Abstract
We investigated the actions of the endogenous opioid tetra-peptide endomorphin 1, a selective μ-opioid receptor agonist, on oxytocin and vasopressin cell activity in vivo and in vitro.
The activity of antidromically-identified supraoptic nucleus cells were recorded from urethane-anaesthetized female rats. The firing rates of both oxytocin and vasopressin cells were reduced by intracerebroventricular endomorphin 1 (5–100 pmol); this inhibition was prevented by intravenous naloxone (5 mg kg−1).
A second group of rats was infused intracerebroventricularly with endomorphin 1 (27 pmol min−1) over 5 days. The firing rates of oxytocin and vasopressin cells in endomorphin 1 pre-treated rats were similar to those of endomorphin 1 naïve rats, indicating tolerance to the inhibitory effects of endomorphin 1. Intravenous naloxone induced similar modest and transient increases in the firing rate of oxytocin cells in endomorphin 1 pre-treated rats and endomorphin 1 naïve rats, indicating that endomorphin 1, unlike the μ-opioid alkaloid agonist, morphine, does not induce μ-opioid dependence in these cells.
In vitro, whole-cell current clamp recordings were made from supraoptic nucleus cells in superfused coronal hypothalamic slices from young female rats. Endomorphin 1 (100 nM) inhibited the firing rate of oxytocin cells but had no significant effect on vasopressin cells at up to 10 μM. Inhibition of oxytocin cells was reversed by naloxone, and remained when synaptic transmission was blocked by superfusion with low Ca2+/Co2+-containing medium.
Thus, endomorphin 1 directly inhibits oxytocin cells but inhibits vasopressin cells by indirect actions. Chronic endomorphin 1 administration induces μ-opioid tolerance in oxytocin and vasopressin cells but not μ-opioid dependence in oxytocin cells.
Keywords: Dependence, hypothalamus, magnocellular neurosecretory cell, morphine, naloxone, μ-opioid receptor, posterior pituitary, supraoptic nucleus, tolerance
Introduction
Magnocellular neurosecretory cells of the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus project to the posterior pituitary where they secrete oxytocin or vasopressin directly into the systemic circulation (Hatton, 1990). Opioid receptor mechanisms participate in the regulation of the electrical activity of oxytocin and vasopressin neurones (Russell et al., 1995; Brown et al., 2000a). In vivo studies suggest that oxytocin cells are inhibited by μ- and κ-opioid agonists while vasopressin cells are inhibited only by κ-opioid agonists (Pumford et al., 1993a), but in vitro electrophysiological studies have shown that both μ- and κ-opioids inhibit SON cells (Wakerley et al., 1983; Inenaga et al., 1990; 1994). However, oxytocin and vasopressin cells were not distinguished in these investigations.
Oxytocin cells develop tolerance and dependence when chronically exposed to morphine, a μ-opioid alkaloid. Tolerance is seen as a decreased sensitivity to the inhibitory actions of morphine while dependence is unmasked as a rebound hyper-excitation following withdrawal of morphine. In late pregnancy, the activity of oxytocin neurones is restrained by tonic activation of μ-receptors (Douglas et al., 1995) and it has been proposed that release of this endogenous opioid restraint may contribute to the increased activity of oxytocin cells at parturition (Russell et al., 1995).
Although the products of the three opioid peptide genes (pro-dynorphin, pro-enkephalin A and pro-opiomelanocortin) are well described, until recently no endogenous opioids have been characterized that exhibit high selectivity for the μ-receptor over δ- and κ-receptors. Two novel endogenous tetra-peptides have now been identified that have high affinity and selectivity for the μ-receptor, endomorphin 1 and endomorphin 2 (Zadina et al., 1997). The affinity of the endomorphins for the μ-receptor is almost equal to that of the most potent synthetic μ-receptor agonist, D-Ala(2)-N-MePhe(4)-Gly(5)-ol]enkephalin (DAMGO), and they exhibit a greater selectivity for the μ-receptor over δ- and κ-opioid receptors compared to DAMGO. Behavioural studies have shown antinociceptive effects of endomorphins in mice (Zadina et al., 1997; Stone et al., 1997). Endomorphin 1 is found in various regions of the rat brain, including the hypothalamus (Martin-Schild et al., 1999).
In vitro, oxytocin and vasopressin SON cells can be distinguished with intracellular or patch clamp electrodes by their electrophysiological properties (Stern & Armstrong, 1995). We have therefore tested the action of endomorphin 1, as a highly selective μ-opioid peptide agonist, on the electrical activity of identified oxytocin and vasopressin SON cells in vitro, and we compared the effects of the κ-opioid agonist U50,488H on vasopressin cells. We have also determined the acute effects of endomorphin 1 on oxytocin and vasopressin cells in vivo and whether chronic central administration of endomorphin 1 is able to induce μ-opioid dependence in oxytocin neurones.
Methods
Animal preparation for in vivo studies
Virgin female Sprague-Dawley rats (232–319 g) were anaesthetized with urethane (ethyl carbamate, 1.25 g kg−1 i.p.), and implanted with a jugular venous cannula for drug injection. A double-barrelled 28 gauge guide cannula was placed stereotaxically into the left lateral cerebral ventricle (0.6 mm caudal and 1.6 mm lateral to bregma and 4.1 mm below the surface of the skull). The double-barrelled cannula allowed increasing doses of endomorphin 1 to be administered from separate syringes without removal and replacement of intracerebroventricular (i.c.v.) infusion cannulae to avoid interference with electrophysiological recording of the firing rate of SON cells. The ventral surface of the brain was then exposed transpharyngeally (Leng, 1981) for extracellular recording of the activity of SON cells with a glass microelectrode filled with 0.9% NaCl (resistance 20–40 MΩ), identified by antidromic stimulation via an electrode placed on the neurohypophysial stalk (Lincoln & Wakerley, 1974).
Extracellular recordings were made from single cells. Oxytocin cells were characterized by their continuous firing pattern and by an excitatory response to intravenous (i.v.) cholecystokinin-8-sulphate (CCK; 20 μg kg−1 i.v.) (Renaud et al., 1987). Vasopressin cells were distinguished by their phasic firing pattern or, where necessary, by their lack of an excitatory response to systemic CCK. Discriminated spikes were recorded and their firing rates measured using conventional software (Spike2®, Cambridge Electronic Design, U.K.).
Endomorphin 1 (in increasing doses of 5–100 pmol in 5–10 μl) was injected via the i.c.v. cannula, with at least 15 min between injections. Naloxone was administered (5 mg kg−1, i.v.), followed by a repeat injection of endomorphin 1 at least 5 min later. To test whether endomorphin 1 could inhibit strongly excited activity of oxytocin and vasopressin cells, some rats were given an i.p. injection of hypertonic saline (1.5 M, 4 ml kg−1) on completion of the transpharyngeal surgery to produce a sustained hyperosmotic stimulation.
