Abstract
In normal mice, the distribution of adrenergic, cholinergic, some peptidergic, and neuronal nitric oxide synthase (nNOS)-containing nerves were investigated. Functional in vitro correlates were obtained. An in vivo model was developed in which erectile haemodynamics in response to drugs or nerve-stimulation were studied.
Immunoreactivities for vesicular acetylcholine transporter protein (VAChT), nNOS-, and vasoactive intestinal polypeptide (VIP), co-existed in nerve fibres and terminal varicosities. Immunoreactivities for neuropeptide Y (NPY) and tyrosine hydroxylase (TH) were found in the same nerve structures.
Chemical sympathectomy abolished TH- and NPY-IR nerve structures in cavernous smooth muscle bundles. The distribution of calcitonin gene-related peptide (CGRP)-, nNOS-, VAChT- and VIP-IR nerve structures was unchanged.
In endothelial cells of the central and helicine arteries, veins and venules, intense immunoreactivity for endothelial NOS (eNOS) was observed. No distinct eNOS-IR cells were found lining the cavernous sinusoids.
In vitro, nerve-induced relaxations were verified, and endothelial NO/cyclic GMP-mediated relaxant responses were established. VIP and CGRP had small relaxant effects. A functioning adenylate cyclase/cyclic AMP pathway was confirmed.
Neuronal excitatory responses were abolished by prazosin, or forskolin. VIP and CGRP counteracted contractions, whereas NPY and scopolamine enhanced excitatory responses.
In vivo, erectile responses were significantly attenuated by L-NAME (50 mg kg−1) and facilitated by sildenafil (200 μg kg−1).
It is concluded that the mouse is a suitable model for studies of erectile mechanisms in vitro and in vivo.
Keywords: Penile erection, nitric oxide, cyclic GMP, cholinergic, peptides, nerves, relaxation, sildenafil, in vivo, mouse
Introduction
Penile erection is regulated by coordinated activities in the central and peripheral nervous systems (Andersson & Wagner, 1995). In humans and many animals, the erectile response has been shown to be dependent on nitric oxide (NO), released from nerves, but also from vascular endothelium. In mice, the NO-producing enzyme, NO-synthase (NOS), has been located to the dorsal penile nerve and its branches in the mouse penis (Burnett et al., 1996) as well as in intrinsic nerves of the erectile tissue (Hedlund et al., 2000a). Upon release from nerves, NO activates soluble guanylyl cyclase (sGC) in adjacent smooth muscle cells, resulting in an increased production of cyclic GMP, and cyclic GMP conveys signals by modification of ion channels, phosphodiesterases, and protein kinases (Lincoln & Cornwell, 1993). In mouse corpus cavernosum (CC) smooth muscle, cyclic GMP-dependent protein kinase I (cyclic GKI) has been shown to be of major importance for erectile function (Hedlund et al., 2000a). However, other signalling pathways have also been implicated in smooth muscle relaxant functions in the erectile tissue (Andersson & Wagner, 1995). Immunoreactivity for the vesicular acetylcholine transporter protein (VAChT) has been demonstrated in nerve terminals exhibiting close relations to tyrosine hydroxylase (TH)-immunoreactive (-IR) nerve fibres, an arrangement favouring a cholinergic modification of the release of noradrenaline (NA) from adjacent adrenergic nerves. Muscarinic receptor-mediated release of relaxant factors from vascular endothelium has been proposed as a cholinergic proerectile effect (Andersson & Wagner, 1995). Vasoactive intestinal polypeptide (VIP)-IR nerve fibres have been demonstrated in CC tissue, and it is believed that VIP, by receptor-mediated activation of the adenylyl cyclase/cyclic AMP system, relaxes isolated CC tissue, and is able to induce erectile responses in vivo (Andersson & Wagner, 1995). NOS-IR nerves have been shown also to contain immunoreactivity for VIP and VAChT, and these nerves have been suggested to represent a distinct population of penile parasympathetic cholinergic nerves (Hedlund et al., 1999; 2000b).
