Abstract
The aim of the present study was to investigate the effects of extracellular application of some sterically-hindered phenols, namely 3-t-butyl-4-hydroxyanisole (BHA), 3,5-di-t-butyl-4-hydroxyanisole (DTBHA) and the dimer of BHA, 2,2′-dihydroxy-3,3′-di-t-butyl-5,5′-dimethoxydiphenyl (DIBHA), on the whole-cell Ca2+ current (ICa) of freshly isolated smooth muscle cells from the guinea-pig gastric fundus, in the presence of a range of Ca2+ concentrations (1 – 5 mM) using the patch-clamp technique. The influx of Ca2+ had characteristics of L-type ICa (ICa(L)).
BHA as well as DTBHA inhibited ICa(L) in a concentration-dependent manner, during depolarization to 10 mV from a holding potential of −50 mV. Bath application of BHA (50 μM) and DTBHA (30 μM) decreased ICa(L) by 48.9% and 45.2%, respectively. This inhibition was only partially reversible. In contrast, DIBHA (up to 50 μM) was devoided of effects on ICa(L).
BHA inhibition of ICa(L) was voltage-dependent and inversely related to the external concentration of Ca2+. On the other hand, DTBHA inhibition was only voltage-dependent.
BHA and DTBHA shifted the voltage range of the steady-state inactivation curve to more negative potentials by 8 mV at the mid-potential of the curve, without affecting the activation curve. Furthermore, BHA and DTBHA did not modify the time-course of the current decay.
We conclude that the inhibition of ICa(L) by BHA and DTBHA is qualitatively similar to that of a Ca2+ channel blocker and is characterized by the stabilizing effect of the inactivated state of the channel.
Keywords: BHA, antioxidant phenol derivatives, Ca2+ channel blocker, gastric fundus smooth muscle, whole-cell L-type Ca2+ current
Introduction
Several attempts have been made to integrate via a unifying hypothesis the widely recognized events concerning the molecular mechanism of ischaemia-reperfusion injury. This is based on accumulation of cytosolic Ca2+ and increase in radical oxygen species production (Kukreja & Hess, 1992; Opie, 1992). According to the most recent data, radical oxygen species initiate Ca2+-mediated functional changes or cell damage by acting on the sarcoplasmic reticulum and promoting Ca2+ entry into the cytosol (Viner et al., 1996; Peers, 1997). The exposure of vascular (Roveri et al., 1992; Krippeit-Drews et al., 1995) as well as intestinal smooth muscle cells (Bielefeldt et al., 1997) to radical oxygen species (oxidative stress) induces a rapid and significant increase in intracellular Ca2+ concentration to abnormally high and likely cytotoxic levels. Ca2+ influx into the cell and Ca2+ release from intracellular stores contribute predominantly to this phenomenon.
A successful strategy aimed at preventing or reducing oxidative stress damage can be envisaged with use of free radical scavenging drugs, which can also protect endogenous antioxidant systems, thus regulating the redox status of the cell. These antioxidant compounds have been fully assessed in their capacity to prevent stress induced apoptosis (Jabs, 1999; Dalton et al., 1999). Alternatively, compounds which safeguard the cell Ca2+ homeostasis can be used. Therefore, molecules combining the antioxidant property with the capability to reduce/limit the increase in intracellular Ca2+ concentration, might be valuable drugs against the oxidative stress injury, representing a double-edged defence for preventing cell Ca2+ overload subsequent to oxidative stress, which characterizes the ischaemia-reperfusion injury. Interestingly, in this context, it has been observed in our laboratory that 3-t-butyl-4-hydroxyanisole (BHA) and structurally related phenol derivatives, besides the well-known antioxidant properties, exhibit a marked myorelaxant activity and cause the activation of the skeletal sarcoplasmic reticulum Ca2+-ATPase (Sgaragli et al., 1993a,1993b; Fusi et al., 1998a; 1999a,1999b). The most active of them, i.e. 3,5-di-t-butyl-4-hydroxyanisole (DTBHA), had been proposed to be used as a prototype compound for the synthesis of drugs useful for preventing tissue damage caused by ischaemia-reperfusion (Sgaragli et al., 1993a). In both gastric and vascular smooth muscle preparations, BHA reduces the contraction induced by different stimuli (Fusi et al., 1998b; 2000). Since Ca2+ plays a pivotal role in smooth muscle contraction, these data suggest that a reduction of Ca2+ entry through plasmalemma Ca2+-channels could be one of the mechanisms by which BHA counteracts an increase in cytosolic free Ca2+ concentration and hence muscle contraction. However, electrophysiological analysis of the effects of BHA or DTBHA on voltage-dependent Ca2+ channels of smooth muscle cells has not yet been performed. In the present study we analyse the action of BHA and DTBHA on L-type inward Ca2+ channel current (ICa(L)) in isolated single smooth muscle cells of the guinea-pig fundus, using the whole-cell patch-clamp technique. Freshly dissociated cells and physiological Ca2+ concentrations were used in order to make a closer comparison with the experiments previously performed on isolated longitudinal gastric fundus strips (Fusi et al., 1998b). The results presented here support the hypothesis that these phenol derivatives block voltage-dependent ICa(L).