Chronic endomorphin 1 infusion
Rats were prepared for chronic i.c.v. endomorphin 1 infusion using a modification of a procedure to induce morphine dependence previously described (Rayner et al., 1988). Briefly, virgin female Sprague-Dawley rats (233–364 g, n=10) were anaesthetized with 5% halothane in a mixture of O2 and N2O (both flow rates at ca. 500 ml min−1). A 21 gauge stainless steel cannula was implanted into the right lateral cerebral ventricle (3.0 mm caudal, 2.0 mm lateral to bregma and 4.5 mm below the surface of the skull). These co-ordinates allowed the chronic infusion cannula to be placed in the right lateral cerebral ventricle without obstruction by the pedestal of the double-barrelled acute i.c.v. guide cannulae. The cannula was attached via polythene tubing to a sub-cutaneous (s.c.) Alzet model 2001 mini-osmotic pump (Charles River U.K. Ltd., U.K.) for chronic endomorphin 1 infusion. The pump and tubing contained endomorphin 1 dissolved in sterile saline to deliver 1 μg h−1 (27 pmol min−1) at 1 μl h−1 over 5 days. Following surgery, the animals were housed individually with free access to food and water. Five days later, the rats were anaesthetized with urethane and prepared for electrophysiological recording from identified SON cells as described above.
In vitro electrophysiology
Female Sprague-Dawley rats (ca. 100–200 g) were decapitated under halothane anaesthesia and the brain was quickly removed and placed in ice-cold oxygenated artificial cerebrospinal fluid (aCSF; composition (in mM): NaCl 124, KCl 5, KH2PO4 1.2, MgSO4 1.3, CaCl2 2.4, NaHCO3 26, D-Glucose 10; saturated with 95% O2 and 5% CO2). The brain was trimmed and hypothalamic slices (350 μm thickness) were cut coronally with a Vibraslice (Campden Instruments). The slices were trimmed with a scalpel blade to isolate the hypothalamus containing the SON, and they were pre-incubated in aCSF (room temperature) for more than 1 h before recording. Then, the slices were transferred to an interface-type chamber that was continuously perfused with oxygenated aCSF (flow rate, 1.5 ml min−1, 35°C) throughout the experiment. In some experiments, low Ca2+/Co2+-containing medium (0.5 mM CaCl2, 0.5 mM CoCl2) was used to block synaptic activity (Jahnsen, 1980).
Whole-cell current clamp recordings were made from SON cells using glass microelectrodes, resistances 5–15 MΩ, filled with the following solution, (mM): potassium gluconate 130, KCl 10, HEPES 10, MgCl2 2, K2ATP 2, pH 7.3 (adjusted using KOH). Current clamp recordings of membrane potential were made with a conventional microelectrode amplifier (Axoclamp-2B, Axon Instruments Inc., U.S.A.), and data were recorded on tape and digitized using pClamp6 software (Axon Instruments Inc., U.S.A.). Tight (2–5 GΩ) seals were formed on SON cells by applying gentle suction to the recording pipette while observing the potential response to a hyperpolarizing current pulse (200 ms, 1 mV). Whole-cell recording was obtained by applying a short pulse of negative pressure. Once stable recording conditions were achieved, electrophysiological identification of SON cells was performed as described previously (Doi et al., 1998a). SON cells were identified electrophysiologically as either vasopressin or oxytocin cells on the basis of their responses to long-duration (1.2 s), small amplitude (100–300 pA) hyperpolarizing current pulses applied with the membrane potential held 10 mV depolarized above resting membrane potential (Stern & Armstrong, 1995). Oxytocin cells were characterized by a membrane potential sag during the hyperpolarizing pulse, and a burst of action potentials on termination of the pulse; vasopressin cells were identified by the lack of these features. In some SON cells we further verified the identification of the cells at the end of the experiments with the same method in the presence of tetrodotoxin (TTX; 0.5 μM) to eliminate action potentials; the membrane potential sag and depolarizing after-potential were clearer to observe in these conditions. Changes in input resistance following drug application were not measured because of the disturbance by this procedure of the firing rate.
Opioid drugs (endomorphin 1, the opioid antagonist naloxone, or the κ-opioid agonist, U50,488H) were added to the superfusate, and applied for 2 min.
Drugs
Endomorphin 1 (Tyr-Pro-Trp-Phe-NH2) (Peninsula Laboratories (U.K.) or Tocris Cookson Ltd. (U.K.)) was dissolved in distilled water and aliquots stored frozen. The aliquots were diluted with aCSF to the appropriate concentration immediately before use. Naloxone hydrochloride and TTX were from Sigma (U.K.), and U50,488H (trans-(±)-3,4-dichloro-N-methyl-N-(2-[1-pyrrolidinyl]cyclohexyl)-benzeneacetamide methanesulphonate) from Tocris Cookson Ltd.
Data analysis
In vivo, the firing rate (spikes s−1) of each neurone was measured in 10 s bins. The mean firing rate was calculated for the 5 min before and after each injection. In vitro, the activity of SON cells was assessed by measurement of firing rate and membrane potential. Firing rate was analysed in 10 s bins. Changes in the firing rate (action potentials or spikes s−1) were compared between firing rate (averaged over 1 min) under basal (pre-drug) conditions and 1 min after application of a drug. For individual cells, sustained changes in firing rate of more than 20% were considered significant. Data are expressed as means±s.e.mean. Statistical analyses were performed using SigmaStat® (Jandel Scientific, Germany).
Results
In vivo studies
Effects of i.c.v. endomorphin 1 administration on the firing rates of oxytocin and vasopressin cells
The spontaneous firing rates of 60 antidromically-identified magnocellular neurosecretory cells recorded from 11 female Sprague-Dawley rats was 2.8±0.3 spikes s−1. Seventeen cells were identified as oxytocin cells (2.8±0.5 spikes s−1 spontaneous firing rate) from their excitatory response to 20 μg kg−1, i.v. CCK (1.5±0.4 spikes s−1 increase over 5 min) and 25 as vasopressin cells (3.8±0.5 spikes s−1 spontaneous firing rate) from their phasic firing pattern or, where necessary, by their lack of an excitatory response to systemic CCK (0.8±0.3 spikes s−1 decrease over 5 min; n=17). The remaining 18 cells could not be classified as either oxytocin or vasopressin cells as they were either silent (n=5) or recordings from these cells were lost prior to systemic CCK injection (n=13).
Nine oxytocin cells (3.7±0.5 spikes s−1 spontaneous firing rate) tested with acute i.c.v. endomorphin 1 administration (5–100 pmol) exhibited a rapid, short-lasting (ca. 3–5 min) dose-dependent inhibition (Figure 1A, C1). Similarly, seven vasopressin cells (5.3±1.2 spikes s−1 spontaneous firing rate) were also inhibited by 5–100 pmol endomorphin 1 i.c.v. (Figure 1B, C2). Two-way repeated measures (RM) analysis of variance (ANOVA) of the basal and post-endomorphin 1 firing rates over 5 min (Figure 1C) revealed significant effects of endomorphin 1 (P<0.0001), significant differences between the responses of oxytocin and vasopressin cells (P=0.005) and a significant interaction between the dose of endomorphin 1 and cell type (P=0.01). Thus, oxytocin cells were more strongly inhibited by endomorphin 1 than vasopressin cells and both oxytocin and vasopressin cells showed dose-dependent inhibition.
Figure 1.
