internalization, cells were washed twice with a glucose-free phosphate buffered saline solution of the following composition (mM): NaCl 137, KCl 2.6, Na2HPO4 8.0, K2HPO4 1.75, CaCl2 0.9 MgCl2 0.5. The cells were then left to incubate in phosphate buffered saline (PBS) (pH 7.3 with 0.2 mg ml−1 bacitracin) with or without antimycin A (50 nM) and deoxyglucose (50 mM) for 30 min. Then, 0.05 ml of 0.5 nM [125I]-[Tyr11]-SRIF was added to each well and left to incubate for increasing periods of time. Incubation was terminated by the removal of assay buffer and the acid wash step outlined previously. The effect of increasing concentrations of competing unlabelled agonists on surface binding was examined by co-incubation of increasing concentrations of agonist with 0.03 nM [125I]-[Tyr11]-SRIF for 15 min followed by the washing procedure described above.
Radioligand recycling
Cells were incubated with [125I]-[Tyr11]-SRIF for 15 min and treated with acid-wash buffer for 20 min at 15°C, before being incubated at 37°C with fresh media (pH 7.4) containing excess SRIF (1 μM) for various time points. All of the media was collected from the wells and counted in a γ counter. To determine the proportion of radioactivity localized internally, 0.25 ml 1% v v−1 Triton X-100 was added to each well and left for 60 min before being removed and counted in a γ counter.
Immunofluorescence of HA tagged receptors
Both CHOsst2-tag and CHOsst4-tag cells were grown to confluency in 75 cm2 flasks, trypsinised and resuspended in 40 ml of DMEM/Nut Mix : F12. A 10 μl drop of cell suspension was put onto the centre of a glass coverslip (which had been sterilized in absolute ethanol, and air dried) in a 6-well plate and then returned to the incubator for 10 min. This was followed by the addition of 2 ml of media to each well, after which cells were left to equilibrate overnight.
The plates were washed with 2×2 ml of assay buffer and left to equilibrate for 10 min before being treated with agonist (SRIF, 1 μM) for 10, 30 or 60 min. The incubation was terminated by the removal of media and fixation with 3% formaldehyde in PBS for 15 min. The coverslips were washed with 3×2 ml PBS before being treated for 10 min with 0.2% v v−1 Triton X-100 to permeabilize the cells. Coverslips were further washed with PBS and incubated in PBS containing 3% horse serum, 1% bovine serum albumin (PBS/HS/BSA) to reduce non-specific binding. Monoclonal anti-HA antisera (dilution 1 : 1000) was added and left to incubate for 60 min. After washing (PBS/HS/BSA 3×2 ml) for 10 min the cells were incubated with cy-3 conjugated anti-mouse antisera (dilution 1 : 1000) for 60 min. After further washing (PBS×6×2 ml) for 10 min, the coverslips were dried and treated with anti-fade before being fixed onto glass slides. Slides were examined by laser scanning confocal microscopy. The confocal images were printed on an Epson Stylus Colour 800 printer.
Measurement of cell surface receptor expression by radio-immunoassay
Cell surface receptor expression was calculated by use of an immunoassay. The principle behind this study is that the amount of specifically bound [125I]-sheep anti-mouse IgG, fragment (F(ab′)2) is directly proportional to the amount of HA-tagged receptor. Initial experiments were performed to calculate the time of association, using low concentrations of the F(ab′)2 fragment. Cells expressing the human sst4-tag receptor were seeded into 24-well plates, at a density of 5000 cells per well and incubated overnight. Media was aspirated from the plate and the cells fixed with 3% formaldehyde solution for 10 min, before being washed rapidly with 3×1 ml PBS. Cells were incubated with 0.3 ml anti-HA antisera (1 : 500) in PBS/BSA/HS for 2 h. Wells were then washed with 3×1 ml PBS followed by the addition of 0.2 ml PBS/BSA/HS. Cells were treated with increasing concentrations (0.14, 0.24, 0.52, 1.0 and 1.9 μg ml−1) of [125I]-F(ab′)2 fragment and left to equilibrate for 4 h. Media was then aspirated and the plates washed with 3×1 ml PBS/BSA/HS before being extracted in Triton-X (1% v v−1) overnight. Samples were counted using a γ-counter. Non-specific binding was determined by performing parallel [125I]-F(ab′)2 binding experiments on wild-type CHO-K1 cells.
Results were fitted to a single site binding equation B=BmaxD / D+KD (where B=F(ab′)2 fragment specifically bound and D=Initial F(ab′)2 fragment added) using Graph Pad Prism to give indications of Bmax. Estimates of Bmax were then converted to the number of binding sites from the specific activity data of the [125I]-F(ab′)2 fragment supplied by the manufacturer (5.90 μCi/μg).
Statistical analysis
Unless otherwise stated, all values are means±s.e.mean from at least three experiments performed in duplicate. All pEC50 values and Hill slopes were determined from individual experiments by non-linear regression, using a four parameter logistic equation (Graph Pad Prism). Statistical comparisons of responses performed using Student's t-test, results were noted to be significantly different when P<0.05.
Drugs and reagents
Unless otherwise stated, all reagents were from Sigma. Tissue culture media were from Life Technologies, Paisley, U.K. and tissue culture ware from Costar. SRIF was obtained from Peninsular Laboratories Europe Ltd. (St. Helen's, Merseyside). NNC-296100 was synthesized by Dr J. Murray's team (GlaxoWellcome Chemistry Unit, University of Cambridge, U.K.). L-362855 (c[Aha-Phe-Trp-D-Trp-Lys-Thr-Phe]) was custom synthesized by Protein Research Consultants (University of Exeter, U.K.).
The Anti-HA primary antibody was from Babco, (Cambridge, U.K.). The Cy-3 anti-mouse IgG was from Amersham, U.K. Horse serum and Vectashield, anti-fade mounting medium was from Vector Laboratories (California, U.S.A.). [125I]-Sheep anti-mouse IgG F(ab′)2 fragment was from NEN Life sciences (Boston, U.S.A.).
