Abstract
In this study, we examined whether morphine dependence was inhibited by rolipram, a cyclic AMP selective phosphodiesterase inhibitor in mice, since a role for the cyclic AMP systems in the development of morphine dependence has been reported.
Mice, which received morphine (10 mg kg−1 s.c.) twice a day for 5 days showed withdrawal syndromes such as jumping, rearing and forepaw tremor following naloxone challenge (5 mg kg−1 i.p.) on the 6th day.
Such mice exhibited a significant elevation of cyclic AMP levels in the thalamus compared to control mice. However, co-administration of rolipram (1 mg kg−1 i.p.) with morphine for 5 days significantly attenuated the severity of the withdrawal syndrome and the increase in the cyclic AMP levels after the administration of naloxone.
In naïve mice, acute morphine treatment (10 mg kg−1 s.c.) decreased cyclic AMP levels in the thalamus and cerebral cortex 10 min later. The decrease of cyclic AMP levels induced by acute morphine treatment was blocked by co-administration of rolipram (1 mg kg−1 i.p.). However, acute rolipram did not affect the naloxone-precipitated morphine withdrawal syndrome.
These results suggest that the elevation of the cyclic AMP levels is involved in the development of morphine withdrawal syndrome and that blockade of the morphine-induced reduction of cyclic AMP levels by chronic rolipram inhibits the development of dependence and the behavioural and biochemical changes induced by naloxone. Furthermore, rolipram may be a useful drug for attenuating the development of morphine dependence.
Keywords: Cyclic AMP, morphine dependence, phosphodiesterase inhibitor, rolipram
Introduction
The clinical use of morphine is limited in practice by its tendency to cause tolerance and dependence with prolonged or repeated administration. Many researchers have reported the changes of opioid receptors and second messengers (e.g. cyclic AMP) in opiate tolerance and dependence by using biochemical and molecular techniques. Opioid dependence has been demonstrated to be associated with changes in the cyclic AMP systems in in vitro experiments. In neuroblastoma x glioma (NG108) cells, acute treatments with morphine and other opiates have been shown to inhibit adenylate cyclase activity resulting in a decrease of cyclic AMP levels (Sharma et al., 1975; Traber et al., 1975). However, upon repeated exposure to morphine, the adenylate cyclase activity and cyclic AMP levels return to control levels in the tolerant state and increase above the control levels during withdrawal (Sharma et al., 1975; Traber et al., 1975; Benalal & Bachrach, 1985). These findings have provided the basis for a ‘cyclic AMP hypothesis' for the development of morphine dependence: that increases in adenylate cyclase activity and hence cyclic AMP levels in the brain, represent biochemical correlates of morphine dependence (Kuriyama et al., 1978; Collier, 1980). The activity is thought to be up-regulated as a compensatory response to chronic opioid receptor mediated inhibition of adenylate cyclase (see review, Nestler, 1997). Thus, the transient inhibition of adenylate cyclase activity might be important to the development of morphine dependence (Beitner et al., 1989). However, little is known about how and when the cyclic AMP levels change in vivo.
The cyclic AMP levels are regulated by the activities of adenylate cyclase and phosphodiesterases (PDEs; Thompson, 1991). In addition, it has been reported that some non-selective PDE inhibitors (i.e. theophylline, caffeine and 3-isobutyl-1-methylxanthine) and forskolin, a lipid soluble analogue of cyclic AMP, induce the behaviour that resembles morphine withdrawal syndrome in naïve rats and potentiate the naloxone-precipitated morphine withdrawal syndrome in morphine dependent rats (Collier & Francis, 1975; Francis et al., 1975; Ho et al., 1975; Rasmussen et al., 1990). It is reported that PDEs have at least seven isozymes, and within the different gene families there is considerable sequence similarity across species (Manganiello et al., 1995). Non-selective inhibitors prevent the effects of not only cyclic AMP selective PDE (PDE 4) but other type PDEs, such as Ca2+-dependent, photoreceptor and cyclic GMP selective PDEs (Beavo & Reifsnyder, 1990; Beavo et al., 1994).
Although we have shown that co-administration of non-selective PDE inhibitors and cyclic AMP-related drug with morphine block naloxone-precipitated morphine withdrawal syndrome and increase of cyclic AMP level in brain (Nabeshima et al., 1997; Itoh et al., 2000; Hamdy et al., 2001), it is not enough to clarify the role of cyclic AMP in morphine dependence. We speculated that the drugs which increase cyclic AMP levels can inhibit morphine dependence, since the transient inhibition of adenylate cyclase activity may be critical point to develop morphine dependence.
Rolipram, one of the best-known PDE 4 inhibitors (Schwabe et al., 1976; Wachtel, 1983; Schneider, 1984), was clinically tested as an antidepressant (Wachtel, 1983) before the discovery of its potent PDE 4 inhibitory activity (Davis, 1984). Rolipram exerts a multiplicity of effects in the central nervous system, peripheral cells and tissues. For example, rolipram reduces neuronal damage following cerebral ischemia (Kato et al., 1995; Block et al., 1997) and attenuates the memory deficits (Imanishi et al., 1997), and furthermore, it might be an useful drug for an anti-asthma or anti-inflammation (Torphy & Undem, 1991).
