Abstract
We have shown earlier that activation of metabotropic glutamate (mGlu) receptors using a group I-specific mGlu receptor agonist, (RS)-3,5-dihydroxyphenylglycine (DHPG), can induce long-term depression (LTD) in the CA1 region of the hippocampus. In an attempt to determine the signal transduction mechanisms involved in this form of synaptic plasticity, we have tested the effects of a range of inhibitors on DHPG-induced LTD.
In vitro grease-gap electrophysiological recordings were performed in the rat hippocampal CA1 region.
We have found that DHPG-induced LTD is resistant to the two potent protein kinase C (PKC) inhibitors, Gö 6976 (10 μM) and Gö 6983 (10 μM), the potent and selective protein kinase A (PKA) inhibitor, KT 5720 (10 μM), and the potent broad spectrum kinase inhibitor, staurosporine (10 μM).
In contrast, non-selective inhibitors of protein phosphatases (PP1 and PP2A), okadaic acid (1 μM) or calyculin A (1 μM), facilitated DHPG-induced LTD. However, an inhibitor of protein phosphatase 2B, FK 506 (1 μM), did not influence this process.
The PP1/PP2A protein phosphatase inhibitors, but none of the other agents tested, also inhibited (S)-α-methyl-4-carboxyphenylglycine (MCPG)-induced reversal of DHPG-induced LTD.
These data suggest that activation of neither PKC nor PKA is involved in DHPG-induced LTD. They do, however, suggest that the process is under regulation by protein phosphorylation and dephosphorylation.
Keywords: Glutamate; metabotropic glutamate (mGlu) receptor; (RS)-3,5-dihydroxyphenylglycine (DHPG); hippocampal slice; long-term depression; synaptic plasticity; protein kinases; protein phosphatases; okadaic acid; calyculin A
Introduction
The mechanisms responsible for generating LTD, like its counterpart, long-term potentiation (LTP), are under intense investigation as these forms of synaptic plasticity are thought to underlie learning and memory formation (Bear & Abraham, 1996). It is now established that, under certain conditions, activation of mGlu receptors is required for the induction of two forms of LTD–depotentiation (LTD of pre-established LTP) and de novo LTD (LTD of naïve inputs)–in the CA1 region of the hippocampus (e.g., Bashir et al., 1993; Bolshakov & Siegelbaum, 1994; Oliet et al., 1997), since their induction can be blocked by the mGlu receptor antagonist, MCPG. In addition, forms of LTD can be induced by activation of mGlu receptors using the broad-spectrum mGlu receptor agonist, (1S,3R)-1-amino-cyclopentane-1,3-dicarboxylate ((1S,3R)-ACPD) (Bolshakov & Siegelbaum, 1994; O'Mara et al., 1995; Overstreet et al., 1997).
Recently, we have shown that the activation of group I mGlu receptors (probably mGlu5 receptors), by application of a specific agonist, DHPG (Ito et al., 1992), can induce LTD in the CA1 region of the adult rat hippocampus (Palmer et al., 1997). This form of LTD was enhanced when the tissue was made hyperexcitable by omitting Mg2+ ions from the superfusate. The depression persisted for as long as recordings were maintained (up to 3 h) even though DHPG had washed out of the system within circa 15 min (Palmer et al., 1997). An unusual property of DHPG-induced LTD is that when it has been established (i.e., DHPG has washed out of the system), it can be reversed by MCPG and then re-established by washing out MCPG (Palmer et al., 1997). Although DHPG-induced LTD is sensitive to NMDA receptor antagonists, it does not occlude with NMDA receptor-dependent LTD induced by low frequency stimulation (Palmer et al., 1997). We have now commenced an investigation into the potential signal transduction mechanisms involved in DHPG-induced LTD. In previous studies, we found that a Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor, KN-62, facilitated DHPG-induced LTD (Schnabel et al., 1999a). In a further study, we observed no effects of two potent PKC inhibitors, chelerythrine and Ro 31-8220, or of two inhibitors of Ca2+ release from intracellular stores, thapsigargin and cyclopiazonic acid, on this process (Schnabel et al., 1999b).