The development of genetically modified animals, for which the mouse is the species of choice, is receiving attention in most fields of research. The use of such mice will most probably be a valuable additional tool for the study of molecular mechanisms involved in erectile function and various forms of dysfunction. NO/cyclic GMP-mediated inhibitory responses have been shown to be absent in CC tissue from cyclic GKI deficient mice, and a preserved cyclic AMP signalling pathway did not compensate for the erectile dysfunction or the reduced fecundity in these animals (Hedlund et al., 2000a). In mice lacking neuronal NOS (nNOS), the NO-producing enzyme, a compensatory increase in the endothelial isoform of NOS was suggested to account for a preserved mating behaviour with erections (Burnett et al., 1996).
Mouse erectile tissue has been used previously for in vitro studies of erectile mechanisms (Burnett et al., 1996; Gocmen et al., 1997; 1998). Monitoring of intracavernous pressure in the mouse in vivo has been described by Sezen & Burnett (2000). However, to the best of our knowledge, a thorough characterization of the erectile tissue from mice has not been made. The aim of the present study was therefore to describe the innervation patterns of adrenergic, cholinergic, some peptidergic, and NOS-containing nerves, and to present functional correlates in an isolated CC preparation from normal mice. In addition, we present a model in which erectile haemodynamics, in response to drugs or nerve-stimulation, were studied in vivo by intracavernous pressure (ICP) measurements.
Methods
Animals
Ninety-five adult male NMRI mice (Möllegård, Denmark) were used. Prior to experiments, the mice were kept and cared for in standard cages under clean conditions in separate quarters in a 12 – 12 h light – darkness cycle with free access to water and pellets. The experiments performed were approved by the Animal Ethics Committee of Lund University.
Morphological studies
Chemical sympathectomy
As previously described for the rat (Hedlund et al., 1999), chemical sympathectomy was performed in six mice which were given two doses (100 mg.kg−1) of 6-hydroxydopamine (6-OHDA; Sigma Chemical Co, St Louis, MO, U.S.A.).
Tissue handling
For immunocytochemistry (see below), 20 mice were anaesthetized and perfusion-fixed as previously described (Hedlund et al., 1999). The mouse penis (Figure 1) was removed by cutting the crura of the CC at the point of adhesion to the lower pubic bone, and the CC were then dissected free and immersion-fixed, and further prepared for immunohistochemical staining (Hedlund et al., 1999).
Figure 1.
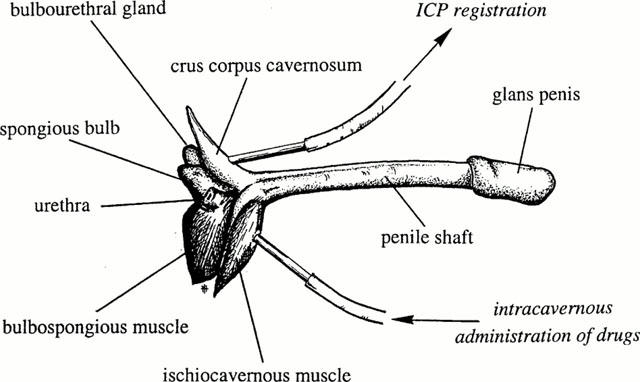
The mouse penis and technical landmarks for the registration of intracavernous pressure in vivo.
Immunocytochemistry
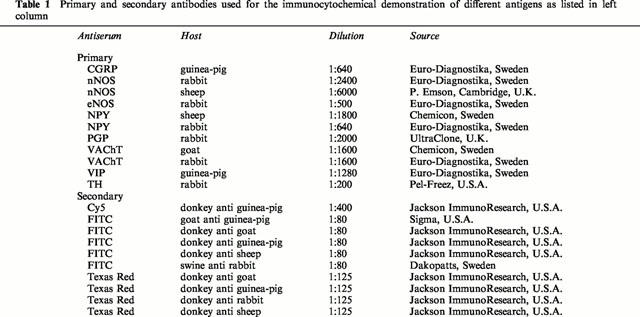
Cryostat sections (8 μm) were further processed and subsequently incubated (+4°C, 24 h) with rabbit antisera against protein gene product 9.5 (PGP), VAChT, nNOS, endothelial NOS (eNOS), neuropeptide Y (NPY), or TH, or sheep antisera to nNOS or NPY, or guinea-pig antisera to VIP- or calcitonin gene-related peptide (CGRP), or goat antiserum to VAChT. The main protocol followed was the the same as described previously (Hedlund et al., 1999).