Results
Ligand binding characteristics of human sst4 receptors
In CHOsst4 cell membranes, SRIF (pIC50 8.82±0.02) caused a concentration-dependent inhibition of specific [125I]-[Tyr11]-SRIF binding (pKD 8.80±0.05 and Bmax 9.38± 0.46 pmol mg−1 protein). Specific [125I]-[Tyr11]-SRIF binding was reduced both by sodium (140 mM) and GTP (100 μM) by 61.0±1.9% and 30.0±1.6%, respectively.
When ligand dissociation experiments were performed on CHOsst4 membranes in the presence of sodium chloride (140 mM) and GTP (100 μM) at pH 5.0, 15°C, over 90% of bound ligand dissociated in 20 min (Figure 1).
Figure 1.
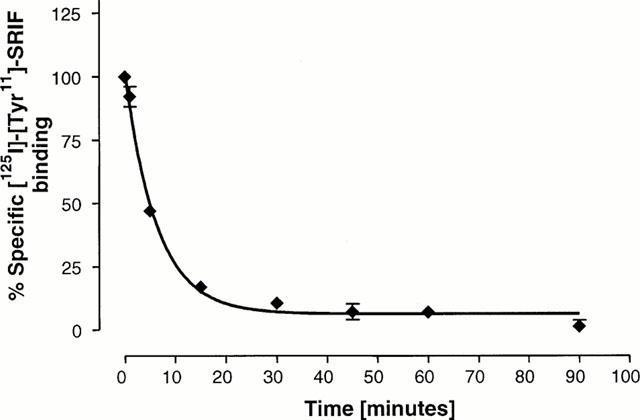
Dissociation of [125I]-[Tyr11]-SRIF (0.03 nM) from CHOsst4 membranes. Association was allowed to proceed for 60 min at 37°C, with dissociation being initiated by the addition of excess cold-SRIF (1 μM) in buffer containing GTP (100 μM) and NaCl (140 mM) at pH 5.0 and 15°C. Data is expressed as a percentage of total SRIF bound under non-dissociating conditions. Data shows the mean of three experiments±s.e.mean.
Ligand internalization
CHOsst4 cells internalized [125I]-[Tyr11]-SRIF in a time-dependent manner and reached a plateau after 60 min at 37°C (Figure 2) (t1/2=19.0±3.9 min−1, k=0.040±0.01). Cell density was 1.97±0.23×105 cells per well, leading to a maximal ligand internalization of 1.4±0.1×104 molecules of [125I]-[Tyr11]-SRIF per cell. Internalization of [125I]-[Tyr11]-SRIF in wild-type CHO-K1 cells was less than 1% of that observed in CHOsst4 cells. Pre-treatment (10 min) with hypertonic sucrose (0.5 M) significantly reduced the amount of ligand detected internally at 60 minutes to 1.74±0.07×103 molecules per cell, representing an inhibition of 88%. In ligand binding experiments on CHOsst4 membrane homogenates, addition of 0.5 M sucrose only reduced specific [125I]-[11Tyr]-SRIF binding by 27.8±7.3%. The effect of temperature upon ligand internalization was investigated by performing the experiment at 4°C, which reduced the amount of ligand internalized at 60 min by 84%, to 2.3±0.8×103 molecules per cell.
Figure 2.
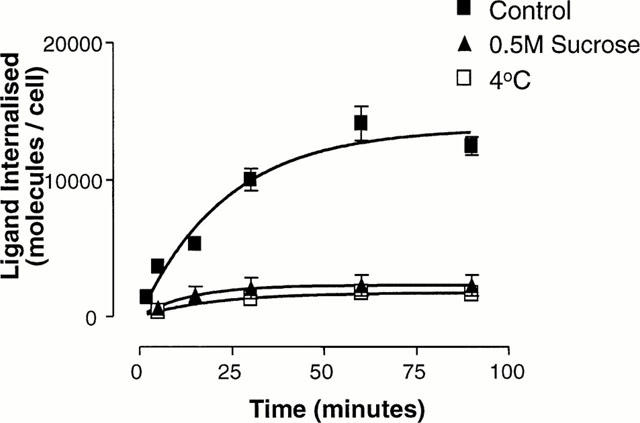
Effect of sucrose and decrease in temperature on internalization of [125I]-[Tyr11]-SRIF (0.1 nM) in CHOsst4 cells. Cells were incubated for various times in DMEM/HEPES at 4°C or 37°C (control) or in the presence of 0.5 M sucrose before being washed thoroughly with DMEM/HEPES/MES (pH 5.0) to remove surface bound radioactivity. Cells were solubilized before being transferred to vials for counting. Data represents the mean±s.e.mean of three experiments in duplicate.
In order to assess the effect of ATP-depletion upon ligand internalization, cells were incubated in glucose-free PBS, in the presence or absence of antimycin A (50 nM) and deoxyglucose (50 mM) (Figure 3). Internalization of SRIF was time-dependent reaching steady state at 60 min (Figure 3) (5.83±0.07×103 molecules per cell), and was inhibited by 73% in the presence of antimycin A and deoxyglucose (1.61±0.03×103 molecules per cell). Specific [125I]-[Tyr]-SRIF binding on membrane homogenates from CHOsst4 cells was 95% that of control value, in the presence of antimycin A (50 nM) and deoxyglucose (50 mM).
Figure 3.
