Abstract
Vanilloid receptors (VR1) were cloned from human and rat dorsal root ganglion libraries and expressed in Xenopus oocytes or Chinese Hamster Ovary (CHO) cells.
Both rat and human VR1 formed ligand gated channels that were activated by capsaicin with similar EC50 values. Capsaicin had a lower potency on both channels, when measured electrophysiologically in oocytes compared to CHO cells (oocytes: rat=1.90±0.20 μM; human=1.90±0.30 μM: CHO cells: rat=0.20±0.06 μM; human=0.19±0.08 μM).
In CHO cell lines co-expressing either rat or human VR1 and the calcium sensitive, luminescent protein, aequorin, the EC50 values for capsaicin-induced responses were similar in both cell lines (rat=0.35±0.06 μM, human=0.53±0.03 μM).
The threshold for activation by acidic solutions was lower for human VR1 channels than that for rat VR1 (EC50 pH 5.49±0.04 and pH 5.78±0.09, respectively).
The threshold for heat activation was identical (42°C) for rat and human VR1.
PPAHV was an agonist at rat VR1 (EC50 between 3 and 10 μM) but was virtually inactive at the human VR1 (EC50>10 μM).
Capsazepine and ruthenium red were both more potent at blocking the capsaicin response of human VR1 than rat VR1.
Capsazepine blocked the human but not the rat VR1 response to low pH. Capsazepine was also more effective at inhibiting the noxious heat response of human than of rat VR1.
Keywords: Capsaicin, capsazepine, VR1, pain response, proton activation, heat activation, intracellular calcium
Introduction
Capsaicin, the pungent vanilloid agent in chilli peppers, activates a subset of somatic and visceral primary afferent sensory neurones, notably the polymodal nociceptors (see Bevan & Szolcsanyi, 1990). These have conduction velocities in the C- and Aδ-fibre range (see Szolcsanyi, 1993), are responsive to noxious mechanical, thermal, and chemical stimuli and convey nociceptive signals from the periphery to the spinal cord. A vanilloid receptor (VR1) that is activated by capsaicin, low pH solutions and temperatures greater than 42°C has been cloned from rat dorsal root ganglia (Caterina et al., 1997; Tominaga et al., 1998). VR1 is a non-selective cation channel, with high permeability for divalent cations, and is localized to a subset of primary afferent neurones (Caterina et al., 1997; Helliwell et al., 1998). A second VR1 homologue termed the Vanilloid Receptor-like protein-1 (VRL-1) (Caterina et al., 1999) does not respond to capsaicin or protons but is sensitive to heat at high temperatures with a threshold of about 52°C and it is possible that this channel may play a role in the physiological response to noxious heat in vivo.
Studies in transgenic mice lacking the VR1 receptor provide strong evidence for a role of VR1 in the sensation of noxious heat in vivo. As well as losing vanilloid-evoked pain behaviour, these mice showed reduced thermal hyperalgesia in models of inflammatory pain, whereas responses to mechanical stimuli were normal (Caterina et al., 2000; Davis et al., 2000).
In addition to its sensitivity to capsaicin, protons and heat, rat VR1 is activated by endogenous lipoxygenase products that are likely to be expressed in inflamed tissues (Hwang et al., 2000) and high concentrations of anandamide, an endogenous activator of the cannabinoid receptor, also activate rat and human VR1 (Zygmunt et al., 1999; Smart et al., 2000). Other inflammatory agents such as PGE2 which do not directly activate VR1, sensitize the vanilloid receptor to other noxious stimuli (Kress et al., 1997; Vyklicky et al., 1998). Furthermore, the hyperalgesic neurotrophin, nerve growth factor (NGF), also acutely sensitizes the response of adult rat sensory neurons to capsaicin, as well as having the longer term effect of upregulating the expression of the receptor itself (Bevan & Winter, 1995; Shu & Mendell, 1999). VR1 is therefore able to detect and integrate multiple pain stimuli, especially in inflamed conditions when products of lipoxygenase and cyclo-oxygenase pathways as well as NGF are present at elevated concentrations. It is likely that drugs that interfere with VR1 function will be analgesic.
A number of other exogenous activators of vanilloid receptors have been identified. These include resiniferatoxin (RTX) the ultrapotent agonist isolated from the plant Euphorbia resinifera and its non pungent analogue, Phorbol 12-phenylacetate 13 acetate 20-homovanillate (PPAHV). Studies with these compounds have suggested that their interaction with the receptor may differ significantly from that of capsaicin (Walpole et al., 1996; Liu et al., 1998; Szallasi et al., 1999; Jerman et al., 2000).
Here, we describe the cloning of a functional human VR1 homologue from human dorsal root ganglion (DRG) and a comparison of its pharmacology with that of the rat VR1. Both receptors were expressed in Xenopus oocytes and in Chinese Hamster ovary (CHO) cells and characterized electrophysiologically and by monitoring intracellular calcium concentration changes either in cell populations with aequorin luminescence or in individual cells by ratiometric imaging of fura 2 fluorescence. Both receptors responded to capsaicin, protons and heat. Although capsaicin had similar potency at the two receptors, significant pharmacological differences were found between the rat and human VR1.
Methods
Hank's balanced salt solution (HBSS), phosphate buffered saline (PBS) and all cell culture reagents were obtained from Gibco BRL. Geneticin (G418), ruthenium red, and capsaicin were obtained from Sigma. All restriction enzymes were obtained from New England Biolabs. Viewplates were obtained from Packard Instruments Ltd. Capsazepine and coelenterazine h were synthesized at Novartis. Phorbol 12-phenylacetate 13-acetate 20-homovanillate (PPHAV) was obtained from Alexis.
Cloning
Human DRG RNA was purchased from Analytical Biochemical Services (MA, U.S.A.). Rat DRG RNA was prepared from dorsal root ganglia that were isolated from adult male Sprague-Dawley rats which had been killed by CO2 asphyxiation using a Home Office approved procedure and were frozen on dry-ice. RNA was extracted by the method of Chomczynski & Sacchi (1987). Poly A+ RNA was purified by oligo dT chromatography (Aviv & Leder, 1972).
