Abstract
The influence of the oestrous cycle and gender on responses of isolated pressurized mesenteric arteries to acute 17 β-oestradiol was investigated.
All vessels, pre-contracted with 60 mM KCl or 10 μM U46619 (9,11 dideoxy-11α, 9α-epoxy methano-prostaglandin), exhibited concentration-dependent vasodilatory responses to 17 β-oestradiol (3–30 μM). The largest responses were seen in vessels from female rats in pro-oestrous (38.9±5.4% U46619 max and 63.1±4.0% KCl max for 30 μM oestradiol), the smallest from animals in di-oestrous (20.1±3.7% U46619 and 50.1±4.5% KCL–both P<0.05 cf pro-oestrous (all n=8)). Responses of vessels from male rats were similar to those from pro-oestrous rats (41.5±9.1% U46619 (n=10) and 54.9±2.9% KCl (n =8)).
All responsees were unaffected by inhibition of nitric oxide synthase (NOS).
Female rats in pro-oestrous had the highest plasma concentrations of 17 β-oestradiol and testosterone (40.76±4.73 pg ml−1 and 0.29±0.05 ng ml−1 respectively (n=8)) while those in di-oestrous had the lowest (15.24±3.94 pg ml−1 for oestradiol and 0.08±0.03 ng ml−1 for testosterone (n=8)). In male rats the concentration of oestrogen was 10.29±1.21 pg ml−1 (n=7) while that of testosterone was 3.15±0.36 ng ml−1 (n=7).
Incubation of arteries isolated from male rats and from female rats in pro-oestrous and di-oestrous with testosterone (1 μM, 3 h) significantly enhanced the subsequent vasodilatory responses to acute 17 β-oestradiol. Following incubation, the responses to 17 β-oestradiol were similar in all groups.
These observations suggest that gender and the oestrous cycle may influence the vascular responses to acute 17 β-oestradiol administration.
Keywords: 17 β-oestradiol, gender, oestrous cycle, vascular
Introduction
It is well established that pre-menopausal women, and post-menopausal women receiving oestrogen replacement therapy, have a reduced incidence of cardiovascular disorders compared to men of similar ages (Bush et al., 1987; Stampfer et al., 1991). These observations suggest that oestrogens have a cardiovascular protective effect. The mechanisms responsible for these effects are multiple and whilst oestrogenic modulation of the lipid profile is certainly an important factor (Knop, 1988) it can account for only 25–50% of the observed reduction in risk suggesting that additional mechanisms may be involved (Bush et al., 1987). It has been proposed that one such factor may be a direct vasodilatory effect of oestrogens on the vasculature.
Oestrogens have two major effects on the vasculature which appear to be dependent on the exposure time to the hormone (Austin., 2000). Chronic oestrogen, for example due to hormone replacement therapy or long-term experimental exposure, has been shown to increase endothelium-dependent vasodilatory responses to both acetylcholine (ACh) (Gilligan et al., 1994; Vedernikov et al., 1997) and to flow (Lieberman et al., 1994; Cockell & Poston, 1997). These effects have been shown to be dependent on nitric oxide (NO) and appear to result from a genomic increase in the synthesis of NO synthase (Weiner et al., 1994). Effects on NO, due to chronic oestrogen exposure, also appear to be responsible for differences in myogenic tone observed in small arteries from male and female rats and in arteries from females treated with oestradiol (Wellman et al., 1996; Geary et al., 1998). In contrast, however, we and others have demonstrated that acute administration of 17 β-oestradiol directly relaxes vascular smooth muscle via non-genomic mechanisms which are independent of NO or other endothelial factors (Shaw et al., 2000). Thus, while chronic effects of oestrogens appear to be endothelium-dependent and genomic in nature, the acute effects of oestrogens on contractility seem to be endothelium-independent involving non-genomic mechanisms. The mechanisms involved in these acute effects are, however, poorly understood.
Gender differences in the acute effects of oestrogens have been observed. In human coronary arteries, for example, a significantly greater relaxation to 17 β-oestradiol occurred in vessels from female patients (Chester et al., 1995), a difference which persisted after endothelium removal. In isolated tail arteries (McNeill et al., 1996) and aorta (Le Tran et al., 1997) from female rats, however, gender differences in the acute vasodilatory effects of 17 β-oestradiol were abolished by endothelial removal. This suggests that, although acute oestradiol may relax arteries via endothelium-independent mechanisms, the vessel sensitivity to such applications may be influenced by gender-specific alterations in endothelial function. In contrast, however, others demonstrated that the vasodilatory responses to acute 17 β-oestradiol were greater in endothelium-denuded aorta from male rats compared to those from females (Crews & Khalil, 1999a). Thus, while there may be ‘cross talk' between the acute and chronic effects of oestrogens, such that the chronic hormonal status of the subject may influence subsequent responses to 17 β-oestradiol, these effects appear contrasting and are poorly understood. One of the potential flaws in many of these studies is that possible effects of hormonal variations within the menstrual or oestrus cycle on responses have been ignored. Gender differences have generally been attributed to differences in circulating oestrogen levels, however, levels of other sex hormones, which themselves have been shown to alter vascular contractility (Crews & Khalil, 1999b), may also be important.
In this study we have, therefore, investigated the responsiveness of mesenteric resistance arteries isolated from female rats in different stages of oestrous, and from male rats, to acute application of 17 β-oestradiol. All responses were related to plasma concentrations of 17 β-oestradiol, testosterone and progesterone. The role of the endothelium in responses was also studied.
Methods
Animals
Male and female Wistar Kyoto rats (200–250 g) were used in all experiments. Female rats exhibited normal oestrous cycles, the oestrous stage being determined by histological examination of a vaginal smear (Hafez, 1970).
