Abstract
Contractile responses to short trains of nerve stimulation have been characterized in small, medium and large arteries from the rat mesenteric circulation (5th – 6th, 2nd – 3rd and 1st order, respectively). In addition, sources of calcium for smooth muscle contraction have been investigated.
Nerve stimulation (10 pulses at 10 Hz) evoked reproducible contractions. The P2 receptor antagonist suramin (100 μM) reduced constrictions by 65.3±7.4, 82.7±3.3 and 3.1±6.1% in small, medium and large arteries respectively. The α-adrenoceptor antagonist prazosin (0.1 μM) reduced responses by 32.6±2.6, 27.0±1.5 and 97.0±1.9% respectively.
The L-type calcium channel antagonist nifedipine (1 μM) reduced nerve-evoked contractions by 2.8±3.3, 10.0±3.7 and 13.5±2.7% in small, medium and large arteries respectively. When the adrenergic component of contraction was blocked by prazosin (0.1 μM) nifedipine reduced responses by 4.6±7.9, 14.3±2.0 and 3.0±1.9% respectively.
Contractile responses to exogenous α,β-meATP were unaffected by the depletion of calcium stores with cyclopiazonic acid (30 μM). This indicates that mobilization of calcium from internal stores is not required for P2X receptor mediated smooth muscle contraction.
We conclude that for neurogenic responses, the P2X receptor mediated component of constriction dominates in small mesenteric arteries (3rd – 6th order) while in large arteries (1st order) noradrenaline mediates contraction. For P2X receptor mediated responses all the calcium required for smooth muscle contraction enters the cell directly through P2X receptor channels.
Keywords: P2X receptors, artery, smooth muscle, calcium
Introduction
Peripheral arterial tone is under the control of the sympathetic nervous system. ATP and noradrenaline are co-stored and co-released from sympathetic nerves and mediate vasoconstriction by acting at arterial P2X receptors and α-adrenoceptors (Sneddon & Burnstock, 1984; von Kugelgen & Starke, 1985). The relative contribution of each transmitter toward the overall contractile response is variable depending on the preparation, species and stimulation parameters used e.g. Bao & Stjarne (1993); Kennedy et al., (1986); Sjoblom-Widfeldt (1990). We have recently shown differences, depending on the size of the vessel, in the sensitivity of P2X receptors in rat mesenteric arteries to exogenously applied agonists (Gitterman & Evans, 2000). This raised questions about the role of P2X receptors in mediating nerve-evoked contractions in different diameter mesenteric arteries. There is some indication that the size of the purinergic component of contraction is dependent on the diameter of the vessel, with the smallest arteries having the largest purinergic component of constriction (Evans & Surprenant, 1992; Ramme et al., 1987). This is of interest as small arteries and arterioles are particularly important in determining vascular resistance and hence systemic blood pressure. The majority of work has however focused on medium and large arteries (e.g. Angus et al. (1988); Sjoblom-Widfeldt (1990)) with comparatively little research investigating small resistance arteries (Evans & Surprenant, 1992; Morris, 1999; Phillips et al., 1998). The use of different vascular beds and different species has also made direct comparisons between large and small vessels difficult. In addition, the parameters of stimulation (frequency and train length) used can have a profound effect on contractile responses (Sjoblom-Widfeldt, 1990). We were interested in determining the relative importance of purinergic and adrenergic constriction using stimulation parameters that are thought to approximate to the symapthetic firing rate in vivo. Studies investigating the discharge pattern of sympathetic neurones in vivo have found that neuronal activity occurs in regular short bursts of high frequency firing rather than long sustained trains (Johnson & Gilbey, 1996). In this study we have used parameters of stimulation that reflect such a pattern of activity.
The trigger for contraction of smooth muscle is a rise in the cytosolic level of calcium. For noradrenaline it is known that activation of postjunctional α-adrenoceptors leads to the production of IP3 and release of calcium from intracellular stores. For P2X receptors calcium influx is essential but the sources of calcium and whether additional amplification of the signal occurs is unclear. ATP released from sympathetic nerve terminals leads to the activation of postjunctional P2X receptors and direct influx of calcium (Benham & Tsien, 1987). The depolarization of the smooth muscle cell in response to P2X receptor activation opens voltage-dependent calcium channels thus providing an additional source of calcium influx. In large arteries the purinergic component of contraction has been shown to be sensitive to the L-type calcium channel blocker nifedipine (Bulloch et al., 1991; Omote et al., 1989; Surprenant et al., 1983). By contrast, studies in small resistance arteries of the guinea-pig submucosa (Galligan et al., 1995), established that nifedipine had no effect on contractile responses evoked by α,β-methylene ATP (α,β-meATP). This suggests that in small vessels, sufficient calcium enters the smooth muscle cell directly through the P2X receptor channel to produce contraction. It is however unclear whether the nifedipine resistance seen in response to exogenous agonists is also seen in nerve-evoked contractions. Moreover, in certain smooth muscle calcium entry is only the first step in producing a rise in the level of intracellular calcium (Ganitkevich & Isenberg, 1992). The initial signal of calcium entry can often trigger a process of amplification whereby further calcium is released from ryanodine-sensitive intracellular stores (Gregoire et al., 1993). This process is termed calcium induced calcium release (CICR). It is unclear whether CICR is involved in achieving the necessary rise in intracellular calcium for P2X receptor-mediated contraction of vascular smooth muscle.