Here, we have extended our investigation into the possible role of protein kinases in DHPG-induced LTD in several ways. We have determined the effects of (i) Gö 6983 (Gschwendt et al., 1996) since this is a potent inhibitor of several isoforms of PKC (α, β, γ, δ, ξ), including those upon which Ro 31-8220 and chelerythrine have not been tested; (ii) Gö 6976 (Gschwendt et al., 1996), since this is a potent inhibitor of an isoform of PKC (μ) that is relatively insensitive to the action of the above-mentioned PKC inhibitors; (iii) a selective PKA inhibitor, KT 5720, and (iv) a potent, broad-spectrum kinase inhibitor, staurosporine. In addition, we have studied the effects of three inhibitors of protein phosphatases (PPs); two inhibitors of PP1/PP2A, okadaic acid and calyculin A, and an inhibitor of PP2B (calcineurin), FK 506. Finally, we have tested the effects of these agents on the ability of MCPG to reverse pre-established DHPG-induced LTD. We have applied a grease-gap recording technique (Blake et al., 1988) since this approach was used for most of our earlier studies of DHPG-induced LTD (Palmer et al., 1997; Fitzjohn et al., 1998; Schnabel et al., 1999a,1999b). However, a similar effect has also been observed using microelectrode recording techniques (Fitzjohn et al., 1998; 1999; Cambodeca et al., 1999; Huber et al., 2000). Our data do not support a role for PKC or PKA in DHPG-induced LTD. They do, however, suggest that DHPG-induced LTD directly involves, or is regulated by, phosphorylation, the dephosphorylation of which may involve PP1 and/or PP2A.
Methods
Grease-gap recordings were obtained from the CA1 region of rat hippocampal slices, which had been obtained from adult female Wistar rats (approximately 8–10 weeks of age), as previously described (Palmer et al., 1997). Each slice, from which area CA3 had been removed, was placed on a glass coverslip, on the surface of an inclined temperature controlled unit (maintained at 28–30°C). The slice was partially covered with absorbent paper and superfused with a Mg2+-free artificial cerebrospinal fluid (aCSF) consisting of (mM): NaCl 124, KCl 3, NaHCO3 26, NaH2PO4 1.25, CaCl2 2, D-glucose 10 (bubbled with 95% O2/5% CO2), at a rate of 2 ml min−1. The Schaffer collateral-commissural pathway was repeatedly stimulated with single shocks delivered every 30 s. The stimulus intensity used was that which evoked a slope of circa 40% of the maximum. The slope of field excitatory postsynaptic potentials (fEPSPs) was plotted versus time on-line and subsequently analysed off-line using programs written in-house (Anderson & Collingridge, 1997). Data are presented as mean±s.e.mean. Statistical analysis was performed using Student's t-test or ANOVA followed by Dunnett's test as appropriate. n signifies the number of times a given experiment was performed with each experiment using a slice from a different rat.
DHPG and MCPG were both obtained from Tocris Cookson (Bristol, U.K.), and were dissolved in distilled water and equimolar NaOH, respectively. Gö 6983, Gö 6976, okadaic acid, 1-norokadaone, KT 5720, FK 506 and calyculin A were all purchased from Calbiochem and dissolved in DMSO. Staurosporine was supplied by Sigma and dissolved in DMSO.
Results
DHPG-induced LTD
Consistent with previous studies (Palmer et al., 1997; Schnabel et al., 1999a,1999b), application of DHPG (100 μM, 10 min) caused a large, initial depression of synaptic transmission with only partial reversibility upon washout (n=15). A stable response was obtained approximately 15 min after washout of DHPG. When the data for all 15 slices were pooled, DHPG was found to induce LTD of 34±5% (P<0.0001; quantified 30 min following the washout of DHPG). A single example and pooled data are shown in Figure 1A,B.
Figure 1.
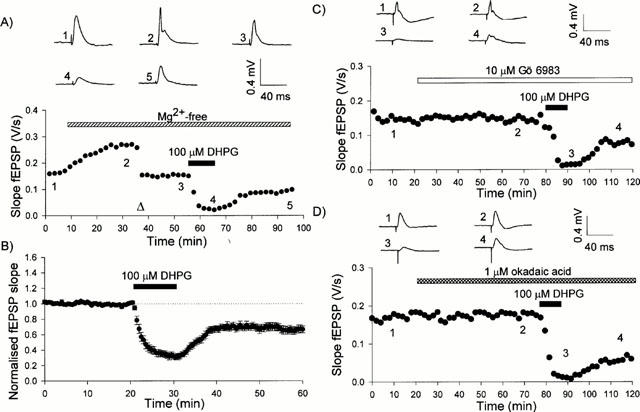
(A) Shows a representative control experiment of DHPG-induced LTD. The graph in the lower portion of (A) plots the average slope of four successive responses versus time and illustrates the effect of omitting Mg2+ from the superfusate. The stimulus intensity was reduced at the time indicated by the arrowhead. Drugs were administered for the times indicated by the bars. The numbered traces in the upper portion of (A) are averages of four successive responses obtained at the times indicated on the graph in the lower portion of (A). (B) Pooled data for all 15 experiments in which DHPG (100 μM, 10 min) was applied under these conditions. (C) A representative experiment of DHPG-induced LTD in the presence of Gö 6983. The graph in the lower portion of (C) plots the average slope of four successive responses versus time. The numbered traces in the upper portion of (C) are averages of four successive responses obtained at the times indicated on the graph in the lower portion of (C). (D) Shows a representative experiment of DHPG-induced LTD in the presence of okadaic acid. The graph in the lower portion of (D) plots the average slope of four successive responses versus time. The numbered traces in the upper portion of (D) are averages of four successive responses obtained at the times indicated on the graph in the lower portion of (D).