Blood pressure measurement
Rats were placed in animal restrainers (International Market Supply, Congleton, Cheshire, U.K.) and their tails were warmed with hot water to dilate the tail artery. A tail cuff was placed at the base of the tail, this was sequentially inflated and deflated using LE 5001 pressure meter (IMS) and blood pressure and heart rate determined. This was repeated each day until the animals became accustomed to the procedure; reproducible readings of blood pressure could then be determined. In female rats the oestrous stage was determined each day and recordings made at each of the three different stages. For each rat a minimum of five readings were taken at each stage of oestrous.
Steroid hormone plasma concentration measurement
Following blood pressure recording, a 0.5 ml sample of blood was taken from the tail artery of each rat. This procedure was repeated in female rats in each stage of oestrous. The plasma concentration of 17 β-oestradiol, testosterone and progesterone was determined using radioimmunoassay kits; estradiol-2, diria-testok and diria-progK respectively (Diasorin s.r.l., Wokingham, Berkshire, U.K.).
Vessel isolation and cannulation
Both male rats and female rats at each stage of oestrous were used for isolated tissue studies. Rats were killed by stunning and exsanguination. This was carried out immediately after determination of oestrous state. The mesentery was removed and placed in ice-cold physiological salt solution (PSS) of the following composition (mM): NaCl 119, KCl 4.7, MgSO4.7H2O 1.2, NaHCO3 25, KHPO4 1.17, K2EDTA 0.03, glucose 5.5, CaCl2.2H2O 1.6, pH 7.4. A 4th order mesenteric artery was dissected out and placed in the chamber of a pressure myograph (Living Systems Instrumentation, Burlington, VT, U.S.A.) containing ice-cold PSS. Each vessel was cannluated onto two glass micropipettes as previously described (Izzard et al., 1996), pressurized to 50 mmHg using a pressure servo-control unit (Living Systems), and checked to ensure the absence of leaks. The PSS was warmed to 37°C, bubbled with 95% air/5% CO2 and the vessel allowed to equilibrate for 30 min. Lumen diameters were continuously measured using a video dimension analyser (Living Systems).
Experimental protocol
Following equilibration, each vessel was subjected to a ‘run-up' procedure consisting of three 2 min exposures to 60 mM KCl. Vessels were then contracted by addition of 60 mM KCl (KCl being isosmotically substituted for NaCl) or 10 μM U46619 (9,11 dideoxy-11α, 9α-epoxy methano-prostaglandin), all drugs being added to the superfusate. Each vessel was then subjected to increasing concentrations of 17 β-oestradiol from 0.01–30 μM and the response at each concentration allowed to reach a steady state. Vessels were then washed with PSS and tone allowed to return to baseline. Solubility limitations of 17 β-oestradiol prevented the effect of higher concentrations being investigated.
Investigation of the role of nitric oxide in responses to 17 β-oestradiol
The integrity of the endothelium was tested in each vessel by observing the relaxation response to 10 μM carbachol following pre-contraction with 60 mM KCl or 10 μM U46619. Vessels which showed a relaxation of <30% were considered to possess a damaged endothelium and were discarded from the study.
To investigate the involvement of NO in the vasodilatory responses to 17 β-oestradiol, concentration-response curves were constructed in the presence of 10 μM NW-nitro-L-arginine (L-NNA) an inhibitor of NO synthase. We have previously shown that this concentration almost completely inhibits the vasodilatory response to carbachol in depolarized rat mesenteric arteries (Shaw et al., 2000). Vessels were incubated with the inhibitor for 30–40 min prior to experimentation and responses compared to those obtained in the same vessels in the absence of the inhibitor. Previous studies revealed no significant differences between first and second curves to 17 β-oestradiol following 30–40 min incubation time (Shaw et al., 2000).
All experimental protocols were completed within 6 h of determination of oestrous state and killing of animal. Over this time period concentration-response curves to ACh were similar at the start and end of the experimental protocol (responses to 10 μM ACh being 31.2±3.5 and 32.9±6.8% KCl respectively (n=3)) even after the construction of concentration curves to 17 β-oestradiol. This makes it unlikely, in our opinion, that any residual oestradiol did not precipitate changes that were genomic.
Effect of in vitro incubation with testosterone on responses to 17 β-oestradiol
In a separate set of experiments, concentration-response curves to 17 β-oestradiol were constructed on mesenteric arteries from male rats, and female rats in pro-oestrous and di-oestrous, pre-contracted with 10 μM U46619. Following washing, arteries were incubated with 1 μM testosterone for 3 h after which concentration-response curves to 17 β-oestradiol were repeated. Control experiments, whereby tissues were incubated with vehicle alone for 3 h, were also performed. A concentration of 1 μM was chosen as we have previously shown that this concentration itself had little effect on arterial tone with noticeable relaxations only being observed at concentrations >10 μM (Sekrahan et al., 1999). Experiments on pressurized mesenteric arteries confirmed this, responses to testosterone being 0, 2.8±1.7, 6.1±2.4, 16.5±6.5, 85.4±2.1 and 97.5±1.36% KCl for 1, 3, 10, 30, 100 and 300 μM testosterone respectively.
Drugs and chemicals
All drugs and chemicals were obtained from Sigma. A stock solution of 17 β-oestradiol was made by dissolving first in 100% ethanol and diluting in PSS (1 in 2000) to give a concentration of 100 μM. A stock solution of testosterone was made by dissolving first in 100% ethanol and diluting in PSS (1 in 100) to give a concentration of 100 μM. A 10 mM stock solution of U46619 was made by dissolving in a mixture of 100% ethanol and 1 mg kg−1 sodium carbonate (1 : 2). Carbachol, ACh and L-NNA were dissolved in PSS.
