Abstract
Specific mechanisms, providing reuptake of cathecholamine and amino acid neurotransmitters (e.g. serotonin and glutamate) into cells of the central nervous system are well known, whereas neuronal uptake of neuropeptide transmitters have not previously been reported.
In the present study we present evidence for uptake of the 37 amino acid neuropeptide, calcitonin gene-related peptide (CGRP) into perivascular terminals of capsaicin sensitive nerve fibres, innervating the guinea-pig basilar artery.
Release of CGRP from perivascular nerve terminals was obtained by capsaicin-induced vanilloid receptor-stimulation and detected as CGRP receptor-mediated dilation of isolated segments of the guinea-pig basilar artery.
Following three repeated capsaicin challenges, CGRP-depleted segments were incubated with CGRP. This caused significant reappearance of capsaicin-induced vasodilatory responses. These responses were dependent on duration and concentration of the preceding CGRP incubation and were inhibited by the CGRP receptor antagonist, CGRP8–37.
The CGRP-re-depletion was significantly reduced when CGRP8–37 was present during the preceding CGRP incubation. Thus, presynaptic CGRP receptors are likely to be involved in neuronal CGRP uptake.
Incubating the artery segments with 125I-CGRP allowed subsequent detection of capsaicin-induced 125I-release.
Immunohistochemical experiments showed that only terminal CGRP is subject to capsaicin-induced depletion in vitro, whereas CGRP-immunoreactivity endures in the nerve fibres.
Keywords: Calcitonin gene-related peptide (CGRP), CGRP receptor, capsaicin, vanilloid receptor, guinea-pig basilar artery, neuropeptide uptake, neurotransmitter uptake, sensory nerve fibres, vasodilatation
Introduction
The potent vasodilatory substance, calcitonin gene-related peptide (CGRP) is present in sensory nerve fibres surrounding arteries in the central nervous system and periphery where the substance is often co-localized with substance P and neurokinin A (Lundberg et al., 1985; Uddman et al., 1985). CGRP is stored in vesicles and released by exocytosis (Lundberg, 1996) upon electrical (Markowitz et al., 1987; Buzzi et al., 1991) or chemical stimulation (Mason et al., 1984; Kallner & Franco-Cereceda, 1998; Holzer, 1991b; Kilo et al., 1997). CGRP, released from perivascular sensory nerve fibres, causes potent CGRP receptor-mediated vasodilatation of various vascular beds (Brain et al., 1985; Edvinsson et al., 1987) and may in addition modulate the processes of neurogenic inflammation, which are initiated by the other sensory transmitters, substance P and neurokinin A (Feuerstein et al., 1995; Kilo et al., 1997). The most likely molecular equivalent of the vascular CGRP receptors is a G-protein coupled receptor (Aiyar et al., 1996) which requires the RAMP1 subunit for activity (McLatchie et al., 1998).
An increased activity of CGRP-containing trigeminovascular nerve fibres has been correlated to the pathophysiology of migraine (Buzzi et al., 1991) either during attacks (Goadsby et al., 1990; Goadsby & Edvinsson, 1993) or as a general imbalance in migraine patients (Ashina et al., 2000). Therefore, clinical potentials of CGRP receptor-antagonists in the treatment of migraine have been addressed (Doods et al., 2000).
Capsaicin, the active substance in chilli peppers (see Holzer, 1991b; Szallasi & Blumberg, 1999) potently and selectively causes release of CGRP from sensory nerve terminals both in vitro and in vivo (Duckles & Levitt, 1984; Duckles, 1986; Holzer, 1991a; Saito & Goto, 1986; Wharton et al., 1986).
The mechanism of capsaicin-induced CGRP depletion involves binding of capsaicin to vanilloid 1 receptors (VR1) (Caterina et al., 1997). Capsaicin-association to VRs triggers Ca2+ influx and elevated intracellular calcium levels in turn stimulate CGRP-release. The vanilloid 1 receptor is in addition to capsaicin stimulated by heat, hydrogen ions, lactate (Franco-Cereceda & Liska, 2000; Franco-Cereceda, 1988) and the endogeneous cannabinoid, anandamide (Zygmunt et al., 1999). A hypoxic reflectory release of CGRP which has been suggested in myocardium (Kallner, 1998; Dai et al., 2000; Franco-Cereceda & Liska, 2000) and in cerebral arteries (McCulloch et al., 1986) may be due to stimulation of this receptor as well. The capsaicin-like substance, capsazepine is a specific vanilloid receptor antagonist (Urban & Dray, 1991; Caterina et al., 1997).
It has previously been demonstrated that CGRP, rather than SP and NKA, is responsible for the capsaicin-induced vasodilatation of guinea-pig basilar artery (Franco-Cereceda & Rudehill, 1989; O'Shaughnessy et al., 1993; Jansen-Olesen et al., 1996).
The aim of this study was to investigate whether CGRP-depleted perivascular nerve terminals of the isolated guinea-pig basilar artery can be reloaded during incubation with exogenous CGRP. Possible reload was detected as a recovery of CGRP mediated vasodilatory responses to capsaicin.
Methods
In vitro pharmacological experiments
Procedure for standard start
Guinea-pigs (250–500 g, Hvidesten, Statens Serum Institut, Denmark) were killed by pentobarbitone (500 mg kg−1 (i.p.)) and the brains were rapidly transferred to a physiological salt solution (PSS). Each basilar artery was carefully dissected out and divided into eight segments of ∼0.5 mm for the investigation of CGRP- or capsaicin-induced vasodilatation. Each segment was mounted on two parallel pins of 150 μm in diameter in a myograph (Model 610M, Danish Myo Tecnology, Denmark) in order to continuously determine the vessel tension. The PSS solution in the myographs was continuously aerated with 95% O2 and 5% CO2 to maintain a constant physiological pH. The mounted segments were allowed to equilibrate for 30 min in PSS at 36°C. A similar equilibration period was repeated after each physical or pharmacological challenge described below.
Initially, the distance between the sets of pins was normalized to equalize 0.9×l100 (l100 is the distance between the pins when the transmural pressure equalizes 100 mHg) and the corresponding optimal normalized circular artery diameter, Do was calculated (Mulvany & Halpern, 1977). To activate and verify vasoconstrictive function, the artery segments were depolarized twice by 125 mM potassium in a modified PSS buffer (KPSS).