Methods
Cell isolation procedure
Smooth muscle cells were freshly isolated by collagenase treatment of gastric fundus strips obtained from male guinea-pigs (350 – 450 g) (Fusi et al., 1998b), previously anaesthetized with Ketavet® (Gellini, Italy), decapitated and exsanguinated. The tissue specimens were digested for 22 – 30 min at 37°C in 2 ml of nitrate-rich digestion solution (see below for composition) containing 2 mg collagenase (type IA), 2.5 mg BSA and 3 mg soybean trypsin inhibitor, bubbled with 95% O2 – 5% CO2. Thereafter, the cells were mechanically dispersed, in a modified Kraftbrüne (KB) solution (Isenberg & Klockner, 1982), with a plastic pipette. The cells were used for experiments within 10 h after isolation. During this time they were stored at 4°C in KB solution containing BSA.
An aliquot of the cell suspension (40 – 150 μl) was transferred to a small recording chamber (250 μl) mounted on the stage of an inverted phase-contrast microscope (TE300, Nikon, Japan) and monitored with a video camera (JVC TK-1280E). Immediately after harvest, the smooth muscle cells were relaxed and elongated in shape (Bean et al., 1986), 3 – 10 μm wide and 200 – 400 μm long. They responded to mechanical and pharmacological stimulation (e.g. 1 μM ACh) (Nakazawa et al., 1987) by contracting and changing the texture of the cell surface from a smooth contour to one overwhelmed by bulbous and mound-like evaginations, as previously described (Fay & Delise, 1973).
After adhesion of the cells to the glass bottom of the chamber (20 min), they were continuously superfused by means of a peristaltic pump (LKB 2132), at a flow rate of 500 μl min−1, with external solution. Electrophysiological responses were tested at room temperature (22 – 24°C) only in cells that were phase dense.
Whole-cell patch clamp recording
The whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) was employed to voltage-clamp the smooth muscle cells. Recording electrodes were pulled from borosilicate glass capillaries (WPI, Berlin, Germany) and fire polished to give a pipette resistance of 2 – 5 MΩ when filled with the internal solution. A low-noise, high-performance Axopatch 200B (Axon Instruments, Burlingame, CA, U.S.A.) patch-clamp amplifier, driven by an IBM computer in conjunction with an A/D, D/A board (DigiData 1200 A/B series interface, Axon Instruments) were used to generate and apply voltage pulses to the clamped cells and to record the corresponding membrane currents. Current signals were low-pass filtered at 1 kHz before being stored on the computer hard disk. Long-lasting, nifedipine-blockable, inward currents through L-type Ca2+ channels, in 1, 2.5 or 5 mM Ca2+-containing external solution, were measured over a range of test potentials (250 ms) from −50 to 50 mV from a holding potential (Vh) of −80 or −50 mV. Nifedipine-insensitive inward currents were not recorded in these cells, as previously observed by Lammel et al. (1991). Data were obtained after the current amplitude had been stabilized (usually 10 min after the whole-cell configuration was obtained). The ICa(L) did not run down over the next 50 to 60 min, under these conditions.
Steady-state inactivation curves were obtained using the double-pulse protocol. Various levels of conditioning potentials were applied for 5 s, followed by a short (5 ms) return to the holding potential to assign the channels to either the closed or inactivated state, and then a test pulse of 10 mV was delivered to evoke the current.
Activation curves were derived from the current-voltage relationships (see Figure 2). Conductance (G) was calculated from the equation G=ICa / (Em – ECa), where ICa is the peak current elicited by depolarizing test pulses to the various potentials and ECa is the reversal potential (obtained from the extrapolated current-voltage curves in Figure 2). Gmax is the maximal Ca2+ conductance (calculated at potentials above 10 mV). The points for G/Gmax were plotted against the membrane potential as a relative amplitude.
Figure 2.
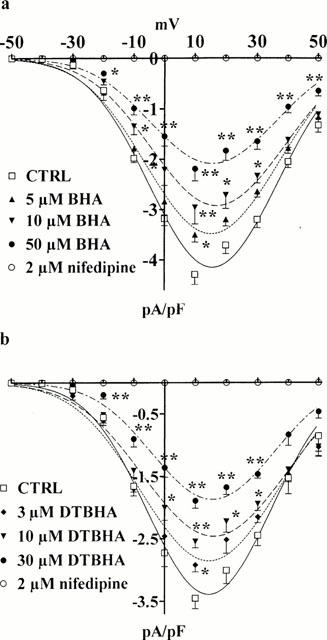
Concentration-dependent inhibition of ICa(L) by BHA and DTBHA. Current-voltage relationships of the peak ICa(L) measured, from Vh −50 mV, in the absence (CTRL) or presence of various concentrations of BHA (a) and DTBHA (b). ICa(L) suppression by 2 μM nifedipine is also shown. *P<0.05, **P<0.01, Dunnett's post test. Data points are means±s.e.mean derived from 3 – 8 cells.