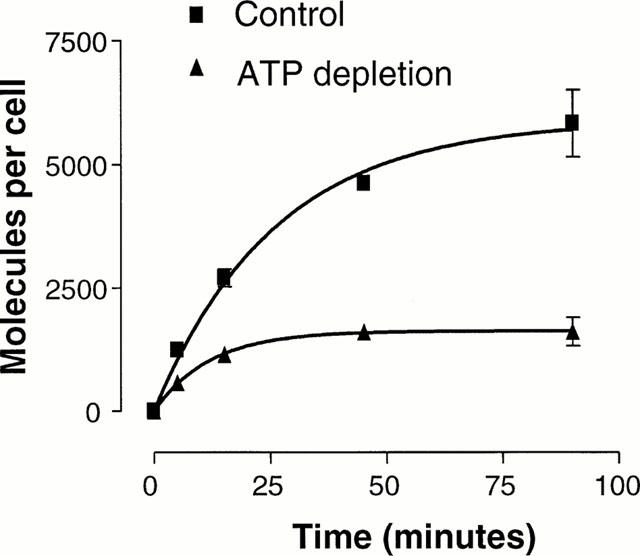
Effect of ATP depletion on internalisation of [125I]-[Tyr11]-SRIF (0.1 nM) in CHOsst4 cells. Cells were incubated for various times in PBS at 37°C with or without antimycin(50 nM)/deoxyglucose (50 mM) before being washed thoroughly with cold DMEM/HEPES/MES (pH 5.0) to remove surface bound radioactivity. Cells were solubilized before being transferred to vials for counting. Data represents the mean±s.e.mean of three experiments in duplicate.
Ligand internalization was inhibited by co-incubation with increasing concentrations of cold SRIF and other somatostatin agonists (Figure 4). Of the analogues tested, SRIF was the most potent at blocking internalization (pIC50 7.74±0.03). Two other somatostatin analogues, NNC-269100 (pIC50 6.50±0.10) and L-362855 (pIC50 6.27±0.11) also blocked internalization, albeit with lower potencies than observed with SRIF.
Figure 4.
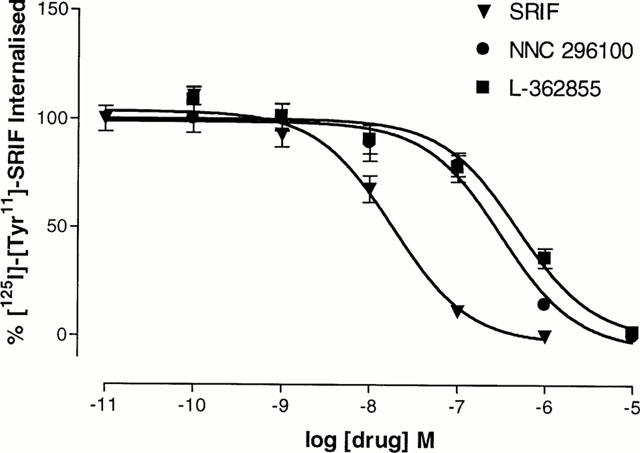
Inhibition of internalization of [125I]-[Tyr11]-SRIF (0.03 nM) in CHOsst4 cells by incubation with unlabelled somatostatin analogues. Cells were incubated in the presence of both the unlabelled analogue and [125I]-[Tyr11]-SRIF for 15 min. After washing to remove surface bound ligand, cells were solubilized and transferred to vials for counting. Data represents the mean±s.e.mean of three experiments in duplicate.
Ligand recycling
In order to determine the fate of the internalized ligand, the recycling of [125I]-[Tyr11]-SRIF was assessed using a ‘pulse-chase' protocol. Following a 15-min period of internalization and 20-min acid-wash, cells were warmed back to 37°C in fresh media in the presence of excess cold SRIF (1 μM) for increasing periods of time. Ligand recycling was rapid (Figure 5), (t1/2=3.95±0.71 min−1, k=0.190±0.038). Indeed, after 45 min, only 6.8±0.6% of internalized radioactivity remained within the cells.
Figure 5.
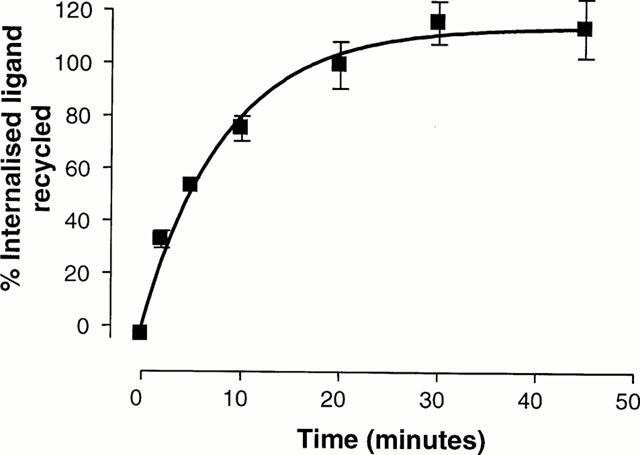
Measurement of recycled radioligand following incubation with 0.1 nM [125I]-[Tyr11]-SRIF for 15 min at 37°C and washed thoroughly with cold DMEM/HEPES/MES (pH 5.0) to remove surface bound radioactivity. Cells were then warmed to 37°C in fresh buffer containing 1 μM SRIF for various times. Buffer was collected and transferred to vials for counting, after which cells were solubilized and transferred to vials for estimation of residual internalized ligand. Data represent the mean of three experiments±s.e.mean in duplicate.
Radioligand binding and function in HA-tagged CHOsst4 cells
CHOsst4-tag cells were shown to have similar pharmacology to the native sst4 receptor (see Smalley et al., 1998). Thus, in CHOsst4-tag cell membranes, SRIF (pIC50 8.83±0.04; Hill slope 1.06±0.1) caused a concentration-dependent inhibition of specific [125I]-[Tyr11]-SRIF binding. It was calculated that [125I]-[Tyr11]-SRIF had estimated equilibrium dissociation constants (KD) of 1.48±0.14 nM and that the density of specific binding sites (Bmax) was 15.2±1.15 pmol mg−1 protein. The SRIF-analogues; NNC-269100 (pIC50 7.88±0.08; Hill slope 0.83±0.1) and L-362855 (pIC50 7.30±0.05; Hill slope 0.79±0.1) also inhibited specific [125I]-[Tyr11]-SRIF binding with affinity estimates similar to those previously obtained in untagged CHOsst4 cells (pIC50 values of 8.0 and 7.4 respectively; see Smalley et al., 1998). In microphysiometer studies, CHOsst4-tag cells were shown to have similar pharmacology (data not shown) to previously published data on the native receptor with respect to desensitization and agonist-induced increases in extracellular acidification rate (Smalley et al., 1998).
