Abstract
The endogenous cannabinoid agonist, anandamide produced a modest contractile response in guinea-pig isolated bronchus compared with the vanilloid receptor agonist capsaicin. The contractile response to both anandamide and capsaicin was inhibited by the vanilloid receptor antagonist, capsazepine. Furthermore, the NK2-selective antagonist, SR48968 but not the NK1-selective antagonist, SR140333 inhibited contractile responses to anandamide.
The contractile response to anandamide was abolished in tissues desensitized by capsaicin. However, anandamide failed to cross-desensitize the contractile response to capsaicin.
The contractile response to anandamide was not significantly altered in the presence of the CB1 receptor antagonist, SR141716A, nor the amidase inhibitor, phenylmethylsulphonyl fluoride (PMSF) but was significantly increased in the presence of the neutral endopeptidase inhibitor, thiorphan.
The cannabinoid agonist, CP55,940 failed to significantly attenuate the excitatory non-adrenergic non-cholinergic (eNANC) response in guinea-pig airways. In contrast, the ORL1 receptor agonist, nociceptin, significantly inhibited this response.
The results demonstrate that anandamide induces a modest contractile response in guinea-pig isolated bronchus that is dependent upon the activation of vanilloid receptors on airway sensory nerves. However, cannabinoid receptors do not appear to play a role in this regard, nor in regulating the release of neuropeptides from airway sensory nerves under physiological conditions.
Keywords: Cannabinoids, anandamide, sensory nerves, airway, guinea-pig, capsaicin, non-adrenergic non-cholinergic
Introduction
The archetypal vanilloid receptor agonist capsaicin is known to activate a ligand-gated channel that was recently cloned (Caterina et al., 1997) and the similarity in structure between the cannabinoid receptor agonist anandamide and the vanilloid agonist, olvanil, led to the discovery that anandamide is an activator of vanilloid receptors (Zygmunt et al., 1999; Smart et al., 2000). This substance is thus a potential candidate for the much sought after endogenous vanilloid receptor agonist (Szallasi & Blumberg, 1999) that joins a growing list of endogenous activators/modulators of the vanilloid receptor, including inflammatory mediators (Stucky et al., 1998; Vyklicky et al., 1998), hydrogen ions (Caterina et al., 1997; Vyklicky et al., 1998), heat (Caterina et al., 1997; Tominaga et al., 1998), arachidonic acid (Manzini et al,. 1989; Manzini & Meini, 1991), lipoxin A4 (Meini et al., 1992), products of the lipoxygenase pathway (Hwang et al., 2000), and prostacylin (Mapp et al., 1991). In contrast, anandamide has recently been shown to inhibit the release of neuropeptides from sensory nerves in the rat via a CB1-dependent mechanism (Richardson et al., 1998a,1998b) indicating that anandamide may have both inhibitory and excitatory actions on sensory nerves.
We have previously shown that bronchial hyperresponsiveness mediated by platelet activating factor (Spina et al., 1991) and 15-hydroperoxyeicosatetraenoic acid (Riccio et al., 1997) in naïve rabbits and allergen challenge in immunized rabbits (Riccio et al., 1993) is abolished following chronic treatment with capsaicin. This is also consistent with numerous studies showing that bronchial hyperresponsiveness induced by toluene diisocyanate, virus, and allergen in guinea-pigs is also attenuated by capsaicin treatment (reviewed in Spina et al., 1998). Together these studies indicate that sensory nerves may be a common pathway by which many stimuli can induce bronchial hyperresponsiveness and consistent with the hypothesis that ‘hyperalgesia' of airway sensory nerves may contribute toward this phenomenon (Adcock & Garland, 1993; Spina et al., 1998). The finding that inflammatory mediators (Stucky et al., 1998; Vyklicky et al., 1998), hydrogen ions (Caterina et al., 1997; Vyklicky et al., 1998), heat (Caterina et al., 1997; Tominaga et al., 1998) and arachidonic acid metabolites (Manzini et al., 1989; Manzini & Meini, 1991; Mapp et al., 1991; Meini et al., 1992; Hwang et al., 2000) can activate the vanilloid receptor suggest that these agents may be potential endogenous mediators that are released during an inflammatory response, and can sensitize airway sensory nerves and induce bronchial hyperresponsiveness.
Guinea-pig isolated bronchus is a useful biological readout of neuropeptide release from airway sensory nerves and we have shown that various pharmacological agents including the phosphodiesterase (PDE)4 inhibitor, Ro-201724 (Spina et al., 1995); the phosphatase 1/2A inhibitor, okadaic acid (Harrison et al., 1997) and the ORL1-receptor agonist, nociceptin (Shah et al., 1998) attenuated the eNANC response in this preparation. We have therefore investigated whether anandamide stimulates vanilloid receptors on airway sensory nerves and the role of cannabinoid receptors in modulating the eNANC response in guinea-pig airways.
Methods
Tissue preparation
Male albino guinea-pigs (300–400 g) were killed by cervical dislocation and the lungs removed and placed in cold (4°C) Krebs-Henseleit solution aerated with 95% O2 and 5% CO2. Main bronchial rings (2 mm) were suspended under 0.5 g tension, in 12 ml organ baths in Krebs-Henseleit solution aerated with 95% O2 and 5% CO2 at 37°C, containing the cyclo-oxygenase inhibitor, indomethacin (5 μM). Changes in tension were measured, via a FTO3C transducer, and recorded using Maclab (version 3.3.8). Tissues were allowed to equilibrate for 40 min with changes in Krebs-Henseleit solution being made at 10 min intervals. Methacholine (1 and 100 μM) was added cumulatively to the bath to establish the sensitivity of the tissue and after the contractile response had reached plateau, the tissues were washed for 10 min and allowed to equilibrate for a further 30 min.