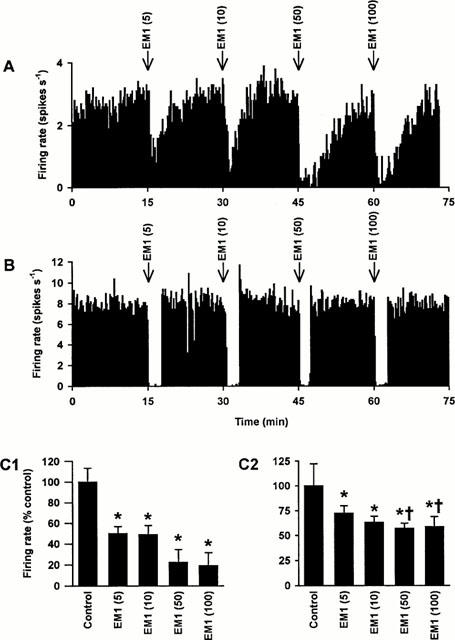
Effects of i.c.v. endomorphin 1 on the activity of oxytocin and vasopressin cells in urethane-anaesthetized rats. (A) The firing rate (averaged in 10 s bins) of a SON oxytocin cell (excited after 20 μg kg−1 CCK i.v.; not shown) recorded from a urethane-anaesthetized rat. This cell was inhibited by endomorphin 1 (5, 10, 50 and 100 pmol, i.c.v.; EM1). (B) The firing rate (averaged in 10 s bins) of a SON vasopressin cell recorded from a urethane-anaesthetized rat. Again, this cell was inhibited by endomorphin 1 (5, 10, 50 and 100 pmol, i.c.v.). (C) The panels show the firing rate (+s.e.mean, averaged over 5 min) of SON oxytocin (C1; n=9) and vasopressin (C2; n=7) cells recorded from rats injected with endomorphin 1 (5, 10, 50 and 100 pmol, i.c.v.). Two-way RM ANOVA revealed significant effects of endomorphin 1 (P<0.0001), significant differences between the responses of oxytocin and vasopressin cells (P=0.005) and a significant interaction between the dose of endomorphin 1 and cell type (P=0.01). *P<0.05 versus control and †P<0.05 versus the equivalent endomorphin 1 dose in oxytocin cells; Student-Newman-Keuls post-hoc tests.
Effects of i.c.v. endomorphin 1 administration on the firing rates of oxytocin and vasopressin cells in hypertonic saline-stimulated rats
As expected, the firing rates of oxytocin cells (6.3±0.8 spikes s−1, n=6) and vasopressin cells (6.6±0.5 spikes s−1, n=6) were greater than in rats not given hypertonic saline (2.2 and 1.7 fold, respectively, see above). Endomorphin 1 (5, 10 or 50 pmol i.c.v.) clearly inhibited five of six osmotically stimulated oxytocin cells (n=2, 2, 1; by between 0.9 and 3.3 spikes s−1; one cell was unaffected by the lowest dose; overall group effect, P=0.01, paired t-test) but only two of six vasopressin cells were inhibited, each by the 5 pmol dose (n=4, 1, 1; with no overall group effect, P=0.43, paired t-test).
Effects of i.v. naloxone on i.c.v. endomorphin 1 actions
Since the effects of i.c.v. endomorphin 1 on the firing rates of oxytocin and of vasopressin cells were indistinguishable between hypertonic saline and non-hypertonic saline treated rats (P=0.51 and P=0.22 for oxytocin and vasopressin cells respectively, t-tests), the results from these sets of rats were pooled for analysis of the effects of endomorphin 1 before and after naloxone administration. Injection of naloxone (5 mg kg−1, i.v.) did not alter the firing rate of 11 oxytocin (0.4±0.3 spikes s−1 increase from 4.0±0.7 spikes s−1; P=0.23, paired t-test; Figure 2A,C1) and six vasopressin cells (0.1±0.3 spikes s−1 increase from 3.8±1.3 spikes s−1; P=0.84, paired t-test; Figure 2C2).
Figure 2.
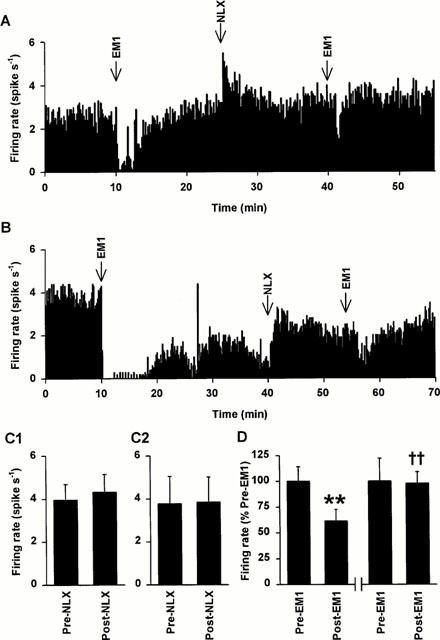
Effects of i.v. naloxone on endomorphin 1 inhibition of oxytocin and vasopressin cell activity in urethane-anaesthetized rats. (A) The firing rate (averaged in 10 s bins) of a SON oxytocin cell (excited after 20 μg kg−1 CCK i.v.; not shown) recorded from a urethane-anaesthetized rat. This cell was inhibited by endomorphin 1 (100 pmol, i.c.v.; EM1). Administration of naloxone (NLX, 5 mg kg−1, i.v.) caused little change in the firing rate of the cell (typical of 11 cells) but markedly reduced its responsiveness to a second injection of the same dose of endomorphin 1 (typical of five cells). (B) The firing rate (averaged in 10 s bins) of a SON vasopressin cell (inhibited after 20 μg kg−1 CCK i.v.; not shown) recorded as in (A) (but in a rat given i.p. 1.5 M NaCl on completion of surgery). This cell was inhibited by endomorphin 1 (500 pmol, i.c.v.) but naloxone (NLX, 5 mg kg−1, i.v.) both reversed the continuing inhibition following the endomorphin 1 injection, and markedly reduced the inhibition from a second injection of the same dose of endomorphin 1. (C) The mean firing rate (+s.e.mean) of SON oxytocin (C1; n=11) and vasopressin (C2; n=6) cells 5 min before and after naloxone injection (NLX, 5 mg kg−1, i.v.). There was no significant effect of naloxone on the firing rates of oxytocin or vasopressin cells (P=0.23 and 0.84, respectively, paired t-tests). (D) The mean firing rate (+s.e.mean) of SON oxytocin cells 5 min before (Pre-EM1) and after (post-EM1) endomorphin 1 injection (5, 10, 50 or 100 pmol, i.c.v.; n=5) 15 min before (left hand panel) and at least 5 min after (right hand panel) naloxone (5 mg kg−1, i.v.). One-way RM ANOVA revealed a significant effect of EM1 (P=0.008). **P<0.01 versus preEM1/preNLX and ††P<0.01 versus postEM1/preNLX; Student-Newman-Keuls post-hoc tests.