Lack of effect of PKC and PKA inhibitors on DHPG-induced LTD
The PKC inhibitor, Gö 6983 (10 μM), superfused for 60 min before and during the application of 100 μM DHPG, had no effect on basal synaptic transmission. It also failed to affect the peak depression of synaptic transmission induced during the application of DHPG or the stable depression induced following washout of DHPG (Figures 1C and 2A). Thus, the stable depression induced 30 min following washout of DHPG was 43±8% of control values (n=6). The other kinase inhibitors tested, Gö 6976 (10 μM), KT 5720 (10 μM) and staurosporine (10 μM), were similarly without effect on basal synaptic transmission, on the peak level of depression or on the stable level of depression induced by DHPG. Thus, 30 min following washout of DHPG, the level of LTD was 33±6% (n=5), 31±9% (n=6) and 41±4% (n=7), respectively (Figure 2B,C,D).
Figure 2.
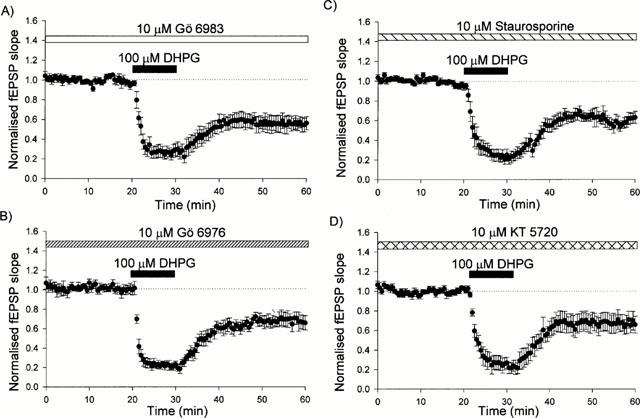
DHPG induces LTD in the presence of Gö 6983, Gö 6976, staurosporine or KT 5720. (A) Pooled data for all experiments where DHPG (100 μM, 10 min) was applied in the presence of 10 μM Gö 6983 (n=6). (B) Pooled data for all experiments in which DHPG (100 μM, 10 min) was applied in the presence of 10 μM Gö 6976 (n=5). (C) Pooled data for all experiments in which DHPG (100 μM, 10 min) was applied in the presence of 10 μM staurosporine (n=7). (D) Pooled data for all experiments in which DHPG (100 μM, 10 min) was applied in the presence of 10 μM KT 5720 (n=6).
Protein phosphatase inhibitors facilitate DHPG-induced LTD
The PP1/PP2A inhibitor, okadaic acid, had no effect on either basal synaptic transmission or on the peak depression induced by DHPG. However, in the presence of 1 μM okadaic acid there was much less recovery from the depression induced by DHPG, such that 30 min following washout, the level of depression was 52±5% (n=11; Figures 1D and 3A). This effect was concentration-dependent since the level of depression induced 30 min following washout of 0.3 μM okadaic acid (33±5 %; n=5) was similar to control values (Figure 4). In contrast, in the presence of the inactive analogue of okadaic acid, 1-norokadaone (1 μM), the size of LTD was comparable with control conditions (22±8%, n=5; Figure 3B). Calyculin A (1 μM) had similar effects to okadaic acid. Thus, it had no effect on basal synaptic transmission or on the peak depression induced by DHPG. However, 30 min following washout of DHPG, the level of depression (59±9%; n=7) was considerably greater than in controls (Figure 3C).
Figure 3.