Exogenous administration of substances
Tissues were incubated in the presence of the neural endopeptidase inhibitor, thiorphan (10 μM) for a period of 30 min. Bronchomotor tone was measured following administration of anandamide, R-(+)-methanandamide (1–100 μM), 2-arachidonylglycerol (0.1–100 μM) and capsaicin (0.01–10 μM). In other experiments, the contractile response to anandamide was evaluated in the absence or presence of the vanilloid receptor agonist, capsazepine (10 μM), the CB1 receptor antagonist SR141716A (1 μM), the amidase inhibitor, PMSF (50 μM), the NK1-receptor antagonist, SR140333 (1 μM) and the NK2-receptor antagonist, SR48968 (100 nM). In other experiments, the response to anandamide was evaluated in the absence or presence of the neutral endopeptidase inhibitor, thiorphan (10 μM). Responses to the cannabinoid agonists were expressed as a percentage of the maximal response to capsaicin (10 μM).
Desensitization studies
In other experiments, tissues were exposed to capsaicin (10 μM) in the absence of thiorphan (to facilitate removal of endogenously released neuropeptides from the biophase) and the contractile response allowed to achieve plateau. The tissues were incubated with capsaicin for a period of 20 min and then repeatedly washed over a 45–60 min period. A second response to capsaicin was performed in order to confirm that desensitization had occurred. Tissues were then incubated in the presence of thiorphan and then anandamide was added to the bath in increasing concentrations, 30 min later. Following completion of the dose response curve to anandamide, capsaicin (10 μM) was again added. Control tissues were treated in a similar fashion except that they were exposed to ethanol (0.01%).
In further experiments, tissues were treated with anandamide (100 μM) in the absence of thiorphan for a period of 50 min. The tissues were washed repeatedly over a 30–60 min period and a second application of anandamide was then performed. Using this protocol, the tissues failed to respond to anandamide (100 μM). Tissues were then incubated with thiorphan for a 30 min period and a concentration-response curve to capsaicin was then performed.
Electrical field stimulation studies
Guinea-pig isolated main bronchi were placed between two platinum electrodes and electrically stimulated (3 Hz, 15 s, 0.5 ms pulse width, 40 V, I=750 mAmp). Tissues were incubated for 30 min in the presence of atropine (1 μM), the non-selective β-antagonist, propranolol (0.1 μM) and the neutral endopeptidase inhibitor, thiorphan (10 μM), and electrically stimulated (S1). The resulting eNANC response returned to baseline after 30 min. Tissues were incubated with the cannabinoid receptor agonist, CP55,940 (1 and 10 μM) and the ORL1 receptor agonist, nociceptin (1 μM) 10 min prior to the second electrical stimulation (S2). The effect of agonist or vehicle on the eNANC response is expressed as per cent control (i.e. S2/S1).
Analysis of results
Results from all experiments are expressed as mean±standard error of mean where n denotes the number of animals. Where appropriate, differences between mean values were assessed using Student's paired or non-paired t-test. Differences between treatments were also assessed using analysis of variance (ANOVA). Differences between mean values were considered significant if P<0.05.
Drugs
Atropine, indomethacin, methacholine, (-)-propranolol, thiorphan (Sigma-Aldrich Chemical Co., Dorset, U.K.); anandamide, R-(+)-methanandamide, CP55,940, phenylmethylsulphonyl fluoride (PMSF) (Tocris Cookson, Bristol, U.K.); SR141716A, SR140333, SR48968 (Sanofi Recherche, Montpellier Cedex, France); 2-arachidonylglycerol (RBI, St Louis, Missouri, U.S.A.). Composition of Krebs-Henseleit solution (mM): NaCl 117.6, NaHCO3 25, Glucose 11.1, KH2PO4 1.03, MgSO4.7H2O 0.57, KCl 5.4 and CaCl2 2.5. Unless otherwise specified all drugs were prepared in Krebs-Henseleit solution. Stock concentrations of indomethacin (0.01 M) and thiorphan (0.01 M) were prepared in 0.5% Na2CO3 and 5% Na2CO3 respectively. Anandamide obtained from Tocris Cookson came prepared as a 10 mg ml−1 emulsion in soya oil/water (1 : 4) and dilutions made in Krebs-Henseleit solution. Stock concentrations of CP55,940, SR141716A and PMSF (0.01 M) and dilutions were prepared in dimethylsulphoxide (DMSO) such that bath concentrations of DMSO did not exceed 0.2%. Stock concentrations of capsaicin (0.1 M), SR48968 and SR140333 (1 mM) were prepared in ethanol. The resulting bath concentration of ethanol did not exceed 0.1%.
Results
Effect of anandamide on bronchomotor tone
The cannabinoid agonist anandamide, produced a concentration-dependent contraction of guinea-pig isolated bronchus (Figure 1). The contractile response to anandamide (pD2=5.26±0.05, per cent capsaicin Emax=41.6±5.8, n=8) was significantly less than that observed for capsaicin (Figure 2, P<0.05 ANOVA). Capsaicin induced a concentration-dependent contraction of guinea-pig bronchial tissue yielding a contractile potency (pD2=−log10 EC50) of 6.594±0.174, n=7) and maximum response (g tension) of 1.13±0.11. The synthetic analogue R-(+)-methanandamide (pD2= 4.84±0.05, per cent capsaicin Emax=32.2±7.2, n=7) also induced a concentration-dependent contraction of guinea-pig isolated bronchus that was also significantly less potent than capsaicin (P<0.05, Figure 2). In contrast, the cannabinoid agonist, 2-arachidonylglycerol failed to significantly increase baseline tone in guinea-pig bronchial preparations (n=3). The vehicle for anandamide (soya oil/water, 0.1%) or methanandamide (ethanol, 0.1%) failed to alter baseline tone in guinea-pig isolated bronchus.
Figure 1.