Each segment was then preconstricted by PGF2α, 3×10−6 M for 15–30 min and was subsequently challenged by 10−5 M acetylcholine as part of the standard procedure. Vasodilatory responses to acetylcholine confirmed that neither endothelium nor smooth muscle cells had been damaged during the mounting procedure.
In subsequent vasomotor studies, artery segments were preconstricted by 3×10−6 M PGF2α before they were challenged for 10 min by either capsaicin or human α-CGRP. Between repeated capsaicin- or CGRP-challenges, artery segments were allowed to rest for 1 h and each repeated preconstriction were preceded by challenging the artery segments with 125 mM potassium. When either vanilloid or CGRP receptor antagonists was present during the agonistic challenge, those were added to the tissue bath 10 min before the preconstrictor.
In studies of CGRP re-depletion, artery segments were initially challenged three times by capsaicin (10−5 M) in order to obtain CGRP-depleted nerve terminals. Subsequently, segments were incubated with human α-CGRP and re-challenged by capsaicin an additional 1–3 times. The amount of time elapsed from the washout of the CGRP incubation solution until the fourth capsaicin challenge was carried out amounted to ∼30 min.
Uptake and release of 125I-CGRP
A functional depletion of CGRP from terminals of the guinea-pig basilar artery was induced by three repeated capsaicin (10−5 M) challenges of preconstricted artery segments. CGRP-depleted artery segments were then incubated in PSS containing human α-CGRP (10−7 M) and 125I-CGRP (18.5 kBq, 0.25 pmol CGRP) for 20 min. This group of artery segments was subject to at least 10 extensive washouts prior to the fourth capsaicin challenge. The amount of 125I was determined (γ-emission) in 100 μl aliquots of the buffer every 5 min before and during the capsaicin (10−5 M) challenge of the artery segments. Half of the segments were subject to the fourth capsaicin challenge following 10 min of preconstriction, whereas the remaining segments were challenged by capsaicin following 25 min of preconstriction. To verify whether the released radioactivity was protein bound the protein in selected aliquots was precipitated by 5% trichloroacetic acid in the presence of 1% bovine serum albumin. The supernatant was discharged after centrifugation (10 min at 3000 r.p.m.) and the amount of radioactivity in the precipitate was detected.
Verification of vanilloid- and CGRP receptor mediated capsaicin-induced vasodilatation
Three groups of artery segments were preconstricted and challenged six times by capsaicin (10−5 M) for 10 min. The preconstricted arteries were challenged by capsaicin either alone or in the presence of either α-CGRP8–37 (10−6 M) or capsazepine (3×10−6 M). Antagonists were added to the tissue baths during only the first, fourth and sixth capsaicin treatment. Following the third capsaicin treatment the artery segments were incubated by human α-CGRP (10−7 M) for 20 min.
Determination of time and concentration dependency of CGRP re-depletion
Different groups of artery segments were challenged six times by capsaicin as described above. Following the third capsaicin treatment segments were incubated by human α-CGRP 10−7, 10−8 or 10−9 M for 20, 5 or 2 min.
Assessment of mechanisms of the CGRP uptake
Three groups of artery segments were challenged six times by capsaicin as described above. Following the third capsaicin treatment, segments were incubated by forskolin (10−6 M), human α-CGRP (10−8 M) or by human α-CGRP (10−8 M) in the presence of α-CGRP8–37 (10−6 M).
Verification of reproducible repeated responses to exogenous CGRP
It has previously been demonstrated that the tachyphylaxis to repeated cumulative CGRP applications in artery segments is avoided if each CGRP application is preceded by challenging the artery segments by 125 mM potassium (Sheykhzade & Nyborg, 1998). In the present study, artery segments were preconstricted and challenged four times by 10−8 M CGRP. Each preconstriction was preceded by a 125 mM potassium challenge and the artery segments were allowed to rest for 1 h between two CGRP administrations.
Another group of artery segments were preconstricted and challenged by 10−8 M CGRP once before and once after six repeated capsaicin challenges were performed. The second CGRP challenge was performed alone or in the presence of either the CGRP receptor antagonist, α-CGRP8–37 or the vanilloid receptor antagonist, capsazepine.
Two groups of artery segments were challenged three times by capsaicin as described above. Following the third capsaicin treatment, segments were incubated by either 10−9 or 10−7 M human α-CGRP for 20 min. Subsequently, the vasodilatory responses to human α-CGRP (10−9 M) were determined.
Chemicals and solutions
For the pharmacological experiments, concentrated solutions of PGF2α (Prostaglandin F2α; Dinoprost®, Upjohn, Sweden), CGRP (human α-CGRP; Bachem, Switzerland), CGRP8–37 (human α-CGRP8–37: Schafer-N, Copenhagen, Denmark), Forskolin (RBI, U.S.A.), acetylcholine (acetylcholine chlorid, Sigma, Denmark), Capsaicin (Sigma-RBI, U.S.A.), Capsazepine (Sigma-RBI, U.S.A.), 125I-CGRP (3-[125I] iodohistidyl10) CGRP (human), 74 TBq mmol−1 (Amersham Pharmacia Biotech, U.K.) were used.
The solutions of capsaicin and forskolin were prepared in ethanol whereas capsazepine was dissolved in methanol. The remaining substances were dissolved in double distilled water. Aliquots of each concentrate were stored at −20°C. Final concentrations of the organic solutes in the tissue baths did not exceed 0.1%. The remaining substances were dissolved in distilled water, aliquoted and stored at −20°C.
PSS (Physiological salt solution) contained (mM): NaCl 119, KCl 4.7, CaCl2 1.5, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, EDTA 0.027, glucose 5.5 (pH 7.4 was obtained by continuous equilibration with a gas stream of 95% O2 and 5% CO2).
KPSS (Potassium rich physiological salt solution) contained (mM): KCl 123.7, CaCl2 1.50, MgSO4 1.17, KH2PO4 1.18, EDTA 0.027, NaHCO3 0.025, glucose 5.5 (pH 7.4 was obtained by continuous equilibration with a gas stream of 95% O2 and 5% CO2). This solution is a modified PSS solution where NaCl is substituted by KCl on an equimolar basis.