Analysis of data
All results are expressed as mean±standard error of the mean (s.e.mean) with n representing number of animals. Results are normalized, where appropriate, as a percentage of the change in diameter observed to 60 mM KCl or 10 μM U46619. Differences between groups were compared by analysis of variance and Student's t-test (paired or unpaired) or the Student–Neuman-Keuls test for multiple comparisons. Results were considered to be significantly different from one another if P<0.05.
Results
Plasma hormone concentrations
The plasma concentrations of 17 β-oestradiol in female rats in all three stages of oestrous examined and in male rats are shown in Figure 1. Female rats had a significantly higher plasma concentration of 17 β-oestradiol when they were in pro-oestrous (n=8) than when they were in oestrous (P<0.05) or di-oestrous (P<0.01). These were also significantly higher than concentrations observed in male rats (P<0.001) (n=7). Female rats had the lowest levels of circulating oestrogen, a level similar to that observed in male rats, when they were in di-oestrous. Circulating plasma levels of testosterone fluctuated in a manner similar to oestradiol throughout the oestrous cycle. Levels of testosterone in male rats (n=7) were significantly higher than female rats in all stages of oestrous (P<0.001) (n=8).
Figure 1.
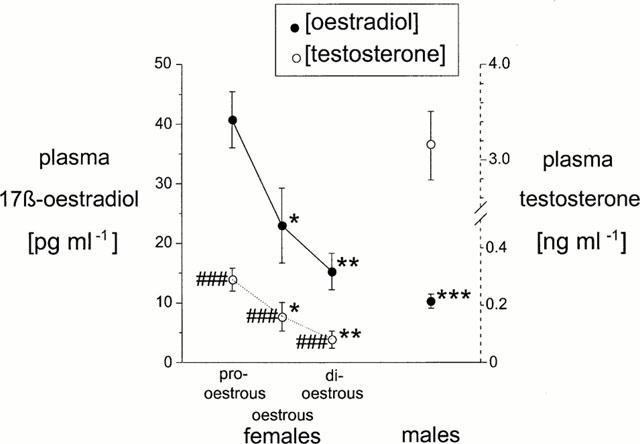
Plasma concentrations of 17 β-oestradiol and testosterone in female rats during pro-oestrous, oestrous and di-oestrous (n=8) and in male rats (n=7). *P<0.05 vs pro-oestrous, **P<0.01 vs pro-oestrous, ***P<0.001 vs pro-oestrous, ###P<0.001 vs males.
Plasma concentrations of progresterone did not fluctuate significantly during the oestrous cycle being 39.03±4.19 ng ml−1 (n=7), 37.79±2.79 ng ml−1 (n=8) and 40.43±3.08 ng ml−1 (n=8) during pro-oestrous, oestrous and di-oestrous respectively. The levels in male rats (3.66±1.05 ng ml−1) were, however, significantly lower than those in females during all stages of oestrous (P<0.001).
Blood pressure
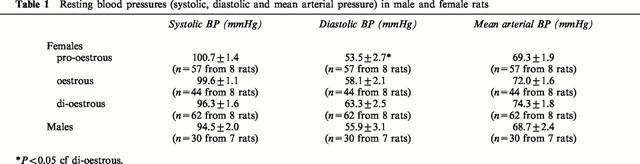
Diastolic blood pressure was lowest in female rats in pro-oestrous and highest in those in di-oestrous (P<0.05). A similar trend was also observed in the mean arterial pressures (MAP) although this did not reach a level of significance. Male rats had MAP and diastolic blood pressures similar to those observed in pro-oestrous females (Table 1).
Table 1.
Resting blood pressures (systolic, diastolic and mean arterial pressure) in male and female rats
Resting arterial diameters
The mean resting diameter of pressurized (50 mmHg) mesenteric arteries taken from female rats at pro-oestrous, oestrous and di-oestrous and male rats were not significantly different from each other being: 262±8 μm (n=11), 271±11 μm (n=8), 260±10 μm (n=11) and 247±6 μm (n=26) respectively.
Contractions to KCl and U46619
Sixty mM KCl and 10 μM U46619 induced maintained contractions in all tissues studied. The changes in diameter to KCl were: 160.0±5.9 μm (n=8) (61.6±2.3% resting diameter (rd)), 173.8±10.3 μm (n=8) (64.1±2.5% rd), 178.1±13.8 μm (n=8) (68.0±2.2% rd) and 155.0±6.7 μm (n=8) (61.2±1.8% rd) and to U46619 were: 173.6±9.6 μm (n=11) (66.0±3.6% rd), 167.5±7.0 μm (n=8) (66.2±2.4% rd), 165.0±9.9 μm (n=11) (63.9±2.7% rd) and 140.0±8.0 μm (n=10) (59.8±2.9% rd) for vessels from pro-oestrous, oestrous and di-oestrous females and males respectively. These were not significantly different from each other.
Effect of 17 β-oestradiol on mesenteric arterial diameter
17 β-oestradiol, studied over the range 0.01–30 μM, elicited a concentration-dependent relaxation in both KCl and U46619 pre-contracted mesenteric arteries taken from female rats at each stage of oestrous and in those taken from male rats. Responses were rapid (i.e. <5 min) and were only observed at high (i.e. >3 μM) concentrations of oestrogens as we have previously reported (Shaw et al., 2000) (see Figure 2 for example).
Figure 2.