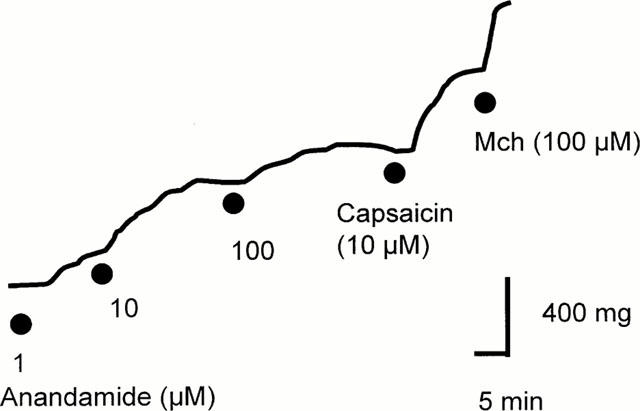
Line representation of the contractile response to increasing concentrations of anandamide. Mch; Methacholine.
Figure 2.
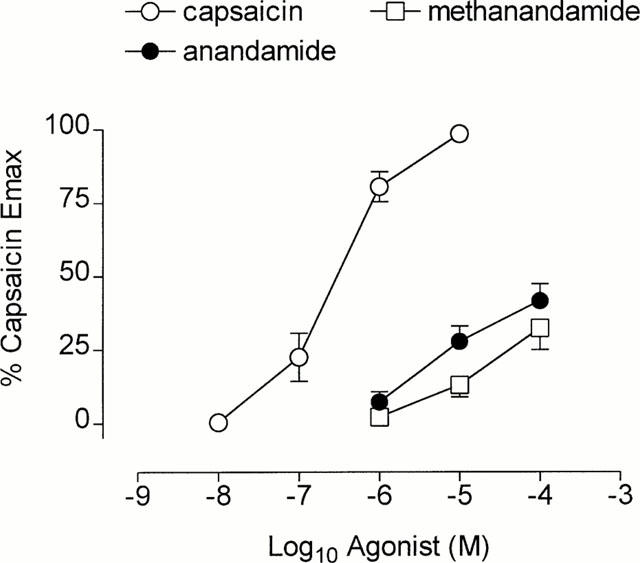
Cumulative concentration effect curves to capsaicin (n=7), anandamide (n=8) and R-(+)-methanandamide (n=7). Each point represents the mean and vertical lines represent standard error of the mean.
The vanilloid receptor antagonist capsazepine (10 μM) significantly attenuated the contractile response to capsaicin (pD2 control, 6.88±0.88 vs capsazepine 5.97±0.11, n=6 each, P<0.05; Figure 3a) which yielded an apparent KB value of −5.85±0.12. Similarly, the contractile response to anandamide was also antagonized by capsazepine (pD2 control, 5.21±0.14 vs capsazepine 4.64±0.18, n=5, P<0.05, Figure 3b), yielding an apparent KB value of −5.42±0.18.
Figure 3.

Cumulative concentration effect curves to (a) capsaicin (n=6 each) and (b) anandamide (n=6 each) in the absence or presence of the vanilloid receptor antagonist, capsazepine (10 μM). Each point represents the mean and vertical lines represent standard error of the mean.
Acute desensitization of guinea-pig isolated bronchus with capsaicin, abolished the contractile response to anandamide (Figure 4a) and capsaicin (Figure 4b). In contrast, repeated application of anandamide (100 μM), while inducing desensitization to itself, failed to significantly alter the contractile potency to capsaicin (pD2; control; 6.83±0.11 vs anandamide treated 6.79±0.06, n=5 each, P>0.05).
Figure 4.
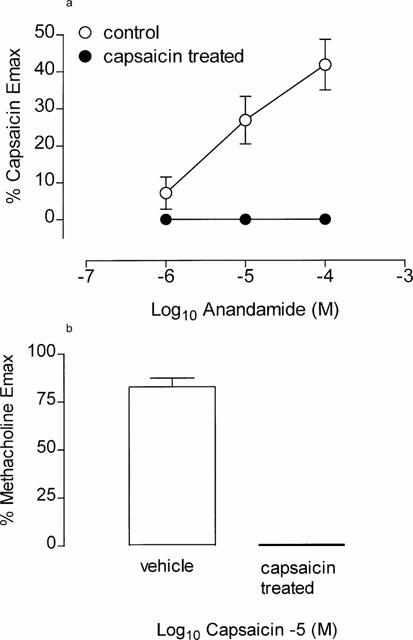
(a) Cumulative concentration effect curves to anandamide in vehicle treated (n=4) and capsaicin-desensitized tissues (n=4). (b) Bar graph representing the contractile response to maximum concentration of capsaicin (10 μM) in vehicle treated and capsaicin-desensitized tissue (n=4 each) following the anandamide concentration-effect curve. Each point represents the mean and vertical lines represent standard error of the mean.
The CB1 selective antagonist, SR141716A (1 μM; Figure 5a) failed to significantly alter the contractile potency to anandamide (control: pD2=5.25±0.10, per cent capsaicin Emax=49.6±8.3, n=5 vs SR141716A: pD2=5.24±0.24, per cent capsaicin Emax=46.7±9.4, n=5, P>0.05). Similarly, the amidase inhibitor PMSF (50 μM; Figure 5b) failed to significantly augment the contractile response to anandamide (control: pD2=5.21±0.08, per cent capsaicin Emax=37.4±83.7, n=8 vs PMSF: pD2=5.23±0.10, per cent capsaicin Emax= 46.5±6.0, n=8, P>0.05). In contrast, the neutral endopeptidase inhibitor, thiorphan (10 μM), significantly augmented the contractile response to anandamide (Figure 5c, P<0.01, ANOVA) (anandamide 100 μM; per cent capsaicin Emax= 19.6±2.8, n=6 vs thiorphan; 34.0±4.0, n=6, P<0.05).