In this study, we examined (i) whether morphine withdrawal syndrome was affected by co-administration of rolipram and (ii) whether the changes of cyclic AMP levels are involved in the development of morphine dependence in mice by using behavioural and biochemical techniques.
Methods
Animals
Male ddY mice (Nihon SLC Co. Ltd., Shizuoka, Japan), 6 weeks of age, were used. The animals were housed in a controlled environment (23±1°C, 50±5% humidity) and were allowed food and water ad libitum. The room lights were off between 0700 and 1900 h.
All experiments were performed in accordance with the Guidelines for Animal Experiments of the Nagoya University School of Medicine, the Guiding Principles for the Care and Use of Laboratory Animals approved by the Japanese Pharmacological Society, and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
Rolipram (Meiji Seika Co. Ltd., Tokyo, Japan), morphine hydrochloride (Shionogi Pharmaceutical Co. Ltd., Osaka, Japan) and naloxone hydrochloride (Sigma Co. St. Louis, U.S.A.) were used. Rolipram was dissolved in 2% dimethoxysulphoxide (DMSO; Sigma) and the other drugs were dissolved in saline.
Behavioural test
Morphine dependence
To develop morphine dependence, mice received morphine (10 mg kg−1 s.c.) with or without rolipram twice daily for 5 days. Rolipram (0.01–1 mg kg−1 i.p.) was administered 30 min before the morphine treatment. On the 6th day, naloxone (5 mg kg−1 i.p.) was administered 2 h after the administration of morphine. Twenty minutes before the naloxone treatment, mice were placed in a transparent acrylic cylinder (20 cm diameter, 35 cm high) to habituate to their environment. Immediately after the naloxone challenge, each mouse was placed gently again in the cylinder, and then, the frequency of naloxone-precipitated withdrawal signs (jumping, rearing and forepaw tremor) was counted for 15 min.
Tail-flick test
A standardized tail-flick apparatus (TAIL FLICK UNIT 7350, Ugo Basile, Italy) with a radiant heat source connected to an automatic timer was used to assess the antinociceptive (analgesic) response. The tail-flick latency was measured from the start of the heat stimulus applied to the distal 2 cm of the tail until the animal exhibited a flick of the tail. The intensity of the stimulus was adjusted to achieve baseline latencies (pre-value) between 2.5 and 4.0 s. Cut-off time (15 s) was used to minimize damage to the tail. The pre-value was obtained prior to injection. Antinociceptive effects of morphine were determined 60 min after its administration on the first treatment of the 1st, 3rd and 5th day.
Locomotor activity
Each animal was placed gently in a transparent acrylic cage (30×45×45 cm), then locomotor activity was measured for 90 min immediately after rolipram treatment using digital counters with infrared sensors (Scanet SV-10; Toyo Sangyo, Toyama, Japan). Morphine was administered 30 min after rolipram treatment.
Rearing and forepaw tremor
Twenty minutes before the observation, mice were placed in a transparent acrylic cylinder (20 cm diameter, 35 cm high) to habituate to their environment. Sixty minutes after rolipram treatment, each mouse was placed gently again in the cylinder, and then the rearing and forepaw tremor were counted for 15 min. All behavioural tests were assessed by an observer ‘blind' to the treatment.
Measurement of cyclic AMP contents in the mouse brain
Cyclic AMP contents were assayed as described previously (Yamada et al., 1996). To prevent the loss of cyclic AMP after decapitation (Schneider, 1984), each mouse was killed by focused micowave irradiation for 1.5 s at 5 kW (Toshiba Microwave Applicator TMW-6402A, Toshiba, Tokyo, Japan) 5 min after the naloxone challenge. Brains were removed rapidly, cerebral cortex and thalamus were dissected out according to the method of Glowinski & Iversen (1966) and to the mouse brain atlas of Franklin & Paxinos (1997) on an ice-cold plate. All brain tissues were stored at −80°C until assayed. Each tissue was homogenized with cold 6% (w v−1) trichloroacetic acid at 2–8°C to give a 10% (w v−1) homogenate, and centrifuged at 2000×g for 15 min at 4°C. The supernatant was washed with five volumes of water-saturated diethylether four times. The upper ether layer was discarded after each wash. The aqueous extract remaining was dried at 40°C. Then the dried extract was dissolved in assay buffer, cyclic AMP levels were determined using enzymeimmumossay kit as described by the manufacturer (Pharmacia Ammersham, U.S.A.).
Data analysis
The results are expressed as the mean±s.e.mean. Statistical significance was determined by the Dunnett multiple comparisons test. P<0.05 was taken as a significant level of difference.
Results
Effects of repeated co-administration of rolipram with morphine on naloxone-precipitated morphine withdrawal syndrome
The effects of repeated co-administration of rolipram with morphine on naloxone-precipitated morphine withdrawal syndrome are shown in Figure 1. As shown in the (Repeated Rolipram 0+Repeated Morphine 10+Naloxone 5)-group, repeated administration of morphine (10 mg kg−1) significantly increased the numbers of jumping, rearing and forepaw tremor after naloxone challenge (5 mg kg−1). In mice co-administered rolipram with morphine repeatedly, the signs of withdrawal syndrome were significantly reduced in a dose dependent manner (See (Repeated Rolipram 0.01–1+Repeated Morphine 10+Naloxone 5)-group).