Receptor internalization–confocal microscopy of HA-tagged CHOsst4 cells
Internalization of sst4-tag receptors labelled with Cy-3 conjugated antibodies was investigated by confocal microscopy. Visualization of fixed, permeabilized, CHOsst4-tag cells showed no differences between control and SRIF (1 μM, 10, 30 or 60 min) treated cells (Figures 6A and B) with no evidence of immunostained endocytic vesicles, characteristic of internalized receptor within the cytoplasm. In contrast, in CHOsst2-tag cells treated with SRIF (1 μM, 60 min) internalized immunoreactivity was clearly visible (Figure 6C and D).
Figure 6.
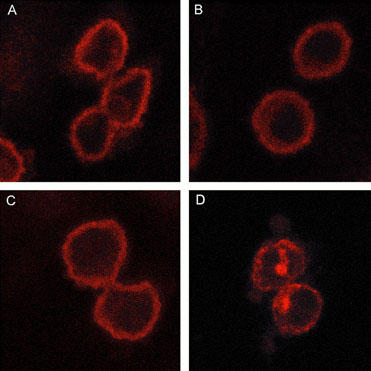
Confocal microscope pictures of control CHOsst4-tag cells (A), CHOsst4-tag cells after 60 min SRIF treatment (1 μM) (B), control CHOsst2-tag cells (C), and CHOsst2-tag cells after 60 min SRIF treatment (1 μM) (D).
Surface receptor expression in HA-tagged CHOsst4 cells
Surface receptor expression was estimated by the use of a radio-immunoassay. This involved labelling the CHOsst4 cells with anti-HA primary antibody, which was then detected using a [125I]-conjugated sheep anti-mouse IgG F(ab′)2 fragment. Initial experiments were performed to calculate the time of association of low concentrations of IgG F(ab′)2 fragment (0.04 μg ml−1) on CHOsst4 tag cells and showed that the [125I]-F(ab′)2 binding had reached steady state by 4 h. Saturation curves were then constructed by incubating with increasing concentrations of F(ab′)2 fragment (0.14, 0.24, 0.52, 1.0 and 1.9 μg ml−1) and incubating for 4 h. Values of cell surface receptor expression were 4.9±0.4×105 receptors per cell (mean±s.e.mean of four estimations obtained from two separate experiments).
CHOsst4 tag cells internalized [125I]-[Tyr11]-SRIF with the same kinetics as cells expressing the native receptor (t1/2=15.8±0.9 min−1, k=0.043±0.03). The extent of ligand internalization was similar to cells expressing the native receptor and led to a maximal internalization of 1.8±0.2×104 molecules of [125I]-[Tyr11]-SRIF per cell at equilibrium. From the calculated values of [125I]-[Tyr11]-SRIF internalized, it was calculated that in HA-tagged CHOsst4 cells, approximately 3.5% of surface receptor was internalized after 1 h SRIF treatment.
Discussion
Of the five G-protein coupled somatostatin receptors, members of the SRIF2 group, (sst1 and sst4 receptors), have been little studied. The recent identification of selective ligands for sst1 (CH-275; see Liapakis et al., 1996) and sst4 receptors (NNC-269100; see Ankersen et al., 1998) should aid our understanding of the function of these receptors. In addition, the expression of the individual receptors in cell lines, has allowed the receptors to be studied in isolation. In an earlier study, we demonstrated a highly selective and prolonged desensitization of human sst4 receptors in response to SRIF, but not the SRIF-analogue L-362855 (Smalley et al., 1998). It was of interest to investigate the operational characteristics of human sst4 receptors further, since it has been shown the rat sst4 receptor, which has 86% sequence homology to the human receptor (Demchyshyn et al., 1993), does not desensitize (Kreienkamp et al., 1998). We therefore studied the endocytosis and recycling properties of human sst4 receptors to determine whether or not SRIF-induced receptor internalization could account for the desensitization we have observed. Two approaches were adopted, the quantitative measurement of the internalization of [125I]-[Tyr11]-SRIF, as well as confocal microscopy to visualize the internalization of the epitope (HA)-tagged human sst4 receptor. This was necessary since previous studies, using differing experimental approaches, led to different conclusions. A confocal microscopy study on cells expressing rat sst4 receptors reported that they were not subject to agonist-induced internalization, which was taken as the explanation for these receptors failing to desensitize (Roth et al., 1997; Kreienkamp et al., 1998). However, Hukovic et al. (1996) have shown that CHO-K1 cells expressing human sst4 receptors internalized [125I]-LTT SRIF-28 but did not determine whether the receptor was subject to desensitization. Since we have previously demonstrated a marked SRIF-induced desensitization in CHO-K1 cells expressing human sst4 receptors (Smalley et al., 1998), it was important to ascertain whether these reported differences reflected species differences, or whether they could be explained by the differing sensitivities of the methods used to study internalization.
Internalization of radioligand was assessed by incubating cells with ligand and washing with low pH buffer to remove all surface-bound ligand, followed by solubilization and counting to determine the remaining ‘internalized' radioactivity. As most peptide ligands bind with high affinity to their receptors it was essential to demonstrate ligand dissociation under appropriate conditions, particularly at low temperatures, which are needed to inhibit any subsequent recycling. Over 90% of surface bound [125I]-[Tyr11]-SRIF dissociated from human sst4 receptors at 15°C, pH 5.0 in 20 min. Previous studies have demonstrated that washing the cells at pH 5.0 does not compromise cellular viability (Koenig et al., 1997). Incubation of CHOsst4 cells with 0.1 nM [125I]-[Tyr11]-SRIF for increasing periods of time followed by acid-wash led to a time-dependent increase in the amount of internalized, cell-associated radioactivity. Internalization was shown to be sst4 receptor-dependent since it could be inhibited by cold SRIF and wild-type CHO-K1 cells internalized less than 1% of the radioactivity detected in CHOsst4 cells. Although the proportion of [125I]-[Tyr11]-SRIF degraded throughout the experiment has not been determined, previous studies suggest that in CHO-K1 cells little degradation of the radioligand takes place extracellularly (Koenig et al., 1998). Furthermore, the inclusion of excess cold-SRIF in the recycling experiments is likely to competitively saturate any proteolytic pathways.