Calculations and statistical analyses
The degree of vasodilatation, induced by CGRP or capsaicin was calculated in percentage of the PGF2α precontraction. For comparison of the responses to different capsaicin or CGRP challenges within one artery group, paired t-tests were performed. When comparing capsaicin-induced responses in different groups of arteries, an initial normalization of the vasodilatory responses was performed: The response to the third capsaicin treatment was defined as a segment-specific CGRP-depleted response. For each individual segment the amount of vasodilatation induced by the third capsaicin challenge was subtracted from the remaining responses before evaluating differences within groups with an unpaired t-test. For the evaluation of reproducibility between repeated CGRP applications, two-way ANOVAs using CGRP application number as column factor and individual segments as row factor were performed.
All statistical analyses were performed using GraphPad Prism 3.0 and differences were considered significant when P<0.05 and symbolized by * (*: P<0.05, **: P<0.01, ***: P<0.001).
Immunocytochemical experiments
Four artery segments were isolated from the myographs at four different time points of the capsaicin-induced CGRP-depletion experiments described above. (1) before the first capsaicin challenge, (2) after the third capsaicin challenge, (3) after the CGRP incubation and (4) after completion of six capsaicin challenges. The segments were fixed in 4% paraformaldehyde for >24 h and the succeeding immunohistochemical studies were performed on free floating segments that were opened to expose the lumen. Initially, segments were rinsed in TBS (75 mM Tris-HCl, 150 mM NaCl, pH 7.6) for 10 min at 20°C and thereafter in TBS containing 0.1% Triton X-100 (Sigma, Denmark) for 10 min. Subsequently, the tissue pieces were incubated in TBS containing 1% Triton X-100 and 10% porcine serum (Dako, Denmark) for 30 min before rabbit anti-CGRP ‘89337' (1 : 3000) (Thulesen et al., 1994) was added and allowed to incubate for 16 h at 4°C. The artery segments were washed three times in TBS containing 0.1% Triton X-100 and CGRP immunoreactivity was detected following three incubations each separated by three washings: (1) Biotinylated anti-rabbit IgG (Dako, Denmark, 1 : 200 in TBS containing 1% Triton X-100, 1 h at 20°C), (2) Biotinyl-tyramide (NEN Dupharma, Denmark, 5 min at 20°C as prescribed by the supplier) and (3) Texas Red coupled streptavidin (Amersham, Denmark, 1 : 200 in TBS containing 1% Triton X-100, 1 h at 20°C). Before and after the biotinyl-tyramide treatment, segments were challenged by ABC-reagent (Dako, Denmark). Segments were coverslipped and CGRP immunoreactivity was finally visualized as fluorescent emission under the microscope.
Results
Uptake and release of 125I-CGRP
Addition of 18.5 kBq (0.25 pmol) 125I-CGRP to the tissue bath (5 ml) during the incubation with 10−7 M CGRP allowed a subsequent capsaicin-induced release of 125I from the isolated artery segment (Figure 1). The specificity of the capsaicin-induced 125I-release was evaluated by comparing the time courses of 125I-release during capsaicin challenge and those during prolonged preconstriction (Figure 1A). Significant increases in mean 125I-release were demonstrated in the artery segments undergoing capsaicin challenge at 25, 30 and 35 min as compared to those undergoing prolonged preconstriction (P=0.0087; 0.0012 and 0.0004, respectively). No difference between the two artery groups was observed in the time interval from 0–20 min (Figure 1A). Furthermore, the amount of capsaicin-induced 125I-release was not different in artery segments preconstricted for different amounts of time (Figure 1B).
Figure 1.
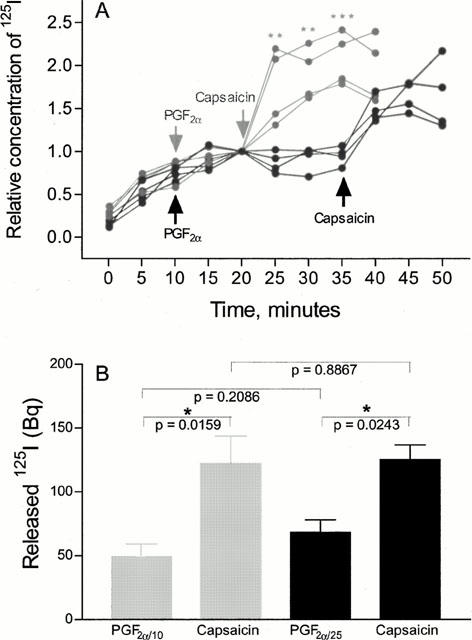
Capsaicin-induced 125I-release following incubation of artery segments by 125I-CGRP. (A) The relative 125I-release into the tissue bath before the application of preconstrictor, during the preconstriction and during the capsaicin challenge is represented for each of eight experiments. The grey and black symbols represent segments, which were preconstricted for 10 and 25 min, respectively before capsaicin was added. By unpaired t-tests, the mean relative 125I-release from segments, undergoing capsaicin challenge was compared to the mean relative amount of 125I released from segments undergoing prolonged preconstriction. Significance of the differences at specific time points is indicated by * (**:P<0.01, ***:P<0.001). (B) Amounts of radioactivity (Bq) released from the two groups of arteries are presented as mean±s.e.mean of four segments from two animals. Grey and black bars represent segments, preconstricted for 10 and 25 min, respectively. Significances of differences in the 125I-contents in the tissue baths are indicated above the bars.
The results presented in Figure 1 cannot be distinguished from results obtained from segments which have not undergone challenges by capsaicin. Thus, the apparent CGRP redepletion is not dependent on preceding CGRP depletion.
The mean amount of 125I, released into the tissue bath during 15 min of capsaicin challenge equals 65±11 Bq (mean±s.e.mean of eight experiments) which corresponds to ∼0.9 fmol 125I-CGRP. Assuming equal uptake and re-depletion characteristics of labelled and unlabelled CGRP, the total amount of CGRP, released into the tissue bath during 15 min of capsaicin challenge amounts 1.8 pmol, corresponding to ∼3.6×10−10 M CGRP in the 5 ml tissue bath. Protein precipitation confirmed that the radioactivity released both before and during the capsaicin challenge was in fact protein bound.
Pharmacological evidence for CGRP uptake and re-depletion
When isolated artery segments were challenged with capsaicin three times in a row, the vasodilatory responses were significantly decreased in a consecutive manner (Figure 2A). This scenario verified functional CGRP depletion in possible combination with vanilloid receptor desensitization.
Figure 2.