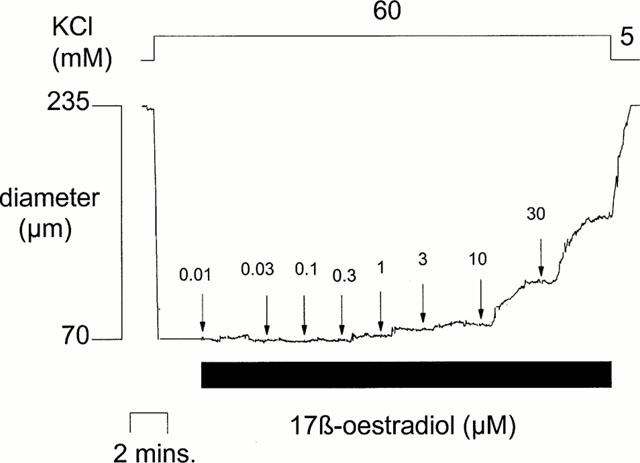
The effects of cumulative additions of 17 β-oestradiol (0.01–30 μM) on the diameter of an isolated pressurized rat mesenteric artery pre-contracted by 60 mM KCl.
Arteries pre-contracted with U46619
Significant differences were observed in the magnitude of the vasodilatory responses of arteries taken from female rats in different stages of oestrous (see Figure 3A). The relaxations to 17 β-oestradiol were largest in arteries taken from rats in pro-oestrous (n=8) and smallest in those taken from rats in di-oestrous (n=8). These responses appeared to correlate with both the plasma levels of 17 β-oestradiol and the plasma levels of testosterone (Figures 1 and 3), the greatest relaxations being observed in arteries isolated from females when plasma levels of the two hormones are highest. In arteries from male rats, however, large relaxations to 17 β-oestradiol were observed. These were comparable to those observed in female rats in pro-oestrous (Figures 1 and 3).
Figure 3.
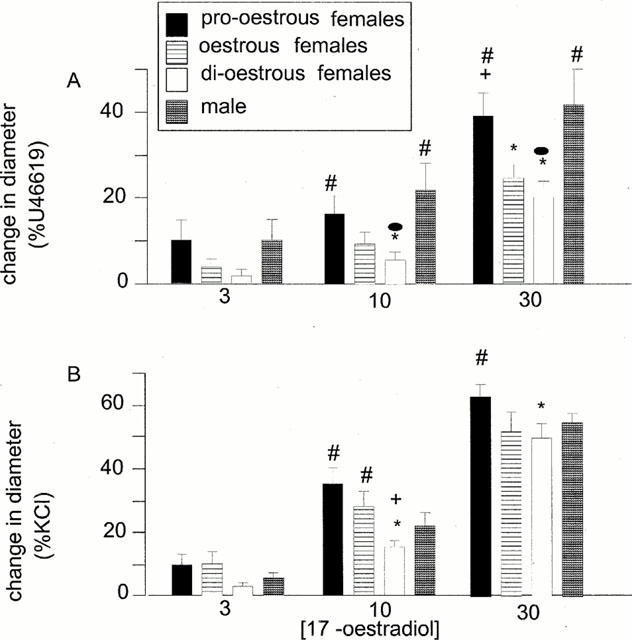
The effects of 17 β-oestradiol on the diameter of isolated mesenteric arteries, pre-contracted with either U46619 (10 μM) (A) or KCl (60 mM) (B), taken from female rats in pro-oestrous, oestrous or di-oestrous (all n=8) or from male rats (n=8 for KCl, n=10 for U46619). *P<0.05 vs pro-oestrous females, +P<0.05 vs oestrous females, #P<0.05 vs di-oestrous females, •P<0.05 vs males.
60 mM KCl pre-contracted arteries
Similar trends to the above were observed in arteries pre-contracted with 60 mM KCl, isolated arteries from female rats showing greatest vasodilatory responses to 17 β-oestradiol when taken from animals in the state of pro-oestrous when levels of plasma oestrogen and testosterone were highest compared to other oestrous states (Figures 1 and 3) (for all groups studied n=8). The variations in responses to 17β-oestradiol during the oestrus cycle were smaller in magnitude than those in tissues pre-contracted with U46619 (Figure 3). Relaxations of tissues from animals in all stages of oestrous were, however significantly greater (P<0.05) in tissues pre-contracted with KCl than those pre-contracted with U46619. Although the magnitude of the vasodilations to 30 μM 17 β-oestradiol produced in male rats was higher than those observed in vessels from female rats in oestrous and di-oestrous these differences did not reach statistical significance.
Involvement of nitric oxide
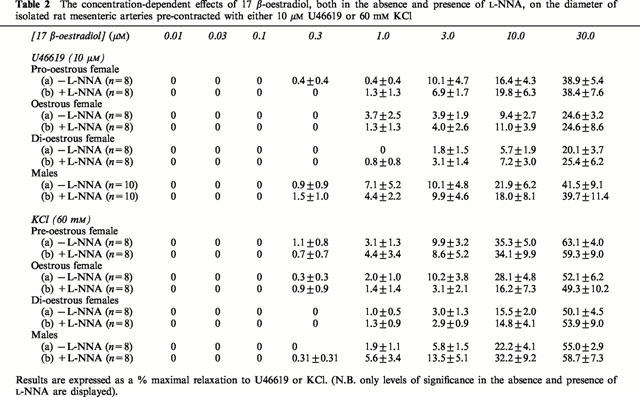
Incubation with 10 μM L-NNA had no effect on contraction to KCl or U46619 in mesenteric arteries from female rats at the three stages of oestrous or male rats. The changes in diameter to KCl were: 192.5±16.0 μm (n=8) (73.6±5.4% rd), 167.5±10.2 μm (n=8) (73.6±2.2% rd), 179.4±11.0 μm (n=8) (75.8±1.9% rd) and 163.8±11.1 μm (n=8) (65.0±4.0% rd) and for U46619 pre-contracted arteries were: 153.1±14.9 μm (n=8) (64.9±4.9% rd) 159.4± 13.7 μm (n=8) (66.0±4.4% rd), 158.1±10.2 μm (n=8) (68.4±3.0% rd) and 154.5±11.0 μm (n=10) (65.7±4.7% rd) in pro-oestrous, oestrous and di-oestrous females and males respectively in the presence of L-NNA. These were not significantly different to the changes in diameter reported previously in the absence of the inhibitor. Similarly, L-NNA had no effect on the vasodilatory response of arteries to 17 β-oestradiol. This was true for those arteries taken from females (all stages of oestrous) and males and in those contracted with U46619 or KCl (Table 2).