The internalization of [125I]-[Tyr11]-SRIF was reduced by a number of treatments such as low temperature, ATP depletion (through treatment with antimycin A/deoxyglucose) and hypertonic sucrose, all of which inhibit agonist-induced receptor endocytosis (see Koenig et al., 1998). The reduction in internalized radioactivity observed after treatment with hypertonic sucrose and antimycin A/deoxyglucose could not be accounted for by reductions in cell surface binding since they had little effect on specific [125I]-[Tyr11]-SRIF binding in membrane preparations. The inhibition of internalization by hypertonic sucrose is thought to be indicative of a clathrin-coated pit mediated internalization process (Daukas & Zigmond, 1985). We have demonstrated that the majority of ligand internalization is sensitive to hypertonic sucrose, leading to a reduction in internalization of 88%. Clathrin-coated pit formation has been implicated in the internalization of a number of receptors, in particular muscarinic (Koenig & Edwardson, 1996), μ-opioid (Koch et al., 1998), GABAA (Tehraini & Barnes, 1997) and substance P (Garland et al., 1994). Internalization of [125I]-[Tyr11]-SRIF was also inhibited by co-incubating with increasing concentrations of unlabelled somatostatin analogues. The relative potencies of these analogues at blocking internalization were consistent with the rank order of affinities in binding studies and potency in microphysiometer studies (Smalley et al., 1998). Thus unlabelled somatostatin was the most potent analogue in both binding and in blocking internalization, whereas the putative sst4-selective agonist, NNC-296100, and L-362855 were weaker. The potencies for inhibition of internalization were about 10 fold weaker than in binding studies. This is probably a reflection of the fact that membrane binding is performed in the absence of NaCl and GTP and consequently the receptor is likely to be in its high-affinity conformational state. In contrast, internalization studies are performed in whole cells where the receptor is associated with an excess of GTP and is likely to be found in a low affinity conformational state (Kenakin, 1993; Koenig et al., 1997).
To determine the rate of ligand recycling, cells were allowed to internalize [125I]-[Tyr11]-SRIF, acid wash treated and incubated at 37°C in the presence of excess unlabelled SRIF (1 μM). Unlabelled SRIF was included to prevent recycling and re-internalization of [125I]-[Tyr11]-SRIF (see Koenig et al., 1998). Ligand recycling occurred quickly, with a half-life of 3.9±0.7 min. The fast rate of recycling observed for these cells suggests that ligand is not being sorted into late endosomes, characteristic of slower intracellular processes and may thus be confined to early endosomes, leading to rapid recycling (Sakai et al., 1998).
Having studied the endocytosis and recycling of ligand in CHOsst4 cells, changes in HA-epitope-tagged hsst4 receptor localization were examined using immunofluorescence confocal microscopy. Radioligand binding experiments confirmed that the affinities of SRIF and two SRIF analogues, NNC-296100 and L-362855 were the same at both the tagged and untagged human sst4 receptor. Additionally, functional microphysiometry studies (data not shown) demonstrated that the CHOsst4-tag cells had similar pharmacological profiles to published data from CHOsst4 cells (Smalley et al., 1998). After treatment with SRIF for increasing periods of time there appeared to be no internal immunofluorescence detected suggesting that there was little, if any receptor remaining internalized. In contrast, parallel experiments on CHOsst2-tag cells, showed that treatment with SRIF led to a marked redistribution of surface receptor, and the appearance of internal immunostaining indicative of internalized receptors.
In order to investigate the paradox between ligand and receptor internalization, we estimated the cell surface receptor expression in the HA-tagged CHOsst4 cells using a radioimmunoassay which gave a level of expression of 4.9×105 receptors per cell. Assuming that one receptor is endocytosed per molecule of [125I]-[Tyr11]-SRIF, 1.8±0.2×104 molecules per cell are internalized by CHOsst4-tag cells following 1 h incubation and under state conditions. This suggests that only about 3.5% of surface human sst4 receptors are inside following 1 h exposure to SRIF. This could explain why there are discrepancies in surface receptor binding measured by radioimmunoassay, and receptor endocytosis measured by confocal microscopy in rat sst4 receptors. Similar findings have been reported for the human sst1 receptor. Work by Nouel et al. (1997) reported that in COS-7 cells, expressing hsst1 receptors, internalized radioligand was measured, but there was no evidence of endosome associated fluorescent-SRIF. The fact that both the sst4 and sst1 receptors are members of the SRIF2 family is intriguing and may suggest that there is some common structural motif(s) that restricts internalization and consequent endocytosis. A sequence motif has been recently identified on the C-terminal tail of the rat sst4 receptor that negatively regulates endocytosis (Kreienkamp et al., 1998). The receptors can be made to endocytose by mutating threonine-331 to alanine. Although the human and rat sst4 receptors have 86% sequence homology the ETT motif (residues 330–332) in the rat receptor is not present in the human counterpart, which instead has the motif EGA. The apparent low internalization efficiency of human sst4 receptors makes it likely that this is not responsible for the marked receptor desensitization. Human sst4 receptors have multiple phosphorylation sites in the third intracellular loop and carboxyl terminus (Xu et al., 1993; Rohrer et al., 1993), which may be important in SRIF-mediated receptor desensitization (Mayor et al., 1987; Hipkin et al., 1997).