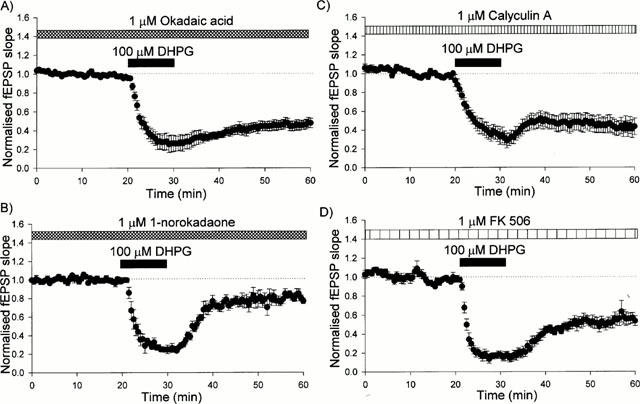
DHPG-induced LTD is enhanced in the presence of okadaic acid or calyculin A. (A) Pooled data for all experiments in which DHPG (100 μM, 10 min) was applied in the presence of 1 μM okadaic acid (n=11). (B) Pooled data for all experiments in which DHPG (100 μM, 10 min) was applied in the presence of 1 μM 1-norokadaone (n=5). (C) Pooled data for all experiments in which DHPG (100 μM, 10 min) was applied in the presence of 1 μM calyculin A (n=7). (D) Pooled data for all experiments in which DHPG (100 μM, 10 min) was applied in the presence of 1 μM FK 506 (n=6).
Figure 4.
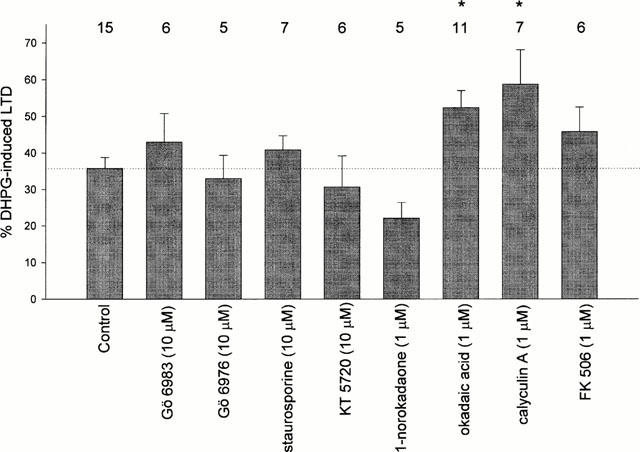
A summary of DHPG-induced LTD under the conditions tested. The histogram illustrates the mean±s.e.mean percentage LTD induced by DHPG (100 μM, 10 min). The measurement was made after a plateau effect was achieved (20–30 min following washout of DHPG). The number above each bar indicates the number of slices (=rats) tested. Control experiments were interlaced with various experimental conditions. In all cases, DHPG induced significant LTD (P<0.05), and in the presence of okadaic acid (1 μM) or calyculin A (1 μM) this was significantly larger than control values (P<0.05). The statistics were performed using Student's t-test or ANOVA followed by Dunnett's test as appropriate. *P<0.05.
The PP2B inhibitor, FK 506 (1 μM), also had no effect on basal synaptic transmission or on the peak depression induced by DHPG. In contrast to the PP1/PP2A inhibitors, FK 506 (Liu et al., 1991; Fruman et al., 1992; Antoni et al., 1993), also had little effect on DHPG-induced LTD (Figure 3D). The data from all of these experiments are summarized in Figure 4.
Effects of kinase and phosphatase inhibitors on MCPG-induced reversal of DHPG-induced LTD
In a subset of the experiments we applied MCPG to determine whether its ability to reverse pre-established DHPG-induced LTD was sensitive to any of the kinase or phosphatase inhibitors used. In agreement with previous studies (Palmer et al., 1997; Schnabel et al., 1999a,1999b), under control conditions 1 mM MCPG reversed the level of depression by circa 80% (Figure 5). A similar level of reversal was observed in the presence of Gö 6983, Gö 6976, KT5720, FK506 or 1-norokadaone. In contrast, the ability of MCPG to reverse DHPG-induced LTD was attenuated in the presence of either okadaic acid or calyculin A (Figure 5).
Figure 5.