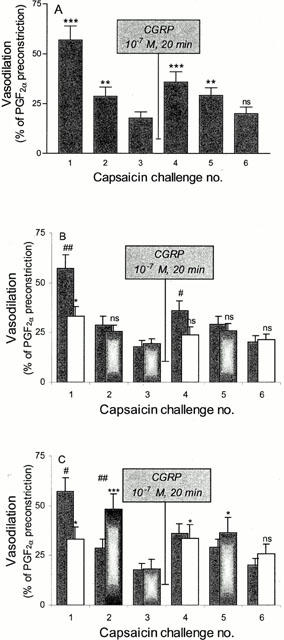
Capsaicin-induced vasodilatations in the absence and presence of vanilloid and CGRP receptor antagonist. The amounts of vasodilatation induced by repeated capsaicin challenges are shown as bars and given in per cent of PGF2α preconstriction (mean±s.e.mean) of 15–22 segments. Capsaicin-induced vasodilatory responses shown in grey bars represent controls (n=22) (A, B and C) and responses shown in light bars indicate that CGRP8–37 (10−6 M) (n=22) (B) or capsazepine (3×10−6 M) (n=15) (C) were present during capsaicin challenge number 1, 4 and 6. When antagonist was not present during a selected capsaicin challenge, the corresponding bar was marked by grey tones. For the two antagonist-treated artery groups, the amount of vasodilatation induced by capsaicin challenge number 1, 2, 4, 5 and 6 were compared to that induced by the third capsaicin challenge by paired t-tests. Levels of significance are indicated above the bars (*:P<0.05, ***:P<0.001). Differences in vasodilatation obtained in the antagonist-treated artery groups were compared to values of vasodilatation in the control group and significances are indicated above the corresponding control bar (#:P<0.05, ##:P<0.01).
Following the third capsaicin challenge, artery segments were incubated with 10−7 M CGRP and the vasodilatory response to the subsequent fourth capsaicin challenge significantly increased as compared to the third capsaicin challenge (P=0.0002). Again, successive and significant decreases in the responses to repeated capsaicin challenges were observed and a significant decrease in the vasodilatory effect upon the sixth capsaicin challenge was demonstrated as compared to that of the fourth (P=0.0032).
Verification of vanilloid 1 and CGRP receptor mediated capsaicin-induced vasodilatation
Both the vanilloid and CGRP receptor antagonists inhibit the capsaicin-induced vasodilatory responses. When artery segments were pre-incubated with either CGRP8–37 (10−6 M) or capsazepine (3×10−6 M) the capsaicin-induced vasodilatations during the first challenge were significantly blocked (P=0.0084 and P=0.0189, respectively) (Figure 2B,C). The responses induced by the second capsaicin challenge was not significantly different when comparing the control and the CGRP8–37-treated group (P=0.7901). However, the response to the second capsaicin challenge was significantly increased (P=0.039) when capsazepine was present during the first challenge.
In control segments (Figure 2A), the responses to the fourth capsaicin treatment were elevated as compared to the responses to the third capsaicin challenge (P=0.0002). Again, the CGRP receptor antagonist significantly inhibited the response (P=0.0191) whereas no significant inhibition by the vanilloid 1 receptor antagonist was observed (P=0.7244). A tendency, however, towards increased degree of vasodilation was noticed for the fifth as compared to the fourth capsaicin challenge, when capsazepine was present during the fourth capsaicin challenge.
In all three artery groups, the re-depleted responses to the sixth capsaicin challenge were not different from the responses induced by the third capsaicin challenge. Furthermore, the presence of CGRP8–37 or capsazepine had no effect on the sixth response indicating non-vanilloid and non-CGRP receptor mediated vasodilatation (Figure 2B,C).
Determination of time and concentration dependency of CGRP re-depletion
The capsaicin-induced CGRP re-depletion is dependent on the concentration of CGRP and on the duration of the preceding CGRP incubation (Figure 3). The capsaicin-induced CGRP-release following incubation by 10−7 CGRP was not significantly different from that following incubation by 10−8 M. The release following incubation by 10−9 M was significantly lower than that following incubation by 10−7 M (Figure 3A).
Figure 3.
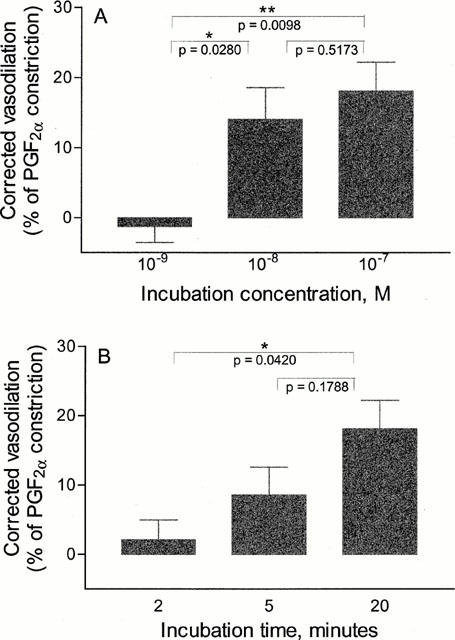
Correlation between CGRP incubation time and concentration and capsaicin-induced CGRP-depletion. The corrected vasodilatations of the fourth capsaicin-challenge following incubation for 20 min with either 10−9, 10−8 or 10−7 M CGRP (A) or by 10−7 M CGRP for either 2, 5 or 20 min (B) are shown in bars and represented as mean values±s.e.mean of 7–22 segments. The corrected vasodilatation equals the amount of vasodilatation induced by the fourth capsaicin challenge subtracted by the amount of vasodilatation induced by the corresponding third capsaicin challenge and is given in per cent of PGF2α preconstriction. The differences between mean values were analysed by unpaired t-test and the corresponding P-values are given in the figures.
No significant difference in the subsequent release was seen when CGRP incubation with 10−7 M was carried out for 5 as compared to 20 min (Figure 3B). However, reducing the incubation time to 2 min caused a significant decrease in the amount of subsequent CGRP-release.
Investigating possible roles of CGRP receptors for the CGRP uptake
The vasodilatory response of the fourth capsaicin challenge succeeding incubation with 10−8 M CGRP was significantly blocked when 10−6 M CGRP8–37 was present during the CGRP incubation (Figure 4).
Figure 4.