For the simultaneous demonstration of three antigens, some sections were incubated with rabbit antiserum to VAChT, rinsed, and incubated with sheep antiserum to nNOS, rinsed, and incubated with antisera raised in guinea-pig to VIP. After rinsing, the sections were incubated with FITC-conjugated donkey anti rabbit IgG, rinsed and incubated with TR-conjugated donkey anti sheep IgG, rinsed and incubated with Cy5-conjugated donkey anti guinea-pig IgG.
All antisera (primary and secondary) used are listed in Table 1. Control experiments and the characteristics of the respective primary antibodies have been previously described (Hedlund et al., 1999). As cross reactions to antigens sharing similar amino acid sequences cannot be completely excluded, the structures demonstrated are referred to as CGRP-, nNOS-, NPY-, PGP-, TH-, VAChT-, and VIP-IR (immunoreactive).
Table 1.
Primary and secondary antibodies used for the immunocytochemical demonstration of different antigens as listed in left column
The immunoreactive structures were evaluated as described previously (Hedlund et al., 1999). In double and triple immunolabelled sections, when three or more varicosities with intervening fibre segments showed similar patterns when examined in the two fluorophores, the corresponding nerve terminals were regarded as displaying coinciding profiles.
Confocal microscopy
Sections were inspected by confocal microscopy using an MRC-1024 laser scanning confocal equipment (Bio-Rad, Hemel Hempstead, U.K.) attached to an Eclipse E800 upright microscope (Nikon, Tokyo, Japan). Fluorochromes were illuminated with three different wavelengths of light (488 nm, 568 nm and 647 nm) and emitted light was detected in three separate photo multiplier tubes after filtration through a 605±32 nm (red light), a 522±32 nm (green light), and a 680±32 nm (far red light) bandpass filter.
Functional in vitro studies
Isometric tension measurements
For the functional experiments, 19 mice were sacrificed by carbon monoxide asphyxia followed by exsanguination. The penises were immediately taken out as described above and placed in chilled Krebs solution (for composition see below). As previously described for the rat (Hedlund et al., 1999), the tunica albuginea was carefully opened from its proximal extremity of the CC towards the penile shaft and the erectile tissue within the CC was microsurgically dissected free. One crural strip preparation (0.3×0.3×3 mm) was obtained from each CC. All preparations were used immediately after removal.
The procedures for mounting, of the preparations and recording of isometric tension and electrical field stimulation (EFS) were the same as described previously (Hedlund et al., 1999).
Experimental procedure
During an equilibration period of 40 min, tension was adjusted until a mean stable tension of 1.37±0.08 mN (n=34, N=19) was obtained. In order to verify the contractile ability of the preparations, a K+ solution (124 mM) was added to the organ baths at the end of the equilibration period. The mean contractile response amounted to 1.23±0.23 mN (n=34, N=19). Concentration-response curves for NA were performed in control animals (n=6, N=6). The agonist concentration used (3×10−6 M) corresponded to the approximate EC70 value, and produced stable and reproducible contractions. The effects of NO, carbachol, VIP, NPY, CGRP and forskolin were investigated in NA-contracted preparations.
Frequency-response relationships were investigated at supramaximum voltage in all preparations stimulated electrically, and the effects of prazosin, VIP, NPY, CGRP, and forskolin on electrically-induced excitatory responses were studied. A preparation was regarded as stable when the amplitude of three consecutive electrically-induced contractions did not differ by more than 5%. The investigated drugs were then added cumulatively. The degree of inhibition was expressed as a percentage of the contraction elicited prior to the addition of the lowest concentration of the drugs. Inhibitory responses to transmural stimulation of nerves were investigated in NA-activated preparations. The degree of relaxation was expressed as percentage of the NA-induced contraction. Some preparations were pretreated for 20 min with the NO synthesis inhibitor NG-nitro-L-arginine (L-NOARG; 10−4 M), or the guanylyl cyclase inhibitor, ODQ (10−6 M). Effects of EFS were then investigated as described above.