Lambda ZAP express cDNA libraries were made with a cDNA synthesis kit (Stratagene) according to the manufacturers instructions. The rat and human lambda ZAP expressing DRG cDNA libraries were screened with a cDNA 989 bp probe which hybridized to part of the coding domain of rat VR1 (Helliwell et al., 1998). The human library was screened at low stringency (2× standard saline citrate (SSC) at 45°C) and the rat library was screened at high stringency (0.2×SSC at 65°C). Positive cDNA clones were excised as pBKCMV plasmid clones, in E. coli and sequenced on an ABI 377 DNA sequencer (PE Applied Biosystems). Only the longest clone was selected from the human library and sequenced, but several clones were isolated and sequenced from the rat library. A rat VR1 clone, which contained the entire protein coding domain was used for further studies. The sequence of the human VR1 has been deposited with Genbank with the accession number: AJ 272063.
In vitro transcription
Copy RNA was prepared from Not1 linearized pBKCMV DNA, with T3 RNA polymerase with a Stratagene mRNA capping kit. The RNA was precipitated with ethanol and rinsed before being resuspended in 10 μl of distilled water at 1 mg ml−1.
Oocyte preparation, injection and recording
Female Xenopus laevis were anaesthetized with tricaine by a Home Office approved procedure and their ovaries removed. Following defolliculation with collagenase (type 1, Sigma) in divalent cation free media (mM: NaCl 82.5, KCl 2.5, Na2HPO4 1.2, HEPES 5, adjusted to pH 7.5 with NaOH) mature stage V and VI oocytes were injected with approximately 50 nl of RNA (∼1 mg ml−1) and maintained at 18°C in ND96 solution (mM: NaCl 96, KCl 2, MgCl2 1, CaCl2 1.8, HEPES 5, sodium pyruvate 2.5, adjusted to pH 7.5 with NaOH), supplemented with 50 μg ml−1 gentamycin, until required.
Recordings were made from oocytes bathed in ND96 solution pH 7.4 under two-electrode voltage clamp, 3–5 days following RNA injection, using a Geneclamp 500 amplifier and pClamp software (Axon Instuments). Electrodes were pulled from standard borosilicate glass (Clark Electromedical Instruments) and had resistance of about 0.2 MΩ when filled with 3 M KCl. Capsaicin-induced currents were elicited at room temperature, at a holding potential of −60 mV unless otherwise stated. Oocytes were microperfused with ND96 and drug-containing solutions.
Generation of human and rat VR1 expressing CHO cell lines
Cells lines expressing aequorin have been used previously to measure calcium fluxes (see Brini et al., 1995 for a description of this technique).
An aequorin-expressing host cell line was generated. The aequorin gene was subcloned into pXMT3 mammalian expression plasmid which encodes the dihydrofolate reductase gene. The aequorin cDNA and the pXMT3 plasmid were gifts from Dr K. Stoekli and Dr S. Geisse, respectively, at Novartis, Basle. CHO-DUKX cells were co-transfected with the aequorin-pXMT3 construct and pCEP4 plasmid (Invitrogen) which contains a hygromycin resistance gene. The transfected cells were selected by growth in MEM medium containing 200 μg ml−1 hygromycin (Gibco BRL) followed by further selection in MEM alpha medium without nucleosides supplemented with dialysed foetal calf serum (Gibco BRL). Cell lines were cloned from the selected population by limiting dilution.
The 3.3 kb ratVR1 cDNA insert was prepared for expression in mammalian cells by removing it from the pBK-CMV plasmid with EcoR1 and Not1 digestion and ligating it into pIRESneo (Clontech). The human VR1 cDNA insert of 3.5 kb was similarily subcloned into the same expression vector. The VR1-pIRESneo constructs were transfected into the CHO-aequorin host cell line by means of Lipofectamine Plus (Gibco BRL). Following selecion in medium containing 700 μg ml−1 G418, the cell populations were cloned by limiting dilution. Resulting clones were screened in the aequorin assay and the highest expressing clones of each species were chosen for further study.
Aequorin assay
Aequorin- and VR1- expressing cells were grown in 96-well viewplates (Packard) and incubated at 37°C with 20 μM coelenterazine h and 30 μM reduced glutathione in 50 μl of medium per well. At the start of the assay, the medium containing coelenterazine h was removed and cells were washed once with 100 μl of HBSS containing 75 μM CaCl2 and buffered to pH 7.4 with 10 mM HEPES. This was replaced with 100 μl of the same buffer containing test compounds where appropriate. Cells were incubated for 10 min at room temperature. They were then placed in the measuring chamber of a luminometer (Wallac Microbeta Jet). Agonists, with the exception of PPAHV, were injected in a volume of 20 μl HBSS at 6 fold final concentration. Because of its low solubility, PPAHV was added at twice the final concentration to 50 μl of cell-containing solution giving a final volume of 100 μl. Unless otherwise stated in the text, luminescence signals were integrated over 10 s for the rat VR1 expressing cells and 20 s for the human VR1 expressing cells, because of the different kinetics of response at the two receptors. Control experiments were carried out on the host CHO-aequorin cell line which did not express VR1. Measurements were made in quadruplicate. Results were expressed as mean±s.e.mean and curve fitting was performed with Microsoft Origin software.
In initial experiments with solutions containing 1.25 mM calcium, the magnitude of the calcium signal evoked by capsaicin was sufficient to deplete most of the aequorin. In order to avoid such saturation effects, aequorin measurements with capsaicin as an agonist were carried out in buffer containing 75 μM calcium. The presence of unused aequorin was confirmed at the end of the assay by measuring the signal produced by addition of 1% Triton X-100. In low pH experiments, assay solutions with 75 μM calcium gave a very small response to a pH 5 stimulus compared to the signal evoked by 3 μM capsaicin. A proton-induced signal of a similar magnitude to capsaicin was obtained when solution containing 1.25 mM calcium was used so all pH experiments were conducted in this higher calcium solution.