Five of the oxytocin cells from naloxone-treated rats were tested with i.c.v. endomorphin 1 (5–100 pmol) 15 min before and at least 5 min after naloxone (5 mg kg−1, i.v.). The dose of endomorphin 1 used in each rat was the same before and after naloxone injection and was the minimum that caused a clear inhibition (>1 spike s−1) of the firing rates of each of the oxytocin cells before naloxone administration. The change in firing rate of oxytocin cells following the post-naloxone injection of endomorphin 1 (0.0±0.4 spikes s−1 decrease) was significantly less than the reduction following the pre-naloxone injection of endomorphin 1 (2.5±0.5 spikes s−1 decrease; P=0.009, paired t-test; n=5; Figure 2D). Vasopressin cells were less strongly inhibited by endomorphin 1 than oxytocin cells. Four vasopressin cells were inhibited by i.c.v. endomorphin 1 (by 1.3±0.8 spikes s−1), and after naloxone, endomorphin 1 had no effect on these individual cells (mean decrease 0.4±0.2 spikes s−1). However, for a single vasopressin cell in a hypertonic-saline treated rat, i.c.v. injection of 500 pmol endomorphin 1 profoundly reduced the activity (by 3.5 spikes s−1) before naloxone, but was much less effective after naloxone (0.5 spikes s−1 reduction; Figure 2B). Antagonism of the effects of endomorphin 1 by naloxone confirmed an action mediated by opioid receptors.
Effects of chronic i.c.v. endomorphin 1 administration on the spontaneous activity of oxytocin and vasopressin cells
The spontaneous firing rate of 40 magnocellular cells recorded from 10 rats receiving chronic i.c.v. endomorphin 1 (27 pmol min−1 over 5 days) was 3.3±0.4 spikes s−1. The spontaneous firing rate of 14 cells categorized as oxytocin cells by their excitation following i.v. CCK (1.3±0.3 spikes s−1 increase in firing rate over 5 min; similar to that seen in endomorphin 1 naïve rats; P=0.72, Student's t-test) was 2.7±0.6 spikes s−1 (Figure 3B1). The spontaneous firing rate of 15 cells that were categorized as vasopressin cells due to their phasic firing pattern or, where necessary, by their lack of excitation by i.v. CCK (1.1±0.6 spikes s−1 decrease in firing rate over 5 min) was 3.9±0.6 spikes s−1 (Figure 3B2). These spontaneous activities were similar to those of cells recorded from endomorphin 1 naïve rats (P=0.89 and P=0.93 for oxytocin and vasopressin cells, respectively; Student's t-tests). Eleven cells could not be identified as either oxytocin or vasopressin cells as they were silent (n=4) or the recordings were lost prior to i.v. CCK administration (n=7). The proportions of oxytocin, vasopressin and non-categorized cells recorded were similar in endomorphin 1 naïve and endomorphin 1 pre-treated rats (P=0.78, Chi-square test). These results indicate that oxytocin and vasopressin cells develop tolerance to chronic i.c.v. endomorphin 1.
Figure 3.
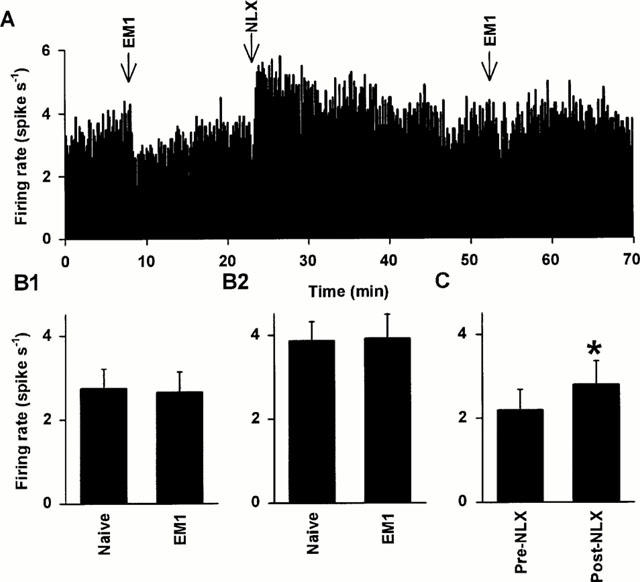
Effects of chronic i.c.v. endomorphin 1 on the activity of oxytocin and vasopressin cells in urethane-anaesthetized rats. (A) The firing rate (averaged in 10 s bins) of a SON oxytocin cell (excited after 20 μg kg−1 CCK i.v.; not shown) recorded from a urethane-anaesthetized rat infused with i.c.v. endomorphin 1 for 5 days. This cell was inhibited by acute injection of endomorphin 1 (100 pmol, i.c.v.), but to a lesser extent than in endomorphin 1 naïve cells (Figure 2A,D). Similarly to its effects on endomorphin 1 naïve cells, naloxone (5 mg kg−1, i.v.) caused only a small increase (relative to pre-EM1) in the firing rate of the cell, and markedly reduced its responsiveness to a second injection of the same dose of endomorphin 1. (B) The spontaneous firing rates (+s.e.mean) of SON oxytocin (B1) and vasopressin (B2) cells recorded from urethane-anaesthetized endomorphin 1 naïve (oxytocin: n=17; vasopressin: n=25) and endomorphin 1 pre-treated (EM1; oxytocin: n=14; vasopressin: n=15) rats. There were no significant differences in the initial firing rates of oxytocin cells, or of vasopressin cells, between endomorphin 1 naïve and endomorphin 1 pre-treated rats, indicating that both cell types were tolerant to the inhibitory effects of i.c.v. endomorphin 1. (C) The mean firing rate of SON oxytocin cells recorded from endomorphin 1 pre-treated rats (n=9) 5 min before and after naloxone injection (NLX, 5 mg kg−1, i.v.), showing a small increase in firing rate after naloxone (*P<0.05; paired t-test).
Nine of the 14 oxytocin cells recorded from endomorphin 1 pre-treated rats were challenged with naloxone (5 mg kg−1, i.v.). The firing rate of these cells was 2.2±0.5 and 2.8±0.6 spikes s−1 before and after naloxone administration, respectively (P=0.03, paired t-test). Two-way RM ANOVA of the effects of naloxone on endomorphin 1 naïve and chronic endomorphin 1 pre-treated oxytocin cells revealed a significant effect of naloxone (P=0.02) but no effect of endomorphin 1 pre-treatment (P=0.11) nor any interaction between the effects of naloxone and endomorphin 1 pre-treatment (P=0.52; Figure 3A,C). Thus, there was no evidence for naloxone-precipitated withdrawal excitation of oxytocin cells in rats given i.c.v. endomorphin.
Since we have previously shown that vasopressin cells do not develop μ-opioid dependence in response to chronic i.c.v. morphine administration (Russell et al., 1995; Brown et al., 2000a), we did not systematically test the effects of naloxone on vasopressin cells recorded from endomorphin 1 pre-treated rats.
In vitro studies
Stable whole-cell recordings were made from 24 SON cells in total. These cells all had a membrane potential more negative than −45 mV and mean input resistance was 171.3±8.9 MΩ (range: 110–253 MΩ). Using the electrophysiological criteria described in the Methods, before the application of peptides, we identified 11 of 24 as vasopressin cells and 13 of 24 as oxytocin cells. The mean resting membrane potential of oxytocin cells was −51.7±1.5 mV and that of vasopressin cells was −53.5±1.8 mV. The mean spontaneous (basal) firing rate of oxytocin cells was 4.9±0.7 spikes s−1 and that of vasopressin cells was 6.2±0.6 spikes s−1.