Figure 5.
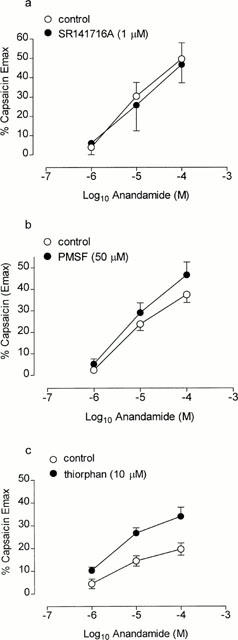
Cumulative concentration effect curves to anandamide in the absence or presence of (a) SR141716A (n=5 each), (b) phenylmethylsulphonyl fluoride (PMSF, n=8 each) and (c) thiorphan (n=6 each). Each point represents the mean and vertical lines represent standard error of the mean.
The NK1-receptor antagonist, SR140333 (1 μM) failed to significantly alter the contractile response to capsaicin (pD2, per cent methacholine Emax; control, 7.31±0.13, 74.6±7.9, n=6 vs SR140333, 7.34±0.16, 68.4±2.6, n=6, P>0.05, Figure 6a). In contrast, the NK2-selective antagonist, SR48968 (100 nM) significantly reduced the contractile response to capsaicin (per cent methacholine Emax, capsaicin 10 μM; 41.6±10.8, n=6, P<0.05 of control, Figure 6b). In further experiments, SR140333 failed to alter the contractile response to anandamide (pD2, per cent capsaicin Emax; control, 5.71±0.11, 37.0±4.3, n=6 vs SR140333; 5.71±0.16, 37.6±6.4, n=6, P>0.05, Figure 6c). In the presence of SR48968, the contractile response to anandamide was significantly reduced (per cent capsaicin Emax, anandamide 100 μM; 4.1±1.7, n=6, P<0.05 c.f. control, Figure 6d).
Figure 6.
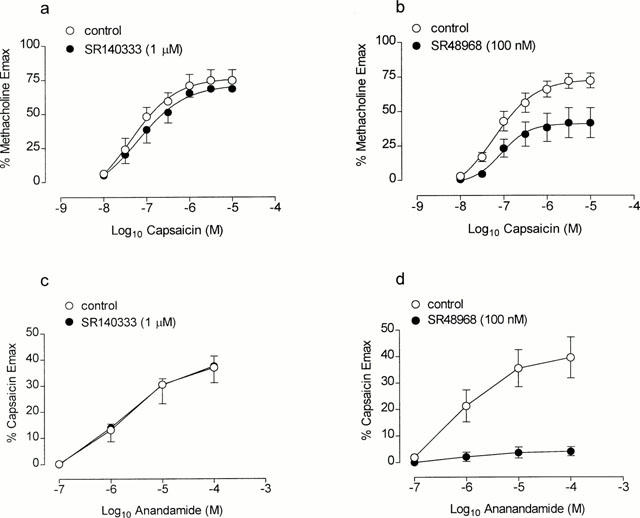
Cumulative concentration effect curves to capsaicin (a,b, n=5–6) and anandamide (c,d, n=6) in the absence or presence of the NK1- (a,c) and NK2- (b,d) selective antagonist, SR140333 (1 μM) and SR48968 (100 nM), respectively. Each point represents the mean and vertical lines represent standard error of the mean.
Electrical field stimulation
EFS (3 Hz) of guinea-pig isolated main bronchi induced a contractile response, 35±3% (n=9) of the maximum response to methacholine (100 μM; 1.24±0.13 g tension). In vehicle controls, no significant reduction in the contractile response (eNANC) to repeated EFS was observed (per cent methacholine Emax; 35±4, n=9, P>0.05 c.f. control). The cannabinoid receptor agonist CP55,940 (1 and 10 μM) did not attenuate the eNANC contractile response (Figure 7). In contrast, the ORL1-receptor agonist, nociceptin (1 μM) significantly inhibited the eNANC response in this preparation (P<0.05 c.f. control; Figure 7). Neither CP55,940 (1 and 10 μM) or vehicle (DMSO, 0.1% and 0.2% respectively) induced contraction of guinea-pig bronchial preparations.
Figure 7.
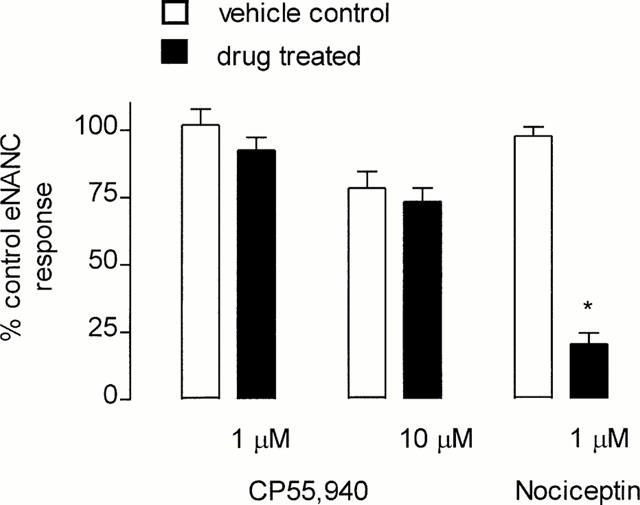
Bar graph representing the eNANC response as a percentage of control values. Tissues were either treated with vehicle or the CB1-selective agonist CP55,940 (1 μM n=9; 10 μM n=10) or the ORL1-receptor agonist, nociceptin (n=4). Vertical lines represent standard error of the mean. *P<0.05 c.f. vehicle control.