Figure 1.
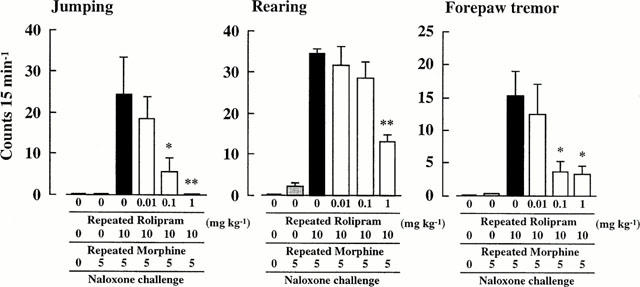
Effects of repeated co-administration of rolipram with morphine on naloxone-precipitated morphine withdrawal syndrome in mice. n=6–10. *P<0.05, **P<0.01 vs (Repeated Rolipram 0+Repeated Morphine 10+Naloxone 5)-group.
We checked the effects of acute rolipram on some behaviours. Acute rolipram at the range 0.01–1 mg kg−1 did not affect the locomotor activity and the frequency of rearing behavior and forepaw tremor compared with (Rolipram 0)-group, although at 10 mg kg−1, rolipram induced forepaw tremor, head weaving, hypolocomotion and sniffing, significantly (data not shown).
Effects of repeated co-administration of rolipram with morphine on cyclic AMP levels in the thalamus and cerebral cortex of morphine dependent mice
The cyclic AMP contents of the thalamus and the cerebral cortex of the (Repeated Rolipram 0+Repeated Morphine 0+Naloxone 0)-group was 247.0±37.2 and 469.8±51.3 pmol g−1 tissue, respectively. No significant difference was observed in the cyclic AMP levels of the thalamus and the cerebral cortex between (Repeated rolipram 0+Repeated Morphine 0+Naloxone 0)-group and (Repeated Rolipram 0+Repeated Morphine 10+Naloxone 0)-group (data not shown). The cyclic AMP levels in the thalamus of morphine dependent mice increased after naloxone challenge (see (Repeated Rolipram 0+Repeated Morphine 10+Naloxone 5)-group)), to over twice the control levels (Figure 2A). This increase in cyclic AMP levels was attenuated by the repeated co-administration of rolipram (1 mg kg−1) with morphine (see (Repeated Rolipram 1+Repeated Morphine 10+Naloxone 5)-group). Repeated administration of rolipram alone had no effect on the cyclic AMP levels in the thalamus (see (Repeated Rolipram 1+Repeated Morphine 0+Naloxone 5)-group). Similar phenomenon was observed in the cerebral cortex (Figure 2B), but not significant.
Figure 2.
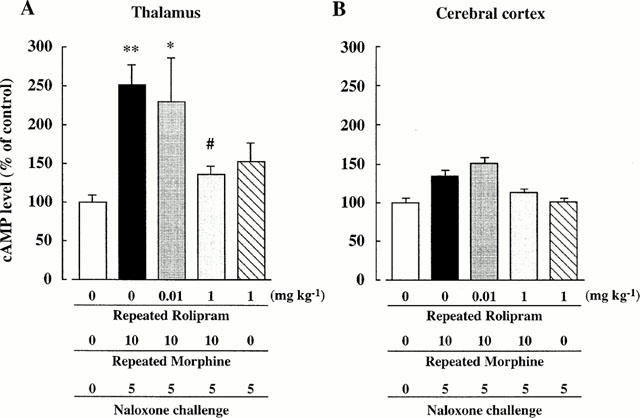
Effects of repeated co-administration of rolipram with morphine on the cyclic AMP levels in the brain of morphine dependent mice. The cyclic AMP levels in the thalamus (A) and the cerebral cortex (B) of the (Repeated Rolipram 0+Repeated Morphine 0+Naloxone 0)-group are 247.0±37.2 and 469.8±51.3 pmol g−1 tissue, respectively. n=5–7. *P<0.05, **P<0.01 vs (Repeated Rolipram 0+Repeated Morphine 0+Naloxone 0)-group, #P<0.05 vs (Repeated Rolipram 0+Repeated Morphine 10+Naloxone 5)-group.
Effects of acute morphine and rolipram on cyclic AMP levels in the thalamus and cerebral cortex of naïve mice
To clarify whether rolipram directly affects the change of cyclic AMP levels induced by acute morphine (10 mg kg−1), we investigated the effects of rolipram (1 mg kg−1) on the acute morphine-induced reduction of cyclic AMP levels in naive mice. The cyclic AMP contents of the thalamus and the cerebral cortex in the control mice was 309.8±58.1 and 558.6±83.9 pmol g−1 tissue, respectively. Ten minutes after the morphine administration, cyclic AMP levels in the thalamus decreased significantly compared with control. However, 20 and 30 min after morphine administration, cyclic AMP levels returned to the control levels. In the cerebral cortex, there was only a tendency for the cyclic AMP levels to decrease at 10 min and the to recover at 20 and 30 min (Figure 3A).
Figure 3.