Electrophysiological recording
Human and rat VR1-expressing CHO cells grown on Poly-D-Lysine coated glass coverslips were studied under whole-cell voltage clamp. Steady state current was recorded with membrane potential held at +30 mV to minimise the effects of desensitisation (Yeats et al., 1992; Bevan & Docherty, 1993; Liu & Simon, 1996) in a standard solution (mM, 140 NaCl, 5 KCl, 10 glucose, 10 HEPES, 2 CaCl2, 1 MgCl2) adjusted to pH 7.4 with NaOH, to which capsaicin was added. All solutions contained 0.1% dimethyl sulphoxide (DMSO) as a solvent for capsaicin. Recordings were made with borosilicate glass patch pipettes (2–6 MΩ) filled with; (mM) 130 CsCl, 1 CaCl2, 10 EGTA, 1 MgCl2, 10 HEPES) adjusted to pH 7.4 with CsOH with an Axoclamp 200A amplifier and pClamp6 software (Axon instruments). Solution changes, including capsaicin application were made using a U-tube system (Bevan & Winter, 1995). Experiments were carried out at room temperature and only one cell was used from each coverslip to minimize desensitization.
Imaging of intracellular calcium levels [Ca2+]i
Cells grown on Poly-D-Lysine coated glass coverslips were loaded with fura-2 AM (5 μM, 45 min), washed and incubated at room temperature for at least a further 30 mins. Coverslips were placed in a laminar flow perfusion chamber (Warner Instrument Corp.) and constantly perfused with HEPES-buffered saline (2 mM Ca2+) via a local perfusion pipette through which drugs and heated solutions were also applied. Heat stimulations consisted of a linear increase (1°C s−1) in perfusate temperature from 25 to 50°C. Perfusate temperature was controlled by a regulated Peltier device and was monitored at the tip of the perfusion pipe by a miniature thermocouple. Images of fura-2 loaded cells with the excitation wavelength alternating between 340 and 380 nm were captured with a cooled CCD camera (Photometrics). Following subtraction of background fluorescence, the ratio of fluorescence intensity at the two wavelengths was calculated. Ratio levels in groups of 20–40 individual cells were analysed using ImageMaster sofware (PTI, NJ, U.S.A.). Over the range of calcium concentrations measured, the ratio values increased linearly with [Ca2+]. The magnitude of responses is quoted as the maximum ratio increase over the basal ratio levels immediately prior to stimulation, representing mean data from at least three separate experiments on three different passages of CHO cells.
Statistical analysis
Experimental results are expressed as mean±s.e.mean and the statistical significance of any differences between pairs were determined using two-tailed Student's t-test, unless otherwise stated. Differences between multiple samples were analysed using ANOVA.
Results
Cloning
Full-length cDNA clones were identified from human and rat DRG libraries by screening with part of the coding domain of the rat VR1 (Helliwell et al., 1998). The human clone had a 3463 base pair insert which encoded a novel 839 amino acid protein that gave a predicted protein sequence with 85% identity to the rat VR1 (Figure 1), and 47 and 48% identity to the rat and human VRL-1 proteins respectively. Comparison of the human and rat VR1 amino acid sequences (Figure 1) revealed that the ankyrin repeats and transmembrane domains are conserved and that the C-terminus is more highly conserved than the N-terminus.
Figure 1.

Alignment of the putative protein sequences of human and rat VR1. The predicted sequences of the human (above, this paper) and rat (below, Caterina et al., 1997, Accession number AF029310) were aligned using the GCG GAP program (University of Wisconsin, Genetics Computer Group). Solid vertical bars indicate identity, a colon indicates strong similarity, a period indicates weak similarity and a gap indicates no similarity. Putative ankyrin repeats are underlined and predicted transmembrane domains are in bold and underlined. The human VR1 sequence has been deposited in the EMBL database and been assigned accession number AJ272063.
Properties of receptors expressed in oocytes
To determine whether the human cDNA clone encodes a vanilloid receptor, RNA was transcribed and expressed in Xenopus oocytes. Under two-electrode voltage clamp at a holding potential of −60 mV, oocytes expressing the human VR1 clone showed a capsaicin-evoked inward current. A log(concentration)-response curve was constructed for both the rat and human VR1 clones (Figure 2a) by sequential application of increasing concentrations of capsaicin. The curves were essentially superimposable with EC50 values of 1.9 μM (rat 1.9±0.2 μM, n=6; human 1.9±0.3 μM, n=10). Current-voltage relationships of the capsaicin-evoked responses were generated by stepping to different membrane potentials from the holding value of −60 mV. The current elicited at each potential was normalized to that recorded at +80 mV. As shown in Figure 2b, the currents evoked by capsaicin acting on both human and rat VR1 receptors showed similar outwardly rectifying current-voltage relationships.
Figure 2.
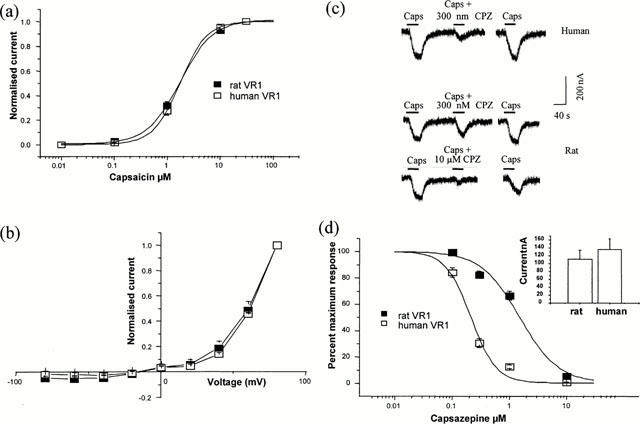
The human VR1 clone codes for a functional capsaicin receptor. Human and rat VR1 were expressed in oocytes. (a) Capsaicin log (concentration)-response curve for the human clone (n=10) compared to that for the rat (n=6). Currents recorded at −60 mV by sequential application of increasing concentrations of capsaicin and normalized to the response elicited by 30 μM capsaicin. (b) Current voltage relationships produced using voltage steps from the holding value of −60 mV in the presence of 1 μM capsaicin, normalized to the current at +80 mV. Leak current in the absence of capsaicin subtracted (n=8, human; n=3, rat). (c) Reversible antagonism of the response to 1 μM capsaicin by 300 nM or 10 μM capsazepine as indicated. Wash period of 5 min indicated by gap in trace. (d) Percentage inhibition of the response to 1 μM capsaicin by increasing concentration of capsazepine, expressed as a percentage of the response in the absence of antagonist (n=12 human; n=9 rat). The inset in (d) shows mean currents with 1 μM capsaicin (values, 136±27 nA (n=12), and 112±23 nA (n=9)). This shows that the differences seen with capsazepine are not due to differences in expression level.