The aims of this study are twofold: (1) to systematically compare neurogenic vasoconstriction in small, medium and large arteries from the rat mesenteric bed, using stimulation parameters thought to approximate to conditions found in vivo and (2) to determine the relative roles of P2X receptors, L-type voltage-gated calcium channels and CICR in mediating the increase in intracellular calcium required for smooth muscle contraction in response to nerve stimulation.
Methods
Adult male Wistar rats (250 – 300 g) were killed by cervical dislocation or CO2 asphyxiation followed by femoral artery exsanguination. A portion of the gut with attached mesenteric arcade was removed and mesenteric arteries were dissected; large vessels correspond to the superior mesenteric artery, medium-sized vessels were from second or third order branches and small vessels correspond to fifth or sixth order arteries.
Medium and large artery rings were mounted in a Mulvany myograph using standard procedures (Lagaud et al., 1996) (internal diameters 252±5 μm, range 231 – 315 μm, n=23, and 549±28.0 μm, range 408 – 705 μm, n=19, respectively); changes in arterial tone were recorded and analysed using a MacLab data acquisition system. Small arteries were dissected carefully cleaning away all connective tissue and pinned out (stretched to approximately 150% of their resting length) in a Sylgard coated organ bath (volume 2 ml). The organ bath was placed on the stage of an inverted microscope and changes in external arterial diameter were analysed using Diamtrak software as previously described (Neild, 1989). Small arteries were studied using diamtrak video imaging microscopy because they were too small to be mounted in a myograph. In order to demonstrate that the different experimental methodologies had no bearing on our results we have previously compared the behaviour of medium-sized arteries using the two systems. The contractile responses to α,β-meATP and KCl were very similar in each case, producing almost identical concentration-response relationships when using either myography or Diamtrak (Gitterman & Evans, 2000). We are confident that the results we obtain with the two systems are directly comparable. The outside diameter of the vessels was 109±6 μm, range 80 – 193 μm, n=33 (wall thickness accounts for ∼40% giving a mean internal diameter of ∼66 μm).
Nerve stimulation
Tissues were superfused with Ringer's solution (composition in mM) NaCl 120, Glucose 11, NaHCO3 22, KCl 5, NaH2PO4 1, CaCl2 2.5, pH to 7.3 with NaOH, kept at 35°C – 37°C for myography and 32°C – 34°C for Diamtrak and continually gassed with 95% O2 and 5% CO2. Magnesium was omitted from the solution as this has previously been found to increase the amplitude of contractile responses to nerve stimulation (Ramme et al., 1987).
For myograph experiments, perivascular nerves were electrically stimulated by two platinum electrodes mounted in the jaws either side of the vessel. In Diamtrak studies, arteries were stimulated through a blunt glass microelectrode filled with bath solution and placed in close proximity to the artery about 1 mm from the diameter recording site. Electrical stimulation was delivered through an Applegarth Electronics stimulator (Oxford, U.K.); parameters were 10 pulses at 1 – 50 Hz (usually 10 Hz), 0.2 – 0.3 ms pulse width at 10 – 40 V. Stimulation delivered every 6 min gave reproducible responses. In each experiment 0.3 μM tetrodotoxin was applied to the preparation to confirm that contractile responses were neurogenic in origin. Antagonists were only applied once contractile responses were stable and applications continued until stable responses were obtained in the presence of the antagonist.
Agonist experiments
Tissues were superfused with a physiological saline solution (PSS; composition in mM): NaCl 150, KCl 2.5, HEPES 10, CaCl2 2.5, MgCl2 1, pH to 7.3 with NaOH. Experiments were conducted at 35°C – 37°C for myography and 32°C – 34°C for Diamtrak. Drugs were added to the superfusate at the required final concentration. Reproducible responses to agonists were obtained when applications were separated by 30 min intervals. In experiments testing the effect of nifedipine and CdCl2 both antagonists were pre-superfused for 10 min prior to being added together with the agonist. In each case, contractions evoked by PSS containing 60 mM KCl (with a proportionally reduced concentration of NaCl) were used as a positive control to test for antagonist function.