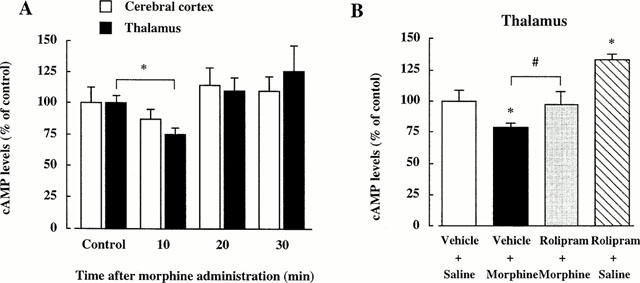
Effects of acute morphine (10 mg kg−1) on the cyclic AMP levels in the mouse brain (A) and effects of rolipram (1 mg kg−1) on the decrease of cyclic AMP levels induced by morphine in the thalamus (B). (A) The control cyclic AMP levels in non-treated mice are 309.8±58.1 and 558.6±83.9 pmol g−1 tissue in the thalamus and cerebral cortex, respectively. n=6–7. *P<0.05 vs corresponding control. (B) The cyclic AMP levels in the thalamus of (Vehicle+Saline)-group are 322.8±48.3 pmol g−1 tissue. n=5–6. *P<0.05 vs (Vehicle+Saline)-group, #P<0.05 vs (Vehicle+Morphine)-group.
To investigate the effects of acute rolipram on the reduction of cyclic AMP levels induced by morphine, the amount of cyclic AMP in the thalamus of mice co-administered rolipram (1 mg kg−1) with morphine (10 mg kg−1) was determined. Rolipram alone elevated the cyclic AMP levels significantly. Pretreatment with rolipram attenuated the morphine-induced decrease of the cyclic AMP levels to the control levels (Figure 3B).
Effects of repeated co-administration of rolipram with morphine on the morphine tolerance in antinociception
As shown in Figure 4, there was no difference among groups in the tail-flick latency before the drug treatments (Pre-value). Morphine (10 mg kg−1) prolonged significantly the tail flick latency on the 1st day. Rolipram alone affected neither the antinoiception nor nociception (data not shown). When rolipram (0.01–1 mg kg−1) was co-administered with morphine (10 mg kg−1), the antinociceptive effects of morphine were not affected on the 1st day.
Figure 4.
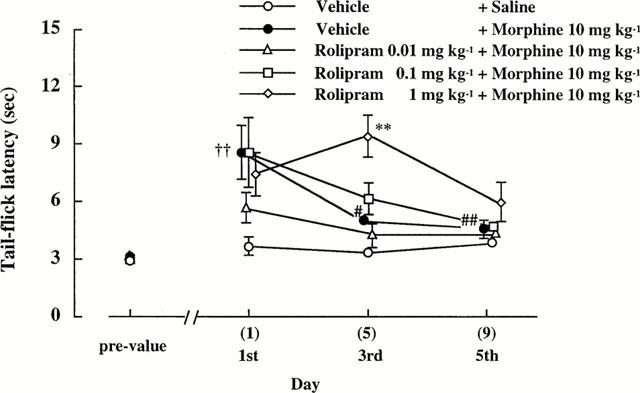
Effects of repeated co-administration of rolipram with morphine on the morphine tolerance in antinociception. The numbers in the parenthesis indicate the number of injections. n=6–10. ††P<0.01 vs (Vehicle+Saline)-group, **P<0.01 vs (Vehicle+Morphine)-group, #P<0.05, ##P<0.01 vs the corresponding 1st treatment.
By repeated morphine treatment the tail-flick latencies on the 3rd and 5th day were shorter compared to those on the 1st day, indicating a development of tolerance to antinociception on the 3rd and 5th day. There was no difference in the tail-flick latency in the (Rolipram 1 mg kg−1+Morphine 10 mg kg−1) group between the 1st day and 3rd day, but not 5th day, suggesting that co-administration of rolipram (1 mg kg−1) with morphine prevented the development of tolerance to antinociception induced by morphine up to the 3rd day.