Potassium current was blocked with tetraethylammonium (TEA) in the external solution and Cs+ in the internal solution. Peak ICa(L) values were corrected for the leak using 2 μM nifedipine, which blocked completely and specifically ICa(L) (see Figures 1 and 2).
Figure 1.
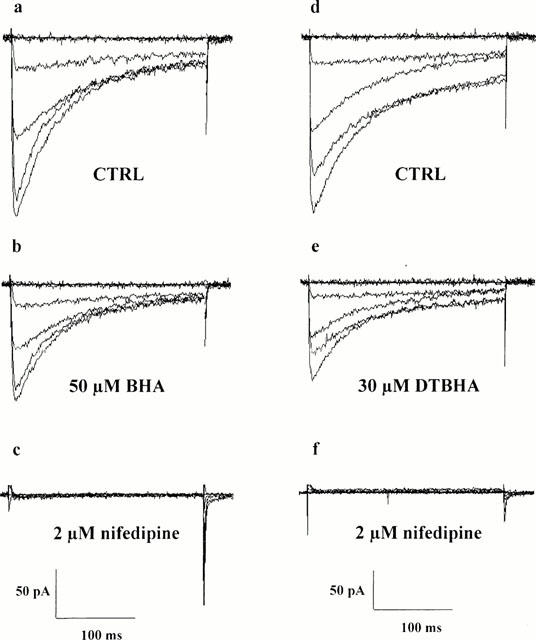
Inhibitory action of BHA, DTBHA and nifedipine on ICa(L). Representative recordings illustrating whole-cell ICa(L) in gastric fundus cells in external solution containing 2.5 mM Ca2+. The current traces were elicited with 250 ms depolarizing voltage-clamp pulses from a Vh of −50 mV to test potentials of −60 to 40 mV in increments of 20 mV. (a – c) Effect of BHA on whole-cell ICa(L): control (CTRL) current traces (a), 6 min after bath application of 50 μM BHA (b) and 2 min after bath application of 2 μM nifedipine (c). (d – f) Effect of DTBHA on whole-cell ICa(L): control (CTRL) current traces (d), 6 min after bath application of 30 μM DTBHA (e) and 2 min after bath application of 2 μM nifedipine (f).
Solutions and chemicals
Digestion solution contained the following compounds (in mM): NaCl 55, NaNO3 65, KCl 5, Na-pyruvate 5, glucose 10, taurine 10, HEPES 10 and MgCl2 1.2; pH was adjusted to 7.4 with NaOH.
The modified KB solution contained 1 mg BSA and (in mM): NaCl 105, KH2PO4 7, KCl 5, glucose 5, taurine 10, HEPES 10, MgCl2 1.6, Na-pyruvate 2.5, creatine 1.7, oxalacetate 2, Na2ATP 1.5 and EGTA 0.1; pH was adjusted to 7.25 with NaOH.
To isolate the ICa(L), the pipette was filled with a high Cs+ solution (internal solution) of the following composition (in mM): CsCl 105, HEPES 10, EGTA 11, MgCl2 2, CaCl2 1, Na-pyruvate 5, succinic acid 5, oxalacetic acid 5, Na2ATP 3, phosphocreatine 5 and cyclic AMP 0.005, pH was adjusted to 7.4 with CsOH. The free Ca2+ concentration in this solution was estimated to be less than 4 nM.
The bath solution (external solution) contained (in mM): NaCl 135, KCl 5.6, Na-pyruvate 5, glucose 20, TEA 30, HEPES 10 and MgCl2 1.2; pH was adjusted to 7.4 with NaOH. In order to obtain low or elevated Ca2+-containing external solutions, Na+ was replaced isosmotically with Ca2+.
The osmolarity of the external solution was adjusted to 330 mosmol, that of internal to 300 mosmol (osmotic strength of 8.02 and 7.29 atm, respectively) (Stansfeld & Mathie, 1993) with use of an osmometer (Osmostat OM 6020, Menarini Diagnostics, Italy).
The chemicals used were: collagenase, BHA, DTBHA and nifedipine (Sigma Chimica, Italy). 2,2′-Dihydroxy-3,3′-di-t-butyl-5,5′-dimethoxydiphenyl (DIBHA), was synthesized by direct oxidation of BHA as described elsewhere (Sgaragli et al., 1980). DIBHA, BHA and DTBHA, dissolved directly in dimethylsulphoxide (DMSO), and nifedipine, dissolved in ethanol, were diluted at least 1000 times in the external solution, before use. The resulting concentrations of DMSO and ethanol (below 0.1%) did not alter the currents (data not shown). In order to limit both the amount of solvents in the external solution and to avoid any precipitation of the phenols, the maximum concentrations tested were 50 μM for BHA and DIBHA, and 30 μM for DTBHA, respectively. Final drug concentrations are stated in the text.