In experiments determining the relative role of intracellular calcium stores caffeine (10 mM) was applied to evoke responses by releasing calcium from the sarcoplasmic reticulum. When contractions were reproducible, 30 μM cyclopiazonic acid (CPA) was applied in zero calcium PSS for 15 min to deplete calcium stores. In the continued presence of CPA, the artery was then returned to normal calcium PSS for 90 s and caffeine subsequently applied. Responses to caffeine were abolished after this treatment and intracellular calcium stores considered to be fully depleted. The same depletion protocol was used to assess the contribution of intracellular calcium stores to contractions evoked by α,β-meATP.
Data analysis
Data are expressed as mean±s.e.mean throughout and n=number of arteries, number of animals. When more than one vessel was used from one animal, the data were averaged. The average value per animal was then used in calculating the overall mean and standard error. Differences between means were tested using either a two sample or paired, two-tailed t-test, as appropriate. A P value of <0.05 was considered statistically significant.
Drugs
α,β-methylene ATP (α,β-meATP), caffeine, CdCl2, cyclopiazonic acid (CPA), nifedipine, prazosin and suramin (Sigma, U.K.).
Results
Frequency response relationship
Electrical stimulation of rat mesenteric arteries evoked reproducible constrictions in all vessels tested. Responses were fast, monophasic and transient, and arterial tone/diameter rapidly returned to the initial baseline value after stimulation ceased. In order to verify that the properties of sympathetic vasoconstriction were comparable in all sizes of artery, frequency response relationships were constructed. A short burst of 10 pulses was applied at frequencies ranging from 1 – 50 Hz (Figure 1). Relative contractile responses were very similar for all vessel sizes up to a frequency of 20 Hz (n=5 – 6 arteries from 3 – 6 animals), and only a slight divergence was seen at higher frequencies of stimulation (30 and 50 Hz). For the remaining experiments we chose to stimulate with 10 pulses at 10 Hz as these parameters always gave a robust, near maximal response and reflect the short bursts of sympathetic activity recorded under physiological conditions (Johnson & Gilbey, 1996).
Figure 1.
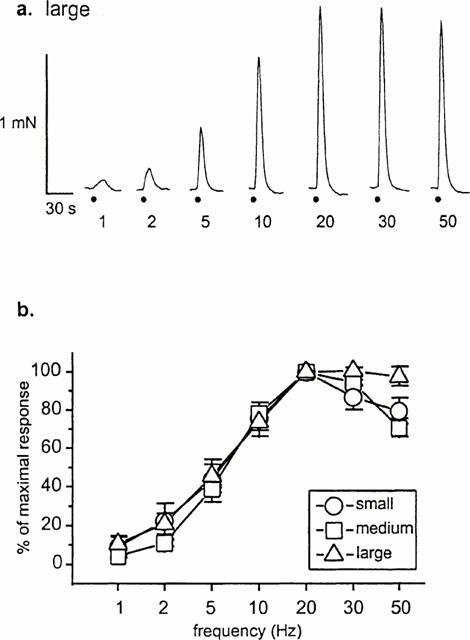
Nerve-evoked contractions of rat mesenteric arteries to 10 pulses of stimulation at a range of frequencies. (a) Responses of medium-sized mesenteric arteries; contractions are rapid and transient. Nerve stimulation is indicated by the circle. (b) Frequency-response relationships showing similar responses for all three sizes of artery.
Relative amplitude of purinergic and adrenergic neurogenic responses
To determine whether the proportion of purinergic and adrenergic components of sympathetic vasoconstriction changed with vessel diameter, the effects of selective antagonists were tested. The P2 receptor antagonist suramin (100 μM) inhibited the purinergic component of constriction and substantially reduced responses in both small and medium-sized arteries (pA2 5.2 for small and medium arteries, see Gitterman & Evans (2000) but had virtually no effect in large vessels (Figure 2); responses were reduced by 65.3±7.4, 82.7±3.3 and 3.1±6.1% (n=6 arteries from 3 – 5 animals) in small, medium and large arteries respectively. Prazosin (0.1 μM), a potent and selective antagonist of α adrenergic receptors, had only a limited effect on constrictions in small and medium sized arteries, reducing contractile responses by 32.6±2.6 and 27±1.5% (n=4 – 6 arteries from 3 – 4 animals) respectively. Contractions in large arteries were abolished by prazosin (reduced by 97.0±1.9%, n=6 arteries from three animals). When the two antagonists were applied concomitantly, responses to nerve stimulation were abolished in all arteries tested (n=6 arteries from 3 – 5 animals).
Figure 2.