Discussion
Since the cyclic AMP pathway is known to be important to morphine dependence, and cyclic AMP levels are regulated by adenylate cyclases and PDEs, in this study, we examined the behavioural and biochemical effects of repeated co-administration of rolipram, a cyclic AMP selective PDE (PDE 4) inhibitor, with morphine on naloxone-precipitated morphine withdrawal syndrome. The mice repeatedly co-administered vehicle (2% DMSO) with morphine started to exhibit the major withdrawal behaviours (jumping, rearing and forepaw tremor) within a few min with the peak of withdrawal expression at around 5 min after the naloxone administration. Generally, morphine dependent mice show various withdrawal signs, e.g. jumping, forepaw tremor, rearing, diarrhoea, teeth chattering, weight loss and ptosis. To induce more severe withdrawal symptoms including autonomic signs, a high concentration of naloxone (5 mg kg−1) was used as reported previously (Geary & Wooten, 1985; Neal & Sparber, 1986; Kamei et al., 1995). We could observe jumping, forepaw tremor and rearing as major three withdrawal signs, but we found no other clear signs of withdrawal even in morphine dependent mice as some previous reports have mentioned (Bianchetti et al., 1986; Maldonado et al., 1992). That there were no changes in autonomic signs may be due to our fixed low dose (10 mg kg−1, twice a day) of morphine as opposed to the gradual increases up to 100 mg kg−1 in other experiments (e.g. Maldonado et al., 1996; Ledent et al., 1999). Repeated co-administration of rolipram with morphine reduced the expression of the major withdrawal behaviors in a dose dependent manner. We also observed modest effects of rolipram (0.1 mg kg−1) on naloxone-precipitated morphine withdrawal syndrome. Although the numbers of jumping and forepaw tremor were suppressed by co-administration of rolipram at a dose of 0.1 mg kg−1, the numbers of rearing behaviour were not. The reasons why major three withdrawal behaviours were not suppressed by 0.1 mg kg−1 of rolipram are unknown. One possible explanation is follows: Jumping and rearing behaviours are induced in the same period, and the posture of rearing behaviour is similar to that of jumping. Thus, we could not differentiate the effects of co-administration of rolipram (0.1 mg kg−1) on rearing behaviour. Furthermore, naloxone-precipitated morphine withdrawal syndrome was not affected by acute rolipram treatment on the 6th day (data not shown), indicating that rolipram (0.01–1 mg kg−1) does not have effects on the expression of naloxone-precipitated morphine withdrawal syndrome. These findings confirmed our previous results (Nabeshima et al., 1997; Itoh et al., 2000; Hamdy et al., 2001) and suggest that naloxone-precipitated morphine withdrawal syndrome is mediated via the cyclic AMP pathway, and that cyclic AMP systems are involved in the development of morphine dependence.
We measured the cyclic AMP contents of the mouse brain 5 min after naloxone treatment, when the mice were jumping and rearing, two of the most severe withdrawal signs. A significant elevation in the cyclic AMP levels in the thalamus was observed in mice co-administered vehicle (2% DMSO) with morphine. Although the lower dose of rolipram (0.01 mg kg−1) which failed to affect the major withdrawal behaviours, did not change the increase in cyclic AMP levels induced by morphine withdrawal, the increase was significantly attenuated by the repeated co-administration of rolipram (1 mg kg−1) with morphine. Cyclic AMP levels was increased significantly by rolipram alone (1 mg kg−1) in this study but only 25–50% increase compared with control. The cyclic AMP levels of mice showing the severe morphine withdrawal was more than twice of that in the control mice. It seems there is a threshold which mice express the morphine withdrawal. Thus, the increased cyclic AMP levels induced by rolipram (1 mg kg−1) may not be sufficient to induce the withdrawal syndrome although the cyclic AMP levels were elevated. These results indicate that the effects of rolipram on the biochemical change in the cyclic AMP levels parallel and are consistent with the effects of rolipram on the behavioural change. It is suggested that certain regions of the brain are involved in the development of morphine dependence, such as the locus coeruleus (Aghajanian, 1978; Maldonado et al., 1992; see review by Nestler, 1997), amygdala (Calvino et al., 1979; Terwillliger et al., 1991) and thalamus (Wei et al., 1972; 1973; Tremblay & Charton, 1981; Terwilliger et al., 1991). In addition, the cerebral cortex and thalamus are rich in μ receptors and both regions are terminal and intermedia areas for noradrenergic neurons associated with drug addiction. In this study, marked changes in the cyclic AMP levels were observed in the thalamus.
To elucidate the mechanism of the effect of rolipram, mice received acute rolipram and/or morphine and cyclic AMP levels were measured. A decrease in the cyclic AMP levels was observed following acute morphine treatment early on 10 min in the brain, especially, the thalamus. Additionally, the decrease was transient and 20 min later the levels recovered to or above the control. Our in vivo experiment confirmed previous in vitro findings that culture cells exposed to morphine show a decrease of adenylate cyclase activity but gradually recover up to or above normal activity (Sharma et al., 1975; Traber et al., 1975). Further, naloxone treatment after chronic morphine exposure produces a dramatic increase in the cyclic AMP levels in the culture cells, which is thought to reflect the morphine withdrawal state in vitro. Thus, Nestler has proposed the ‘cyclic AMP hypothesis' for opiate addiction, dependent and withdrawal states (Nestler, 1997). In this study the mice that expressed naloxone-precipitated morphine withdrawal signs showed remarkably increased cyclic AMP levels in the thalamus. It can be hypothesized that the transient decreae in cyclic AMP levels evoked by acute morphine is one of the initial and most important molecular steps in the development of opiate dependence, since rolipram blocked the acute morphine-induced decrease of cyclic AMP levels. The changes in adenylate cyclase activity and cyclic AMP levels could possibly occur via a negative feedback pathway, and serve to trigger the up-regulation of the cyclic AMP system that is observed in opiate dependent animals.