After control measurements, each cell was exposed to drugs by perfusing the experimental chamber with a drug-containing external solution.
Curve fitting and statistics
Acquisition and analysis of data were accomplished using SWT software (Shkodrov, 1995) and GraphPad Prism version 3.02 (GraphPad Software, San Diego, CA, U.S.A.). Data are reported as means±s.e.mean. Statistical analysis and determinations of significance with ANOVA (followed by Dunnett or Bonferroni post tests) and Student's t-test for paired or unpaired samples, as appropriate, were performed using GraphPad InStat version 3.02 (GraphPad Software). In all comparisons, P<0.05 was considered significant.
The current-voltage relationships were constructed using the peak values (leakage corrected) from the original traces of currents.
Results
Inhibitory action of BHA and DTBHA on ICa(L)
ICa of freshly dispersed guinea-pig gastric fundus smooth muscle cells induced by step depolarizations from Vh −50 or −80 mV were maximally activated within 5 – 10 ms after depolarization with peak activation occurring at about 10 mV, and slowly inactivated (Figure 1a,d). Threshold activation (occurring at about −30 mV), blockade by nifedipine and the time course of the current evoked at 10 mV (fitted by a mono-exponential function, time constant (τ): 71.7±8.4 ms at Vh −50 mV and 63.6±4.5 ms at Vh −80 mV, respectively; n=10) were not modified by changing the Vh from −50 to −80 mV. Based on their voltage dependence and their sensitivity to blockade by nifedipine (Figure 1c,f), these currents reflected the activity of ICa(L).
Figures 1 and 2 show the effects on current-voltage relationship of BHA and DTBHA. Both compounds significantly inhibited the peak inward current at all potentials in a concentration-dependent manner. This inhibition, however, was not accompanied by a significant shift of the current-voltage curve along the voltage axis. BHA and DTBHA, at the maximum concentrations tested (50 and 30 μM, respectively) inhibited ICa(L) evoked by depolarization step to 10 mV by 48.9 and 45.2%, respectively. Furthermore, BHA and DTBHA washout gave rise only to a partial recovery of the current (50 – 60%, data not shown).
ECa (62.7±1.9 mV, n=15; obtained from the extrapolated current-voltage curves in Figure 2) was not affected by BHA (62.1±2.0 mV, n=5) or DTBHA (60.9±3.2 mV, n=6), respectively.
DIBHA, the dimer of BHA, up to a final concentration of 50 μM, however, did not affect ICa(L) (data not shown).
Ca2+- and voltage-dependence of the inhibitory effect of BHA and DTBHA
To test the hypothesis that BHA and DTBHA may compete with Ca2+ within the channel pore, the dependence of their inhibition on extracellular Ca2+ concentration was examined. When the concentration of Ca2+ in the external solution was raised from 1 to 2.5 and 5 mM, the current-voltage relationships from Vh −50 and −80 mV were shifted to the right and maximum ICa(L) increased significantly (Figure 3a,b). BHA inhibition of ICa(L) evoked by depolarization step to 10 mV was inversely correlated to the external concentration of Ca2+, decreasing, in fact, from 76.3 to 44.9 and 24.0% at Vh −50 mV and from 53.5 to 24.5 and 10.9% at Vh −80 mV, respectively (Figure 3c,d). DTBHA inhibition however was reduced, although to a lesser extent, only at Vh −50 mV (inhibition decreased from 68.6 to 52.4 and 54.2%) but not at Vh −80 mV (46.7, 47.0 and 40.5% of inhibition at 1, 2.5 and 5 mM Ca2+, respectively) (Figure 3e,f). Furthermore, BHA and DTBHA antagonized the ICa(L) with potencies that depended upon the Vh. In fact, inhibition of the current at 10 mV evoked from Vh −80 mV was lower than that observed at Vh −50 mV, at the three Ca2+ concentrations used (Figure 3c – f and above).
Figure 3.