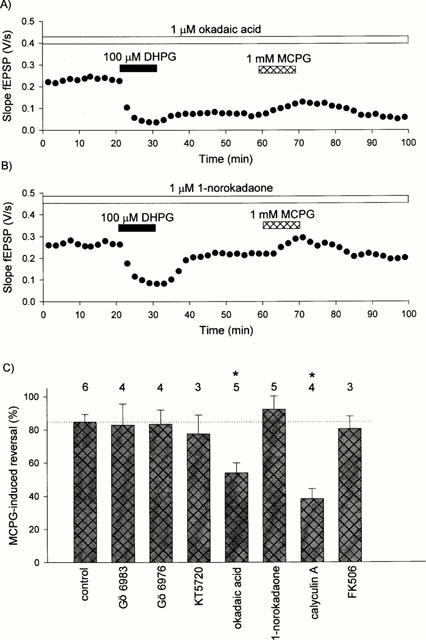
Effects of various treatments on MCPG-induced reversal of DHPG-induced LTD. (A) A single example to show a small reversal of DHPG-induced LTD by MCPG in the presence of okadaic acid. (B) A single example showing practically full reversal of DHPG-induced LTD by MCPG in the presence of 1-norokadaone. (C) Pooled data for all the experimental manipulations. *P<0.05 (ANOVA followed by Dunnett's test).
Discussion
The results of the present study, combined with those from our previous work (Schnabel et al., 1999b) strongly suggest that neither PKC nor PKA is involved in DHPG-induced LTD. However, the observation that two inhibitors of protein phosphatases, okadaic acid and calyculin A (Ishihara et al., 1989), potentiate DHPG-induced LTD suggests that some form of phosphorylation is involved in the induction, or a facilitation, of this process and that this phosphorylation is actively opposed by dephosphorylation by PP1 or PP2A.
No evidence for a role of PKC in DHPG-induced LTD
Our previous findings that neither chelerythrine nor Ro 31-8220 affected DHPG-induced LTD implied that PKC was not involved in DHPG-induced LTD. However, at that time we could not exclude the involvement of an isoform of PKC that is resistant to the actions of these inhibitors and so we have extended the range of PKC inhibitors used. The concentration (10 μM) of Gö 6976 and Gö 6983 used here is many fold higher than the effective IC50 values of these agents in vitro (<0.01 μM) and so, even taking account of the higher ATP concentrations in slices than in the in vitro assays used to determine their potency, full inhibition of their target PKC isoforms seems likely (Martiny-Baron et al., 1993; Gschwendt et al., 1996). Furthermore, in a parallel study using Gö 6983 we were able to fully block a synaptically-mediated effect in hippocampal slices at a concentration of 0.1 μM (Bortolotto & Collingridge, 2000). Gö 6983 and Gö 6976 have been shown to be potent (sub-micromolar) inhibitors of various PKC isoforms (α, β, γ, δ and ξ) and (α, β1 and μ), respectively whereas Ro 31-8220 has additionally been shown to be an inhibitor of the ε isoform (Martiny-Baron et al., 1993; Wilkinson et al., 1993; Gschwendt et al., 1996). Therefore, although we cannot totally discount the involvement of a different isoform of PKC, the lack of action of the tested PKC inhibitors makes it extremely unlikely that PKC is involved in the induction of DHPG-induced LTD.
This conclusion is not readily compatible with the observation that DHPG-induced LTD in the dentate gyrus is blocked by the one PKC inhibitor (bisindolylmaleimide I) tested in this region (Cambodeca et al., 1999). In addition, it has been reported that a peptide inhibitor of PKC (PKC19–36) inhibits synaptically-induced mGlu receptor-dependent LTD in the CA1 region of the hippocampus (Oliet et al., 1997). There are a number of possible explanations for these differences. First, the effects of bisindolylmaleimide I and PKC19–36 may be due to non-specific actions of these inhibitors. Secondly, the critical PKC isoform involved in mGlu receptor-dependent LTD may be sensitive to these two agents but not to Gö 6976, Gö 6983, Ro 31-8220, chelerythrine or staurosporine. Thirdly, multiple forms of mGlu receptor-dependent LTD may exist, some but not all of which require the activation of PKC.
No evidence for a role of PKA in DHPG-induced LTD
Since neither PKC nor release of Ca2+ from intracellular stores (Schnabel et al., 1999b) seem to be involved in DHPG-induced LTD in the CA1 region of the hippocampus, we tested an inhibitor of PKA, KT 5720 (Kase et al., 1987), on this parameter as PKA has been implicated in synaptically-induced LTD (Brandon et al., 1995; Qi et al., 1996). This inhibitor was also without any effect, despite being used at a concentration 10 fold higher than that which is effective in hippocampal slices (Lee et al., 2000). Consistent with this finding, another PKA inhibitor, H89, had no effect on DHPG-induced LTD in the dentate gyrus (Cambodeca et al., 1999). Finally, the observation that a high concentration of staurosporine had no discernible action also argues against a role of PKA, as well as some other protein kinases, including PKC, myosin-light-chain kinase and certain tyrosine kinases (Tamaoki et al., 1986; Nakano et al., 1987; Watson et al., 1988; Elliott et al., 1990; Yanagihara et al., 1991).