Table 2.
The concentration-dependent effects of 17 β-oestradiol, both in the absence and presence of L-NNA, on the diameter of isolated rat mesenteric arteries pre-contracted with either 10 μM U46619 or 60 mM KCl
Effect of in vivo incubation of isolated arteries with testosterone on responses to 17 β-oestradiol
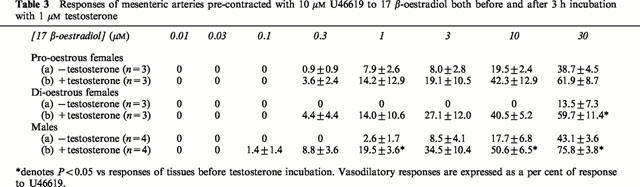
Incubation of arteries from pro-oestrous and di-oestrous female and male rats with 1 μM testosterone for 3 h had no effect on the contractions elicited by U46619. The changes in diameter in pro-oestrous and di-oestrous female and male animals were: 188.3±6.0 μm (n=3) (72.9±3.6% rd), 136.7±3.3 μm (n=3) (53.1±2.3% rd) and 136.3±6.3 μm (n=4) (54.8±1.8% rd) and 140.0±22.9 μm (n=3) (56.4±4.8% rd), 113.3±24.6 μm (n=3) (44.8±10.0% rd) and 103.8±13.9 μm (n=4) (40.8±4.5% rd) in vessels in the absence and presence of testosterone respectively. Incubation also had no effect on the resting diameter of vessels in any group (combined effect=0.96±1.11% increase, n=12).
Testosterone increased the relaxations of all vessels from all groups to 17 β-oestradiol. In the presence of testosterone there was no significant difference in the relaxations observed to 17 β-oestradiol between arteries from pro-oestrous and di-oestrous females and males (Table 3). Control incubation for 3 h with vehicle in male animals had no effect on responses to the oestrogen (these were: 1.7±1.0, 9.1±3.6, 18.3±4.5 and 39.7±8.3% U46619 (n=4) before and 5.3±4.1, 6.1±3.6, 18.9±4.4 and 41.9±4.1% U46619 (n=4) after control incubation for 1, 3, 10 and 30 μM 17 β-oestradiol respectively).
Table 3.
Responses of mesenteric arteries pre-contracted with 10 μM U46619 to 17 β-oestradiol both before and after 3 h incubation with 1 μM testosterone
Discussion
The results of the present study suggest that levels of circulating sex hormones can influence vascular responsiveness to subsequent acute application of 17 β-oestradiol. In all tissues acute application of 17 β-oestradiol resulted in concentration-dependent vasodilatory responses in isolated rat mesenteric arteries similar to those reported previously (Jiang et al., 1991; Shaw et al., 2000). Within the oestrous cycle, the largest responses were observed in vessels taken from rats in pro-oestrous while the smallest were observed in vessels taken from animals in di-oestrous. Vessels taken from rats in oestrous exhibited intermediate responses. These trends were similar to those observed with diastolic, and mean, blood pressure measurements in whole animals although a significant difference was only observed with diastolic blood pressure between female rats in pro and di-oestrous. Thus, normal fluctuations in hormone levels during the oestrous cycle may influence the responsiveness to subsequent acute exposure to 17 β-oestradiol. The responses of vessels from male rats, pre-contracted with U46619 at least, were similar to those of female rats in pro-oestrous. The findings are in contrast to previous studies which have demonstrated enhanced responsiveness to acute 17 β-oestradiol in isolated vessels from female compared to male rats (Chester et al., 1995; McNeill et al., 1996; Le Tran et al., 1997) or in which no gender differences were found (Jiang et al., 1991). In other studies, however, responses to 17 β-oestradiol were greater in endothelium-denuded arteries from male rats compared to those from females (Crews & Khalil, 1999a). The reasons for these differences is unclear. Previous studies also did not consider the influence of cyclical hormonal variations in females on the subsequent responses to oestrogen.