Experimental in vivo procedure
Fifty-five mice were anaesthetized with pentobarbital sodium (50 mg kg−1, Sigma Chemical Co, St Louis, MO, U.S.A.) and ketamine (Ketalar®, Parke Davis, Barcelona, Spain; 10 mg kg−1) given intraperitoneally (i.p.). During the experiment, the mice breathed spontaneously and were placed on a thermally isolating blanket. Body temperature and circulatory volume were kept optimal by frequent i.p. administration of body-warm saline, which prior to nerve stimulation was removed. Through a lower midline abdominal incision, access was given to the femoral artery and the pelvic viscera. A stretched heparinized (100 IE ml−1) polyethylene catheter (Clay Adams PE-10, Parsippany, NJ, U.S.A.) was introduced into the femoral artery. With a midline incision in the perineum, the base of the penis, enclosed by the striated bulbospongious and ischiocavernous muscles, was made visible. By blunt dissection, the ischiocavernous muscle covering the CC was divided on one side, and entrance to the underlying tunica albuginea of the crus of the CC was given. A 27 gauge needle attached to a heparinized (100 IE.ml−1) polyethylene catheter, was inserted into the crus of the CC. In some of the mice, another 27 gauge needle was placed into the other crus for administration of drugs (Figure 1). Continuous direct measurements of mean arterial and ICP were performed with pressure transducers (OHMEDA, Model P23 XL-1, Singapore) and registered by a Grass Polygraph 7E (Grass Instrument Co, MA, U.S.A.).
By moving intestine, seminal vesicles and bladder aside, the cavernous nerve, situated on the lower lateral portion of the prostate, was visualized. Electrical stimulation of nerves was performed with a slender bipolar platinum contact electrode. Square wave pulses were delivered with a duration of 1.0 ms by a Grass S48 stimulator (Grass Instrument Co, MA, U.S.A.). Each stimulation had a duration of 60 s. Prior to the next stimulation, a resting interval of 15 min was allowed. Frequency-response relationships were determined at different voltage amplitudes. In some animals, sildenafil, L-nitro-arginine-methyl-ester (L-NAME), or forskolin were administered systemically or into the CC. Submaximal voltage, producing approximately 70% of maximal ICP, were used when investigating the effects of drugs on nerve-induced erectile responses. Before stimulation, basal ICP (BICP) was noted. During tumescence, the peak ICP (PICP) and time (T80) for the ICP to reach 80% of maximal increase (PICP-BICP) were recorded. At this point, the increase in ICP per second (ΔT80) was evaluated. After stimulation, during detumescence, the time (D20) and the rate (ΔD20) for ICP to decrease to 20% of maximal increase were determined (Figure 2). Circumcision was performed in some animals in order to evaluate penile lengthening and cuffing of the glans penis during erectile responses.
Figure 2.
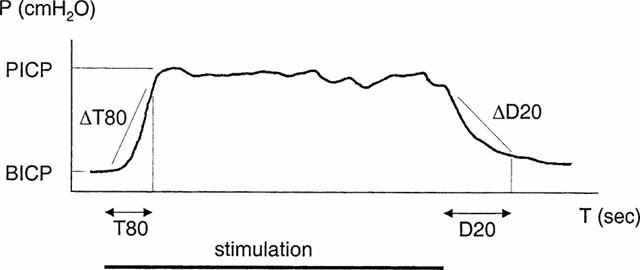
Typical curve showing the increase in intracavernous pressure (ICP) induced by nerve stimulation in vivo. During stimulation, the time for ICP to reach 80% of maximal increase (peak ICP – basal ICP) was recorded (T80). At this point, the increase per second (ΔT80) was evaluated. After stimulation, the time for and the rate by which a decrease to 20% of maximal pressure occurred (D20 and ΔD20) were determined.