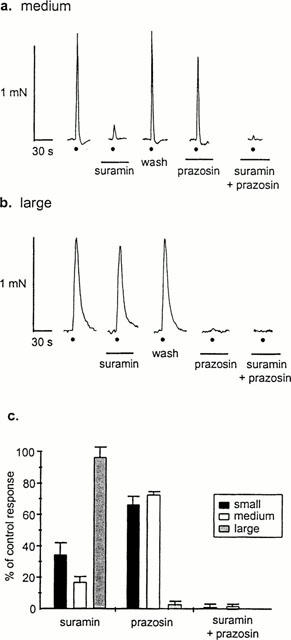
The purinergic component of constriction dominates in small and medium arteries while the noradrenergic component dominates in large. Effects of suramin (100 μM) and prazosin (0.1 μM) alone and in combination on responses to 10 pulses of stimulation at 10 Hz in medium (a) and large (b) arteries. Application of both antagonists abolishes responses in all arteries. Circle indicates electrical stimulation and bars indicate periods of antagonist application; traces without annotation are control responses. (c) Histogram shows percentage of control response in the presence of antagonist. Data are mean contarctions±s.e.mean (n=4 – 6 arteries from 3 – 5 animals).
Role of voltage-gated calcium channels
We have previously demonstrated that calcium influx is essential for P2X receptor-mediated constrictions in all sizes of mesenteric artery (Gitterman & Evans, 2000). Calcium influx in response to P2X receptor activation can occur either directly through the P2X receptor channel or through voltage-gated calcium channels. To investigate the role of the voltage-dependent calcium channels in neurogenic vasoconstriction, the effect of the selective L-type calcium channel antagonist nifedipine was tested on nerve-evoked contractions. Nifedipine (1 μM) had only a small effect on responses, with constrictions in small, medium and large vessels being reduced by only 2.8±3.3, 10.0±3.7 and 13.5±2.7% (n=5 – 6 arteries from 3 – 4 animals), respectively (Figure 3). To examine the effect of nifedipine on just the purinergic component of the response, prazosin was first applied to block adrenergic transmission. Under these conditions, nifedipine also reduced responses only slightly: by 4.6±7.9, 14.3±2.0 and 3.0±1.9% (n=4 – 6 arteries from 3 – 4 animals) respectively.
Figure 3.
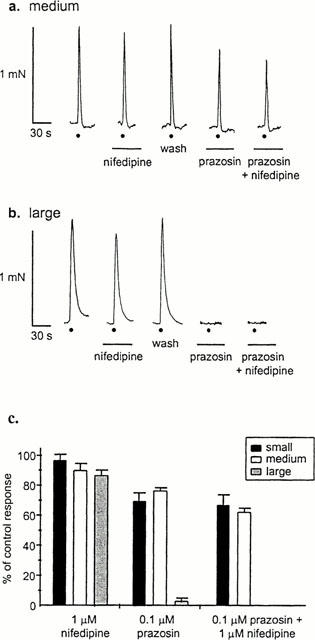
Nifedipine has only a small effect on nerve evoked constrictions. Typical responses in medium (a) and large (b) arteries. Nifedipine (1 μM) has little effect, both when applied alone, and when prazosin (0.1 μM) is first applied to block the adrenergic component of constriction. Circle indicates electrical stimulation and bars indicate period of antagonist application; traces without annotation are control responses. (c) Data are mean responses±s.e.mean (n=4 – 6 arteries from 3 – 5 animals) expressed as per cent of control.
In control experiments, responses to 60 mM KCl were abolished by nifedipine (1 μM) in medium and large vessels but only reduced by 52.8±6.9% (n=4 – 5 arteries from three animals) in small arteries (Figure 4). This nifedipine-resistant component of constriction to 60 mM KCl in small vessels suggested that other calcium channels may be present. We therefore also tested the effect of cadmium, a non-selective blocker of all voltage-dependent calcium channels. CdCl2 (300 μM) abolished contractions to 60 mM KCl in all arteries (n=4 – 5 arteries from three animals).
Figure 4.
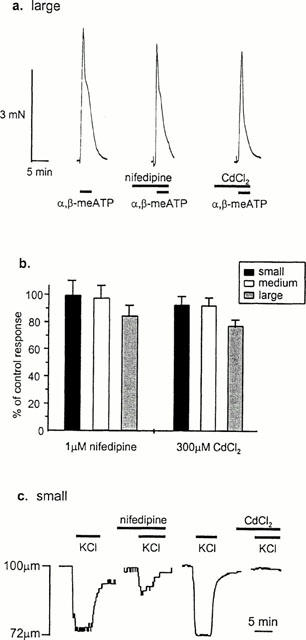
The calcium channel blockers nifedipine and cadmium have little effect on contractions to exogenous application of a P2X receptor agonist (a) Typical responses from a large artery showing the effect of nifedipine (1 μM) and CdCl2 (300 μM) on responses to an EC50 concentration of α,β-meATP. (b) Histogram shows mean data±s.e.mean (n=4 – 6 arteries from 3 – 4 animals) for small, medium and large arteries. (c) Traces showing nifedipine resistance of control responses to 60 mM KCl in small arteries. The component of constriction resistant to nifedipine (1 μM) is abolished by CdCl2 (300 μM). Periods of drug application are indicated by bar.