The competitive antagonist capsazepine inhibited the capsaicin-evoked current at both receptors but, as shown in Figure 2c, capsazepine was more potent at the human than the rat receptor. To compare this difference in more detail capsazepine concentration-inhibition curves were constructed for the response to 1 μM capsaicin (Figure 2d). Although there are not enough data points on the curves to calculate precise IC50 values, capsazepine is clearly more potent at the human than the rat VR1. Fitting curves to the data points allowed us to estimate approximate IC50 values of 0.24±0.03 and 1.70±0.20 μM (n=9) for human and rat VR1 respectively. Differences in capsazepine potency were not related to different expression levels for human and rat VR1, since capsaicin elicited currents of similar amplitude in oocytes expressing either type of receptors (Figure 2d).
Properties of receptors expressed in CHO cells
For a more extensive pharmacological analysis, human and rat VR1 were expressed in mammalian CHO cells and their properties examined either electrophysiologically or by measuring the evoked changes in [Ca2+]i.
Agonists
When studied electrophysiologically under whole cell voltage clamp (Figure 3a) log(concentration)-response curves to capsaicin (measured at +30 mV to minimize desensitization), had similar EC50 values for the human (0.19±0.08 μM, n=4) and rat (0.20±0.06 μM, n=4) VR1 (P>0.5).
Figure 3.
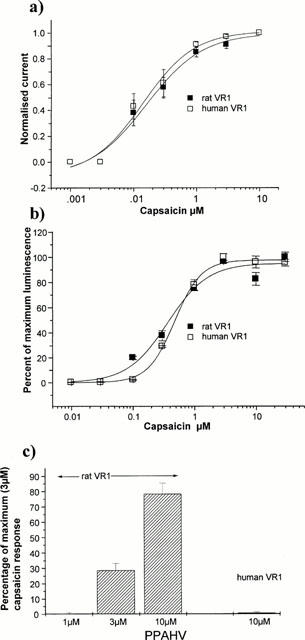
Agonism of capsaicin and PPAHV in rat- and human-VR1 expressing CHO cells measures using aequorin. (a) Capsaicin log(concentration)-response curves for CHO cells stably expressing human and rat VR1 clones. The current elicited by increasing concentrations of capsaicin was measured at +30 mV and normalized to that elicited by 10 μM capsaicin for each cell (n=4 for each). EC50 values, 0.20±0.06 μM rat and 0.19±0.08 μM human. (b) CHO cells expressing aequorin and either human or rat VR1 were challenged with a range of capsaicin concentrations and the calcium signal averaged over the first 10 s for rat VR1 and the first 20 s for human VR1 cells. Graphs represent mean of three separate log (concentration) response experiments. EC50 values were similar using this protocol, 0.53±0.03 μM for human VR1 and 0.35±0.06 μM for rat VR1. Results are expressed as the percentage of maximal response. (c) CHO cells expressing aequorin and rat VR1 were challenged with 1, 3 and 10 μM of PPAHV and the luminescence was averaged over the first 10 s. CHO cells expressing aequorin and human VR1 were challenged with 10 μM PPAHV and the luminescence was averaged over the first 20 s. Results are expressed as the percentage of maximal response to 3 μM capsaicin of cells assayed in parallel.
Figure 3b shows log(concentration)-response curves to capsaicin for both rat and human VR1 in the CHO-aequorin assay. The calculated EC50 values of 0.35±0.06 μM for the rat and 0.53±0.03 μM for the human were not significantly different (P>0.1).
In the aequorin assay, PPAHV showed concentration-dependent agonist activity on the rat VR1 with an EC50 value between 3 and 10 μM but essentially no activity at the human VR1 receptor at 10 μM under the assay condition used (Figure 3c). In electrophysiological studies, a response of the human VR1 to 3 μM PPAHV was detected at −60 mV but this was about 20 fold smaller in magnitude than that of the rat clone when scaled to the response to 300 nM capsaicin (data not shown).
Antagonists
The competitive VR1 inhibitor capsazepine (CPZ) showed a 6 fold higher potency at the human receptor than at the rat receptor when tested against EC50 concentrations of capsaicin in the aequorin assay (Figure 4). The IC50 values of 0.039±0.004 μM and 0.22±0.02 μM were calculated for human and rat expressing VR1 cells respectively. These values were significantly different (P<0.01). The effects of another capsaicin antagonist ruthenium red (RR) were also studied in the aequorin assay where ruthenium red showed a 2.3 fold greater potency at human VR1 (IC50 0.90±0.02 μM) than at the rat VR1 (0.21±0.03 μM, P<0.05).
Figure 4.