Evidence for a role of protein phosphatases in DHPG-induced LTD
Since the attempts to identify a role for serine/threonine protein kinases in DHPG-induced LTD failed, we used a different strategy. We reasoned that if phosphorylation by one or more such kinases was involved in DHPG-induced LTD, then inhibition of serine/threonine protein phosphatases might potentiate DHPG-induced LTD. The finding that both okadaic acid and calyculin A, but not FK 506, had this effect infers that some form of phosphorylation is important in DHPG-induced LTD and that this is regulated by PP1 and/or PP2A, but not by PP2B. These results distinguish DHPG-induced LTD from NMDA receptor-mediated LTD, which is inhibited by these same phosphatase inhibitors (Mulkey et al., 1993; 1994; Hodgkiss & Kelly, 1995). This modulation by PP1/PP2A implicates an unknown protein kinase (PKx), the activation of which mediates or facilitates DHPG-induced LTD.
On the role of CaMKII in DHPG-induced LTD
Based on our earlier observation using a calcium/calmodulin protein kinase inhibitor, KN-62, we suggested that CaMKII may be involved in the regulation of DHPG-induced LTD (Schnabel et al., 1999a). However, CaMKII cannot be the postulated PKx since CaMKII acts to inhibit DHPG-induced LTD, possibly by the mediation of an opposing DHPG-induced LTP process.
On the mechanism of MCPG-induced reversal of DHPG-induced LTD
The observation that neither Gö 6976 nor Gö 6983, together with our previous observations that neither Ro 31-8220 nor chelerythrine, affect MCPG-induced reversal strongly argues against a role for PKC in this effect. Similarly, the observation that KT 5720 was ineffective suggests that PKA is not involved either. However, the finding that the PP1/PP2A, but not the PP2B, phosphatase inhibitors reduced MCPG-induced reversal raises the possibility that activation of one or both of these phosphatases mediates the process.
DHPG-induced LTD may be explained if the level of synaptic transmission is determined by the net activity of some protein kinase and PP1/PP2A. Activation of mGlu receptors by DHPG switches the receptor-effector cascade into a mode that promotes phosphorylation. Application of MCPG is able to temporarily reverse the effect via transient inhibition of this activation, which in turn enables dephosphorylation via PP1/PP2A. The effects of MCPG are mimicked by other mGlu receptor antagonists, such as LY341495 (Fitzjohn et al., 1998) and LY393053 (Fitzjohn et al., 1999), and so it is not a unique property of MCPG. One explanation for these effects is that DHPG application primes mGlu5 receptors so that they become tonically activated by endogenous L-glutamate. A second possibility is that mGlu receptor agonists, such as DHPG, and mGlu receptor antagonists, such as MCPG, switch the mode of activity of mGlu5 receptors (i.e., act as agonists and inverse agonists) with respect to the downstream phosphorylation events.
Concluding remarks
The present observation that inhibition of certain protein phosphatases (PP1/PP2A) elicits an effect similar to inhibition of a protein kinase by KN-62 (probably CaMKII; Schnabel et al., 1999a) indicates that DHPG-induced LTD involves, and is regulated by, specific phosphatases and kinases. The mechanism by which inhibition of protein phosphatases can potentiate DHPG-induced LTD is a matter for speculation. In this context, it has been shown that activation of protein phosphatases reverses desensitization of mGlu5 receptor-mediated events (Alagarsamy et al., 1999). However, this effect is unlikely to account for the present observations since the reported desensitization involved an activation of PKC and was reversed by inhibitors of PP2B. Recently, it has been suggested that DHPG-induced LTD involves rapid protein synthesis since it is antagonized by anisomycin (Huber et al., 2000). Further studies are required to understand the pathways linking mGlu receptor activation to protein synthesis and how this might be regulated by protein kinases and phosphatases.
Acknowledgments
Supported by the MRC and Knoll Ltd./BASF Pharma. We are most grateful to Bill Anderson for providing the computer programmes.