Discussion
We have demonstrated that the cannabinoid agonist, anandamide induces contraction of guinea-pig isolated bronchus via activation of airway sensory nerves, a response that was abolished by acute desensitization with capsaicin, antagonized by the vanilloid receptor antagonist, capsazepine and the NK2-receptor antagonist, SR48968. In contrast, the cannabinoid receptor agonist, CP55,940 and 2-arachidonylglycerol failed to increase bronchomotor tone suggesting that activation of cannabinoid receptors per se, does not induce the release of sensory neuropeptides in this preparation. Furthermore, cannabinoid receptors do not appear to play a role in modulating the release of sensory neuropeptides under physiological conditions in this preparation.
With the recent cloning of the vanilloid receptor (Caterina et al., 1997) there is considerable interest in the discovery of endogenous ligands for this receptor which has important implications for the development of novel analgesic agents (Szallasi & Blumberg, 1999). It has been shown that various inflammatory mediators including bradykinin, serotonin, prostaglandin E2 (Stucky et al., 1998; Vyklicky et al., 1998); heat (Caterina et al., 1997; Tominaga et al., 1998) and arachidonic acid metabolites (Mapp et al., 1991; Meini et al., 1992; Hwang et al., 2000) can activate the vanilloid receptor. Furthermore, acid pH appears to augment the ability of various agents including capsaicin, certain inflammatory mediators and heat to activate this receptor (Caterina et al., 1997; Tominaga et al., 1998; Vyklicky et al., 1998). It has recently been demonstrated that the endogenous cannabinoid, anandamide, induced vasodilation that was not antagonized by the CB1 receptor antagonist SR141716A (Wagner et al., 1999; Zygmunt et al., 1999). Furthermore, anandamide which has structural similarities to a vanilloid receptor agonist, olvanil, provided the impetus to the discovery that this endogenous cannabinoid agonist stimulates the release of neuropeptides from rat blood vessels and activates the cloned vanilloid receptor (VR)-1 receptor (Zygmunt et al., 1999; Smart et al., 2000). Our findings add further weight to this notion since anandamide stimulated contraction of guinea-pig isolated bronchus by a vanilloid receptor- and neuropeptide-dependent mechanism.
The concentration of anandamide required to elicit activation of the vanilloid receptor in the guinea-pig isolated bronchus was considerably greater than those required to activate CB1-receptors in mouse vas deferens (Devane et al., 1992; Pertwee et al., 1995; Lay et al., 2000). A number of factors could account for this difference including active metabolism of anandamide by different tissues (Pertwee et al., 1995), although we can rule out this possibility since in the presence of the amidase inhibitor PMSF, there was no significant augmentation of the contractile response to anandamide. Furthermore, R-(+)-methanandamide which is more resistant to enzymatic degradation (Pertwee et al., 1995) was not a more potent contractile agonist compared with anandamide, although it is less active at the vanilloid receptor (Smart et al., 2000). Alternatively, significant differences in the pharmacological activity of anandamide at CB1-receptors has been observed between species (Lay et al., 2000). This species difference in activity of anandamide also extends to its effects on vanilloid receptors (Zygmunt et al., 1999). We have shown that anandamide is significantly less potent than capsaicin in eliciting contractile responses in guinea-pig isolated bronchus. This is consistent with findings in guinea-pig basilar artery where anandamide is one to two orders of magnitude less potent than capsaicin in mediating relaxation of these vessels (Zygmunt et al., 1999) and at least one to two orders of magnitude less potent in activating the vanilloid receptor expressed in HEK293 cells or in rat DRG neurones compared with capsaicin (Zygmunt et al., 1999; Smart et al., 2000; Hwang et al., 2000). However, anandamide appears to behave as a partial agonist in guinea-pig isolated bronchus, consistent with the data observed for activation of vanilloid receptors in rat DRG neurones (Zygmunt et al., 1999; Smart et al., 2000; Hwang et al., 2000), but not it would appear, in rat vasculature (Zygmunt et al., 1999) or HEK293 cells expressing the human vanilloid receptor (Smart et al., 2000). While it is possible that the actual concentrations of anandamide that are achieved in the biophase may be a limiting factor in some studies, in view of the lipophilicity of this agonist (Smart et al., 2000), this is clearly not a significant factor when studying the effects of anandamide at CB1-receptors in isolated tissues (Pertwee et al., 1995; Lay et al., 2000). Our data support the view that anandamide is less efficacious than capsaicin in activating the vanilloid receptor (Zygmunt et al., 1999; Hwang et al., 2000). Furthermore, we show that the contractile response to capsaicin was unaffected by exposure to anandamide, despite producing desensitization to the contractile response to anandamide itself. In contrast, anandamide did induce cross desensitization to capsaicin in HEK293 cells expressing the human vanilloid receptor, although, this was not an unexpected finding in view of the fact that anandamide behaves as a full agonist in this preparation (Smart et al., 2000). It is clear that different stimuli induce the release of different quantities of neuropeptide from the pool of releasable peptide within sensory nerves (Hakanson et al., 1987). Therefore, it is likely that under the current experimental conditions, anandamide is not a sufficient stimulus to promote complete desensitization of the vanilloid receptor and/or deplete airway sensory nerves of their releasable pool of neuropeptides and to suppress the response to a full agonist like capsaicin. Consistent with this view is the observation that compared with capsaicin, the vanilloid receptor agonist olvanil, is less able to induce desensitization to responses evoked by capsaicin in some preparations (Dray et al., 1990; Wardle et al., 1996).