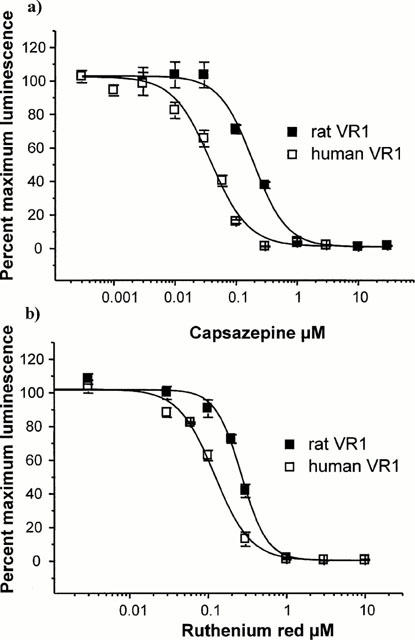
Capsazepine and ruthenium red inhibit capsaicin responses in rat- and human-VR1 expressing CHO cells. (a) Capsazepine inhibited the capsaicin response in CHO cells expressing aequorin and either rat or human VR1. Various concentrations of capsazepine were added to the cells for 10 min prior to the assay, then the response was measured over 10 s (rat VR1) or 20 s (human VR1) after the addition of an EC50 concentration of capsaicin. Capsazepine effectively inhibited both rat VR1 and human VR1 responses to capsazepine (IC50 values of 0.22±0.02 and 0.04± 0.00 μM respectively). Signal was normalized to that produced by the EC50 concentration of capsaicin with no antagonist as 100%, to allow comparison between rat and human VR1 expressing cell lines. (b) Ruthenium red inhibition of capsaicin responses was measured as in (a). Ruthenium red was an effective blocker of capsaicin responses in rat VR1 and human VR1 (IC50 values of 0.22±0.03 and 0.09±0.02 μM respectively).
Protons
Low pH solutions activated both rat and human VR1. A small difference in pH sensitivity was noted between species (Figure 5a). In aequorin-expressing CHO cells, the threshold for activation of the rat receptor was consistently higher than the threshold for the human receptor. Analysis of the individual pH-response curves showed that the EC50 value for the rat VR1 (pH 5.78±0.09, n=11) was significantly higher than the value for the human VR1 (pH 5.49±0.04, n=11, P<0.001).
Figure 5.
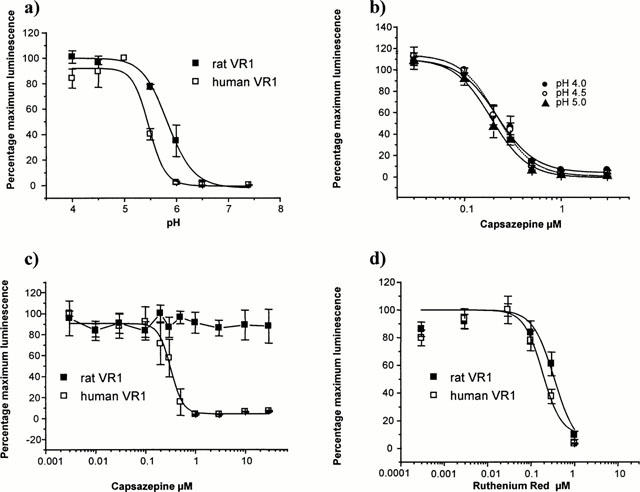
Ability of Ruthenium Red and Capsazepine to block low pH-induced responses in rat- and human-VR1 expressing CHO cells. (a) Low pH solutions stimulate uptake of Ca2+ in CHO cells that express aequorin and either rat or human VR1. Rat VR1 is more sensitive to low pH with a significant stimulation seen at pH 6.0 whereas human VR1 is not activated until pH is dropped to 5.5. CHO cells that express aequorin but not receptor show no calcium signal at low pH. (b) CHO cells that express aequorin and either rat or human VR1were preincubated with a range of capsazepine concentrations and challenged with pH 5 buffer as in Figure 4a. Capsazepine completely inhibited the human VR1 proton induced calcium signal (IC50=0.31±0.02 μM), but was ineffective against the rat VR1. Signal was normalized to pH5 signal with no antagonist as 100% to allow comparison between rat and human VR1 expressing cell lines. (c) CHO cells that express aequorin and human VR1were preincubated with a range of capsazepine concentrations and challenged with pH 4.0, pH 4.5 or pH 5 buffer. Capsazepine was equally effective at blocking the proton-induced response at each pH used. (d) Ruthenium red was effective at blocking pH 5-induced calcium signal in both rat and human VR1 expressing cells.
When a maximally effective acidic pH (pH 5.0) was used to stimulate VR1, capsazepine blocked the calcium response to pH stimulation of human VR1 with an IC50 of 0.32±0.02 μM. In contrast capsazepine did not block the pH5 activation of rat VR1 at concentrations of up to 30 μM (Figure 5b). We tested the effect of increasing the activating proton concentration on the inhibition by capsazepine, to see if the inhibition of the low pH response was surmountable, which would be indicative of competitive inhibition, or non-surmountable, which would be indicative of non-competitive inhibition. Capsazepine showed equal ability to inhibit the responses of human VR1 to pH 4, pH 4.5 or pH 5.0 solutions (see Figure 5c). The IC50 values of 0.22±0.02 μM pH 4; 0.21±0.02 μM pH 4.5; 0.19±0.02 μM pH 5 were not significantly different (P>0.05) which implies that capsazepine was not a competitive inhibitor of the low pH response.
Ruthenium red blocked the low pH activation of cells expressing either rat or human VR1 (Figure 5d) with similar IC50 values (rat 0.34±0.05; human 0.24±0.04, n=4).
Heat
Rat VR1 is activated by heat in the noxious range, from 42–50°C (Tominaga et al., 1998). We investigated the heat response of CHO cells transfected with rat and human VR1 by measuring intracellular calcium concentrations in individual cells using ratiometric imaging of fura-2 (Figure 6). An increase in perfusate temperature from 25 to 50°C (1° s−1) stimulated substantial increases in [Ca2+]i in both human and rat VR1-CHO cells, with similar average magnitudes (average peak ratio increases of 3.8±0.2 (n=318) and 3.7±0.1 (n=239), respectively). These responses were non-linear with temperature. and showed a clear threshold temperature at which the rate of [Ca2+]i rise increased dramatically (Figure 6a,b). This was estimated to be 42.4±0.3°C for human VR1, similar to the estimate of 42.6±0.3°C for rat VR1 (Savidge et al., 2001). These specific heat responses were not seen in non-transfected CHO cells.