While it was perhaps surprising that we had demonstrated ligand internalization but not seen human sst4 receptor endocytosis, similar findings have been reported previously. Thus, human sst4 receptors internalize [125I]-LTT SRIF-28 in CHO-K1 cells (Hukovic et al., 1996), whilst studies with the rat sst4 receptor detected neither changes in receptor distribution by confocal microscopy nor a reduction in surface receptor binding after agonist treatment (Roosterman et al., 1997; Roth et al., 1997). Our study indicates that these discrepancies between ligand internalization and confocal microscopy studies are likely to reflect differences in sensitivity of the two experimental approaches adopted. In [125I]-[Tyr11]-SRIF internalization studies, a large signal (ligand internalized) is measured over a very small baseline (non-specific ligand internalization), whereas in the confocal microscopy studies, small changes in receptor distribution are being detected against a high background of total cell surface receptor expression.
In summary, we have demonstrated that CHOsst4 cells internalize [125I]-[Tyr11]-SRIF in a clathrin coated pit, energy-dependent manner, with subsequent rapid recycling of ligand to the extracellular media. However the extent of receptor internalization appeared small and it was not possible to detect internalized receptor in endosomes by confocal microscopy. There are two possible explanations for this. The first is that receptors are being internalized but if the receptor is recycled as quickly as the agonist, then there will be little change in surface receptor number. The second explanation is simply that the rate of endocytosis is very slow. Using the current available methodology, it is impossible to distinguish between these two mechanisms. We can conclude however, that the marked receptor desensitization which we have observed in CHO-K1 cells expressing human sst4 receptors (Smalley et al., 1998), cannot be accounted for simply by a decrease in surface receptor number. Furthermore, it is possible that the slow rate of endocytosis may be responsible for the apparently rapid agonist-induced desensitization (Smalley et al., 1998). The results from this study support the findings from an immunohistochemical study in rat brain in vivo which has shown that rat sst2 but not sst4 receptors are internalized following intracerebroventricular administration of SRIF (Schreff et al., 2000).
Abbreviations
- BSA
bovine serum albumin
- CHO
Chinese hamster ovary
- DMEM
Dubecco's modified Eagles medium
- GTP
guanosine 5′ triphosphate
- HA
haemoaggglutin
- HEK
human embryonic kidney
- HEPES
N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulphonic acid]
- HS
horse serum
- L-362855
c[Aha-Phe-Trp-D-Trp-Lys-Thr-Phe]
- MES
2-[N-morpholino] ethane sulphonic acid
- NNC-296100
(1-[3-(N-bromopyridin-2-yl)-N-(3,4 dichlorobenzyl)amino]propyl]-3-[3-(1H-imidazol-1-yl)propyl] thiourea
- PEI
polyethylinimine
- PBS
phosphate buffered saline
- SRIF
somatotrophin release inhibitory factor (somatostatin)
References
- ANKERSEN M., CRIDER M., LIU S.Q., HO B., ANDERSEN H.S., STIDSEN C. Discovery of a novel non-peptide somatostatin agonist with sst4 selectivity. J. Am. Chem. Soc. 1998;120:1368–1373. [Google Scholar]
- ARDEN J.R., SEGREDO V., WANG Z., LAMEH J., SADEE W. Phosphorylation and agonist-specific intracellular trafficking of an epitope tagged mu-opioid receptor expressed in HEK-293 cells. J. Neurochem. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- BÖHM S.K., GRADY E.F., BUNNETT N.W. Regulatory mechanisms that modulate signalling by G-protein coupled receptors. Biochem. J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOW J.C., CONDORELLI G., SMITH R.J. Insulin-like growth factor-1 receptor internalisation regulates signalling via the Shc/mitogen activated protein kinase pathway, but not the insulin receptor substrate-1 pathway. J. Biol. Chem. 1998;273:4672–4680. doi: 10.1074/jbc.273.8.4672. [DOI] [PubMed] [Google Scholar]
- DAAKA Y., LUTTRELL L.M., AHN S., DELLA ROCCA G.J., FERGUSON S.G., CARON M.G., LEFKOWITZ R.J. Essential role for G-protein coupled receptor endocytosis in the activation of mitogen activated protein kinase. J. Biol. Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- DAUKAS G., ZIGMOND S.H. Inhibition of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear leukocytes. J. Cell Biol. 1985;101:1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLA ROCCA G.J., MUKHIN Y.V., GARNOVSKAYA M.N., DAAKA Y., CLARK G.J., LUTTRELL L.M., LEFKOWITZ R.J., RAYMOND J.R. Serotonin 5-HT1A receptor mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J. Biol. Chem. 1999;274:4749–4753. doi: 10.1074/jbc.274.8.4749. [DOI] [PubMed] [Google Scholar]
- DEMCHYSHYN L.L., SRIKANT C.B., SUNAHARA R.K., KENT G., SEEMAN P., VAN TOL H.H.M., PANETTA R., PATEL Y.C., NIZNIK H.B. Cloning and expression of a human somatostatin-14 selective receptor variant (Somatostatin receptor 4) located on Chromosome 20. Mol. Pharmacol. 1993;43:894–901. [PubMed] [Google Scholar]
- FAUSSNER A., PROUD D., TOWNS M., BATHON J.M. Influence of the cytosolic carboxyl termini of human B1 and B2 Kinin receptors on receptor sequestration, ligand internalisation and signal transduction. J. Biol. Chem. 1998;273:2617–2623. doi: 10.1074/jbc.273.5.2617. [DOI] [PubMed] [Google Scholar]