The finding that the vanilloid receptor antagonist, capsazepine, inhibited the contractile response to anandamide is further evidence that this substance activates the vanilloid receptor in this preparation. The apparent −pKB for capsazepine against capsaicin-induced contraction was 5.85 (approximately 1.4 μM) which is lower than that previously reported in this tissue (−pKB=5.12) (Belvisi et al., 1992) but higher than that reported in HEK293 cells (7.31) (Smart et al., 2000) and rat hepatic arteries (6.24) (Zygmunt et al., 1999). The apparent pKB for capsazepine against anandamide-induced contraction was also greater than that reported in HEK293 and rat hepatic arteries and this may reflect species differences. We also investigated the role of sensory neuropeptides in the contractile response to anandamide following activation of the vanilloid receptor. The NK2-selective antagonist, SR48968 significantly attenuated the contractile response to capsaicin, while the NK1-receptor antagonist was without effect, consistent with a previous study (Ellis & Undem, 1994). The combination of NK1 and NK2-receptor antagonist resulted in almost complete suppression of the contractile response to capsaicin, thereby implicating a role for both neurokinin A and substance P in the contractile response to capsaicin (Ellis & Undem, 1994). Similarly, the contractile response to anandamide was nearly abolished in the presence of SR48968, a response typical of indirect acting agonists (Black et al., 1980) and supports the view that anandamide is less effective than capsaicin in stimulating neuropeptide release in this preparation.
Neuropeptides including substance P and neurokinin A which are released into the airways from sensory nerves are subject to degradation by neutral endopeptidase in this preparation (Maggi et al., 1990). The contractile response induced by anandamide in bronchial preparations was augmented by the neutral endopeptidase inhibitor, thiorphan. Together these data are consistent with the view that contraction of guinea-pig bronchial preparations is attributable to the release of neuropeptides following activation of the vanilloid receptor on airway sensory nerves. We can rule out a role for cannabinoid receptors in this response since the synthetic agents, WIN55,212-2 and CP55,940 failed to activate vanilloid receptors expressed in HEK293 cells (Smart et al., 2000). Similarly, neither CP55,940 (non-selective) and 2-arachidonylglycerol, recently shown to activate CB2-receptors (Sugiura et al., 2000), did not induce contraction of guinea-pig bronchial preparations, thereby ruling out a role for these receptors in the contractile response to anandamide. Furthermore, we were unable to demonstrate an inhibitory effect of a CB1-selective antagonist on anandamide-induced contraction in guinea-pig isolated bronchus and we employed a concentration of SR141716A (1 μM) that would be sufficient to effectively antagonise (Ki of 12 nM) responses at CB1 receptors (Felder et al., 1998). Whilst it has recently been shown that high concentrations of SR141716A induced a non-specific relaxation of vascular smooth muscle (White & Hiley, 1998), at the concentration employed in the present study, SR141716A was without an inhibitory effect.
An important area of cannabinoid pharmacology is the role played by these agonists as analgesic agents, and in this regard, there is convincing evidence that endogenous cannabinoids alleviate pain perception via stimulation of CB1 and CB2 receptors (Calignano et al., 1998) and that anandamide inhibits neuropeptide release from spinal cord and rat skin (Richardson et al., 1998a,1998b). The effect of anandamide in the respiratory system is controversial, with one study reporting that intravenous administration of anandamide failed to alter respiratory mechanics in conscious guinea-pigs (Stengel et al., 1998). In contrast, anandamide appeared to elicit a dual response in the airways dependent on an intact vagus nerve. Thus, intravenously administered anandamide inhibited bronchoconstriction induced by capsaicin in anaesthetized guinea-pigs, but induced bronchoconstriction in guinea-pigs that had been vagotomized and treated with atropine. Both these effects were mediated via a CB1-receptor dependent mechanism (Calignano et al., 2000). The mediators responsible for the bronchoconstrictor response was not identified, but the role of vanilloid receptors was ruled out as capsazepine did not attenuate the bronchoconstrictor response to intravenously administered anandamide. This finding contrasts with reports showing that anandamide stimulates vanilloid receptors (Zygmunt et al., 1999; Smart et al., 2000; Hwang et al., 2000). The inability to document a neuropeptide-dependent bronchoconstrictor response to anandamide in vivo, is most likely due to degradation of endogenously release neuropeptides by neural endopeptidase, which would tend to limit the concentration of neuropeptides at the level of airway smooth muscle, as observed in this study.
In further experiments, we were unable to demonstrate that cannabinoid receptors are functionally linked to inhibition of neuropeptide release in guinea-pig bronchial preparations. The synthetic cannabinoid agonist CP55,940 did not significantly attenuate the eNANC contractile response in guinea-pig isolated bronchus an observation consistent with the lack of effect of this agonist on contractile responses elicited by parasympathetic stimulation in the trachea of this species (Spicuzza et al., 2000). The concentrations of CP55,940 employed in this study were chosen on the basis that they cause near maximal inhibition of the cholinergic contractile response in guinea-pig small intestine (Pertwee et al., 1996). The reasons for this discrepancy remain to be established, although it is clear that there is a paucity of CP55,940 binding sites in rat and guinea-pig airways (Lynn & Herkenham, 1994; Spicuzza et al., 2000). However, CB1-receptor binding sites may be localized to discrete regions within the lung including nerves (Calignano et al., 2000) and lymphoid tissues (Lynn & Herkenham, 1994). Furthermore, these receptors may only be expressed on a sub-population of nerves as illustrated by a recent study showing co-expression of cannabinoid receptors on 13% of substance P mRNA-containing sensory nerves in the rat dorsal ganglia (Hohmann & Herkenham, 1999). Consistent with this view is the finding that inhibition of the eNANC response in the ileum by cannabinoid agonists was less efficacious and between 10–100-fold less potent compared with the μ-opioid selective agonist D-[Ala2-,N-methyl-Phe4,Gly5-ol]enkephalin, suggesting that CB1-receptor density and/or distribution on sensory nerves is less than for μ-opioid receptors in this preparation (Izzo et al., 1998). Similary, in the present study we have confirmed our previous findings that the ORL1-receptor agonist, nociceptin inhibited the eNANC response (Shah et al., 1998), which serves as a positive control.