Figure 6.
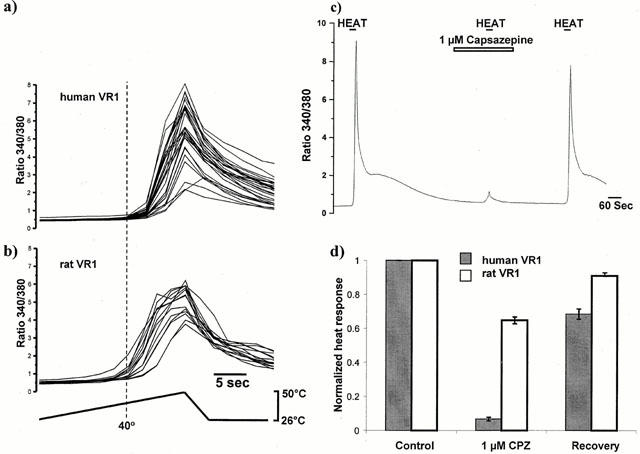
Effect of heat on intracellular calcium concentration ([Ca2+]i- expressed as ratio values) in VR1-expressing CHO cells. (a) human and (b) rat VR1 CHO cells show a rapid increase in [Ca2+]i when the temperature rises above around 40°C. Each trace on graphs (a) and (b) represent a single cell in the same representative experiment. The estimated average threshold temperature at which [Ca2+]i began to increase rapidly showed no difference between rat- and human- VR1. (c) Average [Ca2+]i increases in a group of around 30 human VR1 transfected- CHO cells from a single representative experiment in response to heating to 50°C (as in a)) in the absence and presence of capsazepine (1 μM). Responses to heat in human VR1-CHO showed virtually complete and reversible inhibition by capsazepine (1 μM). (d) Comparison of the effect of 1 μM capsazepine (CPZ) on the heat response in human (filled bars) and rat (open bars)- VR1 CHO cells. Heat responses in the presence of capsazepine, and those following capsazepine washoff for approximately 5 min (‘Recovery') were normalized to an initial response to heat in the same cells (‘Control'). Inhibition of the heat response by capsazepine in rat VR1-CHO is markedly weaker than in human VR1-CHO.
As previously reported (Savidge et al., 2001), 1 and 10 μM capsazepine produced almost complete blockade of responses in rat VR1-CHO stimulated by 100 nM capsaicin (99.8±0.4% inhibition with 10 μM capsazepine and 88±2% with 1 μM capsazepine), but responses to heat were inhibited by only 37±2% (n=119) by 10 μM capsazepine and 28±2% (n=105) by 1 μM capsazepine. In contrast, heat responses in cells expressing human VR1 showed a much greater sensitivity to capsazepine blockade (Figure 6c). At a concentration of 1 μM, capsazepine produced a reversible blockade of heat responses (93±0.8%, n=75), which was significantly greater than the inhibition seen with the same concentration of capsazepine on rat VR1 (Figure 6d, P<0.0001).
Discussion
We have described the cloning of a novel cDNA sequence which, from sequence homology and functional evaluation is the human orthologue of the rat VR1. The protein encoded by this sequence is a vanilloid receptor sensitive to capsaicin and protons, which is consistent with a previous study on cultured human DRG neurones (Baumann et al., 1996) and it is also sensitive to heat. We have generated CHO cell lines stably expressing rat or human VR1, allowing us to carry out a detailed comparison of the pharmacology of these species orthologues for the first time. After submission of this manuscript, Hayes et al. (2000) reported cloning of a human VR1 orthologue. The pharmacology of this receptor expressed in HEK293 cells is consistent with our results.
In the Xenopus oocyte and CHO cell expression systems, the EC50 for capsaicin was the same for both the rat and human VR1 although the potency was lower in the Xenopus oocyte system than in CHO cells. A lower potency for capsaicin at rat VR1 expressed in the Xenopus oocyte system compared to the mammalian cells was also seen by Caterina and colleagues (1997) and this may represent some secondary modification of the receptors that differs in the two expression systems.
Although human and rat VR1 expressing cells had similar EC50 values for capsaicin, they responded differently to PPAHV, which had an EC50 of between 3 and 10 μM at the rat VR1 but was essentially inactive up to a concentration of 10 μM at the human VR1. This compound is probably a full agonist at the rat VR1 as, at 10 μM, it produced approximately 80% of the maximal response seen with capsaicin at the rat receptor; however, the lack of solubility of the compound prevented us from acquiring reliable data at higher concentrations. The failure to evoke robust responses with PPAHV at the human VR1 in either electrophysiological or calcium assay experiments implies a difference in either affinity or efficacy at rat and human VR1. This, in turn, suggests that there are differences between the agonist binding site in the two species.
Studies on the in vivo activity of capsaicin and PPAHV in rat suggest that both compounds are capable of acting as agonists of VR1, although some differences in their action have been noted. PPAHV is less pungent than capsaicin and although both compounds are capable of causing effective desensitization against neurogenic inflammation in adult rats, administration of PPAHV at a dose sufficient to result in desensitization of the neurogenic response does not result in a hypothermia response that is normally seen with capsaicin (Appendino et al., 1996).
Ruthenium red inhibited the responses of both rat and human VR1 to capsaicin and low pH, and has also been shown to inhibit the heat response in rat-VR1 CHO cells (Savidge et al., 2001). In contrast, capsazepine showed species-dependent inhibitory effects. We observed that capsazepine could inhibit capsaicin activation of both human and rat VR1 but that its potency on the human VR1 was about 6 fold greater than on the rat VR1. Furthermore, whilst both the human and rat VR1 channels could be activated by protons, capsazepine blocked the low pH-evoked responses of human but not rat VR1. Capsazepine was only a weak inhibitor of the heat response mediated by rat-VR1, but was highly effective at blocking the heat response mediated by human-VR1.