The possibility therefore exists that, at least in small arteries, voltage-gated calcium channels distinct from L-type channels may be present. As cadmium blocks all voltage-dependent calcium channels, N-type calcium channels in the nerve terminal which are essential for the release of transmitters would also be blocked. We therefore stimulated arterial P2X receptors with applied agonist. Cadmium (300 μM) marginally reduced contractions to an ∼EC50 concentration of α,β-meATP (1 μM for small and medium and 100 μM for large arteries see Gitterman & Evans (2000) in small and medium-sized arteries (reduced by 8.4±5.4 and 9.1±5.0%, respectively; n=4 – 5 arteries from three animals), and reduced contractions by 22.8±4.0% (n=5 arteries from three animals) in large vessels (Figure 4). To determine whether these reductions reflected the proportion of calcium influx through L-type calcium channels, nifedipine was also tested on responses to α,β-meATP. In this case, a similar pattern of results was seen as with cadmium although responses were slightly more resistant to blockade by nifedipine (Figure 4). Contractions were not reduced in small arteries and were reduced by 2.0±8.7 and 15±7.3% (n=4 – 5 arteries from three animals) in medium and large vessels respectively.
Effect of cyclopiazonic acid on purinergic constrictions
To investigate whether calcium-induced calcium release is involved in smooth muscle contraction, we tested the effect of depleting intracellular calcium stores with cyclopiazonic acid (30 μM). Ideally this experiment would have been conducted using nerve-evoked responses. It has however been shown that CICR is involved in transmitter release from sympathetic nerve terminals (Smith & Cunnane, 1996). We therefore tested the effects of CPA on contractions to applied agonists. For these experiments, responses to caffeine (10 mM), which causes contractions by releasing calcium from intracellular stores, were used to control for complete store depletion. The amplitude of responses to caffeine were also used to estimate the size of the intracellular pool of calcium. Responses to 10 mM caffeine were 88.7±7.5, 51.7±10.9 and 30.9±9.4% (n=4 – 7 arteries from 3 – 4 animals) of contractions to 60 mM KCl and 90.8±5.1, 41.8±5.8 and 50.4±19.3% of response to α,β-meATP (10 μM for small and medium 100 μM for large, n=3 – 6 arteries from three animals) in small, medium and large arteries, respectively. Treatment with 30 μM CPA abolished responses to caffeine but did not reduce responses to an EC50 concentration of α,β-meATP (1 μM for small and medium, 100 μM for large, Figure 5). Responses were, as a percentage of control, 101.3±6.4, 101.3±3.5 and 117.3±9.5% (n=4 – 7 arteries from 3 – 4 animals) for small, medium and large arteries, respectively.
Figure 5.
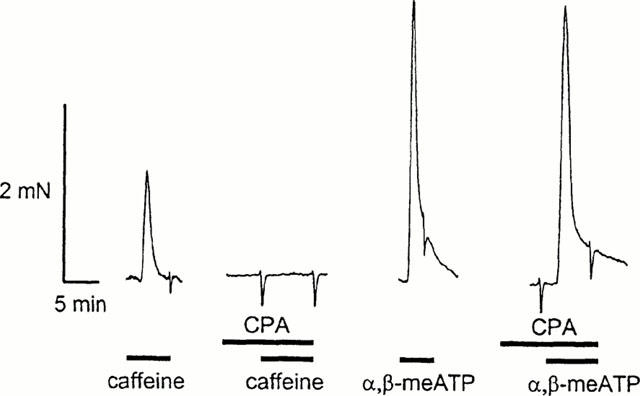
Contractions to α,β-meATP are unaffected by depletion of intracellular calcium stores with CPA. Typical traces of responses in medium arteries. Control contractions evoked by emptying intracellular calcium stores with caffeine (10 mM) are abolished by prior perfusion with CPA (30 μM) while contractions to α,β-meATP (1 μM) remain unaffected.
Discussion
In this study we have shown substantial differences in the relative roles of P2X receptors and α-adrenoceptors in mediating sympathetic control of arterial tone in the rat mesenteric bed depending on the diameter of the vessel. The purinergic component of transmission dominates in small-medium arteries (3rd – 6th order), while contractions in large arteries (1st order) are almost entirely adrenergic. We have also shown that calcium entering the smooth muscle cell directly through the P2X receptor channel is sufficient to mediate contraction with little contribution from L-type voltage dependent calcium channels or CICR.
The variety of stimulation parameters used in studies investigating neurogenic vasoconstriction raises the question as to which pattern of stimulation corresponds to sympathetic transmission in vivo. Johnson & Gilbey (1996) showed that sympathetic neurones innervating rat arteries fire in rhythmic bursts separated by relative silence rather than sustained trains. The rhythm is often dictated by respiration as indicated by activity of the phrenic nerve. The firing rate of sympathetic neurones was found to be between 0.8 and 0.9 Hz, each discharge being a brief burst of multiple action potentials with an intraburst frequency of up to 20 Hz or more (Johnson & Gilbey, 1994). The parameters we chose were designed to represent one such burst of activity. Electrical stimulation of 10 pulses at 10 Hz evoked reproducible contractions in all arteries tested. Responses were neurogenic in origin as they were abolished by treatment with tetrodotoxin. Frequency response relationships were similar for all vessel sizes, indicating that the properties of neurotransmitter release are very similar. This further demonstrates that the different experimental methodologies which were used would not be expected to influence the results obtained.