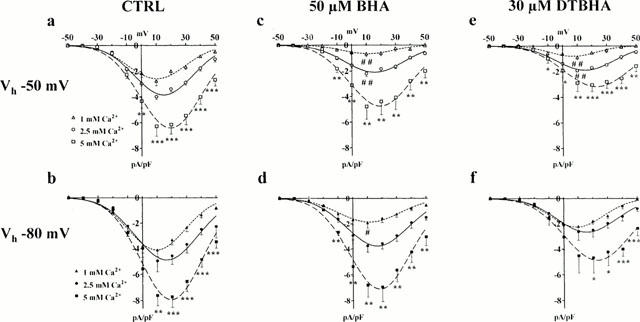
Ca2+- and voltage-dependence of BHA and DTBHA effect. Current-voltage relationships with various concentrations of Ca2+ obtained in the absence (CTRL) (a,b) or presence of 50 μM BHA (c,d) or 30 μM DTBHA (e,f). Gastric smooth muscle cells were voltage-clamped from a Vh of −50 mV (open symbols) or Vh of −80 mV (closed symbols) to different depolarizing steps ranging between −50 to +50 mV. Data points are means±s.e.mean derived from 3 – 12 cells. *P<0.05, **P<0.01, ***P<0.001 (ANOVA) refer to data points over the symbol; #P<0.05, ##P<0.01, indicate statistical significance of data point versus CTRL at the same Vh and Ca2+ concentration (Dunnett's post test).
Effects of BHA and DTBHA on the current decay
The effects of BHA and DTBHA on the time course of current decay were analysed. The current evoked at 10 mV from Vh −50 mV showed a τ of 69.3±5.7 ms (n=17). BHA and DTBHA did not significantly modify τ (75.7±13.0 ms, n=7 for BHA and 58.4±5.3 ms, n=10, for DTBHA, respectively).
Effect of BHA and DTBHA on steady-state inactivation and activation curves for ICa(L)
To elucidate the mechanism underlying BHA and DTBHA inhibition on ICa(L), their effect on the voltage-dependent inactivation of the Ca2+ channels was investigated. BHA and DTBHA significantly shifted the steady-state inactivation curve to more negative potentials (Figure 4). The 50% inactivation potentials, obtained from individual experiments and evaluated by Boltzmann fitting, were −31.58±0.95 (CTRL, n=10), −39.56±1.16 (BHA; n=4, P<0.01, Dunnett's post test) and −37.21±0.88 mV (DTBHA; n=7, P<0.01), respectively. The slope factor (−8.33±0.84 mV, CTRL) was not affected by BHA (−8.76±1.02 mV) on DTBHA (−8.67±0.77 mV).
Figure 4.
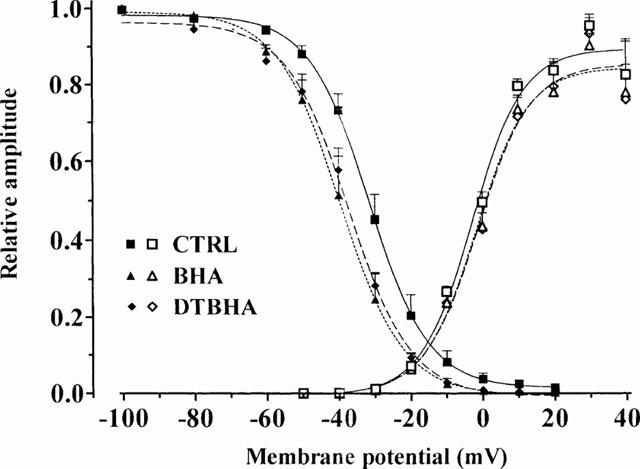
Effect of BHA and DTBHA on activation and inactivation curves. Steady-state inactivation curves (closed symbols), obtained in the absence (CTRL) and presence of 50 μM BHA and 30 μM DTBHA, were fitted by the Boltzman equation. Peak current values were used. The steady-state inactivation curve was obtained using the double-pulse protocol (see Methods section). Amplitude of the current evoked by each test pulse was normalized to maximum ICa(L). Activation curves (open symbols) were obtained from the current-voltage relationships of Figure 2 and fitted to the Boltzmann equation (see Methods section). Relative amplitude is plotted against membrane potential. Each point represents the mean±s.e.mean of 4 – 10 cells.
Activation curves, obtained from the current-voltage relationships of Figure 2 and fitted to the Boltzmann equation, are shown in Figure 4. The 50% activation potential as well as the slope factor obtained from individual experiments (−2.86±1.08 and 7.27±0.98 mV, CTRL; n=10) were not affected by either 50 μM BHA (−1.86±1.35 and 7.43±1.22 mV; n=5) on 30 μM DTBHA (−1.40±2.07 mV and 7.61±1.88 mV; n=6), respectively.
Discussion
The main finding of the present study is that both BHA and its analogue DTBHA inhibit ICa(L) recorded in single smooth muscle cells from guinea-pig gastric fundus. Inhibition of ICa(L) was concentration-related and characterized by a stabilizing effect on the inactivated state of the channel, but did not depend on alteration in permeability (channel selectivity) for Ca2+, since neither BHA nor DTBHA affected the extrapolated ECa.