In the tail-flick test, rolipram at doses of 0.01–1 mg kg−1 which itself does not have any effects on the antinociception, failed to affect the antinocicepton caused by acute morphine treatment on the 1st day. This result is consistent with previous reports that low doses (<3 mg kg−1) of rolipram did not affect the antinociceptive effects of acute morphine (Nicholson et al., 1991) and the pain thresholds are not affected by the treatment of cyclic AMP or dibutyryl cyclic AMP intrathecallly or intracerebroventricularly (Murayama & Hikino, 1985; Wang et al., 1993). Taken together, cyclic AMP levels seem to be irrelevant to the pain. Surprisingly, co-administration of rolipram (1 mg kg−1) with morphine prevented the development of tolerance to the antinociceptive effects of morphine up to the 3rd day, although vehicle with morphine developed the tolerance. No significant reduction of tail-flck latency was observed between the 1st and 5th day in the mice treated with rolipram (1 mg kg−1) and morphine, suggesting that rolipram inhibits the development of morphine tolerance. Rolipram has been reported to enhance the activity of the brain opioid system and increase the sensitivity of opiate receptors as well as some antidepressants (Baraldi et al., 1983; Przewlocki et al., 1985). These effect may be due to a stimulatory effect on noradrenergic transmission by both enhancement of noradrenergic turnover presynaptically (Schoffelmeer et al., 1985) and inhibition of cyclic AMP degradation postsynaptically (Wachtel, 1983). However, these effects also disappeared on the 5th day. As mentioned above, the mechanism might be due to change in the activity of brain opioid system, but this remains to be clarified.
In conclusion, these results suggest that the inhibition of reduction in the cyclic AMP levels induced by acute morphine prevents the behavioural and biochemical changes observed in morphine withdrawal in mice. Rolipram has already been reported to have anti-ischaemic (Kato et al., 1995; Block et al., 1997) and anti-amnesic (Imanishi et al., 1997) effects and is used as an antidepressant in the clinical setting. Additionally, our experiments show that rolipram may be used to attenuate the development of morphine dependence in the clinical field.
Acknowledgments
Many thanks to Meiji Seika Co. Ltd. for supplying rolipram. This work was supported, in part, by a Grant-in-Aid for Scientific Research (10044260) and COE Research from the Ministry of Education, Science, Sports and Culture of Japan, the Foundation of Kyosaidan, the Fund of Hibino Foundation, the Health Sciences Research Grants for Research on Pharmaceutical and Medical Safety from the Ministry of Health and Welfare of Japan, and by Special Coordination Funds for Promoting Science and Technology, Target-oriented Brain Science Research Program from the Ministry of Science and Technoogy of Japan.
Abbreviations
- DMSO
dimethoxysulphoxide
- i.p.
intraperitoneal
- PDE
phosphodiesterase
- s.c.
subcutaneous
References
- AGHAJANIAN G.K. Tolerance of locus neurons to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- BARALDI M., POGGIOLI R., SANTI M, VERGONI A.V., BERTOLINI A. Antidepressants and opiates interactions: Pharmacological and biochemical evidence. Pharmacol. Res. Commun. 1983;15:843–857. doi: 10.1016/s0031-6989(83)80092-7. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A., CONTI M., HEASLIP R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994;46:399–405. [PubMed] [Google Scholar]
- BEAVO J.A., REIFSNYDER D.H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol. Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- BEITNER D.B., DUMAN R.S., NESTLER E.J. A novel action of morphine in the rat locus coeruleus: Persistent decrease in adenylate cyclase. Mol. Pharmacol. 1989;35:559–564. [PubMed] [Google Scholar]
- BENALAL D., BACHRACH U. Opiates and cultured neuroblastoma x glioma cells. Effect on cyclic AMP and polyamine levels and on ornithine decarboxylase and protein kinase activities. Biochem. J. 1985;227:389–395. doi: 10.1042/bj2270389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIANCHETTI A., GUIDICE A., NAVA F., MANARA L. Dissociation of morphine withdrawal diarrhea and jumping in mice by the peripherally selective opioid antagonist SR58002C. Life Sci. 1986;39:2297–2303. doi: 10.1016/0024-3205(86)90660-0. [DOI] [PubMed] [Google Scholar]
- BLOCK F., TONDAR A., SCHMIDT W., SCHWARZ M. Delayed treatment with rolipram protects against neuronal damage following global ischemia in rats. Neuroreport. 1997;8:3829–3832. doi: 10.1097/00001756-199712010-00033. [DOI] [PubMed] [Google Scholar]