Drugs and solutions
A saline solution, containing 154 mM NaCl, and a Krebs solution of the following composition were used (mM): NaCl 119, KCl 4.6, CaCl2 1.5, MgCl2 1.2, NaHCO3 15, NaH2PO4 1.2, glucose 5.5. A high K+ solution (124 mM) was used, in which the NaCl in the normal Krebs solution was replaced by equimolar amounts of KCl was used. The following drugs were used: noradrenaline (NA; Aldrich-Chemie GmbH & Co, Germany), carbachol (carbamylcholine chloride), VIP, NPY, CGRP, forskolin, L-NOARG, L-NAME (Sigma Chemical Co, St Louis, MO, U.S.A.), ODQ (1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one; Tocris Cookson, UK), sildenafil (Pfizer, Sandwich, U.K.). Stock solutions were prepared and then stored at −70°C and subsequent dilutions of the drugs were made with 0.9% NaCl (NA; with ascorbic acid added as an antioxidant). NO was freshly prepared at each experiment. An air-tight glass-beaker, containing 20 ml of distilled water, was deoxygenated for 1 h with helium gas. The beakers were then bubbled with medical NO gas (purity >99.5%) for 15 min until saturated solutions were obtained (3×10−3 M).
Calculations
Student's paired or unpaired two-tailed t-tests were used for statistical comparison of two means. A probability of P<0.05 was accepted as significant. ANOVA with Bonferroni correction was used for the comparison of multiple means. When appropriate, results are given as mean values±standard error of the mean (s.e.m.). Small n denotes the number of strip preparations, and capital N denotes the number of individuals. All statistical calculations were based on N.
Results
Morphological findings
Neuronal NOS-, and TH-IR coarse nerve trunks were uniformly distributed in the erectile tissue. No trunks expressing CGRP-, NPY-, VAChT- and VIP-immunoreactivity could be detected.
The smooth muscle septa, surrounding the cavernous spaces, were supplied with varicose terminals, which ran along the bundles of smooth muscle cells. TH-IR terminals were most numerous (large number), whereas nNOS-, NPY-, VAChT- and VIP-IR terminals were slightly less abundant (moderate number). In contrast, only few CGRP-IR terminals were found.
Arteries in the corpus cavernosum were surrounded by nNOS-, NPY-, TH-, VAChT- and VIP-IR varicose terminals, which formed dense plexuses in the adventitia of the vessels. CGRP-IR varicose terminals also formed similar plexuses, which were less dense than those formed by the other populations of nerve terminals. Generally, CGRP-IR nerve fibres exhibited different profiles than nerve fibres of the other investigated transmitters.
In double and triple stained sections, VAChT-, nNOS-, and VIP-IR nerve fibres and terminal varicosities showed identical coinciding profiles (Figure 3). With confocal laser scanning sectioning, co-existence of immunoreactivities for VAChT, nNOS, and VIP was observed in nerve fibres and varicosities (Figure 4), interspersed in trabecular smooth muscles of the erectile tissue and in the adventitial layers of vessels. TH- and NPY-IR nerve fibres also had coinciding profiles, and terminal varicosities were observed to contain immunoreactivities for TH and NPY (Figure 3). VAChT- and TH-, VAChT- and NPY-, VAChT- and CGRP-, and TH- and VIP- IR terminals sometimes displayed intimate and parallel profiles, although never coinciding.
Figure 3.
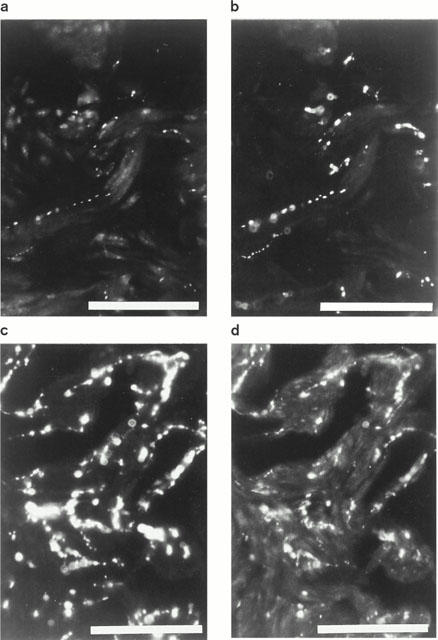
Mouse corpus cavernosum. Double immunohistochemistry. (a) Vesicular acetylcholine transporter (VAChT)-immunoreactive (IR) nerve terminals along smooth muscle bundles. FITC immunofluorescence. (b) Same section as in (a). NO synthase-IR nerve terminals displaying coinciding profiles with VAChT-IR varicosities. (c) Tyrosine hydroxylase (TH)-IR nerve terminals. FITC immunofluorescence. (d) Neuropeptide Y-IR nerve terminals exhibiting coinciding profiles with TH-IR nerve terminals. Same section as in (d). Texas red immunofluorescence. Bars=100 μm
Figure 4.