In gastric fundus smooth muscle cells, current flowing through L-type Ca2+ channel is responsible for the nifedipine-sensitive tonic contractions (Boev et al., 1976). Therefore, the inhibitory action of BHA and DTBHA on L-type Ca2+ channels observed here, and possibly the activation of the sarcoplasmic reticulum Ca2+-ATPase (Fusi et al., 1999a), are likely to contribute to their already described myorelaxing action (Fusi et al., 1998b). Moreover, the decrease of Ca2+ channel activity induced by BHA cannot be ascribed to changes in the intracellular levels of cyclic AMP, as previously hypothesized (Fusi et al., 1998b). In the present experiments, in fact, patch pipettes were filled with 5 μM cyclic AMP. Thus, diffusion of cyclic AMP into the cytosol should guarantee the maintenance of high levels of this nucleotide, thereby buffering the cyclic AMP changes promoted by BHA (Fusi et al., 1998b). Thus, the inhibitory effect of BHA on the ICa(L) under these conditions is not mediated by changes in cyclic AMP, but rather might be the consequence of a direct action on the channel components. To validate this hypothesis, however, more direct studies such as in excized patches are required. Consequently, it is suggested that L-type Ca2+ channel inhibition by BHA contributes to the muscle relaxation elicited by this drug, in addition to other possible cellular effects (i.e. increase in cyclic AMP tissue levels).
The inhibition induced by both BHA and DTBHA on ICa(L), although with a much lower potency, share several basic features which characterize the actions of Ca2+ channel-blockers such as nifedipine and flunarizine (Kuga et al., 1990). First, the extent of the inhibition of ICa(L) by BHA and, to a lesser extent DTBHA, was inversely correlated to the external concentration of Ca2+ (see Karaki, 1987). Second, this inhibition depended on membrane potential (Bean, 1984; Kuriyama et al., 1995). This result can be understood/explained by postulating that more positive voltages favour block, and blocked channels can be restored to the available pool by hyperpolarization that permits drug release from the weakly binding closed-state (McDonald et al., 1994). This voltage dependence of both BHA and DTBHA blockade might be of value, since low concentrations of these drugs could potently block Ca2+ entry into cells that are depolarized (perhaps during anoxia or as a consequence of the hypoxic damage) leaving unaffected the cells with normal, i.e. more negative resting potentials (Bean, 1989). Third, both drugs shifted the steady-state inactivation curve to more negative potentials, leaving unaltered the sigmoid shape of the curves. These data indicate that the effect of BHA and DTBHA is voltage dependent, i.e. is more prominent when the membrane is held at more depolarized potentials, suggesting that drugs may bind more strongly to the inactivated state than to the closed/resting state of L-type Ca2+ channels (see Bean, 1984).
Sarcolemma thiol redox state may be an important determinant of ICa(L) activity since sulfhydryl groups in cysteine residues on L-type Ca2+ channel can undergo redox modulation and in so doing alter channel function. However, analysis of the modulation by reducing and oxidizing agents on both native and recombinant L-type Ca2+ channels has revealed contrasting effects. In fact, inhibition of whole-cell ICa of recombinant human cardiac L-type α1C subunits caused by oxidizing agents (Fearon et al., 1999) appears to be significantly different from up-regulation of ICa(L) in ferret ventricular myocytes caused by thiol oxidation of a putative redox switch (Campbell et al., 1996). The structure of BHA and DTBHA make them both lipophilic (i.e. membrane permeable) and potent antioxidants as well as radical scavengers (Sgaragli et al., 1993b). Also DIBHA, the dimer of BHA, is a lipophilic antioxidant as potent and hydrophobic as BHA; however, in our experimental model it did not influence ICa(L). Thus, it appears that BHA and DTBHA do not influence ICa(L) by virtue of their antioxidant and radical scavenging activity (on the membrane surface and/or from within the membrane) or by partitioning into lipid bilayers (Sgaragli et al., 1977), but rather through a direct effect on the Ca2+ channel.
Many antioxidant, natural products have been shown to elicit diverse pharmacological responses of some therapeutic relevance. Recently, some synthetic antioxidants such as BHA have been reported to activate, at 50 – 100 μM concentrations, signal transduction pathways (e.g. the mitogen-activated PKs) which may lead to the expression of genes for cell protection and/or survival mechanisms (for a review, see Kong et al., 2000). These observations, together with the data presented here, make BHA a prototype molecule of value for the development of new drugs which protect the cell against damages, such as those due to oxidative stress following ischaemia-reperfusion.
In conclusion, this is the first direct demonstration that BHA, as well as its analogue DTBHA, inhibit ICa(L) in single isolated gastric smooth muscle cells. This finding, together with the observational data on gastric muscle strip contractions, provides further insight on the myorelaxing properties of BHA.
Acknowledgments
We wish to thank Dr Lara Vittori for the assistance to some experiments and Dr Luciana Volpi and Prof Alberto Auteri (Istituto di Semeiotica Medica, Facoltà di Medicina e Chirurgia, Universitá degli Studi di Siena) for measurement of solutions osmolarity. This work was supported by MURST Cofin'98 and a grant from Ministero degli Affari Esteri (Rome, Italy) under Law 212 (26-2-1992).