- GARLAND A.M., GRADY E.F., PAYAN D.G., VIGNA S.R., BUNNETT N.W. Agonist-induced internalisation of the substance P (NK1) receptor expressed in epithelial cells. Biochem. J. 1994;303:177–186. doi: 10.1042/bj3030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIPKIN R.W., FRIEDMAN J., CLARK R.B., EPPLER C.M., SCHONBRUNN A. Agonist-induced desensitisation, internalisation, and phosphorylation of the sst2A somatostatin receptor. J. Biol. Chem. 1997;272:13869–13876. doi: 10.1074/jbc.272.21.13869. [DOI] [PubMed] [Google Scholar]
- HOYER D., BELL G.I., BERELOWITZ M., EPELBAUM J., FENIUK W., HUMPHREY P.P.A., O'CARROLL A.-M., PATEL Y.C., SCHONBRUNN A., TAYLOR J.E., REISINE T. Classification and nomenclature of somatostatin receptors. TiPS. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- HOYER D., LUBBERT H., BRUNS C. Molecular pharmacology of somatostatin receptors. Naunyn. Schmiedeberg Arch. Pharmacol. 1994;350:441–453. doi: 10.1007/BF00173012. [DOI] [PubMed] [Google Scholar]
- HUKOVIC N., PANETTA R., KUMA U., PATEL Y.C. Agonist-dependent regulation of cloned human somatostatin receptor types 1–5 (hSSTR1–5): subtype selective internalisation or upregulation. Endocrinology. 1996;137:4046–4049. doi: 10.1210/endo.137.9.8756582. [DOI] [PubMed] [Google Scholar]
- HURST R.D., MODLIN I.M. Use of radiolabelled somatostatin analogues in the identification and treatment of somatostatin receptor-bearing tumours. Digestion. 1993;54:88–91. doi: 10.1159/000201084. [DOI] [PubMed] [Google Scholar]
- IGNATOVA E.G., BELCHEVA M.M., BOHN L.M., NEUMAN M.C., COSCIA C.J. Requirement of receptor internalisation for opioid stimulation of mitogen-activated protein kinase: Biochemical and immunofluorescence confocal microscopic evidence. J. Neurosci. 1999;19:56–63. doi: 10.1523/JNEUROSCI.19-01-00056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANUARY B., SEIBOLD A., WHALEY B., HIPKIN R.W., LIN D., SCHONBRUNN A., BARBER A., CLARK R.B. β 2-Adrenergic receptor desensitisation, internalisation, and phosphorylation in response to full and partial agonists. J. Biol. Chem. 1997;272:23871–23879. doi: 10.1074/jbc.272.38.23871. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Pharmacologic analysis of drug-receptor interaction 1993Raven Press, New York; 69–117.Chapter 32nd Edition [Google Scholar]
- KOCH T., SCHULZ S., SCHRODER H., WOLF R., RAULF E., HOLLT V. Carboxyl-terminal splicing of the rat μ opioid receptor modulates agonist-mediated internalisation and receptor resensitisation. J. Biol. Chem. 1998;273:13652–13657. doi: 10.1074/jbc.273.22.13652. [DOI] [PubMed] [Google Scholar]
- KOENIG J.A., EDWARDSON J.M. Intracellular trafficking of the muscarinic acetylcholine receptor: Importance of subtype and cell type. Mol. Pharmacol. 1996;49:351–359. [PubMed] [Google Scholar]
- KOENIG J.A., EDWARDSON J.M. Endocytosis and recycling of G-protein coupled receptors. TiPS. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- KOENIG J.A., EDWARDSON J.M., HUMPHREY P.P.A. Somatostatin receptors in Neuro2A neuroblastoma: ligand internalisation. Br. J. Pharmacol. 1997;120:52–59. doi: 10.1038/sj.bjp.0700859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOENIG J.A., KAUR R., DODGEON I, EDWARDSON J.M., HUMPHREY P.P.A. Fates of endocytosed somatostatin sst2 receptors and associated agonists. Biochem. J. 1998;336:291–298. doi: 10.1042/bj3360291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREIENKAMP H.J., ROTH A., RICHTER D. Rat somatostatin receptor subtype 4 can be made sensitive to agonist-induced internalisation by mutation of a single threonine (residue 331) DNA Cell Biology. 1998;17:869–878. doi: 10.1089/dna.1998.17.869. [DOI] [PubMed] [Google Scholar]
- LAMBERTS S.W.J., KRENNING E.P., REUBI J.-C. The role of somatostatin and its analogues in the diagnosis and treatment of tumours. Endocrinol. Rev. 1991;12:450–482. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- LAMBERTS S.W.J., CHAYVIALLE J.-A., KRENNING E.P. The visualisation of gastroenteropancreatic endocrine tumours. Digestion. 1993;54 suppl. 1:92–97. doi: 10.1159/000201085. [DOI] [PubMed] [Google Scholar]
- LIAPAKIS G., HOEGER C., REISINE T. Development of a selective agonist at the somatostatin receptor subtype sstr1. J. Pharmacol. Exp. Therap. 1996;276:1089–1094. [PubMed] [Google Scholar]
- MAYOR F., BENOVIC J.L., CARON M.G., LEFKOWITZ R.J. Somatostatin induces translocation of β-adrenergic receptor kinase and desensitises somatostatin receptors in S49 lymphoma cells. J. Biol. Chem. 1987;262:6468–6471. [PubMed] [Google Scholar]
- MOORE R.H., SADOVNIKOFF N., HOFFENBERG S., LIU S., WOODFORD P., ANGELIDES K., TRIAL J., CARSRUD N.D.V., DICKEY B.F., KNOLL B.J. Ligand-stimulated β2-adrenergic receptor internalisation via the constitutive endocytic pathway into rab5-containing endosomes. J. Cell. Sci. 1995;108:2983–2991. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- MOREL G., PELLETIER G., HEISLER S. Internalisation and subcellular distribution of radiolabelled somatostatin-28 in mouse anterior pituitary cells. Endocrinology. 1986;119:1972–1979. doi: 10.1210/endo-119-5-1972. [DOI] [PubMed] [Google Scholar]