It has yet to be established whether endogenous cannabinoids are released in sufficient quantities to activate vanilloid receptors in the airways, although macrophages and neuronal cells are potential sources of this putative endogenous vanilloid receptor agonist (diMarzo et al., 1996). However, under the current experimental conditions, the eNANC response was not modified in the presence of the CB1-selective antagonist, SR141716A suggesting that insufficient quantities, if any, of anandamide are released under physiological conditions in the airways. However, quantifiable amounts of anandamide have been measured in the periaqueductal gray during an inflammatory insult following subcutaneous injection of formalin into the rat hindpaw (Walker et al., 1999), and a significantly lower level of anandamide was detected following removal of calcium from guinea-pig lung membranes (Calignano et al., 2000).
In conclusion, we have demonstrated that the endogenous cannabinoid receptor agonist, anandamide, stimulates eNANC contraction secondary to activation of vanilloid receptors on airway sensory nerves. However, unlike capsaicin, it is a modest activator of this receptor in guinea-pig isolated bronchus.
Abbreviations
- ANOVA
analysis of variance
- DMSO
dimethylsulphoxide
- eNANC
excitatory non-adrenergic non-cholinergic
- PMSF
phenylmethylsulphonyl fluoride
- PDE
phosphodiesterase
- VR
vanilloid receptor
References
- ADCOCK J.J., GARLAND L.G.The contribution of sensory reflexes an ‘hyperalgesia' to airway hyperresponsiveness Airway Hyperresponsiveness: is it really important for asthma 1993Oxford: Blackwell Scientific; 234–255.eds. Page C.P. & Gardner P.J. [Google Scholar]
- BELVISI M.G., MIURA M., STRETTON D., BARNES P.J. Capsazepine as a selective antagonist of capsaicin-induced activation of C-fibres in guinea-pig bronchi. Eur. J. Pharmacol. 1992;215:314–344. doi: 10.1016/0014-2999(92)90054-8. [DOI] [PubMed] [Google Scholar]
- BLACK J.W., JENKINSON D.H., KENAKIN T.P. Antagonism of an indirectly acting agonist: Block by propranolol and sotalol of the action of tyramines on rat heart. Eur. J. Pharmacol. 1980;65:1–10. doi: 10.1016/0014-2999(80)90202-2. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., KATONA I., DESARNAUD F., GIUFFRIDA A., LA RANA G., MACKIE K., FREUND T.F., PIOMELLI D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- CALIGNANO A., LA RANA G., GIUFFRIDA A., PIOMELLI D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DIMARZO V., DEPETROCELLIS L., SEPE N., BUONO A. Biosynthesis of anandamide and related acylethanolamides in mouse J774 macrophages and N-18 neuroblastoma cells. Biochem. J. 1996;316:977–984. doi: 10.1042/bj3160977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAY A., BETTANEY J., RUEFF A., WALPOLE C., WRIGGLESWORTH R. NE-19550 and NE-21610, antinociceptive capsaicin analogues: studies on nociceptive fibres of the neonatal rat tail vein in vitro. Eur. J. Pharmacol. 1990;181:289–293. doi: 10.1016/0014-2999(90)90091-j. [DOI] [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J. Inhibition by capsazepine of resiniferatoxin and capsaicin-induced contractions of guinea-pig trachea. J. Pharmacol. Exp. Ther. 1994;268:85–89. [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., GLASS M., MACKIE K.P., FAHEY K.J., CULLINAN G.J., HUNDEN D.C., JOHNSON D.W., CHANEY M.O., KOPPEL G.A., BROWNSTEIN M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to a stimulation of cAMP accumulation. J. Pharmacol. Exp. Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- HAKANSON R., BEDING B., EKMAN R., HEILIG M., WAHLESTEDT C., SUNDLER F. Multiple tachykinin pools in sensory nerve fibres in the rabbit iris. Neurosci. 1987;4:943–950. doi: 10.1016/0306-4522(87)90049-2. [DOI] [PubMed] [Google Scholar]
- HARRISON S., SPINA D., PAGE C.P. The effect of okadaic acid on non-adrenergic non-cholinergic contraction in guinea-pig isolated bronchus. Br. J. Pharmacol. 1997;121:181–186. doi: 10.1038/sj.bjp.0701114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHMANN A.G., HERKENHAM M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: A double-label in situ hybridization study. Neurosci. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J.,. , LEE S.Y., KANG C.J., JUNG J., CHO S., MIN K.H., SUH Y.-G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLOL M., BORRELLI F., CAPASSO F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid CB1 receptors. Br. J. Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAY L., ANGUS J.A., WRIGHT C.E. Pharmacological characterisation of cannabinoid CB1 receptors in the rat and mouse. Eur. J. Pharmacol. 2000;391:151–161. doi: 10.1016/s0014-2999(00)00062-5. [DOI] [PubMed] [Google Scholar]
- LYNN A.B., HERKENHAM M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: implications for receptor-mediated immune modulation by cannabinoids. J. Pharmacol. Exp. Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., PERRETTI F., MEINI S., MANZINI S., SANTICIOLI P., DEL BIANCO E., MELI A. The effect of thiorphan and epithelium removal on contractions and tachykinin release produced by activation of capsaicin-sensitive afferents in the guinea-pig isolated bronchus. Naunyn-Schmeideberg's Arch. Pharmacol. 1990;341:74–79. doi: 10.1007/BF00195061. [DOI] [PubMed] [Google Scholar]