Previous studies investigating the neurogenic control of arterial tone have revealed varying proportions of purinergic versus adrenergic components depending on the species, tissue and stimulation parameters used (Yang & Chiba, 1999, 2000). Although there is considerable evidence to suggest the purinergic component dominates in small resistance arteries (Evans & Surprenant, 1992; Ramme et al., 1987), no systematic comparisons have been conducted on arteries from one vascular bed. To our knowledge, this is the first study directly comparing neurogenic contractions in three different sizes of artery from the same vascular bed. Selective blockade of purinergic and noradrenergic transmission revealed a P2X receptor mediated component of approximately 70% in small and medium arteries. A similar proportion of purinergic constriction has also been seen in other arteries when similar short trains of stimulation were used (Evans & Cunnane, 1992; Ren et al., 1996; Warland & Burnstock, 1987). If however the duration of stimulation is increased, a substantial adrenergic component of constriction can be produced. We have seen this in small arteries where increasing the duration of stimulation to 100 pulses at 10 Hz more than doubled the adrenergic component (unpublished observations). Similar findings have been made in numerous other preparations (Kennedy et al., 1986; Sjoblom-Widfeldt, 1990; Todorov et al., 1999). In small resistance arteries of the guinea-pig ear, a 30 s train of stimulation at 10 Hz can even produce an entirely adrenergic response (Morris, 1999). However it is unclear what physiological firing patterns these long trains of high frequency correspond to. These examples demonstrate the great influence that stimulation parameters have on the contractile behaviour of blood vessels and underline how the choice of parameters can significantly affect the results obtained. They also highlight the difficulties in comparing data from different studies. Nevertheless there is a clear physiological role for P2X receptor mediated transmission in small and medium arteries when sympathetic nerves fire in short high frequency bursts. The P2X receptor subtype in these arteries corresponds to a P2X1 receptor homomer (Gitterman & Evans, 2000).
By contrast, in large arteries, over 95% of the response to nerve stimulation was adrenergic. Such a high adrenergic component has also been seen in other vessels that represent the main conduit artery of a vascular bed (Bao & Stjarne, 1993) and may be a general characteristic of large arteries. The relative lack of purinergic transmission in large arteries can be accounted for by our previous work. We have shown that P2X receptors in large mesenteric arteries are about 100 times less sensitive to agonists than in smaller vessels (Gitterman & Evans, 2000). The lower innervation density in large arteries (Luff & McLachlan, 1989) may also be a factor. Less release of transmitter combined with a lower sensitivity of P2X receptors in large arteries than in smaller vessels may combine to produce a much smaller purinergic component of contraction in large vessels.
It is known that NPY can be released from sympathetic nerve terminals (Potter, 1988). Under certain conditions using long trains of stimulation NPY has also been shown to mediate part of the contractile response to electrical field stimulation (Phillips et al., 1998). Co-application of prazosin and suramin abolished neurogenic contractile responses in all three sizes of artery demonstrating that NPY does not contribute to the constrictor response. The suramin and prazosin-resistant components of contraction combine to approximately 100% of the response. This suggests that in our experiments, the action of the ATP and noradrenline is additive and that there is no synergistic interaction between the two transmitter systems.
Calcium influx is essential for P2X receptor mediated contraction in rat mesenteric arteries (Gitterman & Evans, 2000). In the present study we have shown that L-type voltage-gated calcium channels do not contribute significantly to contractions evoked by nerve stimulation or to α, β-meATP. This extends on findings made in submucosal arterioles of the guinea-pig ileum (Galligan et al., 1995). Our results suggest that all the calcium influx that occurs in response to P2X receptor activation following nerve stimulation is directly through the P2X receptor channel. Although evidence has been found for the involvement of calcium channels in purinergic vasoconstriction (Bulloch et al., 1991; Omote et al., 1989), these data are generally from large vessels, not small resistance arteries. The non-selective calcium channel blocker cadmium had a very similar effect to nifedipine on P2X receptor mediated contractions and confirms that there is no other voltage dependent calcium channel contributing to calcium influx. Interestingly, there was a discrepancy in the effects of calcium channel blockers on control responses to 60 mM KCl. Nifedipine and cadmium abolished responses in medium and large arteries, in contrast for small arteries there was a nifedipine-resistant component of contraction to 60 mM KCl that was abolished by cadmium. This suggests the presence of a non-L-type calcium channel in small arteries, however it does not appear to be involved in P2X receptor-mediated contraction.