Effects of endomorphin 1 on SON cells
In oxytocin cells, endomorphin 1 caused a clear inhibition of spontaneous firing (Figures 4A and 5A). Figure 5B summarizes the changes in firing rate of five oxytocin cells when 100 nM endomorphin 1 was applied for 2 min. The inhibitory effect of endomorphin 1 appeared from about 10 nM (n=5) and peaked between 100 nM and 1 μM (n=5). In six of 13 oxytocin cells, the inhibition of firing rate was accompanied by a small (∼5 mV) hyperpolarization (Figure 4B). Firing activity of these cells recovered from endomorphin 1 inhibition in less than 30 min after returning to superfusion with aCSF. In 10 of 11 vasopressin cells, 10 μM endomorphin 1 did not inhibit spontaneous firing; the firing rate of the 11th cell was reduced by only 25%. Overall, there was no significant effect of 10 μM endomorphin 1 on the spontaneous firing rate of vasopressin cells (Figure 5C).
Figure 4.
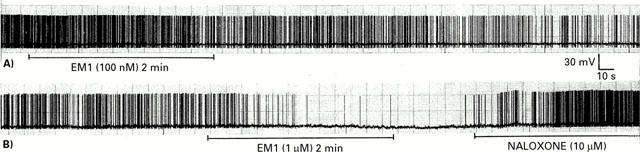
Effects of endomorphin 1 on spontaneous spiking activity of SON oxytocin cells in vitro. Typical original chart records of action potentials recorded with whole cell current-clamp in hypothalamic slices; SON oxytocin cells were identified by their response to a hyperpolarizing pulse as described in the Methods. (A): After stable basal activity was recorded for several minutes, endomorphin 1 (EM1, 100 nM) was added to the superfusate for 2 min. Firing was progressively inhibited, beginning during the endomorphin 1 application, and continuing for at least 5 min after the end of the application. (B): A greater concentration of endomorphin 1 (1 μM) was applied to this cell for 2 min, rapidly causing a small hyperpolarization with near complete inhibition of firing. Application of naloxone (10 μM) 1 min after the end of the endomorphin 1 application caused a rapid reversal of the hyperpolarization and increase in firing rate. This is in contrast to the prolonged inhibition after the end of endomorphin 1 application at a 10 fold lower concentration, without naloxone, seen in (A).
Figure 5.
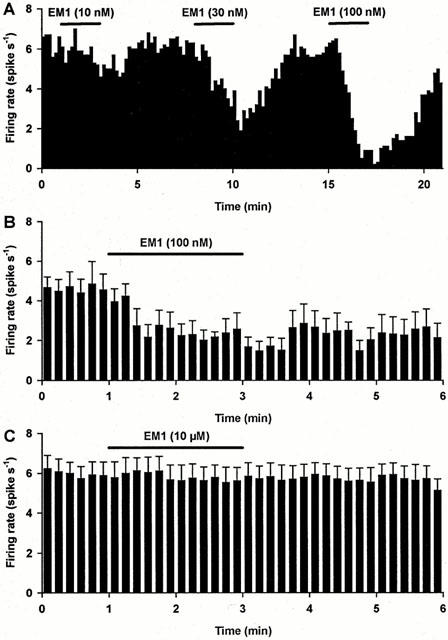
Effects of endomorphin 1 on SON cells in vitro. (A) The firing rate (in 10 s bins) of a SON oxytocin cell (measured with whole cell current-clamp recording in vitro in hypothalamic slices; oxytocin and vasopressin cells were distinguished by their different responses to hyperpolarizing pulses as described in Methods). This cell shows a dose-dependent inhibition of the firing rate by endomorphin 1 (EM1) (typical of three experiments). (B) The mean firing rate (+s.e.mean in 10 s bins) of five oxytocin cells recorded as in (A). For each cell, the firing rate was inhibited by over 50% by 100 nM endomorphin 1. (C) The mean firing rate of 11 vasopressin cells recorded as in (A); there was no significant effect of 10 μM endomorphin 1 on the firing rate of this group of cells (10 cells were unaffected by endomorphin 1, while the firing rate of just one cell was reduced, by 25%).
Effects of naloxone on the inhibition of oxytocin cells by endomorphin 1
To test for mediation by opioid receptors, the effects of the opioid antagonist naloxone on the inhibitory action of endomorphin 1 on oxytocin cells was investigated. Ten μM naloxone was applied 1 min after endomorphin 1 application was stopped, long before any spontaneous recovery of firing was seen in other experiments (Figure 4). This concentration of naloxone is sufficient to antagonize opioid agonists, without affecting the spontaneous firing of SON cells (Doi et al., 1998a; data not shown). Naloxone rapidly reversed the inhibition of firing by endomorphin 1 (n=6; Figures 4B and 6A).
Figure 6.
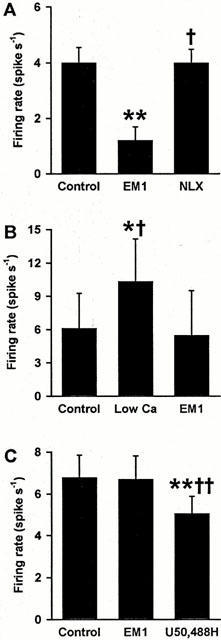
Endomorphin 1 inhibits oxytocin, but not vasopressin, cells in vitro. (A) The mean firing rate of six oxytocin cells averaged over 1 min before (Control), the second minute during 100 nM–10 μM endomorphin 1 (EM1) superfusion and during the second minute of subsequent 10 μM naloxone (NLX) superfusion. Naloxone reversed endomorphin 1 inhibition of oxytocin cell activity (see Figure 4). (B) The mean firing rate of three oxytocin cells before (Control) synaptic blockade using low Ca2+/Co2+-containing medium (Low Ca) and during 1–3 μM endomorphin 1 superfusion in low Ca2+/Co2+-containing medium. The firing rate of these cells was inhibited from an excited level by endomorphin 1 in the presence of synaptic blockade. (C) The mean firing rate of ten vasopressin cells before (Control) and during 10 μM endomorphin 1 superfusion and during subsequent superfusion of four of the cells with 30 μM U50,488H, showing κ-opioid inhibition of vasopressin cells insensitive to endomorphin 1. *P<0.05 and **P<0.01 versus control; †P<0.05 and ††P<0.01 versus endomorphin 1; one-way RM ANOVA followed by Student-Newman-Keuls tests.
Effects of endomorphin 1 in low Ca2+/Co2+-containing medium
To test the effect of blockade of synaptic transmission, we investigated the effect of endomorphin 1 in low Ca2+/Co2+-containing medium on the activity of oxytocin cells (Figure 6B, n=3). Perfusion with low Ca2+ and Co2+-containing medium was started 2 min before endomorphin 1 application. This increased the firing rate, as noted previously (Doi et al., 1998a). Nevertheless, endomorphin 1 still showed a clear inhibitory effect on firing rate in this condition, similar to that in the normal medium.
Sensitivity of vasopressin cells to U50,488H
As more than 90% of vasopressin cells did not respond to the μ-opioid agonist, endomorphin 1, we tested the sensitivity of some vasopressin cells to the κ-opioid agonist U50,488H, which is known to inhibit vasopressin cells in vitro (Doi et al., 1998a,1998b). After application of 10 μM endomorphin 1 with no response, 30 μM U50,488H was applied for 2 min (n=5) and clearly inhibited the firing rate of vasopressin cells (Figure 6C), indicating that the lack of opioid sensitivity of vasopressin cells was specific to μ- and not κ-opioid receptors.