- MANZINI S., BALLATI L., GEPPETTI P., RUBINI I., MEINI S., PERRETTI F. Arachidonic acid-induced bronchomotor responses are partially mediated by release of sensory neuropeptides from capsaicin-sensitive structures. Br. J. Pharmacol. 1989;98:1077–1079. doi: 10.1111/j.1476-5381.1989.tb12649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANZINI S., MEINI S. Involvement of capsaicin-sensitive nerves in the bronchomotor effects of arachidonic acid and melittin: A possible role for Lipoxin A4. Br. J. Pharmacol. 1991;103:1027–1032. doi: 10.1111/j.1476-5381.1991.tb12295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAPP C.E., FABBRI L.M., BONIOTTI A., MAGGI C.A. Prostacyclin activates tachykinin release from capsaicin-sensitive afferents in guinea-pig bronchi through a ruthenium red-sensitive pathway. Br. J. Pharmacol. 1991;104:49–52. doi: 10.1111/j.1476-5381.1991.tb12383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEINI S., EVANGELISTA S., GEPPETTI P., SZALLASI A., BLUMBERG P.M., MANZINI S. Pharmacologic and neurochemical evidence for the activation of capsaicin-sensitive sensory nerves by lipoxin A in guinea pig bronchus. Am. Rev. Respir. Dis. 1992;146:930–934. doi: 10.1164/ajrccm/146.4.930. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., GRIFFIN G., ABADJI V., MAKRIYANNIS A. Effect of phenylmethylsulphonyl fluoride on the potency of anandamide as an inhibitor of electrically evoked contractions in two isolated tissue preparations. Eur. J. Pharmacol. 1995;272:73–78. doi: 10.1016/0014-2999(94)00618-h. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., NASH J.E., COUTTS A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICCIO M.M., MANZINI S., PAGE C.P. The effect of neonatal capsaicin on the development of bronchial hyperresponsiveness in allergic rabbits. Eur. J. Pharmacol. 1993;232:89–97. doi: 10.1016/0014-2999(93)90732-w. [DOI] [PubMed] [Google Scholar]
- RICCIO M.M., MATSUMOTO T., ADCOCK J.J., DOUGLAS G.J., SPINA D., PAGE C.P. The effect of 15-HPETE on airway responsiveness and pulmonary cell recruitment in rabbits. Br. J. Pharmacol. 1997;122:249–256. doi: 10.1038/sj.bjp.0701379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON J.D., AANONSEN L., HARGREAVES K.M. Antihyperalgesic effects of spinal cannabinoids. Eur. J. Pharmacol. 1998a;345:145–153. doi: 10.1016/s0014-2999(97)01621-x. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.D., KILO S., HARGREAVES K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998b;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- SHAH S., PAGE C.P., SPINA D. Nociceptin inhibits non-adrenergic non-cholinergic contraction in guinea-pig airway. Br. J. Pharmacol. 1998;125:510–516. doi: 10.1038/sj.bjp.0702068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPICUZZA L., HADDAD E.-B., BIRRELL M., CLARKE D., VENKATESAN P., BARNES P.J., BELVISI M.G. Characterization of the effects of cannabinoids on guinea-pig tracheal smooth muscle tone: role in the modulation of acetylcholine release from parasympathetic nerves. Br. J. Pharmacol. 2000;130:1720–1726. doi: 10.1038/sj.bjp.0703497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINA D., HARRISON S., PAGE C.P. Regulation by phosphodiesterase isoenzymes of nonadrenergic noncholinergic contraction in guinea-pig isolated main bronchus. Br. J. Pharmacol. 1995;116:2334–2340. doi: 10.1111/j.1476-5381.1995.tb15074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINA D., HARRISON S., SHAH S. Modulation of sensory nerve function in the airways. Trends Pharmacol. Sci. 1998;19:460–466. doi: 10.1016/s0165-6147(98)01261-9. [DOI] [PubMed] [Google Scholar]
- SPINA D., MCKENNIFF M.G., COYLE A.J., SEEDS E.A., TRAMONTANA M., PERRETTI F., MANZINI S., PAGE C.P. Effect of capsaicin on PAF-induced bronchial hyperresponsiveness and pulmonary cell accumulation in the rabbit. Br. J. Pharmacol. 1991;103:1268–1274. doi: 10.1111/j.1476-5381.1991.tb12335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STENGEL P.W., RIPPY M.K., COCKERMAN S.L., DEVAN W.A., SILBAUGH S.A. Pulmonary actions of anandamide, an endogenous cannabinoid receptor agonist, in guinea-pigs. Eur. J. Pharmacol. 1998;355:57–66. doi: 10.1016/s0014-2999(98)00472-5. [DOI] [PubMed] [Google Scholar]
- STUCKY C.L., ABRAHAMS L.G., SEYBOLD V.S. Bradykinin increases the proportion of neonatal rat dorsal root ganglion neurons that respond to capsaicin and protons. Neurosci. 1998;84:1257–1265. doi: 10.1016/s0306-4522(97)00572-1. [DOI] [PubMed] [Google Scholar]
- SUGIURA T., KONDO S., KISHIMOTO S., MIYASHITA T., NAKANE S., ODAKA T., SUHARA Y., TAKAYAMA H., WAKU K. Evidence that 2-arachidonoylglycerol but not N-Palmitoyethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. J. Biol. Chem. 2000;275:605–612. doi: 10.1074/jbc.275.1.605. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechnisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- VYKLICKY L., KNOTKOVA-URBANCOVA H., VITASKOVA Z., VLACHOVA V., KRESS M., REEH P.W. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. Journal of Neurophysiology. 1998;79:670–676. doi: 10.1152/jn.1998.79.2.670. [DOI] [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., JARAI Z., KUNOS G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- WALKER J.M., HUANG S.M., STRANGMAN N.M., TSOU K., SANUDO-PENA M.C. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLE K.A., FUREY G., SANGER S.J. Pharmacological characterization of the vanilloid receptor in the rat vas deferens. J. Pharm. Pharmacol. 1996;48:285–291. doi: 10.1111/j.2042-7158.1996.tb05918.x. [DOI] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of the cannabinoid receptor antagonist, SR141716A, in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.-H., SORGARD M., DIMARZO M., JULIUS V., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