The responses of isolated arteries from female rats to acute 17 β-oestradiol were greatest in those taken from animals in pro-oestrous. Animals in this stage had higher plasma levels of 17 β-oestradiol compared to those in oestrous or di-oestrous. In male rats, however, which had lower plasma oestrogen concentrations than all groups of females, relaxations to acute 17 β-oestradiol were large and comparable to those observed in female pro-oestrous vessels. However, plasma testosterone levels were highest in females in pro-oestrous rats leading to the suggestion that testosterone may influence the responsiveness to acute 17 β-oestradiol application. In support of this, we were able to demonstrate that, in both males and females (pro-oestrous and di-oestrous), 3 h incubation with exogenous testosterone (1 μM) enhanced responsiveness to subsequent acute 17 β-oestradiol exposure. At this concentration testosterone had no effect on resting diameter or on the diameter of pre-contracted arteries although we have shown that at higher concentrations a vasodilation may be observed (Sekrahan et al., 1999). The direct vasodilatory effects of high concentrations of testosterone on vascular tissues have previously been shown, however, to be gender-independent (Yue et al., 1995). In support of this we found that testosterone incubation elevated the responses of arteries from pro-oestrous females and males to 17 β-oestradiol by similar amounts. In contrast, however, testosterone had a greater effect on responses of vessels from di-oestrous females, which previously were smaller than those of males or pro-oestrous females. Following testosterone incubation the responses to 17 β-oestradiol were similar in all groups. This observation further supports the idea that the concentrations of testosterone in vivo can influence the subsequent responses to acute 17 β-oestradiol. The mechanisms responsible for this remain unclear but the effects of testosterone on vascular tissues appear unlikely to be due to a conversion to oestrogen by aromatase (Yue et al., 1995). No relationship between plasma levels of progesterone and subsequent responses to 17 β-oestradiol were found. We, and others, have previously shown that the acute vasodilatory effects of 17 β-oestradiol are mediated via endothelial-independent mechanisms involving decreases in smooth muscle intracellular calcium ([Ca2+]i) (Han et al., 1995; Shaw et al., 1998) by inhibition of Ca2+-influx through voltage dependent L-type channels (Shan et al., 1994; Nakajima et al., 1995; Kitazawa et al., 1997). A number of studies, however, have suggested that gender differences in the contractile responses to 17 β-oestradiol may involve additional effects on the endothelium (McNeill et al., 1996; Le Tran et al., 1997). These effects appear to involve non-genomic modulation of NOS activity and thus the release of NO (Lantin-Hermoso et al., 1997). In the present study, however, we found that responses to 17 β-oestradiol were unaffected by inhibition of NOS suggesting that the differences reside at the level of the smooth muscle. In support of this, it has previously been shown that the inhibitory effect of 17 β-oestradiol on Ca2+-influx is greater in tissues from male rats compared to those from females (Crews & Khalil, 1996b). In a separate study, however, the authors showed that 17 β-oestradiol was more effective at reducing influx than progesterone or testosterone, the later hormones presumably inhibiting other mechanisms in addition to Ca2+ entry (Crews & Khalil, 1999a). Whilst these studies appear somewhat contradictory they could be explained by interactions between the sex hormones i.e. in males vs females.
In the present study it was found that vessels isolated from female rats, at all stages of oestrous, exhibited significantly greater responses to 17 β-oestradiol if they were pre-contracted with KCl than with U46619. As one of the major mechanisms whereby 17 β-oestradiol acutely relaxes vascular tissues is via an inhibition of Ca2+-influx through voltage dependent Ca2+ channels (Shan et al., 1994; Nakajima et al., 1995; Kitazawa et al., 1997), these differences may be a consequence of a smaller dependence of U46619-induced contractions on such influx than KCl-induced contractions. Such an effect does not, however, explain why relaxations of tissues from male rats to 17 β-oestradiol were independent of the vasoconstrictor agent used especially as others have shown 17 β-oestradiol to have a greater inhibitory effect on Ca2+ influx in tissues from male rats than from females (Crews & Khalil, 1999b). Another contributory factor may be a stimulatory effect of testosterone on thromboxane receptor density (Masuda et al., 1991) although if this were contributing significantly to the present results, differences in the magnitude of contractions to U46619 throughout the oestrous cycle and between male and females would have been expected. It has previously been shown that the direct vasodilatory effect of testosterone is greater in isolated arteries pre-contracted with PGF2α compared to depolarized preparations, an effect which was attributed to effects on K+ channels (Yue et al., 1995). Less variation in the direct effects of 17 β-oestradiol with oestrous in arteries pre-contracted with KCl compared to those contracted with U46619, as observed in the present experiment, may be due to similar actions of elevated testosterone.
These acute vasodilatory responses of isolated rat mesenteric arteries to 17 β-oestradiol are stereospecific, non-genomic and appear to be mediated by interactions at the plasma membrane (Shaw et al., 2000). Although membranous binding of fluorescently-labelled membrane-impermeant oestrogen has been demonstrated in a number of cell types (Pappas et al., 1995; Pyo et al., 1999) the identity of this putative binding site remains unclear but it may be a specific oestrogen receptor (Razandi et al., 1999) or another plasma membranous protein. Support for the latter concept in smooth muscle is provided by the finding that 17 β-oestradiol can bind to, and regulate the activity of, maxi-K channels (Valverde et al., 1999). Although such an action would be expected to cause membrane hyperpolarization leading to a reduction in ICa and hence relaxation we observed similar, or even larger, relaxations to 17 β-oestradiol in depolarized mesenteric arteries compared to those pre-contracted with U46619. Such a mechanism is therefore unlikely to be involved in the acute vasodilatory responses of 17 β-oestradiol in the rat mesenteric vascular bed. Evidence also exists, in T cells at least, that testosterone may also alter Ca2+ influx via non-genomic interactions with the surface membrane (Benten et al., 1997). Whether such a mechanism/binding site exists in vascular smooth muscle is currently unclear, however, testosterone has also been shown to relax arteries via mechanisms which are endothelium-independent and are unaffected by inhibition of classical testosterone receptors (Yue et al., 1995; Costarella et al., 1996).