- CALVINO B., LAGOWSKA J., BEN-ARI Y. Morphine withdrawal syndrome: Differential participation of structures located within the amygdaloid complex and striatum of the rat. Brain Res. 1979;177:19–34. doi: 10.1016/0006-8993(79)90915-6. [DOI] [PubMed] [Google Scholar]
- COLLIER H.O. Cellular site of opiate dependence. Nature. 1980;283:625–629. doi: 10.1038/283625a0. [DOI] [PubMed] [Google Scholar]
- COLLIER H.O., FRANCIS D.L. Morphine abstinence is associated with increased brain cyclic AMP. Nature. 1975;255:159–162. doi: 10.1038/255159b0. [DOI] [PubMed] [Google Scholar]
- DAVIS C.W. Assessment of selective inhibition of rat cerebral cortical calcium independent and calcium dependent phosphodiesterases in crude extracts using deoxycyclic AMP and potassium ions. Biochem. Biophys. Acta. 1984;797:354–362. doi: 10.1016/0304-4165(84)90257-5. [DOI] [PubMed] [Google Scholar]
- FRANCIS D.L., ROY A.C., COLLIER H.O. Morphine abstinence and quasi-abstinence effects after phosphodiesterase inhibitors and naloxone. Life Sci. 1975;16:1901–1906. doi: 10.1016/0024-3205(75)90299-4. [DOI] [PubMed] [Google Scholar]
- FRANKLIN K.B.J., PAXINOS G. The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego; 1997. [Google Scholar]
- GEARY W.A., WOOTEN G.F. Dose effects of naloxone on fixed morphine dependence: Simultaneous behavioural and 2-deoxyglucose study in the rat. Brain Res. 1985;322:69–78. doi: 10.1016/0006-8993(85)90390-7. [DOI] [PubMed] [Google Scholar]
- GLOWINSKI J., IVERSEN L.L. Regional studies of catecholamines in the rat brain. I. the disposition of [3H] norepinephrine, [3H]dopamine and [3H] dopa in various regions of the brain. J. Neurochem. 1966;13:655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- HAMDY M., MAMIYA T., NODA Y., SAYED M., ASSI A.-A., GOMAA A, YAMADA K., NABESHIMA T.Aselective phosphodiesterase IV inhibitor, rolipram blocks both withdrawal behavioral manifestations, and c-Fos protein expression in morphine dependent mice Behav. Brain. Res. 2001(in press) [DOI] [PubMed]
- HO I.K., LOH H.H. , BHARGAVA H.N., WAY E.L. Effects of cyclic nucleotides and phosphodiesterase inhibition on morphine tolerance and physical dependence. Life Sci. 1975;16:1895–1900. doi: 10.1016/0024-3205(75)90298-2. [DOI] [PubMed] [Google Scholar]
- IMANISHI T., SAWA A., ICHIMARU Y., MIYASHIRO M., KATO S., YAMAMOTO T., UEKI S. Ameliorating effects of rolipram on experimentally induced impairments of learning and memory in rodents. Eur. J. Pharmacol. 1997;321:273–278. doi: 10.1016/s0014-2999(96)00969-7. [DOI] [PubMed] [Google Scholar]
- ITOH A., SHIOTANI T., NAKAYAMA S., MAMIYA T., HASEGAWA T., NODA Y., NABESHIMA T. Attenuation of the development of morphine dependence/tolerance by nefiracetam: Involvement of adenosine 3′:5′-cyclic monophosphate system. Behav. Brain Res. 2000;115:65–74. doi: 10.1016/s0166-4328(00)00237-0. [DOI] [PubMed] [Google Scholar]
- KAMEI J., OHSAWA M., SAITOH A., IWAMOTO Y., SUZUKI T., MISAWA M., NAGASE H., KASUYA Y. Modification of μ-opioid agonist-induced locomotor activity and developement of morphine dependence by diabetes. J. Pharmacol. Exp. Ther. 1995;274:700–706. [PubMed] [Google Scholar]
- KATO H., ARAKI T., ITOYAMA Y., KOGURE K. Rolipram, a cyclic AMP-selective phosphodiesterase inhibitor, reduces neuronal dagame follwing cerebral ischemia in the gerbil. Eur. J. Pharmacol. 1995;272:107–110. doi: 10.1016/0014-2999(94)00694-3. [DOI] [PubMed] [Google Scholar]
- KURIYAMA K., NAKAGAWA K., NAITO K., MURAMATSU M. Morphine-induced changes in cyclic AMP metabolism and protein kinase activity in the brain. Jpn. J. Pharmacol. 1978;28:73–84. doi: 10.1254/jjp.28.73. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETIET F., AUBERT J.F., BESLOT F., BOHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- MALDONADO R., STINUS L., GOLD L.H., KOOBG F. Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J. Pharmacol. Exp. Ther. 1992;261:669–677. [PubMed] [Google Scholar]
- MALDONADO R., BLENDY J.A., TZAVARA E., GASS P., ROQUES B.P., HANOUNE J. Reduction of morphine abstinence in mice with a mutation in the gene encoding CREB. Science. 1996;273:657–659. doi: 10.1126/science.273.5275.657. [DOI] [PubMed] [Google Scholar]
- MANGANIELLO C.V., MURATA T., TAIRA M., BELFRAGE P., DEGERMAN E. Perspectives in biochemistry and biophysics diversity in cyclic nucleotide phosphodiesterase insozyme families. Arch. Biochem. Biophys. 1995;322:1–13. doi: 10.1006/abbi.1995.1429. [DOI] [PubMed] [Google Scholar]
- MURAYAMA M., HIKINO H. Effect of cyclic AMP on mesaconitine-induced analgesia in mice. Eur. J. Pharmacol. 1985;108:19–23. doi: 10.1016/0014-2999(85)90278-x. [DOI] [PubMed] [Google Scholar]