Discussion
The present in vivo studies showed a clear inhibition of both SON oxytocin and vasopressin cells by acute i.c.v. endomorphin 1 while the in vitro studies showed a selective inhibition of the spontaneous firing rate of SON oxytocin cells by endomorphin 1, with no effect on more than 90% of vasopressin cells. Since endomorphin 1 is a highly selective μ-opioid agonist (Zadina et al., 1997) and naloxone antagonized its actions, oxytocin cells (or their nearby inputs) express functional μ-opioid receptors. Vasopressin cells were much less sensitive than oxytocin cells to inhibition by endomorphin 1, thus it appears likely that vasopressin cells (or their nearby inputs) may express functional μ-opioid receptors at much lower levels than oxytocin cells. Alternatively, the differences between the effects of endomorphin 1 on the activity of oxytocin and vasopressin cells may result from coupling of the μ-receptor to different intracellular mechanisms in each cell type. Endomorphin 1 effectively inhibited oxytocin cells in a low Ca2+/Co2+-containing medium which blocks synaptic transmission (Jahnsen, 1980), indicating that endomorphin 1 acts (at least in part) directly on oxytocin cells. Although previous studies with morphine also indicate a direct action on oxytocin cells, the expression of μ-receptors on oxytocin cells is weak (Sumner et al., 1990). Nevertheless, there are changes in μ-selective opioid binding in the SON that are correlated with exposure to morphine; during chronic i.c.v. morphine infusion, μ-receptor binding is selectively reduced (Sumner et al., 1990).
In the present in vitro experiments, vasopressin cells not inhibited by the μ-agonist, endomorphin 1, were inhibited by the κ-agonist, U50,488H, showing that endomorphin 1 does not act on κ-receptors at doses of up to 10 μM. It can be inferred that the inhibition of oxytocin cells by endomorphin 1 was not mediated by κ-receptors. Hyperpolarization was evident in about half of the oxytocin cells during endomorphin 1 superfusion, indicating that the direct action of endomorphin 1 is likely to involve activation of a K+ conductance(s). In other cells such conductances are linked to μ-receptors via pertussis toxin-sensitive Gi/o proteins (Chen & Yu, 1994) and pertussis toxin pre-treatment in vivo attenuates morphine actions on oxytocin cells (Pumford et al., 1993b; Brown et al., 2000b).
Although i.c.v. endomorphin 1 inhibited SON vasopressin cells, in vitro endomorphin 1 was ineffective, so it evidently does not act directly on these cells, nor does it act on any local inputs to vasopressin cells contained within the slice preparation. A major input to the magnocellular neurones is from structures in the lamina terminalis, mediating hyperosmotic stimulation (McKinley et al., 1996), in addition to direct action of hyperosmolarity on the magnocellular neurones (Oliet & Bourque, 1993). These structures contain opioid receptors (Mansour et al., 1988), and are a possible site of action of i.c.v. endomorphin 1 to explain its inhibitory actions on vasopressin cells. It is unlikely that this is the major site of action in relation to oxytocin neurones as there is no evidence for selective distribution of inputs from lamina terminalis structures to oxytocin and vasopressin cells, and i.c.v. endomorphin 1 inhibited oxytocin cells more effectively than vasopressin cells, even when this rostral input was strongly excited by the osmotic stimulus. Taking into account the in vitro experiments and previous studies with systemic morphine (Ludwig et al., 1997), an action of endomorphin 1 directly on oxytocin cells is probable. However, an action of i.c.v. endomorphin 1 on brainstem inputs to oxytocin neurones is also likely. Nucleus tractus solitarius (NTS) cells (including A2 noradrenergic cells) project selectively to SON oxytocin cells (Cunningham & Sawchenko, 1991). The release of noradrenaline within the SON, following stimulation of the A2 neurones by systemic CCK, is inhibited by morphine acting presynaptically within the SON (Onaka et al., 1995a,1995b). In addition there are immunocytochemically-identified endomorphin 1 terminals in the NTS (Martin-Schild et al., 1999). However, systemic morphine, sufficient to interrupt parturition by inhibiting oxytocin secretion, does not prevent the activation of NTS cells in parturition (Luckman & Antonijevic, 1993), although it clearly prevents activation of oxytocin cells (Russell et al., 1989; 1991). Thus, the SON is the locus of powerful μ-opioid inhibition of oxytocin cells, probably through a combination of pre-synaptic actions on the NTS input and the direct actions demonstrated in the present in vitro study.
Oxytocin cells develop dependence on morphine after i.c.v. infusion of this drug over 5 days (Russell et al., 1995) and studies of the development of morphine tolerance and dependence in oxytocin cells also indicate direct actions of i.c.v. morphine on these cells since withdrawal excitation of oxytocin cells in morphine-dependent rats is not prevented by ablative interruption of excitatory inputs (Brown et al., 1998a). Here, infusion of 1 μg h−1 of endomorphin 1 did not induce μ-opioid dependence in oxytocin cells since naloxone administration (to induce endomorphin 1 withdrawal) caused only a modest and transient increase in firing rate, similar to that seen in endomorphin 1 naïve rats. This is unlikely to reflect a failure to deliver sufficient doses of endomorphin 1 to inhibit the cells as the dose used (27 pmol min−1) is in the same range as the effective dosage for the acute inhibition of oxytocin and vasopressin cells by i.c.v. endomorphin 1. Thus, it appears that oxytocin cells develop tolerance to chronic endomorphin 1 infusion (since the cells were spontaneously active at similar levels to those in untreated animals), but that this μ-opioid agonist does not induce μ-opioid dependence in these neurones. It has recently been shown that endomorphin 1, but not morphine, causes receptor internalization (Burford et al., 1998; McConalogue et al., 1999); such differences may account for the failure of the endomorphin 1 to induce μ-opioid dependence (Whistler et al., 1999).
While the present study has revealed that a highly selective μ-agonist directly and potently inhibits SON oxytocin cells, but not vasopressin cells, this merely indicates potential functional significance of endomorphin 1. Endomorphin 1 containing perikarya have been visualized in the posterior hypothalamus as well as the NTS (Martin-Schild et al., 1999) but there are no reports of whether endomorphin 1 nerve terminals impinge on oxytocin cells. Nevertheless, endomorphin 1 remains a candidate endogenous opioid peptide capable of inhibiting oxytocin cells. Previous studies with opioid antagonists have revealed that endogenous opioids do not normally tonically inhibit the electrical activity of oxytocin cells in virgin rats, even when strongly stimulated. However, there is one circumstance, apart from morphine dependence (Russell et al., 1995), where naloxone activates the cell bodies of oxytocin cells and thus reveals central opioid inhibition, and this is in late pregnancy (Douglas et al., 1995). In particular, opioid restraint on activation by the noradrenergic brainstem input emerges, and this is through μ-, not κ-, receptors (Douglas et al., 1995). Whether endomorphin 1 is the endogenous opioid responsible for this inhibition in pregnancy is presently speculative.