In the absence of further information regarding the identity of putative oestrogen and testosterone surface binding sites, which modulate the non-genomic effects on contractility, it is difficult to precisely identify how the acute actions of oestrogens may be regulated by chronic hormonal levels. Whilst it is well known that the density of classical oestrogen receptors (modulating genomic oestrogenic effects) may be regulated by oestrogen itself in a positive manner, such that more are present in vascular tissues from pre-menopausal females compared to post-menopausal females or males (Bergqvist et al., 1993), it is unclear whether the membranous binding sites are similarly regulated. It is possible that the acute effects of oestrogen may be mediated by a membrane binding site which co-operatively binds testosterone as has been suggested for sex hormone binding globulin (Knochenhauer et al., 1998) which itself has a membraneous binding site (Rosner et al., 1999). Whilst in vitro studies clearly demonstrate that serum factors are not necessary for the interactions they may indeed enhance them, and thus reduce the concentration required to produce an effect, in vitro. Further study, however, is necessary to elucidate the nature of the binding site(s) responsible for the acute effects of sex hormones on contractility before the interactions may be understood.
In conclusion, therefore, the results of the present study demonstrate that the responsiveness of isolated pressurized rat mesenteric arteries to 17 β-oestradiol is influenced by the hormonal status of the animal. The greatest relaxation to 17 β-oestradiol was observed in arteries taken from female rats in pro-oestrous and the smallest in those from rats in di-oestrous. Animals in pro-oestrous exhibited the highest plasma concentrations of both 17 β-oestradiol and testosterone while those in di-oestrous exhibited the lowest. In male rats, however, where plasma levels of oestradiol were low while those of testosterone were high, responses were of similar magnitude to maximal vasodilations observed in females in pro-oestrous where levels of both hormones were high. In vitro incubation of arteries with testosterone enhanced subsequent acute responses to oestradiol. These observations suggest that, while there may be ‘cross talk' between the chronic and acute effects of 17 β-oestradiol, testosterone may be more important in governing arterial responsiveness to acute oestrogen exposure.
Acknowledgments
We would like to thank Judy Burgess and Professor B. Mawer, Department of Medicine, Manchester Royal Infirmary for measurement of plasma hormone levels. We wish to thank The Wellcome Trust for financial support.
Abbreviations
- ACh
acetylcholine
- [Ca2+]i
concentration of intracellular calcium
- L-NNA
NW-nitro-L-arginine
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- PSS
physiological salt solution
- rd
resting diameter
- s.e.mean
standard error of the mean
- U46619
9,11 dideoxy-11α, 9α-epoxy methano-prostaglandin
References
- AUSTIN C. Chronic and acute effects of oestrogens on vascular contractility. J. Hypertens. 2000;18:1365–1378. doi: 10.1097/00004872-200018100-00003. [DOI] [PubMed] [Google Scholar]
- BENTEN W.P., LIEBERHERR M., SEKERIS C.E., WUNDERLICH F. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett. 1997;407:211–214. doi: 10.1016/s0014-5793(97)00346-3. [DOI] [PubMed] [Google Scholar]
- BERGQVIST A., BERGQVIST D., FERNO M. Estrogen and progesterone receptors in vessel walls. Biochemical and immunochemical assays. Acta. Obstet. Gynecol. Scand. 1993;72:10–16. doi: 10.3109/00016349309013341. [DOI] [PubMed] [Google Scholar]
- BUSH T.L., BARRETT-CONNOR E., COWAN L.D., CRIQUI M.H., WALLACE R.B., SUCHINDRAN C.M., TYROLER H.A., RIFKIND B.M. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circ. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- CHESTER A.H., JIANG C., BORLAND J.A., YACOUB M.H., COLLINS P. Oestrogen relaxes human epicardial coronary arteries through non-endothelium-dependent mechanisms. Coron. Artery Dis. 1995;6:417–422. doi: 10.1097/00019501-199505000-00009. [DOI] [PubMed] [Google Scholar]
- COCKELL A.P., POSTON L. 17 β-estradiol stimulates flow-induced vasodilation in isolated small mesenteric arteries from prepubertal female rats. Am. J. Obstet. Gynecol. 1997;177:1432–1438. doi: 10.1016/s0002-9378(97)70087-5. [DOI] [PubMed] [Google Scholar]
- COSTARELLA C.E., STALLONE J.N., RUTECKI G.W., WHITTIER F.C. Testosterone causes direct relaxation of rat thoracic aorta. J. Pharmacol. Exp. Ther. 1996;277:34–39. [PubMed] [Google Scholar]
- CREWS J.K., KHALIL R.A. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin. Exp. Pharmacol. Physiol. 1999a;26:707–715. doi: 10.1046/j.1440-1681.1999.03110.x. [DOI] [PubMed] [Google Scholar]
- CREWS J.K., KHALIL R.A. Antagonistic effects of 17 β-oestradiol, progesterone and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler. Thromb. Vasc. Biol. 1999b;19:1034–1040. doi: 10.1161/01.atv.19.4.1034. [DOI] [PubMed] [Google Scholar]
- GEARY G.G., KRAUSE D.N., DUCKLES S.P. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am. J. Physiol. 1998;275:H292–H300. doi: 10.1152/ajpheart.1998.275.1.H292. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., QUYYUMI A.A., CANNON R.O. Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circ. 1994;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- HAFEZ E.S.E. Pub. Lea and Febiger, Philadelphia; 1970. Reproduction and breeding techniques for laboratory animals; pp. 115–117. [Google Scholar]