CPA inhibits the sacrolemmal Ca2+-ATPase depletes internal calcium stores and was used to determine the role of CICR in amplifying the calcium rise associated with P2X receptor activation. CPA treatment to deplete calcium stores had no effect on the amplitude of P2X receptor mediated contractions. Responses to caffeine were abolished by CPA treatment demonstrating that all releasable calcium had been depleted from the intracellular stores. The lack of effect of CPA on P2X receptor mediated responses demonstrates that calcium release from intracellular stores is not required for P2X receptor mediated contraction; and combined with the data with nifedipine and cadmium suggests that all the calcium required for contraction enters directly though the P2X receptor channel. The responses to caffeine can also be used to estimate the size of internal calcium stores. Our data show that relative to contractions evoked by KCl, calcium stores are largest in small arteries and become progressively smaller with increasing arterial diameter. This is intriguing as it seems to contradict other studies which have found that the relative importance and size of intracellular calcium stores is greatest in large conduit arteries (van Breemen & Saida, 1989; Ashida et al., 1988).
In summary we have shown that the relative proportion of purinergic versus adrenergic component of contractile responses to nerve stimulation are dependent on artery size. In large arteries the response is essentially adrenergic, while in small and medium arteries, that play a key role in the control of blood pressure, the response is predominantly mediated through the activation of P2X receptors. We have shown that P2X receptor mediated neurogenic contractions are resistant to commonly used anti-hypertensive thereapies i.e. α-adrenoceptor and calcium channel antagonists. This suggests that P2X receptors may be novel targets for the treatment of hypertension.
Acknowledgments
This work was supported by an MRC studentship to D.P.Gitterman and by the Wellcome Trust.
Abbreviations
- α,β-meATP
α,β-methylene ATP
- CICR
calcium induced calcium release
- CPA
cyclopiazonic acid
- IP3
inositol triphosphate
- NPY
uropeptide Y
References
- ANGUS J.A., BROUGHTON A., MULVANY M.J. Role of α-adrenoceptors in constrictor responses of rat, guinea-pig and rabbit small arteries to neural activation. J. Physiol. 1988;403:495–510. doi: 10.1113/jphysiol.1988.sp017260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHIDA T., SCHAEFFER J., GOODMAN W.F., WADE J.B., BLAUSTEIN M.P. Role of sarcoplasmic reticulum in arterial contraction: Comparison of ryanodine's effect in conduit and a muscular artery. Circ. Res. 1988;62:854–863. doi: 10.1161/01.res.62.4.854. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., TSIEN R.W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- BAO J.-X., STJARNE L. Dual contractile effects of ATP released by field stimulation revealed by effects of αβ-methylene ATP and suramin in rat tail artery. Br. J. Pharmacol. 1993;110:1421–1428. doi: 10.1111/j.1476-5381.1993.tb13979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLOCH J.M., MACDONALD A., MCGRATH J.C. Different sensitivities of rabbit isolated blood vessels exhibiting co-transmission to the slow calcium channel blocker, nifedipine. Br. J. Pharmacol. 1991;103:1685–1690. doi: 10.1111/j.1476-5381.1991.tb09847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.J., CUNNANE T.C. Relative contributions of ATP and noradrenaline to the nerve evoked contraction of the rabbit jejunal artery: dependence on stimulation parameters. Naunyn Schmiedeberg's Arch. Pharmacol. 1992;345:424–430. doi: 10.1007/BF00176620. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., SURPRENANT A. Vasoconstriction of guinea-pig submucosal arterioles following sympathetic nerve stimulation is mediated by the release of ATP. Br. J. Pharmacol. 1992;106:242–249. doi: 10.1111/j.1476-5381.1992.tb14323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIGAN J.J., HERRING A., HARPSTEAD T. Pharmacoligical characterization of purinoceptor-mediated constriction of submucosal arterioles in guinea pig ileum. J. Pharm. Exp. Ther. 1995;274:1425–1430. [PubMed] [Google Scholar]