Abbreviations
- DHPG
(R,S)-3,5-dihydroxyphenylglycine
- LTD
long-term depression
- mGlu receptor
metabotropic glutamate receptor
- MCPG
(S)-α-methyl-4-carboxyphenylglycine
- PKA
protein kinase A
- PKC
protein kinase C
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
- PP2B
protein phosphatase 2B
References
- ALAGARSAMY S., MARINO M.J., ROUSE S.T., GEREAU IV R.W., HEINEMANN S.F., CONN P.J. Activation of NMDA receptors reverses desensitisation of mGluR5 in native and recombinant systems. Nature Neurosci. 1999;2:234–240. doi: 10.1038/6338. [DOI] [PubMed] [Google Scholar]
- ANDERSON W.W., COLLINGRIDGE G.L. A data acquisition program for on-line analysis of long-term potentiation and long-term depression. Soc. Neurosci. Abstr. 1997;264:19. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- ANTONI F.A., SHIPSTON M.J., SMITH S.M. Inhibitory role for calcineurin in stimulus-secretion coupling revealed by FK506 and cyclosporin A in pituitary corticotrope tumor cells. Biochem. Biophys. Res. Comm. 1993;194:220–233. doi: 10.1006/bbrc.1993.1808. [DOI] [PubMed] [Google Scholar]
- BASHIR Z.I., JANE D.E., SUNTER D.C., WATKINS J.C., COLLINGRIDGE G.L. Metabotropic glutamate receptors contribute to the induction of long-term depression in the CA1 region of the hippocampus. Eur. J. Pharmacol. 1993;239:265–266. doi: 10.1016/0014-2999(93)91009-c. [DOI] [PubMed] [Google Scholar]
- BEAR M.F., ABRAHAM W.C. Long-term depression in hippocampus. Annu. Rev. Neurosci. 1996;19:437–443. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- BLAKE J.F., BROWN M.W., COLLINGRIDGE G.L. CNQX blocks acidic amino acid induced depolarisations and synaptic components mediated by non-NMDA receptor in rat hippocampal slices. Neuroscience. 1988;89:182–186. doi: 10.1016/0304-3940(88)90378-3. [DOI] [PubMed] [Google Scholar]
- BOLSHAKOV V.Y., SIEGELBAUM S.A. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- BORTOLOTTO Z.A., COLLINGRIDGE G.L. A role for protein kinase C in a form of metaplasticity that regulates the induction of long-term potentiation at CA1 synapses of the adult rat hippocampus. Eur. J. Pharmacol. 2000;12:4055–4062. doi: 10.1046/j.1460-9568.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- BRANDON E.P., ZHUO M., HUANG Y.-Y., QI M., GERHOLD K.A., BURTON K.A., KANDEL E.R., MCKNIGHT G.S., IDZHERDA R.L. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RIβ subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMBODECA N., ROWAN M.J., ANWYL R. Involvement of group 1 mGluR and activation of PKC and tyrosine kinase in the induction of LTD in the dentate gyrus of the hippocampus in vitro. Neuropharmacology. 1999;38:1597–1606. doi: 10.1016/s0028-3908(99)00093-3. [DOI] [PubMed] [Google Scholar]
- ELLIOTT L.H., WILKINSON S.E., SEDGWICK A.D., HILL C.H., LAWTON G., DAVIES P.D., NIXON J.S. K252a is a potent and selective inhibitor of phosphorylase kinase. Biochem. Biophys. Res. Comm. 1990;171:148–154. doi: 10.1016/0006-291x(90)91369-4. [DOI] [PubMed] [Google Scholar]
- FITZJOHN S.M., BORTOLOTTO Z.A., PALMER M.J., DOHERTY A.J., ORNSTEIN P.L., SCHOEPP D.D., KINGSTON A.E., LODGE D., COLLINGRIDGE G.L. The potent mGlu receptor antagonist LY341495 identifies roles for both cloned and novel mGlu receptors in hippocampal synaptic plasticity. Neuropharmacology. 1998;37:1445–1458. doi: 10.1016/s0028-3908(98)00145-2. [DOI] [PubMed] [Google Scholar]
- FITZJOHN S.M., KINGSTON A.E., LODGE D., COLLINGRIDGE G.L. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology. 1999;38:1577–1583. doi: 10.1016/s0028-3908(99)00123-9. [DOI] [PubMed] [Google Scholar]
- FRUMAN D.A., KLEE C.B., BIERER B.E., BURAKOFF S.J. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3686–3690. doi: 10.1073/pnas.89.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GSCHWENDT M., DIETERICH S., RENNECKE J., KITTSTEIN W., MUELLER H.-J., JOHANNES F.-J. Inhibition of protein kinase C μ by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- HODGKISS J.P., KELLY J.S. Only “de novo” long-term depression (LTD) in rat hippocampus in vitro is blocked by the same low concentration of FK 506 that blocks LTD in the visual cortex. Brain Res. 1995;705:241–246. doi: 10.1016/0006-8993(95)01168-4. [DOI] [PubMed] [Google Scholar]