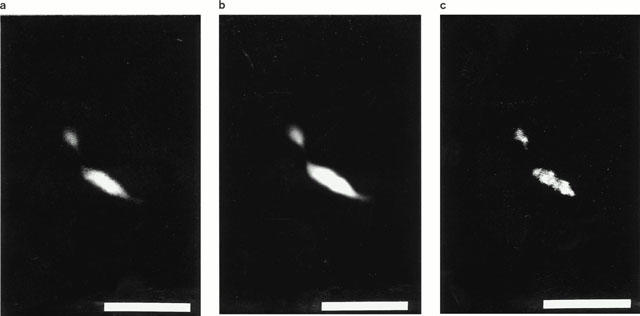
Mouse corpus cavernosum. Triple immunocytochemistry. Confocal microscopy. (a) Vesicular acetylcholine transporter – immunoreactive (IR) nerve terminals. FITC- immunofluorescence. (b) Same section as in (a). NO synthase-IR nerve varicosities. Texas red immunofluorescence. (c) Same section as in (a) and (b). Vasoactive intestinal polypeptide-containing varicosities. Cy-5 immunofluorescence. Each image consists of 19 consecutive sections with 0.30 μm between adjacent sections. Bars=10 μm.
Chemical sympathectomy abolished TH- and NPY-IR nerve structures in smooth muscle bundles of the trabecular erectile tissue. Single TH- and NPY-IR varicose terminals remained in the perivascular plexuses, and in nerve trunks, single TH-IR nerve fibres remained. In contrast, the number and distribution patterns of CGRP-, nNOS-, NPY-, VAChT- and VIP-IR nerve structures were unchanged compared to non-sympathectomized animals.
In endothelial cells, covering the lumen of the central and helicine arteries and veins and venules, intense immunoreactivity for eNOS was observed. Endothelial NOS-IR cells exhibited a non-stained central nucleus with surrounding immunoreactive cytoplasm, which extended sideways into thin cytoplasmic processes. No distinct eNOS-IR cells with central non-stained nuclei and cytoplasmic processes were found lining the cavernous sinusoids.
Functional in vitro studies
Relaxation of noradrenaline-contracted preparations
Spontaneous contractile activity was not observed in any of the mouse isolated CC preparations (n=34, N=19). Reproducible, contractile responses to NA, amounting to 0.52±0.04 mN (n=19, N=12) were obtained.
In NA-contracted preparations, VIP (n=6, N=6) and CGRP (n=5, N=5) had concentration-dependent small relaxant actions, and at 10−6 M, the highest peptide concentration used, mean relaxations of 22±3% and 32±2% was registered.
NO (10−6−10−4 M, n=7, N=7), produced concentration-dependent, rapid and almost complete (97±2%, pIC50 5.8±0.1) relaxations which were significantly counteracted by ODQ (10−6 M). Carbachol-induced (10−6 M) relaxations (54±4 %, n=12, N=12), were significantly attenuated by L-NOARG (10−4 M) or ODQ (10−6 M).
Forskolin, added cumulatively (10−9−10−6 M), produced concentration-dependent relaxations (pIC50 7.1±0.1), which amounted to 98±3% at the highest concentration used (n=7, N=7).
In NA-contracted preparations, EFS generated frequency-dependent and TTX-sensitive (n=9, N=9) relaxant responses. At any investigated frequency, pretreatment with 10−4 M L-NOARG (n=6, N=6), or 10−6 M ODQ (n=5, N=5) abolished the electrically-induced inhibitory activity. Inhibitory responses were unaffected by scopolamine.