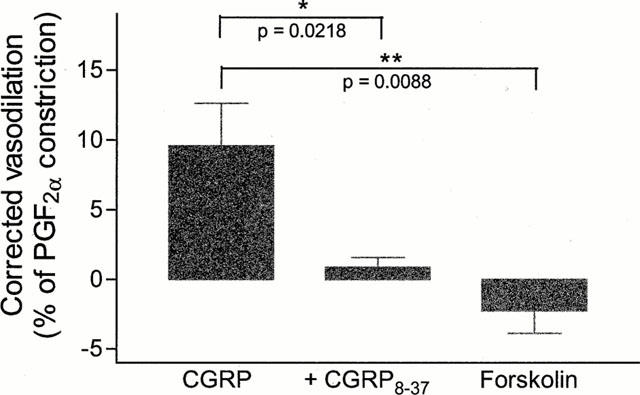
Role of CGRP8–37 and forskolin for re-depletion like scenario. The corrected vasodilatations of the fourth capsaicin challenge following incubation by CGRP (10−8 M) in the absence and the presence of CGRP8–37 (10−6 M) and by forskolin (10−6 M) are represented as mean±s.e.mean of 11–19 segments. The corrected vasodilatation equals the amount of vasodilatation induced by the fourth capsaicin challenge subtracted by the amount of vasodilatation induced by the corresponding third capsaicin challenge and is given in per cent of PGF2α preconstriction. The differences between mean values were analysed by unpaired t-test and the corresponding P-values are given in the figure.
Incubating the artery segments with forskolin, a direct activator of adenylate cyclase, caused no re-depletion-like scenario during repeated capsaicin challenges (Figure 4).
Verification of reproducible responses to repeated administrations of exogenous CGRP
Reproducibility between responses to repeated administration of exogenous CGRP was obtained. The vasodilatory responses to four repeated applications of CGRP (10−8 M) were 88.2±6.1, 75.5±9.0, 76.5±10.1 and 72.6±9.8% (mean±s.e.mean of 11 experiments) of the PGF2α induced preconstriction, respectively. Those responses were not significantly different (P=0.1691).
The CGRP-induced (10−8 M) response was significantly reduced in the presence of CGRP8–37 (10−6 M) (P<0.0001). The amount of vasodilatation obtained by CGRP in the absence and in the presence of CGRP8–37 was 92.8±2.9 and 35.2±3.4% (mean±s.e.mean of seven experiments), respectively. The response to CGRP in the presence of 3×10−6 M capsazepine was 95.8±0.8% (mean±s.e.mean of seven experiments), which was not different from that induced by CGRP alone (P=0.3341).
The degrees of vasodilatation induced by 10−9 M CGRP following incubations by 10−7 and 10−9 M CGRP were not significantly different (P=0.6903), the values being 50.8±8.7 and 56.2±10.1% (mean±s.e.mean of 11–12 experiments), respectively.
Immunohistochemical experiments
Significant perivascular CGRP-immunoreactivity (CGRP-IR) was demonstrated in all four artery representatives independent on the number of preceding capsaicin challenges (Figure 5). No tendency of lower CGRP-IR was observed in capsaicin treated segments compared to controls. The results were reproduced in segments obtained from two animals and no attempt to quantify CGRP-IR was made. Those results indicated that only terminally stored CGRP is released during capsaicin challenge in vitro and that CGRP reserves, which are not readily available for release, exist in the nerve fibres.
Figure 5.
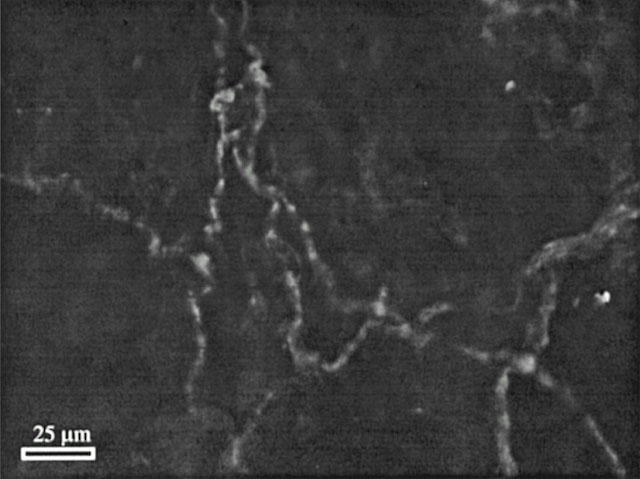
CGRP-IR in perivascular nerve fibres surrounding the guinea-pig basilar artery. Perivascular CGRP-IR is visualized as light fluorescent beaded strings in a segment of guinea-pig basilar artery, which was challenged three times by capsaicin (10−5 M) for 10 min. Thus, CGRP-IR endures in the perivascular nerve fibres upon challenging the artery segments with capsaicin in vitro.
Discussion
In the present study, CGRP was depleted from perivascular terminals of sensory nerve fibres during repeated in vitro capsaicin challenges. Indirect evidence of CGRP reload into these terminals was gained from studies of CGRP re-depletion following CGRP incubation. The mechanisms behind the increased vasodilatory response to the fourth capsaicin challenge (Figure 2A) could be (1) CGRP, which has been reloaded into the perivascular nerve fibres during incubation with CGRP is released; (2) CGRP, which has been mobilized from reserves in the sensory nerve fibres is released; (3) vascular CGRP receptors have been sensitized during the CGRP incubation; or (4) naïve release of CGRP is reduced during incubation with CGRP.
The results of the tracer re-localization experiments support solely the notion of CGRP uptake and re-depletion (Figure 1).
The perivascular nerve fibres contain CGRP-immunoreactivity after challenging the artery segments with capsaicin (Figure 5). Therefore, it should be considered whether the increased response to the fourth capsaicin challenge (Figure 2A) could be due to release of CGRP, which has been mobilized from the nerve fibres. The presented concentration-dependent amounts of re-depletion (Figure 3) excludes that the re-depletion-like scenario is caused by terminal CGRP re-mobilization which has naturally occurred between the third and the fourth capsaicin challenge. However, it could be speculated that CGRP, in a duration- and concentration-dependent manner, mediated terminal CGRP mobilization, e.g. via binding to presynaptic CGRP receptors. As CGRP receptors are coupled to adenylate cyclase (Aiyar et al., 1996; McLatchie et al., 1998), it was investigated, whether the CGRP-release upon the fourth capsaicin stimulation, could be mimicked if the preceding CGRP-incubation was substituted by incubation with a direct activator of adenylate cyclase. No CGRP re-depletion-like scenario could be demonstrated when the artery segments were incubated in forskolin (Figure 4). Strongly supported by results of the tracer re-localization studies, it is therefore not likely, that the re-depletion-like scenario is caused by CGRP-mediated mobilization of terminal CGRP from reserves in the nerve fibres.