- HAN S.Z., KARAKI H., OUCHI Y., AKISHITA M., ORIMO H. 17 β-estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circ. 1995;91:2619–2626. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- IZZARD A.S., BUND S.J., HEAGERTY A.M. Increased wall-lumen ratio of mesenteric vessels from the spontaneously hypertensive rat is not associated with increased contractility under isobaric conditions. Hypertens. 1996;28:604–608. doi: 10.1161/01.hyp.28.4.604. [DOI] [PubMed] [Google Scholar]
- JIANG C.W., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br. J. Pharmacol. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITAZAWA T., HAMADA E., KITAZAWA K., GAZNABI A.K. Non-genomic mechanism of 17 beta-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J. Physiol. 1997;499:497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOCHENHAUER E.S., BOOTS L.R., POTTER H.D., AZZAZ R. Differential binding of estradiol and testosterone to SHBG. Relation to circulating estradiol levels. J. Reprod. Med. 1998;43:665–670. [PubMed] [Google Scholar]
- KNOP R.H. The effects of postmenopausal estrogen therapy on the incidence of arteriosclerotic vascular disease. Obstet. Gynecol. 1988;72:23S–30S. [PubMed] [Google Scholar]
- LANTIN-HERMOSO R.L., RODSENFELD C.R., YUHANNA I.S., GERMAN Z., CHEN Z., SHAUL P.W. Estrogen actively stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am. J. Physiol. 1997;273:L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- LE TRAN Y., FUNG A., FORSTER C. Role of gender and vascular endothelium in rat aorta response to 17 beta-estradiol. Can. J. Physiol. Pharmacol. 1997;75:1393–1397. [PubMed] [Google Scholar]
- LIEBERMAN E.H., GERHARD M.D., UEHATA A., WALSH B.W., SELWYN A.P., GANZ P., YEUNG A.C., CREAGER M.A. Estrogen improves endothelium-dependent, flow-ventilated vasodilation in postmenopausal women. Ann. Intern. Med. 1994;121:936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- MCNEILL A.M., DUCKLES S.P., KRAUSE D.N. Relaxant effects of 17 β-estradiol in the rat artery are greater in females than males. Eur. J. Pharmacol. 1996;308:305–309. doi: 10.1016/0014-2999(96)00374-3. [DOI] [PubMed] [Google Scholar]
- MASUDA A., MATHUR R., HALUSHKA P.V. Testosterone increases thromboxane A2 receptors in cultured rat aortic smooth muscle cells. Circ. Res. 1991;69:638–643. doi: 10.1161/01.res.69.3.638. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA T., KITAZAWA T., HAMADA E., HAZAMA H., OMATA M., KURACHI Y. 17 beta-estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur. J. Pharmacol. 1995;294:625–635. doi: 10.1016/0014-2999(95)00602-8. [DOI] [PubMed] [Google Scholar]
- PAPPAS T.C., GEMETCHU B., WATSON C.S. Membrane estrogen receptors identified by multiple antibody labelling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- PYO K.H., YOUNG L.J., JEONG J., WON B.S., KYU L.H., JO I. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor alpha localized in caveolae. Biochem. Biophys. Res. Commun. 1999;263:257–262. doi: 10.1006/bbrc.1999.1348. [DOI] [PubMed] [Google Scholar]
- RAZANDI M., PEDRAM A., GREENE G.L., LEVIN E.R. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- ROSNER W., HRYB D.J., KHAN M.S., NAKHLA A.M., ROMAS N.A. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J. Steroid Biochem. Mol. Biol. 1999;69:481–485. doi: 10.1016/s0960-0760(99)00070-9. [DOI] [PubMed] [Google Scholar]
- SEKRAHAN P., OTTER D., AUSTIN C. The effects of sex hormones on isolated rat mesenteric artery tone. Clin. Sci. 1999;97:1P. [Google Scholar]
- SHAN J., RESNICK L.M., LIU Q.Y., WU X.C., BARBAGALLO M., PANG P.K. Vascular effects of 17 beta-estradiol in male Sprague-Dawley rats. Am. J. Physiol. 1994;266:H967–H973. doi: 10.1152/ajpheart.1994.266.3.H967. [DOI] [PubMed] [Google Scholar]
- SHAW L., TAGGART M., AUSTIN C. Mechanisms of oestrogen-induced vasodilation in isolated rat small arteries. J. Physiol. 1998;511:171P. [Google Scholar]
- SHAW L., TAGGART M.J., AUSTIN C. Mechanisms of 17 β-oestradiol induced vasodilatation in isolated pressurized rat small arteries. Br. J. Pharmacol. 2000;129:555–565. doi: 10.1038/sj.bjp.0703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMPFER M.J., COLDITZ G.A., WILLETT W.C., MANSON J.E., ROSNER B., SPEIZER F.E., HENNEKENS C.H. Postmenopausal estrogen use and coronary atherosclerosis. Ann. Intern. Med. 1991;108:358–363. [Google Scholar]
- VALVERDE M.A., ROJAS P., AMIGO J., COSMELLI D., ORIO P., BAHAMONDE M.I., MANN G.E., VERGARA C., LATORRE R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- VEDERNIKOV Y.P., LIAO Q.P., JAIN V., SAADE G.R., CHWALISZ K., GARFIELD R.E. Effect of chronic treatment with 17 beta-estradiol and progesterone on endothelium-dependent and endothelium-independent relaxation in isolated aortic rings from ovariectomised rats. Am. J. Obstet. Gynecol. 1997;176:603–608. doi: 10.1016/s0002-9378(97)70555-6. [DOI] [PubMed] [Google Scholar]
- WEINER C.P., LIZOSOAIN I., BAYLISS S.A., KNOWLES R.G., CHARLES I.G., MONCADA S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc. Natl. Acad. Sci. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLMAN G.C., BONEV A.D., NELSON M.T., BRAYDEN J.E. Gender differences in coronary artery diameter involve estrogen, nitric oxide and Ca2+-dependent K+ channels. Circ. Res. 1996;79:1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- YUE P., CHATTERJEE K., BEALE C., POOLE-WILSON P.A., COLLINS P. Testosterone relaxes rabbit coronary arteries and aorta. Circ. 1995;91:1154–1160. doi: 10.1161/01.cir.91.4.1154. [DOI] [PubMed] [Google Scholar]