- NABESHIMA T., NODA Y., ITOH A., MAMIYA T.Prevention of development of dependence to morphine by using cyclic AMP-related compounds in mice The role of adenosine in the nervous system 1997Tokyo: Elsevier; 251–257.ed. Okada, Y. pp [Google Scholar]
- NEAL B.S., SPARBER S.B. Mianserin attenuates naloxone-precipitated withdrawal signs in rats acutely or chronically dependent upon morphine. J. Pharmacol. Exp. Ther. 1986;236:157–165. [PubMed] [Google Scholar]
- NESTLER E.J. Molecular mechanisms of opiate and cocain addiction. Curr. Opin. Neurobiol. 1997;7:713–719. doi: 10.1016/s0959-4388(97)80094-3. [DOI] [PubMed] [Google Scholar]
- NICHOLSON D., REID A., SAWYNOK J. Effects of forskolin and phosphodiesterase inhibitors on spinal antinociception by morphine. Pharmacol. Biochem. Behav. 1991;38:753–758. doi: 10.1016/0091-3057(91)90237-v. [DOI] [PubMed] [Google Scholar]
- PRZEWLOCKI R., LASON W., MAJEED N.H., PREZOLOCKA B. Antidepressants and endogenous opioid peptide system. Neuropeptides. 1985;5:575–578. doi: 10.1016/0143-4179(85)90083-6. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN K., BEITNER-JOHNSON D.B., KRYSTAL J.H. , AGHAJANIAN G.K., NESTLER E.J. Opiate withdrawal and the rat locus coeruleus: behavioural electrophysiological, and biochemical correlates. J. Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER H.H. Brain cAMP response to phosphodiesterase inhibitors in rats killed by microwave irradiation or decapitation. Biochem. Pharmacol. 1984;33:1690–1693. doi: 10.1016/0006-2952(84)90295-8. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N.M., WARDEH G., MULDER A.H. Cyclic AMP facilitates the electrically evoked release of radiolabelled noradrenaline, dopamine and 5-hydroxytryptophan from rat brain slices. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;330:74–80. doi: 10.1007/BF00586712. [DOI] [PubMed] [Google Scholar]
- SCHWABE U., MIYAKE M., OHGA Y., DALY J.W. 4-(3-Cyclopentyloxy-4-methoxyphenyl)-2-pyrrolidone (ZK 62711): A potent inhibitor of adenosine cyclic 3′,5′-monophosphate phosphodiesterases in homogenates and tissue slices from rat brain. Mol. Pharmacol. 1976;12:900–910. [PubMed] [Google Scholar]
- SHARMA S.K., NIREBERG M., KLEE W.A. Morphine receptors as regulators of adenylate cyclase activity. Proc. Natl. Acad. Sci. U.S.A. 1975;72:590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERWILLIGER R.Z., BEITER-JOHNSON D., SEVARINO K.A., CRAIN S.M., NESTLER E.J. A general role for adaptations in G-proteins and the cyclic AMP systems in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- THOMPSON W.J. Cyclic nucleotide phosphodiesterase: Pharmacology, biochemistry and function. Pharmacol. Ther. 1991;51:13–33. doi: 10.1016/0163-7258(91)90039-o. [DOI] [PubMed] [Google Scholar]
- TORPHY T.J., UNDEM B.J. Phosphodiesterase inhibitors: New opportunities for the treatment of asthma. Thorax. 1991;46:512–523. doi: 10.1136/thx.46.7.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRABER J., GULLIS R., HAMPRECHT B. Influence of opiates on levels of adenosine 3′:5′-cyclic monophosphate in neuroblastoma x glioma hybrid cells. Life Sci. 1975;16:1863–1868. doi: 10.1016/0024-3205(75)90292-1. [DOI] [PubMed] [Google Scholar]
- TREMBLAY E.C., CHARTON G. Anatomical correlates of morphine withdrawal syndrome: Differential participation of structures located within the limbic system and striatum. Neurosci. Lett. 1981;23:137–142. doi: 10.1016/0304-3940(81)90030-6. [DOI] [PubMed] [Google Scholar]
- WACHTEL H. Potential antidepressant activity of rolipram and other selective cyclic adenosine 3′, 5′-monophosphate phosphodiesterase inhibitors. Neuropharmacology. 1983;22:267–271. doi: 10.1016/0028-3908(83)90239-3. [DOI] [PubMed] [Google Scholar]
- WANG J.F., REN M.F., XUE J.C., HAN J.S. Cyclic AMP mediates mu and delta, but not kappa opioid analgesia in the spinal cord of the rat. Life Sci. 1993;52:1955–1960. doi: 10.1016/0024-3205(93)90636-h. [DOI] [PubMed] [Google Scholar]
- WEI E., LOH H.H., WAY E.L. Neuroanatomical correlates of morphine dependence. Science. 1972;177:616–617. doi: 10.1126/science.177.4049.616. [DOI] [PubMed] [Google Scholar]
- WEI E., LOH H.H., WAY E.L. Brain sites of precipitated abstinence in morphine-dependent rats. Neuroanatomical correlates of morphine dependence. J. Pharmacol. Exp. Ther. 1973;185:108–115. [PubMed] [Google Scholar]
- YAMADA K., HIRAMATSU M., NODA Y., MAMIYA T., MURAI M., KAMEYAMA T., KOMORI Y., NIKAI T., SUGIHARA H., NABESHIMA T. Role of nitric oxide and cyclic GMP in the dizocilpine-induced impairment of spontaneous alternation behavior in mice. Neuroscience. 1996;74:365–374. doi: 10.1016/0306-4522(96)00161-3. [DOI] [PubMed] [Google Scholar]