The estimated IC50 value for capsazepine blockade of capsaicin at the rat VR1 receptor was 0.22±0.02 μM which is in good agreement with the value of 0.28 μM reported by Caterina et al. (1997) for rat VR1 expressed in Xenopus oocytes and is similar to the value of 0.42±0.05 μM obtained using a calcium uptake assay of native receptors on cultured rat DRG neurones (Bevan et al., 1992a). The value we obtained is approximately 10 times higher than the pKB value of 7.52±0.08 (28 nM) reported by Jerman et al. (2000). The reason for this difference is not clear. In Xenopus oocytes capsazepine was approximately six times more potent at blocking capsaicin activation of the human than the rat receptor, which is the same order of difference seen in the mammalian expression system. The absolute values that we see in the oocyte system are higher than observed in the mammalian expression system and are also higher for rat VR1 than the value observed by Caterina et al. (1997). Different experimental conditions for oocyte recordings could account for the difference between the two groups.
Effect of capsazepine on pH responses of rat- and human- VR1 CHO cells
Capsazepine failed to inhibit the responses of rat VR1 to low pH at concentrations 100 times greater than the IC50 value at the human VR1. This is in contrast to the results of Jerman et al. (2000) who stated that capsazepine blocked low pH activation of rat VR1 expressed in human embryonic kidney cells, although these authors provided no data, and Tominaga et al. (1998) who showed that 10 μM capsazepine reversibly blocked the low pH response of rat VR1 in HEK cells by 79% in calcium-free conditions. Our results are consistent with the findings of earlier studies on rat cultured dorsal root ganglion cells which showed that capsazepine did not inhibit the sustained inward current induced by acidic (pH 6.1 or 5.5) extracellular solutions (Bevan et al., 1992b; Vyklicky et al., 1998). In the current study, the block of proton responses by capsazepine at human VR1 could not be overcome by further lowering the pH (Figure 5c) suggesting that capsazepine does not compete with protons for the same binding site. This implies, in turn, that capsaicin and protons bind to different sites. Oh and colleagues have published evidence that capsaicin binds to an intracellular site on the native rat VR1 (Jung et al., 1999) whereas protons probably exert their effect from outside the cell. A recent study by Julius and colleagues (Jordt et al., 2000) has shown that mutation of glutamate 600 in the putative pore forming region between transmembrane domains 5 and 6, towards the outer side of the membrane, in rat VR1 affects the proton-potentiation of the capsaicin response. Further evidence for different sites of interaction comes from mutation of glutamate 648 in rat VR1 which abolishes the ability of protons to activate the channel while leaving the responses to capsaicin and heat unaffected.
Effect of capsazepine on the heat response of rat- and human- VR1
In our hands 1 μM capsazepine completely inhibited the heat response in CHO cells expressing human VR1, in contrast to rat VR1 expressing cells where 10 μM capsazepine reduced the response by only a third. The weak inhibitory effects of capsazepine on heat responses on cells expressing rat VR1 are similar to the findings of Rang and colleagues that capsazepine could inhibit heat responses only slightly, even at 10 μM, in rat cultured dorsal root ganglion cells (Nagy & Rang, 1999; Savidge et al., 2001). Treede and colleagues (Kirschstein et al., 1999) found a similar degree of blockade in cultured rat dorsal root ganglion cells using the same (10 μM) capsazepine concentration. Thus, the pharmacology of the heat reponse of recombinant rat VR1 appears to reflect that of the native receptor, at least for capsazepine. Tominaga et al. (1998) showed that 10 μM capsazepine produced a more substantial blockade of the heat response of rat VR1 expressed in HEK 293 cells. However, they also saw a much more substantial rundown of the heat response than we observed.
Capsazepine blocks voltage gated calcium channels (Docherty et al., 1997) and also blocks activation of nicotinic acetylcholine receptors (Liu & Simon, 1997). The concentrations at which these effects are seen above 10 μM and therefore do not explain the species selectivity that we describe. Further work will be necessary to determine if the effects of capsazepine on agonist activation and the effects of this compound against protons are due to interactions at separate sites.
Taken together, our data suggest that the site and mechanism of activation of VR1 by capsaicin is different from that for protons and heat. Capsazepine, as well as being a competitive antagonist at the capsaicin binding site, can inhibit the proton activation of human but not rat VR1 in a non-competitive manner. The identification of a species-specific effect of capsazepine will allow us to map the binding site of capsazepine in the human VR1 using a chimeric receptor approach, which in turn will provide important information about the way in which the channel operates and the mechanisms of capsazepine block.
Acknowledgments
We thank Armand Wanner (Novartis, Switzerland) for help with DNA sequencing.