The notion that the apparent secondary release could be caused by vascular hyper-sensitivity towards CGRP was also excluded as a likely explanation, as the response to exogenous CGRP was not dependent on the preceding CGRP incubation concentration.
Thus, based on the present studies of capsaicin-induced CGRP and 125I-CGRP re-depletion, we provide evidence that CGRP is subject to uptake into perivascular nerve fibres of the guinea-pig basilar artery.
Previous studies have demonstrated that CGRP concentrations above 10−10 M elicit detectable vasodilatory responses on isolated segments of guinea-pig basilar artery (Sams et al., 1999). In this study, the capsaicin-induced CGRP-release following CGRP incubation is sufficient to cause significant vasodilatation. Thus, the CGRP concentrations at the receptor sites must be equivalent to the concentrations obtained by tissue bath concentrations above 10−10 M. From the tracer re-localization studies, the extent of the capsaicin-induced CGRP-release was equivalent to 3.6×10−10 M CGRP in the 5 ml tissue bath. Thus, the magnitude of the likely CGRP-re-depletion is consistent in the two types of experiments.
In addition to the specific capsaicin-induced 125I-release, a significant amount of 125I is released into the buffer solution until equilibrium is obtained at ∼20 min (Figure 1). The origin of this 125I has not been determined. However, due to extensive washings prior to measurements of 125I-content, this 125I is not likely to originate from e.g. receptor bound or tissue bath adsorbed 125I-CGRP, yet it may represent leakage of 125I-CGRP from the perivascular fibres. Release of CGRP from non-depolarized artery segments has previously been reported (Booth et al., 2000).
Investigations of the pharmacological responses to CGRP and capsaicin in the presence of the CGRP and vanilloid 1 receptor antagonists, verified post and pre-synaptic inhibition of capsaicin-induced vasodilatation by CGRP8–37 and capsazepine, respectively (Figure 2B,C). A significant inhibition of the vasodilatory response both during depletion of endogenous CGRP and during depletion of reloaded CGRP, could be inhibited by CGRP8–37 (Figure 2B). However, it could not be concluded, whether the CGRP re-depletion was mediated via vanilloid 1 receptor-stimulation as no significant inhibition of the re-depletion could be demonstrated in the presence of capsazepine (Figure 2C). It can be speculated that the inhibition by capsazepine during the first capsaicin challenge and the lack of inhibition during the fourth capsaicin challenge is caused by successive development of tachyphylaxis of capsazepine-sensitive vanilloid receptors. This explanation requires the presence of capsazepine-insensitive vanilloid receptors on the nerve fibres and that a significant shift towards these receptors takes place between the first and the fourth capsaicin challenge. The existence of capsazepine-sensitive and -insensitive vanilloid receptors has previously been suggested (Colquhoun et al., 1995; Griffiths et al., 1996; Liu & Simon, 1998), but it has not been shown whether the capsazepine-sensitive receptors are more susceptible to desensitization than capsazepine-insensitive receptors. Some support of shift in pathway in the present study was gained, as the capsaicin-induced vasodilatory responses seem to shift from a fast to a slow response (data not shown). The distinction between fast and slow capsaicin-induced effects, was first observed by Liu & Simon (1996).
The origin of the observed depleted vasodilatation (Figure 2A, third and sixth capsaicin challenge) has not been determined in the present study, whereas previous works have evaluated direct capsaicin-induced effects on vascular smooth muscle (Jansen et al., 1990; Duckles, 1986). In the present study a vasodilatory effect of the vehicle (0.1% ethanol) cannot be excluded.
The uptake of CGRP during incubations in concentrations above 10−8 M (Figure 3) fits well with previous studies, demonstrating that 10−8 M CGRP induces maximal vasodilatation in segments of guinea-pig basilar artery (Sams et al., 1999). Thus, the presence of CGRP concentrations above 10−8 M has no local physiological relevance and the likely re-use of CGRP seems sound from an energy expenditure point of view. In contrast, 10−9 M CGRP elicits half maximal effects (Sams et al., 1999) and no significant re-depletion could be demonstrated following incubation by 10−9 M CGRP.
The rather fast onset of the CGRP uptake also correlates well with CGRP pharmacology, as the functional equilibrium of CGRP with the guinea-pig basilar artery is accomplished within 3–5 min (Sams et al., 1999). Again, from an energy sparing point of view, a slow uptake could lead to a greater amount of CGRP wasted due to diffusion and breakdown. The threshold for concentration-dependent vasodilatory responses to guinea-pig basilar artery may be the limiting factor for detection of re-depletion following incubations with 10−9 M CGRP.
Neuronal uptake of neuropeptides has not previously been demonstrated whereas specific mechanisms for uptake of amino acid and cathecholamine neurotransmitters (e.g. glutamate, serotonin) are well known. Cellular peptide transport mechanisms have been demonstrated only for di- and tri-peptides via the specific transporters PEPT1 and PEPT2 (Leibach & Ganapathy, 1996; Meredith & Boyd, 2000). The mechanism of the uptake of the 37 amino acid neuropeptide, CGRP remains to be investigated. However, significantly reduced degrees of capsaicin-induced CGRP re-depletion were observed when CGRP8–37 was present during the CGRP incubation (Figure 4). Thus, CGRP uptake was subject to inhibition by CGRP8–37, which suggests that the uptake could be mediated either via a CGRP8–37 sensitive transport mechanism, via a transport mechanism that mediates re-use of both CGRP and CGRP8–37 or via CGRP8–37 sensitive receptors. Possible CGRP uptake via CGRP8–37 sensitive CGRP receptors introduces a role for the CGRP receptor encoding mRNAs, which previously have been demonstrated in CGRP containing sensory ganglia (Edvinsson et al., 1997). Thus, receptor mediated endocytosis of CGRP may be involved in the uptake of CGRP into the sensory terminals.
Recent studies of CGRP receptor trafficking during agonist stimulation revealed that CGRP receptors undergo internalization in HEK293 cells (Kuwasako et al., 2000). Here the CGRP receptor-containing endosomes were targeted to a degradative pathway. It could be speculated that the trafficking of these endosomes in e.g. sensory nerve terminals is different from that in HEK293 cells. For example, endosome-fusion with CGRP-containing vesicles or between endosomes could be expected to form vesicles of readily releasable CGRP. The notion that receptor internalization could represent a pathway for not only re-use of G-protein coupled receptors (Bunemann et al., 1999, Ferguson & Caron, 1998) but in addition for presynaptic re-use of neuropeptides should be investigated further.