- HUBER K.M., KAYSER M.S., BEAR M.F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1256. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- ISHIHARA H., MARTIN B.L., BRAUTIGAN D.L., KARAKI H., OZAKI H., KATO Y., FUSETANI N., WATABE S., HASHIMOTO K., UEMURA D., HARTSHORNE J. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Comm. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- ITO I., KOHDA A., TANABE S., HIROSE E., HAYASHI M., MITSUNAGA S., SUGIYAMA H. 3,5-Dihydroxyphenylglycine: a potent agonist of metabotropic glutamate receptors. NeuroReport. 1992;3:1013–1016. [PubMed] [Google Scholar]
- KASE H., IWAHASHI K., NAKANISHI S., MATSUDA Y., YAMADA K., TAKAHASHI M., MURAKATA C., SATO A., KANEKO M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem. Biophys. Res. Comm. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- LEE H.-K., BARBAROSIE M., KAMEYAMA K., BEAR M.F., HUGANIR R.L. Regulation of distinct AMPA receptor phosphorylation sites during bi-directional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- LIU J., FARMER J.D. , JR, LANE W.S., FRIEDMAN J., WEISSMAN I., SCHREIBER S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- MARTINY-BARON G., KAZANIETZ M.G., MISCHAK H., BLUMBERG P.M., KOCHS G., HUG H., MARMÉ D., SCHÄCHTELE C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- MULKEY R.M., ENDO S., SHENOLIKAR S., MALENKA R.C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:468–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- MULKEY R.M., HERRON C.E., MALENKA R.C. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- NAKANO H., KOBAYASHI E., TAKAHASHI I., TAMAOKI T., KUZUU Y., IBA H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J. Antibiotics. 1987;40:706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- OLIET S.H.R., MALENKA R.C., NICOLL R.A. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- O'MARA S.M., ROWAN M.J., ANWYL R. Metabotropic glutamate receptor-induced homosynaptic long-term depression and depotentiation in the dentate gyrus of the rat hippocampus in vitro. Neuropharmacology. 1995;34:983–989. doi: 10.1016/0028-3908(95)00062-b. [DOI] [PubMed] [Google Scholar]
- OVERSTREET L.S., PASTERNAK J.F., COLLEY P.A., SLATER N.T., TROMMER B.L. Metabotropic glutamate receptor mediated long-term depression in developing hippocampus. Neuropharmacology. 1997;36:831–844. doi: 10.1016/s0028-3908(97)00031-2. [DOI] [PubMed] [Google Scholar]
- PALMER M.J., IRVING A.J., SEABROOK G.R., JANE D.E., COLLINGRIDGE G.L. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- QI M., ZHUO M., SKÅLHEGG B.S., BRANDON E.P., KANDEL E.R., MCKNIGHT G.S., IDZHERDA R.L. Impaired hippocampal plasticity in mice lacking the Cβ1 catalytic subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1571–1576. doi: 10.1073/pnas.93.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNABEL R., KILPATRICK I.C., COLLINGRIDGE G.L. An investigation into signal transduction mechanisms involved in DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology. 1999b;38:1585–1596. doi: 10.1016/s0028-3908(99)00062-3. [DOI] [PubMed] [Google Scholar]
- SCHNABEL R., PALMER M.J., KILPATRICK I.C., COLLINGRIDGE G.L. A CaMKII inhibitor, KN-62, facilitates DHPG-induced LTD in the CA1 region of the hippocampus. Neuropharmacology. 1999a;38:605–608. doi: 10.1016/s0028-3908(98)00229-9. [DOI] [PubMed] [Google Scholar]
- TAMAOKI T., HISAYO N., ISAMI T., KATO Y., MORIMOTO M., TOMITA F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem. Biophys. Res. Comm. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- WATSON S.P., MCNALLY J., SHIPMAN L.J., GODFREY P.P. The action of the protein kinase C inhibitor, staurosporine, on human platelets. Evidence against a regulatory role for protein kinase C in the formation of inositol trisphosphate by thrombin. Biochem. J. 1988;249:345–350. doi: 10.1042/bj2490345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON S.E., PARKER P.J., NIXON J.S. Isoenzyme specificity of bisindolmaleimides, selective inhibitors of protein kinase C. Biochem. J. 1993;294:335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANAGIHARA N., TACHIKAWA E., IZUMI F., YASUGAWA S., YAMAMOTO H., MIYAMOTO E. Staurosporine: an effective inhibitor for Ca2+calmodulin-dependent protein kinase II. J. Neurochem. 1991;56:294–298. doi: 10.1111/j.1471-4159.1991.tb02595.x. [DOI] [PubMed] [Google Scholar]