Electrically-induced responses
Stimulation of preparations at baseline level produced frequency-dependent contractile responses (n=6, N=6), which were abolished by TTX 10−6 M. Prazosin (n=6, N=6) effectively counteracted the contractions produced by EFS, and at 10−7 M, the electrically-induced responses were abolished. The pIC50 value for prazosin amounted to 9.3±0.1. Forskolin also concentration-dependently inhibited contractile responses induced electrically (Figure 5), and at 10−6 M, contractions were not obtained. A pIC50 value of 7.5±0.1 was calculated for forskolin on EFS-induced contractions.
Figure 5.
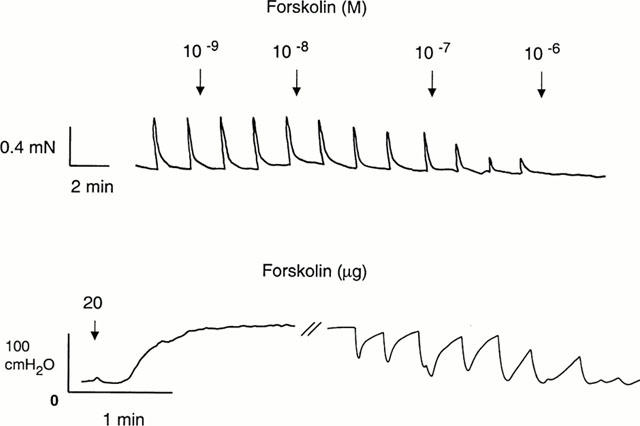
Tracing showing the effects of forskolin on electrical field stimulation-induced contractions in an isolated preparation of mouse corpus cavernosum (top) and on intracavernous pressure in an anaesthetized mouse after intracavernous administration (bottom).
Contractions induced by electrical stimulation were to a limited degree counteracted by VIP (n=6, N=6), and at 10−6 M a mean inhibitory effect of 36±6% was registered. CGRP (n=4, N=4) effectively counteracted EFS-induced contractions with a maximum inhibitory effect of 70±8% at 10−5 M, and with a pIC50 value of 7.7±0.8. NPY enhanced contractions produced by EFS; the effect amounted to 29±7% at 10−6 M (n=4, N=4). Scopolamine (10−11−10−6 M) also enhanced electrically-induced contractions, and at 10−6 M this effect amounted to 68±20% (n=4, N=4). Addition of prazosin, VIP, NPY, CGRP, forskolin or scopolamine did not affect baseline tension in the preparations.
In vivo studies
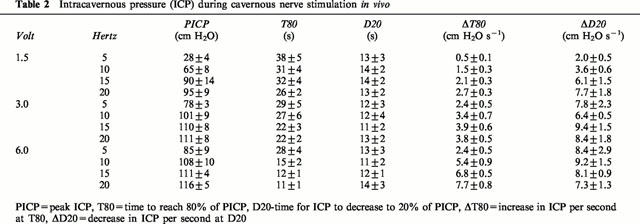
No spontaneous increases in ICP were registered. BICP was 13±1 cm H2O and mean arterial blood pressure 131±3 cm H2O (N=51). Stimulation of the cavernous nerve produced voltage- and frequency-dependent erectile responses, observed as penile tumescence, increases in shaft length of the penis, and engorgement of the glans penis, and simultaneously, reproducible increases in ICP were registered (Table 2). The highest values for PICP were recorded at 6 V and 20 Hz, and amounted to a mean value of 116±5 cm H2O (N=6). At these stimulation parameters, the fastest and most effective increases in ICP were also registered, and as defined by T80 and ΔT80, mean values of 11±1 s and 8±1 cm H2O s−1 were calculated. Corresponding values at e.g. 1.5 V and 5 Hz were 28±4 cm H2O, 38±5 s and 0.5±0.1 cm H2O s−1 (N=6; for PICP P<0.0001, for T80 P<0.001, and for ΔT80 P<0.0001, respectively). During detumescence, after assessment of cavernous nerve stimulation, small values for ΔD20 were observed at the lowest stimulation parameters (Table 2). No statistical differences were found in the values for D20 at any investigated frequency, and mean values were kept between 11 and 14 s for D20.