Throughout this study, a large number of repetitions of each pharmacological experiment have been performed. In the studies of capsaicin-induced CGRP-depletion, both deviations in the density of capsaicin-sensitive nerve fibres and deviations in CGRP responsiveness are expected to induce considerable variation between experiments. For example, vasodilatory responses induced by the initial capsaicin challenge (Figure 2A) range from 5.5–97.6% of the corresponding preconstriction whereas responses to the third and fourth capsaicin challenge range from 0–53.9 and from 2.6–91.7%, respectively. The large deviation between responses to the first capsaicin challenge is the reason why CGRP uptake in the present study was assessed in capsaicin treated segments. Despite the large derivation between segments, a large degree of consistency was observed towards successively reduced responses to capsaicin challenge number 1, 2 and 3 and to capsaicin challenge number 4, 5 and 6. The deviation between the results obtained with 125I-CGRP is lower than that obtained in the pharmacological studies. Furthermore, this approach does not require preceding CGRP depletion for detection.
Capsaicin-induced 125I-CGRP-release and CGRP receptor-mediated vasomotor responses could be obtained following incubation of guinea-pig basilar artery with CGRP-containing solutions. Based on the current knowledge of capsaicin-mediated CGRP depletion we have provided evidence that CGRP is subject to reload into perivascular nerve fibres. In order to interpret the present data as final conclusive evidence, our results should be supported by other methodological approaches and the mechanism of uptake should be further investigated.
Acknowledgments
Niels C. B. Nyborg and Majid Sheykhzade are acknowledged for their helpful discussions. The authors thank Betina Christensen for expert assistance with the pharmacological studies. The work was supported by the Danish Medical Council (9702065) and The Lundbeck Foundation.
Abbreviations
- CGRP
calcitonin gene-related peptide
- PGF2α
Prostaglandin F2α
- RAMP1
receptor-activity-modifying protein 1
References
- AIYAR N., RAND K., ELSHOURBAGY N.A., ZENG Z., ADAMOU J.E., BERGSMA D.J., LI Y. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J. Biol. Chem. 1996;271:11325–11329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- ASHINA M., BENDTSEN L., JENSEN R., SCHIFTER S., OLESEN J. Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain. 2000;86:133–138. doi: 10.1016/s0304-3959(00)00232-3. [DOI] [PubMed] [Google Scholar]
- BOOTH B.P., TABRIZI-FARD M.A., FUNG H. Calcitonin gene-related peptide-dependent vascular relaxation of rat aorta. An additional mechanism for nitroglycerin. Biochem. Pharmacol. 2000;59:1603–1609. doi: 10.1016/s0006-2952(00)00290-2. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D., WILLIAMS T.J., TIPPINS J.R., MORRIS H.R., MACINTYRE I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- BUNEMANN M., LEE K.B., PALS-RYLAARSDAM R., ROSEBERRY A.G., HOSEY M.M. Desensitization of G-protein-coupled receptors in the cardiovascular system. Annu. Rev. Physiol. 1999;61:169–192. doi: 10.1146/annurev.physiol.61.1.169. [DOI] [PubMed] [Google Scholar]
- BUZZI M.G., CARTER W.B., SHIMIZU T., HEATH H., MOSKOWITZ M.A. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991;30:1193–1200. doi: 10.1016/0028-3908(91)90165-8. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway [see comments] Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- COLQUHOUN E.Q., ELDERSHAW T.P., BENNETT K.L., HALL J.L., DORA K.A., CLARK M.G. Functional and metabolic evidence for two different vanilloid (VN1 and VN2) receptors in perfused rat hindlimb. Life Sci. 1995;57:91–102. doi: 10.1016/0024-3205(95)00250-a. [DOI] [PubMed] [Google Scholar]
- DAI W., ZHOU F.W., SONG Q.J., LI Y.J., DENG H.W., XIONG X.M. Protective effects of calcitonin gene-related peptide on guinea-pig cardiac anaphylaxis. Naunyn Schmiedeberg's Arch. Pharmacol. 2000;361:161–165. doi: 10.1007/s002109900171. [DOI] [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., WU D., ENTZEROTH M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUCKLES S.P. Effects of capsaicin on vascular smooth muscle. Naunyn Schmiedeberg's Arch. Pharmacol. 1986;333:59–64. doi: 10.1007/BF00569661. [DOI] [PubMed] [Google Scholar]
- DUCKLES S.P., LEVITT B. Specificity of capsaicin treatment in the cerebral vasculature. Brain Res. 1984;308:141–144. doi: 10.1016/0006-8993(84)90925-9. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., CANTERA L., JANSEN-OLESEN I., UDDMAN R. Expression of calcitonin gene-related peptide1 receptor mRNA in human trigeminal ganglia and cerebral arteries. Neurosci. Lett. 1997;229:209–211. doi: 10.1016/s0304-3940(97)00456-4. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., EKMAN R., JANSEN I., MCCULLOCH J., UDDMAN R. Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J. Cereb. Blood Flow Metab. 1987;7:720–728. doi: 10.1038/jcbfm.1987.126. [DOI] [PubMed] [Google Scholar]
- FERGUSON S.S., CARON M.G. G protein-coupled receptor adaptation mechanisms. Semin. Cell Dev. Biol. 1998;9:119–127. doi: 10.1006/scdb.1997.0216. [DOI] [PubMed] [Google Scholar]
- FEUERSTEIN G., WILLETTE R., AIYAR N. Clinical perspectives of calcitonin gene related peptide pharmacology. Can. J. Physiol. Pharmacol. 1995;73:1070–1074. doi: 10.1139/y95-152. [DOI] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A. Calcitonin gene-related peptide and tachykinins in relation to local sensory control of cardiac contractility and coronary vascular tone. Acta Physiol. Scand. Suppl. 1988;569:1–63. [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., LISKA J. Potential of calcitonin gene-related peptide in coronary heart disease. Pharmacology. 2000;60:1–8. doi: 10.1159/000028339. [DOI] [PubMed] [Google Scholar]