Abbreviations
- BHA
3-t-butyl-4-hydroxyanisole
- DIBHA
2,2′-dihydroxy-3,3′-di-t-butyl-5,5′-dimethoxydiphenyl
- DMSO
dimethylsulphoxide
- DTBHA
3,5-di-t-butyl-4-hydroxyanisole
- ICa(L)
L-type Ca2+ current
- KB
Kraftbrüne
- TEA
tetraethylammonium
- Vh
holding potential
References
- BEAN B.P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc. Natl. Acad. Sci. U.S.A. 1984;81:6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAN B.P. Classes of calcium channels in vertebrate cells. Annu. Rev. Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- BEAN B.P., STUREK M., PUGA A., HERMSMEYER K. Calcium channels in muscle cells isolated from rat mesenteric arteries: modulation by dihydropyridine drugs. Circ. Res. 1986;59:229–235. doi: 10.1161/01.res.59.2.229. [DOI] [PubMed] [Google Scholar]
- BIELEFELDT K., WHITEIS C.A., SHARMA R.V., ABBOUD F.M., CONKLIN J.L. Reactive oxygen species and calcium homeostasis in cultured human intestinal smooth muscle cells. Am. J. Physiol. 1997;272:G1439–G1450. doi: 10.1152/ajpgi.1997.272.6.G1439. [DOI] [PubMed] [Google Scholar]
- BOEV K., GOLENHOFEN K., LUKANOW J.Selective suppression of phasic and tonic activation mechanisms in stomach smooth muscle Physiology of smooth muscle 1976New York: Raven Press; 202–208.ed. Bülbring, E. & Shuba, M.F. pp [Google Scholar]
- CAMPBELL D.L., STAMLER J.S., STRAUSS H.C. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J. Gen. Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALTON T.P., SHERTZER H.G., PUGA A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- FAY F.S., DELISE C.M. Contraction of isolated smooth muscle cells - Structural changes. Proc. Nat. Acad. Sci. U.S.A. 1973;70:641–645. doi: 10.1073/pnas.70.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEARON I.M., PALMER A.C.V., BALMFORTH A.J., BALL S.G., VARADI G., PEERS C. Modulation of recombinant human cardiac L-type Ca2+ channel α1c subunits by redox agents and hypoxia. J. Physiol. 1999;514:629–637. doi: 10.1111/j.1469-7793.1999.629ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUSI F., GORELLI B., VALOTI M., MARAZOVA K., SGARAGLI G.P. Effects of 2,5-di-t-butyl-1,4-benzohydroquinone (BHQ) on rat aorta smooth muscle. Eur. J. Pharmacol. 1998a;346:237–243. doi: 10.1016/s0014-2999(98)00056-9. [DOI] [PubMed] [Google Scholar]
- FUSI F., MARAZOVA K., PESSINA F., GORELLI B., VALOTI M., FROSINI M., SGARAGLI G.P. On the mechanisms of the antispasmodic action of some hindered phenols in rat aorta rings. Eur. J. Pharmacol. 2000;394:109–115. doi: 10.1016/s0014-2999(00)00152-7. [DOI] [PubMed] [Google Scholar]
- FUSI F., TZANKOVA V., GALGANI F., VALOTI M., SGARAGLI G.P. Effect of 3,5-di-t-butyl-4-hydroxyanisole (DTBHA) on sarcoplasmic reticulum Ca2+-ATPase of rat skeletal muscle. Pharmacol. Res. 1999a;39 suppl.:26. doi: 10.1016/s0006-2952(01)00794-8. [DOI] [PubMed] [Google Scholar]
- FUSI F., VALOTI M., FROSINI F., SGARAGLI G.P. 2,5-Di-t-butyl-1,4-benzohydroquinone induces endothelium-dependent relaxation of rat thoracic aorta. Eur. J. Pharmacol. 1999b;366:181–187. doi: 10.1016/s0014-2999(98)00932-7. [DOI] [PubMed] [Google Scholar]
- FUSI F., VALOTI M., PETKOV G., BOEV K.K., SGARAGLI G.P. Myorelaxant activity of 2-t-butyl-4-methoxyphenol (BHA) in guinea pig gastric fundus. Eur. J. Pharmacol. 1998b;360:43–50. doi: 10.1016/s0014-2999(98)00660-8. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., KLOCKNER U. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium”. Pflügers Arch. 1982;395:6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- JABS T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- KARAKI H. Use of tension measurements to delineate the mode of action of vasodilators. J. Pharmacol. Methods. 1987;18:1–21. doi: 10.1016/0160-5402(87)90013-1. [DOI] [PubMed] [Google Scholar]
- KONG A.-N.T., YU R., CHEN C., MANDLEKAR S., PRIMIANO T. Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch. Pharm. Res. 2000;23:1–16. doi: 10.1007/BF02976458. [DOI] [PubMed] [Google Scholar]