The selective inhibition of vasopressin cells by κ-, but not μ-, receptor activation, in vitro may relate to the body of evidence showing that dynorphins (including dynorphin-A1-8, a κ-agonist with μ-opioid activity (Zhang et al., 1998)), are co-secreted products of vasopressin cells (Watson et al., 1982; Whitnall et al., 1983). Dynorphins acting on vasopressin cell κ-receptors may have an auto-inhibitory function. Vasopressin is released from the dendrites of these cells when they are stimulated (Ludwig, 1998) and so co-packaged dynorphins will also be released. There is evidence from the effects of the κ-selective antagonist nor-binaltorphimine, and from κ-receptor desensitization induced by chronic U50,488H exposure, that such an auto-inhibitory κ-opioid action is important in regulating the firing pattern of vasopressin cells (Brown et al., 1998b; 1999; Brown & Leng, 2000). The relative lack of functionally linked μ-receptors on vasopressin cells, as shown in the present study, may prevent interference with this κ-opioid mechanism by locally released μ-opioids.
In conclusion, the selective endogenous μ-opioid peptide, endomorphin 1, directly inhibits oxytocin cells but chronic endomorphin 1 administration does not induce μ-opioid dependence in these cells. The relative lack of direct μ-opioid sensitivity of vasopressin cells may be important in preventing cross-talk between μ- and κ-mechanisms, since the latter are proposed to be important in regulating vasopressin neurone firing pattern, but can also inhibit oxytocin cells.
Acknowledgments
N. Doi was supported by Kaken Pharmaceutical Co. Ltd., Chiba, Japan, C.H. Brown by The Medical Research Council, and J.A. Russell by the Biotechnology and Biological Sciences Research Council.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- ANOVA
analysis of variance
- CCK
cholecystokinin-8-sulphate
- DAMGO
D-Ala(2)-N-MePhe(4)-Gly(5)-ol]enkephalin
- EM1
endomorphin 1
- i.c.v.
intracerebroventricular
- i.p.
intraperitoneal
- i.v.
intravenous
- NLX
naloxone
- NTS
nucleus tractus solitarius
- RM
repeated measures
- s.c.
sub-cutaneous
- SON
supraoptic nucleus
- TTX
tetrodotoxin
- U50,488H
trans-(±)-3,4-dichloro-N-methyl-N-(2-[1-pyrrolidinyl]cyclohexyl)-benzeneacetamide methanesulphonate
References
- BROWN C.H., GHAMARI-LANGROUDI M., LENG G., BOURQUE C.W. Kappa-opioid receptor activation inhibits post-spike depolarising after-potentials in rat supraoptic nucleus neurones in vitro. J. Neuroendocrinol. 1999;11:825–828. doi: 10.1046/j.1365-2826.1999.00419.x. [DOI] [PubMed] [Google Scholar]
- BROWN C.H., JOHNSTONE L.E., MURPHY N.P., LENG G., RUSSELL J.A. Local injection of pertussis toxin attenuates morphine withdrawal excitation of rat supraoptic nucleus neurones. Brain Res. Bull. 2000b;52:115–121. doi: 10.1016/s0361-9230(00)00241-0. [DOI] [PubMed] [Google Scholar]
- BROWN C.H., LENG G. In vivo modulation of post-spike excitability in vasopressin cells by kappa-opioid receptor activation. J. Neuroendocrinol. 2000;12:711–714. doi: 10.1046/j.1365-2826.2000.00547.x. [DOI] [PubMed] [Google Scholar]
- BROWN C.H., LUDWIG M., LENG G. Kappa-opioid regulation of neuronal activity in the rat supraoptic nucleus in vivo. J. Neurosci. 1998b;18:9480–9488. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.H., MURPHY N.P., MUNRO G., LUDWIG M., BULL P.M., LENG G., RUSSELL J.A. Interruption of central noradrenergic pathways and morphine withdrawal excitation of oxytocin neurones in the rat. J. Physiol. 1998a;507:831–842. doi: 10.1111/j.1469-7793.1998.831bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.H., RUSSELL J.A., LENG G. Opioid modulation of magnocellular neurosecretory cell activity. Neurosci. Res. 2000a;36:97–120. doi: 10.1016/s0168-0102(99)00121-2. [DOI] [PubMed] [Google Scholar]
- BURFORD N.T., TOLBERT L.M., SADEE W. Specific G protein activation and mu-opioid receptor internalization caused by morphine, DAMGO and endomorphin 1. Eur. J. Pharmacol. 1998;342:123–126. doi: 10.1016/s0014-2999(97)01556-2. [DOI] [PubMed] [Google Scholar]
- CHEN Y., YU L. Differential regulation by cAMP-dependent protein kinase and protein kinase C of the mu opioid receptor coupling to a G protein-activated K+ channel. J. Biol. Chem. 1994;269:7839–7842. [PubMed] [Google Scholar]
- CUNNINGHAM E.T.J., SAWCHENKO P.E. Reflex control of magnocellular vasopressin and oxytocin secretion. Trends Neurosci. 1991;14:406–411. doi: 10.1016/0166-2236(91)90032-p. [DOI] [PubMed] [Google Scholar]
- DOI N., DUTIA M.B., RUSSELL J.A. Inhibition of rat oxytocin and vasopressin supraoptic nucleus neurons by nociceptin in vitro. Neuroscience. 1998a;84:913–921. doi: 10.1016/s0306-4522(97)00547-2. [DOI] [PubMed] [Google Scholar]
- DOI N., DUTIA M.B., BROWN C.H., LENG G., RUSSELL J.A. Inhibitory actions of nociceptin (orphanin FQ) on rat supraoptic nucleus oxytocin and vasopressin neurones in vitro. Adv. Exp. Med. Biol. 1998b;449:147–151. doi: 10.1007/978-1-4615-4871-3_17. [DOI] [PubMed] [Google Scholar]
- DOUGLAS A.J., NEUMANN I., MEEREN H.K., LENG G., JOHNSTONE L.E., MUNRO G., RUSSELL J.A. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J. Neurosci. 1995;15:5049–5057. doi: 10.1523/JNEUROSCI.15-07-05049.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTON G.I. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog. Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- INENAGA K., IMURA H., YANAIHARA N., YAMASHITA H. Kappa-selective opioid receptor agonists leumorphin and dynorphin inhibit supraoptic neurons in rat hypothalamic slice preparations. J. Neuroendocrinol. 1990;2:389–396. doi: 10.1111/j.1365-2826.1990.tb00423.x. [DOI] [PubMed] [Google Scholar]
- INENAGA K., NAGATOMO T., NAKAO K., YANAIHARA N., YAMASHITA H. Kappa-selective agonists decrease postsynaptic potentials and calcium components of action potentials in the supraoptic nucleus of rat hypothalamus in vitro. Neuroscience. 1994;58:331–340. doi: 10.1016/0306-4522(94)90039-6. [DOI] [PubMed] [Google Scholar]
- JAHNSEN H. The action of 5-hydroxytryptamine on neuronal membranes and synaptic transmission in area CA1 of the hippocampus in vitro. Brain Res. 1980;197:83–94. doi: 10.1016/0006-8993(80)90436-9. [DOI] [PubMed] [Google Scholar]
- LENG G. The effects of neural stalk stimulation upon firing patterns in rat supraoptic neurones. Exp. Brain Res. 1981;41:135–145. doi: 10.1007/BF00236603. [DOI] [PubMed] [Google Scholar]