- FRANCO-CERECEDA A., RUDEHILL A. Capsaicin-induced vasodilatation of human coronary arteries in vitro is mediated by calcitonin gene-related peptide rather than substance P or neurokinin A. Acta Physiol. Scand. 1989;136:575–580. doi: 10.1111/j.1748-1716.1989.tb08704.x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS C.D., ELDERSHAW T.P., GERAGHTY D.P., HALL J.L., COLQUHOUN E.Q. Capsaicin-induced biphasic oxygen uptake in rat muscle: antagonism by capsazepine and ruthenium red provides further evidence for peripheral vanilloid receptor subtypes (VN1/VN2) Life Sci. 1996;59:105–117. doi: 10.1016/0024-3205(96)00267-6. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin as a tool for studying sensory neuron functions. Adv. Exp. Med. Biol. 1991a;298:3–16. doi: 10.1007/978-1-4899-0744-8_1. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991b;43:143–201. [PubMed] [Google Scholar]
- JANSEN I., ALAFACI C., UDDMAN R., EDVINSSON L. Evidence that calcitonin gene-related peptide contributes to the capsaicin-induced relaxation of guinea-pig cerebral arteries. Regul. Pept. 1990;31:167–178. doi: 10.1016/0167-0115(90)90003-f. [DOI] [PubMed] [Google Scholar]
- JANSEN-OLESEN I., MORTENSEN A., EDVINSSON L. Calcitonin gene-related peptide is released from capsaicin-sensitive nerve fibres and induces vasodilatation of human cerebral arteries concomitant with activation of adenylyl cyclase. Cephalalgia. 1996;16:310–316. doi: 10.1046/j.1468-2982.1996.1605310.x. [DOI] [PubMed] [Google Scholar]
- KALLNER G. Release and effects of calcitonin gene-related peptide in myocardial ischaemia. Scand. Cardiovasc. J. Suppl. 1998;49:1–35. [PubMed] [Google Scholar]
- KALLNER G., FRANCO-CERECEDA A. Ion channels involved in the release of calcitonin gene-related peptide by low pH, prostacyclin and capsaicin in the isolated guinea-pig heart. Eur. J. Pharmacol. 1998;352:223–228. doi: 10.1016/s0014-2999(98)00348-3. [DOI] [PubMed] [Google Scholar]
- KILO S., HARDING-ROSE C., HARGREAVES K.M., FLORES C.M. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- KUWASAKO K., SHIMEKAKE Y., MASUDA M., NAKAHARA K., YOSHIDA T., KITAURA M., KITAMURA K., ETO T., SAKATA T. Visualization of the calcitonin receptor-like receptor and its receptor activity-modifying proteins during internalization and recycling. J. Biol. Chem. 2000;275:29602–29609. doi: 10.1074/jbc.M004534200. [DOI] [PubMed] [Google Scholar]
- LEIBACH F.H., GANAPATHY V. Peptide transporters in the intestine and the kidney. Annu. Rev. Nutr. 1996;16:99–119. doi: 10.1146/annurev.nu.16.070196.000531. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J. Neurophysiol. 1996;76:1858–1869. doi: 10.1152/jn.1996.76.3.1858. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. The influence of removing extracellular Ca2+ in the desensitization responses to capsaicin, zingerone and olvanil in rat trigeminal ganglion neurons. Brain Res. 1998;809:246–252. doi: 10.1016/s0006-8993(98)00853-1. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- LUNDBERG J.M., FRANCO-CERECEDA A., HUA X., HOKFELT T., FISCHER J.A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur. J. Pharmacol. 1985;108:315–319. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- MARKOWITZ S., SAITO K., MOSKOWITZ M.A. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J. Neurosci. 1987;7:4129–4136. doi: 10.1523/JNEUROSCI.07-12-04129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON R.T., PETERFREUND R.A., SAWCHENKO P.E., CORRIGAN A.Z., RIVIER J.E., VALE W.W. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature. 1984;308:653–655. doi: 10.1038/308653a0. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH J., UDDMAN R., KINGMAN T.A., EDVINSSON L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MEREDITH D., BOYD C.A. Structure and function of eukaryotic peptide transporters. Cell Mol. Life Sci. 2000;57:754–778. doi: 10.1007/s000180050040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- O'SHAUGHNESSY C.T., WALDRON G.J., CONNOR H.E. Lack of effect of sumatriptan and UK-14,304 on capsaicin-induced relaxation of guinea-pig isolated basilar artery. Br. J. Pharmacol. 1993;108:191–195. doi: 10.1111/j.1476-5381.1993.tb13461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO A., GOTO K. Depletion of calcitonin gene-related peptide (CGRP) by capsaicin in cerebral arteries. J. Pharmacobiodyn. 1986;9:613–619. doi: 10.1248/bpb1978.9.613. [DOI] [PubMed] [Google Scholar]
- SAMS A., YENIDUNYA A., ENGBERG J., JANSEN-OLESEN I. Equipotent in vitro actions of alpha- and beta-CGRP on guinea-pig basilar artery are likely to be mediated via CRLR derived CGRP receptors. Regul. Pept. 1999;85:67–75. doi: 10.1016/s0167-0115(99)00072-5. [DOI] [PubMed] [Google Scholar]
- SHEYKHZADE M., NYBORG N.C. Caliber dependent calcitonin gene-related peptide-induced relaxation in rat coronary arteries: effect of K+ on the tachyphylaxis. Eur. J. Pharmacol. 1998;351:53–59. doi: 10.1016/s0014-2999(98)00290-8. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- THULESEN J., RASMUSSEN T.N., SCHMIDT P., HOLST J.J., POULSEN S.S. Calcitonin gene-related peptide (CGRP) in the nipple of the rat mammary gland. Histochemistry. 1994;102:437–444. doi: 10.1007/BF00269575. [DOI] [PubMed] [Google Scholar]
- UDDMAN R., EDVINSSON L., EKMAN R., KINGMAN T., MCCULLOCH J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985;62:131–136. doi: 10.1016/0304-3940(85)90296-4. [DOI] [PubMed] [Google Scholar]
- URBAN L., DRAY A. Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci. Lett. 1991;134:9–11. doi: 10.1016/0304-3940(91)90496-g. [DOI] [PubMed] [Google Scholar]
- WHARTON J., GULBENKIAN S., MULDERRY P.K., GHATEI M.A., MCGREGOR G.P., BLOOM S.R., POLAK J.M. Capsaicin induces a depletion of calcitonin gene-related peptide (CGRP)-immunoreactive nerves in the cardiovascular system of the guinea-pig and rat. J. Auton. Nerv. Syst. 1986;16:289–309. doi: 10.1016/0165-1838(86)90035-4. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