- KRIPPEIT-DREWS P., HABERLAND C., FINGERLE J., DREWS G., LANG F. Effects of H2O2 on membrane potential and [Ca2+]i of cultured rat arterial smooth muscle cells. Biochem. Biophys. Res. Commun. 1995;209:139–145. doi: 10.1006/bbrc.1995.1481. [DOI] [PubMed] [Google Scholar]
- KUGA T., SADOSHIMA J., TOMOIKE H., KANAIDE H., AKAIKE N., NAKAMURA M. Actions of Ca2+ antagonists on two types of Ca2+ channels in rat aorta smooth muscle cells in primary culture. Circul. Res. 1990;67:469–480. doi: 10.1161/01.res.67.2.469. [DOI] [PubMed] [Google Scholar]
- KUKREJA R.C., HESS M.L. The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc. Res. 1992;26:641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., NABATA H. Pharmacological and physiological significance of ion channels and factors that modulate them in vascular tissue. Pharmacol. Rev. 1995;47:387–573. [PubMed] [Google Scholar]
- LAMMEL E., DEITMER P., NOACK T. Suppression of steady membrane currents by acetylcholine in single smooth muscle cells of the guinea-pig gastric fundus. J. Physiol. 1991;432:259–282. doi: 10.1113/jphysiol.1991.sp018384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDONALD T.F., PELZER S., TRAUTWEIN W., PELZER D.J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- NAKAZAWA K., MATSUKI N., SHIGENOBU K., KASUYA Y. Contractile response and electrophysiological properties in enzymatically dispersed smooth muscle cells of rat vas deferens. Pflügers Arch. 1987;408:112–119. doi: 10.1007/BF00581338. [DOI] [PubMed] [Google Scholar]
- OPIE L. Myocardial stunning: a role for calcium antagonists during reperfusion. Cardiovasc. Res. 1992;26:20–24. doi: 10.1093/cvr/26.1.20. [DOI] [PubMed] [Google Scholar]
- PEERS C. Oxygen-sensitive ion channels. Trends Pharmacol. Sci. 1997;18:405–408. doi: 10.1016/s0165-6147(97)01120-6. [DOI] [PubMed] [Google Scholar]
- ROVERI A., COASSIN M., MAIORINO M., ZAMBURLINI A., VAN AMSTERDAM F.T., RATTI E., URSINI F. Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Arch. Biochem. Biophys. 1992;297:265–270. doi: 10.1016/0003-9861(92)90671-i. [DOI] [PubMed] [Google Scholar]
- SGARAGLI G.P., DELLA CORTE L., PULITI R., DE SARLO F., FRANCALANCI R., GUARNA A., DOLARA P., KOMARYNSKY M. Oxidation of 2-t-butyl-4-methoxyphenol (BHA) by horseradish and mammalian peroxidase systems. Biochem. Pharmacol. 1980;29:763–769. doi: 10.1016/0006-2952(80)90554-7. [DOI] [PubMed] [Google Scholar]
- SGARAGLI G.P., DELLA CORTE L., RIZZOTTI-CONTI M., GIOTTI A. Effects of monocyclic compounds on biomembranes. Biochem. Pharmacol. 1977;26:2145–2149. doi: 10.1016/0006-2952(77)90266-0. [DOI] [PubMed] [Google Scholar]
- SGARAGLI G.P., VALOTI M., FUSI F., PALMI M., MANTOVANI P., DE SANTI M.M., LORENZINI L., TOSI P. Toxic injury to rat gut musculature following intraperitoneal administration of 2-t-butyl-4-methoxyphenol. Eur. J. Pharmacol. 1993a;248:121–129. doi: 10.1016/0926-6917(93)90033-m. [DOI] [PubMed] [Google Scholar]
- SGARAGLI G.P., VALOTI M., GORELLI B., FUSI F., PALMI M., MANTOVANI P. Calcium antagonist and antiperoxidant properties of some hindered phenols. Br. J. Pharmacol. 1993b;110:369–377. doi: 10.1111/j.1476-5381.1993.tb13819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHKODROV G.B. Computer program for performing whole-cell voltage-clamp experiments. Comput. Methods Programs Biomed. 1995;48:241–246. doi: 10.1016/0169-2607(95)01696-1. [DOI] [PubMed] [Google Scholar]
- STANSFELD C., MATHIE A.Recording membrane currents of peripheral neurones in short-term culture Electrophysiology. A practical approach 1993Oxford: IRL Press; 3–28.ed. Wallis, D.I. pp [Google Scholar]
- VINER R.I., HUHMER A.F., BIGELOW D.J., SCHONEICH C. The oxidative inactivation of sarcoplasmic reticulum Ca(2+)-ATPase by peroxynitrite. Free Radic. Res. 1996;24:243–259. doi: 10.3109/10715769609088022. [DOI] [PubMed] [Google Scholar]