- NOUEL D., GAUSRIAULT G., HOULE M., REISINE T., VINCENT J.P., MAZELLA J., BEAUDET A. Differential internalisation of somatostatin in COS-7 cells transfected with SST1 and SST2 subtypes: A confocal microscopic study using novel fluorescent somatostatin derivatives. Endocrinology. 1997;138:296–306. doi: 10.1210/endo.138.1.4834. [DOI] [PubMed] [Google Scholar]
- PIPPIG S., ANDEXINGER S., LOHSE M.J. Sequestration and recycling of β2,–adrenergic receptors permit receptor resensitisation. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- PRESKY D.H., SCHONBRUNN A. Receptor-bound somatostatin and epidermal growth factor are processed differently in GH4C1 pituitary cells. J. Cell. Biol. 1986;102:878–888. doi: 10.1083/jcb.102.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUBI J.-C., LAISSUE J.A. Multiple actions of somatostatin in neoplastic disease. TiPS. 1995;16:110–115. doi: 10.1016/s0165-6147(00)88992-0. [DOI] [PubMed] [Google Scholar]
- ROHRER L., RAULF F., BRUNS, BUETTNER R., HOFSTAEDTER F., SCHULE R. Cloning and characterisation of a fourth human somatostatin receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4196–4200. doi: 10.1073/pnas.90.9.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOSTERMAN D., ROTH A., KREIENKAMP H.J., RICHETR D., MEYERHOF W. Distinct agonist-mediated endocytosis of cloned rat somatostatin receptor subtypes expressed in Insulinoma cells. J. Neuroendocrinol. 1997;9:741–751. doi: 10.1046/j.1365-2826.1997.00632.x. [DOI] [PubMed] [Google Scholar]
- ROTH A., KREIENKAMP H.-J., NEHRING R.B., ROOSTERMAN D., MEYERHOF W., RICHTER D. Endocytosis of the rat somatostatin receptors: Subtype discrimination, ligand specificity, and delineation of carboxy-terminal positive and negative sequence motifs. DNA Cell Biol. 1997;16:111–119. doi: 10.1089/dna.1997.16.111. [DOI] [PubMed] [Google Scholar]
- SAKAI T., MIZUNO T., MIYAMOTO H., KAWASAKI K. Two distinct kinds of tubular organelles involved in the rapid recycling and slow processing of endocytosed transferrin. Biochem. Biophys. Res. Comm. 1998;242:151–157. doi: 10.1006/bbrc.1997.7577. [DOI] [PubMed] [Google Scholar]
- SCHINDLER M., HUMPHREY P.P.A., EMSON P.C. Somatostatin receptors in the central nervous system. Prog. Neurobiol. 1996;50:9–47. doi: 10.1016/0301-0082(96)00030-5. [DOI] [PubMed] [Google Scholar]
- SCHREFF M., SCHULZ S., HANDEL M., KEILHOF G., BRAUN H., PERREIRA G., KLUTZNY M., SCHMIDT H., WOLF G., HOLLT V. Distribution, targetting, and internalization of the sst(4) somatostatin receptor in rat brain. J. Neurosci. 2000;20:3785–3797. doi: 10.1523/JNEUROSCI.20-10-03785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMALLEY K.S.M., FENIUK W., HUMPHREY P.P.A. Differential agonist activity of somatostatin and L-362855 at human recombinant sst4 receptors. Br. J. Pharmacol. 1998;125:833–841. doi: 10.1038/sj.bjp.0702133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMALLEY K.S.M., KOENIG J.A., FENIUK W., HUMPHREY P.P.A. Internalisation characteristics of the human recombinant somatostatin 4 (hsst4) receptor expressed in CHO-K1 cells. Br. J. Pharmacol. 1999;126:125P. doi: 10.1038/sj.bjp.0703896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SROMEK S.M., HARDEN T.K. Agonist-induced internalisation of the P2Y2 receptor. Mol. Pharmacol. 1998;54:485–494. doi: 10.1124/mol.54.3.485. [DOI] [PubMed] [Google Scholar]
- SULLIVAN S.J., SCHONBRUNN A. The processing of receptor-bound [125I-Tyr11] Somatostatin by RINm5F Insulinoma cells. J. Biol. Chem. 1986;261:3571–3577. [PubMed] [Google Scholar]
- TEHRANI M.H.J., BARNES E.M. Sequestration of γ-aminobutyric acidA receptors on clathrin-coated vesicles during chronic benzodiazepine administration in vivo. J. Pharmacol. Exp. Therap. 1997;283:384–390. [PubMed] [Google Scholar]
- VAN GELDER W., HUIJSKES-HEINS M.I.E., CLETON-SOETEMAN M.I., VAN DIJK J.P., VAN EIJK H.G. Iron uptake in blood-brain barrier endothelial cells cultured in iron-depleted and iron-enriched media. J. Neurochem. 1998;71:1134–1140. doi: 10.1046/j.1471-4159.1998.71031134.x. [DOI] [PubMed] [Google Scholar]
- VIEIRA A.V., LAMAZE C., SCHMID S.L. Control of EGF receptor signalling by clathrin-mediated endocytosis. Science. 1996;274:2086–2088. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- VIGUERIE N., ESTEVE J.P., SUSINI C., VAYSSE N., RIBET A. Processing of receptor-bound somatostatin: internalisation and degradation by pancreatic acini. Am. J. Physiol. 1987;252:G535–G542. doi: 10.1152/ajpgi.1987.252.4.G535. [DOI] [PubMed] [Google Scholar]
- WU-WONG J.R., MAGNUSON S.R., OPGENORTH T.J. Endothelin receptors in human astrocytoma U373MG cells: binding, dissociation, receptor internalization. J. Pharm. Exp. Ther. 1995;274:499–507. [PubMed] [Google Scholar]
- YU S.S., LEFKOWITZ R.J., HAUSDORFF W.P. β-adrenergic receptor sequestration. J. Biol. Chem. 1993;268:337–341. [PubMed] [Google Scholar]
- XU Y., SONG J., BERELOWITZ M., BRUNO J.F. Ligand binding and functional properties of the rat somatostatin receptor SSTR4 stably expressed in Chinese hamster ovary cells. Mol. Cell. Neurosci. 1993;4:245–249. doi: 10.1006/mcne.1993.1031. [DOI] [PubMed] [Google Scholar]