- GANITKEVICH V.Y., ISENBERG G. Contribution of Ca2+-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. J. Physiol. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GITTERMAN D.P., EVANS R.J. Properties of P2X and P2Y receptors are dependent on artery diameter in the rat mesenteric bed. Br. J. Pharmacol. 2000;131:1561–1568. doi: 10.1038/sj.bjp.0703760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGOIRE G., LOIRAND G., PACAUD P. Ca2+- and Sr2+ entry induced Ca2+ release from the intracellular Ca2+ store in smooth muslce cells of rat portal vein. J. Physiol. 1993;474:483–500. doi: 10.1113/jphysiol.1993.sp019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON C.D., GILBEY M.P. Sympathetic activity recorded from the rat caudal ventral artery in vivo. J. Physiol. 1994;476:437–442. doi: 10.1113/jphysiol.1994.sp020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON C.D., GILBEY M.P. On the dominant rhythm in the discharges of single postganglionic sympathetic neurones innervating the rat tail artery. J. Physiol. 1996;497:241–259. doi: 10.1113/jphysiol.1996.sp021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY C., SAVILLE V.L., BURNSTOCK G. The contributions of noradrenaline and ATP to the response of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur. J. Pharmacol. 1986;122:291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- LAGAUD G.J.L., STOCLET J.C., ANDRIANTSITOHAINA R. Calcium handling and purinoceptor subtypes involved in ATP-induced contraction in rat small mesenteric arteries. J. Physiol. 1996;492:689–703. doi: 10.1113/jphysiol.1996.sp021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFF S.E., MCLACHLAN E.M. Frequency of neuromuscular junctions on arteries of different dimensions in the rabbit, guinea-pig and rat. Blood Vessels. 1989;26:95–106. doi: 10.1159/000158758. [DOI] [PubMed] [Google Scholar]
- MORRIS J.L. Cotransmission from sympatethic vasoconstrictor neurons to small cutaneous arteries in vivo. Am. J. Physiol. 1999;277:H58–H64. doi: 10.1152/ajpheart.1999.277.1.H58. [DOI] [PubMed] [Google Scholar]
- NEILD T.O. Measurement of arteriole diameter changes by analysis of television images. Blood Vessels. 1989;26:48–52. [PubMed] [Google Scholar]
- OMOTE S., KIGOSHI S., MURAMATSU I. Selective inhibition by nifedipine of the purinergic component of neurogenic vasoconstrction in the dog mesenteric artery. Eur. J. Pharmacol. 1989;160:239–245. doi: 10.1016/0014-2999(89)90496-2. [DOI] [PubMed] [Google Scholar]
- PHILLIPS J.K., MCLEAN A.J., HILL C.E. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentry. Br. J. Pharmacol. 1998;124:1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTTER E.K. Neuropeptide Y as an autonomic neurotransmitter. Pharmacol. Ther. 1988;37:251–273. doi: 10.1016/0163-7258(88)90028-9. [DOI] [PubMed] [Google Scholar]
- RAMME D., REGENOLD J.T., STARKE K., BUSSE R., ILLES P. Identification of the neuroeffector transmitter in jejunal branches of the rabbit mesenteric artery. Naunyn Schmiedeberg's Arch Pharmacol. 1987;336:267–273. doi: 10.1007/BF00172677. [DOI] [PubMed] [Google Scholar]
- REN L.-M., NAKANE T., CHIBA S. Purinergic and adrenergic transmission and their presynaptic modulation in canine isolated perfused splenic arteries. Eur. J. Pharmacol. 1996;295:61–68. doi: 10.1016/0014-2999(95)00654-0. [DOI] [PubMed] [Google Scholar]
- SJOBLOM-WIDFELDT N. Neuro-muscular transmission in blood vessels: phasic and tonic components. An in-vitro study of mesenteric arteries of the rat. Acta Physiologica Scandanavica. 1990;138 supplement 587 [PubMed] [Google Scholar]
- SMITH A.B., CUNNANE T.C. Ryanodine-sensitiv calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J. Physiol. 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEDDON P., BURNSTOCK G. ATP as a co-transmitter in rat tail artery. Eur. J. Pharmacol. 1984;106:149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., NEILD T.O., HOLMAN M.E. Effects of nifedipine on nerve-evoked action potentials and consequent contractions in rat tail artery. Pflugers Arch. Eur. J. Physiol. 1983;396:342–349. doi: 10.1007/BF01063940. [DOI] [PubMed] [Google Scholar]
- TODOROV L.D., MIHAYLOVA-TODOROVA S.T., BJUR R.A., WESTFALL D.P. Differential cotransmission in sympathetic nerves: role of frequency of stimulation and prejunctional autoreceptors. J. Pharm. Exp. Ther. 1999;290:241–246. [PubMed] [Google Scholar]
- VAN BREEMEN C., SAIDA K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu. Rev. Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., STARKE K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. J. Physiol. 1985;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARLAND J.J.I., BURNSTOCK G. Effects of reserpine and 6-hydroxydopamine on the adrenergic and purinergic components of sympathetic nerve responses of the rabbit saphenous artery. Br. J. Pharmacol. 1987;92:871–880. doi: 10.1111/j.1476-5381.1987.tb11393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG X.P., CHIBA S. Perivascular purinergic nerve-induced vasoconstrictions in canine isolated splenic arteries. Jpn. J. Pharmacol. 2000;82:71–73. doi: 10.1254/jjp.82.71. [DOI] [PubMed] [Google Scholar]
- YANG Y.P., CHIBA S. Adrenergic-purinergic interactions on vasoconstrictor responses to periarterial electric nerve stimulation in canine splenic arteries. J. Auton. Pharmacol. 1999;19:139–144. doi: 10.1046/j.1365-2680.1999.00126.x. [DOI] [PubMed] [Google Scholar]