- LINCOLN D.W., WAKERLEY J.B. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J. Physiol. 1974;242:533–554. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCKMAN S.M., ANTONIJEVIC I. Morphine blocks the induction of fos immunoreactivity in hypothalamic magnocellular neurons at parturition, but not that induced in other brain regions. Ann. N.Y. Acad. Sci. 1993;689:630–631. doi: 10.1111/j.1749-6632.1993.tb55612.x. [DOI] [PubMed] [Google Scholar]
- LUDWIG M. Dendritic release of vasopressin and oxytocin. J. Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- LUDWIG M., BROWN C.H., RUSSELL J.A., LENG G. Local opioid inhibition and morphine dependence of supraoptic nucleus oxytocin neurones in the rat in vivo. J. Physiol. 1997;505:145–152. doi: 10.1111/j.1469-7793.1997.145bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSOUR A., KHACHATURIAN H., LEWIS M.E., AKIL H., WATSON S.J. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- MARTIN-SCHILD S., GERALL A.A., KASTIN A.J., ZADINA J.E. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J. Comp. Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- MCCONALOGUE K., GRADY E.F., MINNIS J., BALESTRA B., TONINI M., BRECHA N.C., BUNNETT N.W., STERNINI C. Activation and internalization of the mu-opioid receptor by the newly discovered endogenous agonists, endomorphin 1 and endomorphin-2. Neuroscience. 1999;90:1051–1059. doi: 10.1016/s0306-4522(98)00514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKINLEY M.J., PENNINGTON G.L., OLDFIELD B.J. Anteroventral wall of the third ventricle and dorsal lamina terminalis: headquarters for control of body fluid homeostasis. Clin. Exp. Pharmacol. Physiol. 1996;23:271–281. doi: 10.1111/j.1440-1681.1996.tb02823.x. [DOI] [PubMed] [Google Scholar]
- OLIET S.H., BOURQUE C.W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993;364:341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- ONAKA T., LUCKMAN S.M., ANTONIJEVIC I., PALMER J.R., LENG G. Involvement of the noradrenergic afferents from the nucleus tractus solitarii to the supraoptic nucleus in oxytocin release after peripheral cholecystokinin octapeptide in the rat. Neuroscience. 1995b;66:403–412. doi: 10.1016/0306-4522(94)00609-9. [DOI] [PubMed] [Google Scholar]
- ONAKA T., LUCKMAN S.M., GUEVARA-GUZMAN R., UETA Y., KENDRICK K., LENG G. Presynaptic actions of morphine: blockade of cholecystokinin-induced noradrenaline release in the rat supraoptic nucleus. J. Physiol. 1995a;482:69–79. doi: 10.1113/jphysiol.1995.sp020500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUMFORD K.M., RUSSELL J.A., LENG G. Effects of the selective kappa-opioid agonist U50,488 upon the electrical activity of supraoptic neurones in morphine-tolerant and morphine-naïve rats. Exp. Brain Res. 1993a;94:237–246. doi: 10.1007/BF00230291. [DOI] [PubMed] [Google Scholar]
- PUMFORD K.M., LENG G., RUSSELL J.A. A pertussis toxin-sensitive G protein mediates inhibition by morphine of spontaneous electrical activity of oxytocin neurones in anaesthetized rats. Exp. Brain Res. 1993b;94:247–251. doi: 10.1007/BF00230292. [DOI] [PubMed] [Google Scholar]
- RAYNER V.C., ROBINSON I.C., RUSSELL J.A. Chronic intracerebroventricular morphine and lactation in rats: dependence and tolerance in relation to oxytocin neurones. J. Physiol. 1988;396:319–347. doi: 10.1113/jphysiol.1988.sp016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENAUD L.P., TANG M., MCCANN M.J., STRICKER E.M., VERBALIS J.G. Cholecystokinin and gastric distension activate oxytocinergic cells in rat hypothalamus. Am. J. Physiol. 1987;253:R661–R665. doi: 10.1152/ajpregu.1987.253.4.R661. [DOI] [PubMed] [Google Scholar]
- RUSSELL J.A., GOSDEN R.G., HUMPHREYS E.M., CUTTING R., FITZSIMONS N., JOHNSTON V., LIDDLE S., SCOTT S., STIRLAND J.A. Interruption of parturition in rats by morphine: a result of inhibition of oxytocin secretion. J. Endocrinol. 1989;121:521–536. doi: 10.1677/joe.0.1210521. [DOI] [PubMed] [Google Scholar]
- RUSSELL J.A., LENG G., BICKNELL R.J. Opioid tolerance and dependence in the magnocellular oxytocin system: a physiological mechanism. Exp. Physiol. 1995;80:307–340. doi: 10.1113/expphysiol.1995.sp003850. [DOI] [PubMed] [Google Scholar]
- RUSSELL J.A., LENG G., COOMBES J.E., CROCKETT S.A., DOUGLAS A.J., MURRAY I., WAY S. Pethidine (meperidine) inhibition of oxytocin secretion and action in parturient rats. Am. J. Physiol. 1991;261:R358–R368. doi: 10.1152/ajpregu.1991.261.2.R358. [DOI] [PubMed] [Google Scholar]
- STERN J.E., ARMSTRONG W.E. Electrophysiological differences between oxytocin and vasopressin neurones recorded from female rats in vitro. J. Physiol. 1995;488:701–708. doi: 10.1113/jphysiol.1995.sp021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE L.S., FAIRBANKS C.A., LAUGHLIN T.M., NGUYEN H.O., BUSHY T.M., WESSENDORF M.W., WILCOX G.L. Spinal analgesic actions of the new endogenous opioid peptides endomorphin 1- and -2. Neuroreport. 1997;8:3131–3135. doi: 10.1097/00001756-199709290-00025. [DOI] [PubMed] [Google Scholar]
- SUMNER B.E., COOMBES J.E., PUMFORD K.M., RUSSELL J.A. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience. 1990;37:635–645. doi: 10.1016/0306-4522(90)90095-l. [DOI] [PubMed] [Google Scholar]
- WAKERLEY J.B., NOBLE R., CLARKE G. Effects of morphine and D-Ala, D-Leu enkephalin on the electrical activity of supraoptic neurosecretory cells in vitro. Neuroscience. 1983;10:73–81. doi: 10.1016/0306-4522(83)90081-7. [DOI] [PubMed] [Google Scholar]
- WATSON S.J., AKIL H., FISCHLI W., GOLDSTEIN A., ZIMMERMAN E., NILAVER G., VAN WIMERSMA GREIDANUS T.B. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982;216:85–87. doi: 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]
- WHISTLER J.L., CHUANG H.H., CHU P., JAN L.Y., VON ZASTROW M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- WHITNALL M.H., GAINER H., COX B.M., MOLINEAUX C.J. Dynorphin-A-(1-8) is contained within vasopressin neurosecretory vesicles in rat pituitary. Science. 1983;222:1137–1139. doi: 10.1126/science.6648526. [DOI] [PubMed] [Google Scholar]
- ZADINA J.E., HACKLER L., GE L.J., KASTIN A.J. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- ZHANG S., TONG Y., TIAN M., DEHAVEN R.N., CORTESBURGOS L., MANSSON E., SIMONIN F., KIEFFER B., YU L. Dynorphin A as a potential endogenous ligand for four members of the opioid receptor gene family. J. Pharmacol. Exp. Ther. 1998;286:136–141. [PubMed] [Google Scholar]