Abbreviations
- CGRP
calcitonin gene-related peptide
- CPZ
capsazepine
- CHO
chinese hamster ovary
- cDNA
copy DNA
- DRG
dorsal root ganglion
- NGF
nerve growth factor
- PPAHV
Phorbol 12-phenylacetate 13 acetate 20-homovanillate
- RR
ruthenium red
- VR1
Vanilloid receptor 1
- VRL
Vanilloid receptor like
References
- APPENDINO G., CRAVOTTO G., PALMISANO G., ANNUNZIATA R., SZALLASI A. Synthesis and evaluation of phorboid 20-homovanillates: discovery of a class of ligands binding to the vanilloid (capsaicin) receptor with different degrees of cooperativity. J. Med. Chem. 1996;39:3123–3131. doi: 10.1021/jm960063l. [DOI] [PubMed] [Google Scholar]
- AVIV H., LEDER P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc. Natl. Acad. Sci. U.S.A. 1972;69:1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMANN T.K., BURCHIEL K.J., INGRAM S.L., MARTENSON M.E. Responses of adult dorsal root ganglion neurones in culture to capsaicin and low pH. Pain. 1996;65:31–38. doi: 10.1016/0304-3959(95)00145-X. [DOI] [PubMed] [Google Scholar]
- BEVAN S., DOCHERTY R.J.Cellular mechanisms of the action of capsaicin Capsaicin in the study of pain 1993London: Academic Press; 27–44.ed. Wood, J.N. pp [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S.J., YEATS J. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992a;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEVAN S., RANG H.P., SHAH K. Capsazepine does not block the proton-induced activation of rat sensory neurones. Br. J. Pharmacol. 1992b;107:235P. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEVAN S., SZOLCSANYI J. Sensory neuron-specific actions of capsaicin: Mechanisms and applications. Trends Pharmacol. Sci. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- BEVAN S., WINTER J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J. Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINI M., MARSAULT R., BASTIANUTTO C., ALVAREZ J., POZZAN T., RIZZUTO R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]i) J. Biol. Chem. 1995;270:9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W. J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., ROSEN T.A., TOMINAGA M., BRAKE A.J., JULIUS D. A capsaicin receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SATCCHI Single step method of RNA isolation by acid guanidinium-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DAVIS J.B., GRAY J., GUNTHORPE M.J., HATCHER J.P., DAVEY P.T., OVEREND P., HARRIES M.H., LATCHAM J., CLAPHAM C., ATKINSON K., HUGHES S.A., RANCE K., GRAU E., HARPER A.J., PUGH P.L., ROGERS D.C., BINGHAM S., RANDALL A., SHEARDOWN S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., PIPER A.S. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurons in culture. Br. J. Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES P., MEADOWS H.J., HARRIES M.H., DUCKWORTH M.D., CAIRNS W., HARRISON D.C., CLARKE C., GUNTHORPE M., ELLINGTON K., PRINJHA R.K., BARTON A.J., MEDHURST A.D., SMITH G.D., TOPP S., MURDOCK P., SANGER G.J., TERRETT J., JENKINS O., RANDALL A., BENHAM C.D., GLOGER I.S., DAVIS J.B. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:207–217. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- HELLIWELL R.J.A., MCLATCHIE L.M., CLARKE M., WINTER J., BEVAN S., MCINTYRE P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- HWANG S.W., KWAK J.Y., LEE S.-L., KANG C.-J., JUNG J., CHO S., MIN K.H., SUH Y.G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., PRINJHA R., HARRIES M.H., DAVIS J.B., SMART D. Characterisation using FLIPR of rat vanilloid recepor (rVR1) pharmacology. Br. J. Pharmacol. 2000;130:916–922. doi: 10.1038/sj.bjp.0703390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDT S.-E., TOMINAGA M., JULIUS D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.Y., KANG C.J., KIM W.B., KIM D., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCHSTEIN T., GREFFRATH W., BUSSELBERG D., TREEDE R.D. Inhibition of rapid heat responses in nociceptive primary sensory neurons of rats by vanilloid receptor antagonists. J. Neurophysiol. 1999;82:2853–2860. doi: 10.1152/jn.1999.82.6.2853. [DOI] [PubMed] [Google Scholar]
- KRESS M., REEH P.W., VYKLICKY L. An interaction of inflammatory mediators and protons in small diameter dorsal root ganglion neurons of the rat. Neurosci. Lett. 1997;224:1–4. doi: 10.1016/s0304-3940(97)13450-4. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Similarities and differences in the currents activated by capsaicin, piperine and zingerone in rat trigeminal ganglion cells. J. Neurophysiol. 1996;76:1858–1869. doi: 10.1152/jn.1996.76.3.1858. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci. Lett. 1997;228:29–32. doi: 10.1016/s0304-3940(97)00358-3. [DOI] [PubMed] [Google Scholar]
- LIU L., SZALLASI A., SIMON S.A. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13 acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigeminal ganglion neurons. Neurosci. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- NAGY I., RANG H.P. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. J. Neurosci. 1999;19:10647–10655. doi: 10.1523/JNEUROSCI.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVIDGE J.R., RANASINGHE S.P., RANG H.P. Comparison of intracellular calcium signals evoked by heat and capsaicin in cultured dorsal root ganglion neurons and in a cell line expressing the rat vanilloid receptor, VR1. Neuroscience. 2001;102:177–184. doi: 10.1016/s0306-4522(00)00447-4. [DOI] [PubMed] [Google Scholar]
- SHU X., MENDELL L.M. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci. Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZALLASI A., SZABO T., BIRO T., MODARRES S., BLUMBERG P.M., KRAUSE J.E., CORTRIGHT D.N., APPENDINO G. Resiniferatoxin-type phorboid vanilloids display capsaicin-like selectivity at native vanilloid receptors on rat DRG neurons and at the cloned vanilloid receptor VR1. Br. J. Pharmacol. 1999;128:428–434. doi: 10.1038/sj.bjp.0702810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZOLCSÁNYI J.Actions of capsaicin on sensory receptors Capsaicin in the study of pain 1993London: Academic Press; 1–26.ed. Wood, J.N. pp [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- VYKLICKY L., KNOTKOVA-URBANCOVA H., VITASKOVA Z., VLACHOVA V., KRESS M., REEH P.W. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. J. Neurophysiol. 1998;79:670–676. doi: 10.1152/jn.1998.79.2.670. [DOI] [PubMed] [Google Scholar]
- WALPOLE C.S.J., BEVAN S., BLOOMFEILD G., BRECKENRIDGE R., JAMES I.F., RITCHIE T., SZALLASI A., WINTER J., WRIGGLESWORTH R. Similarities and differences in the structure activity relationships of capsaicin and resiniferatoxin analogues. J. Med. Chem. 1996;39:2939–2952. doi: 10.1021/jm960139d. [DOI] [PubMed] [Google Scholar]
- YEATS J., BEVAN S.J., DOCHERTY R.J. Calcium-dependent and independent desensitization of capsaicin-evoked responses in voltage-clamped adult rat dorsal root ganglion (DRG) neurones in culture. J. Physiol. 1992;446:390P. [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI M.V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