Table 2.
Intracavernous pressure (ICP) during cavernous nerve stimulation in vivo
At submaximal stimulation (3 V, 5 – 10 Hz), intraperitoneally administered L-NAME (N=5, 50 mg kg−1) effectively counteracted the nerve-induced increase in ICP (Figure 6), and PICP decreased from 74±11 cm H2O to 32±8 cm H2O (N=5; P<0.05). Upon stimulation, T80 and δT80 were not significantly altered by L-NAME, and during detumescence no differences in the D20 and ΔD20 values were registered. After intraperitoneal administration of 200 μg kg−1 of sildenafil (N=9, Figure 7), submaximal stimulation increased PICP from 68±6 cm H2O to 90±9 cm H2O (P<0.05). T80 and ΔT80 were not significantly affected by the drug, whereas D20 was significantly (P<0.05) prolonged from 11±1 s to 16±1 s. The value for ΔD20 was not significantly reduced by the drug. Sildenafil, at the investigated concentration, did not affect systemic blood pressure.
Figure 6.
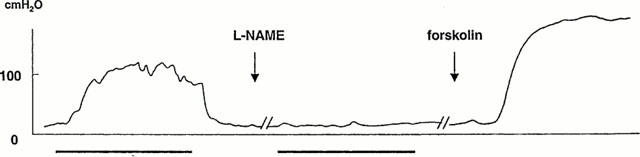
Intracavernous pressure (ICP) changes in the mouse corpus cavernosum in response to cavernous nerve stimulation in vivo. After pretreatment with intraperitoneally administered L-NAME (50 mg kg−1), the erectile response was abolished. The increase in ICP induced by forskolin (5 μg kg−1) was unaffected by L-NAME.
Figure 7.
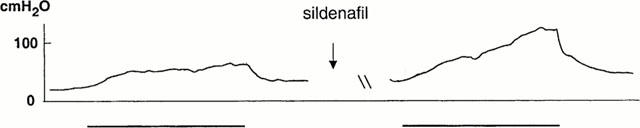
Intracavernous pressure (ICP) changes in the mouse corpus cavernosum in response to cavernous nerve stimulation (3 V, 10 Hz) in vivo. Facilitatory effect of sildenafil (200 μg kg−1).
Intracavernous administration of forskolin (0.2, 2, 20 μg; N=5 – 6 at each concentration) produced concentration-dependent increases in ICP (Figure 5), with the highest PICP of 127±9 cm H2O at 20 μg. The duration of the ICP increase was 4±1, 6±1, and 21±6 min for 0.2, 2, and 20 μg forskolin, respectively. Forskolin did not affect systemic blood pressure. The response to forskolin (5 μg) was not changed after blockade of NO synthesis by L-NAME (50 mg kg−1, N=5; Figure 6).
Discussion
Most neurons originating from the pelvic plexus and supplying the rat penile erectile tissue, have been shown to contain immunoreactivities for NOS and VIP, and are presumed to be cholinergic due to positive acetylcholinesterase staining. VAChT is found in nerves containing acetylcholine, and is considered a special marker for nerves with cholinergic characteristics. In the mouse CC, VAChT-, VIP-, and nNOS-IR nerves were found in moderate numbers, and by double immunolabelling, VAChT- and nNOS-IR, VAChT- and VIP-IR, and nNOS- and VIP-IR nerve fibres showed coinciding profiles. In addition, varicosities with immunoreactivities for VAChT, nNOS, and VIP exhibited identical and overlapping patterns when examined in double filter settings, and co-existence was verified by 3D reconstruction after microscopical confocal laser scanning sectioning. These findings confirm observations made in the CC from rat and cyclic GKI-deficient mice (Hedlund et al., 1999; 2000a), and support that NOS-containing nerves are cholinergic. Immunoreactivity for TH was not found in VAChT-, nNOS-, or VIP-IR nerves, but was observed in close relation to the latter structures. Chemical sympathectomy virtually abolished all TH- and NPY-IR nerve structures. NPY was reported to be present in subpopulations of sympathetic adrenergic nerves, and upon release from these nerves, to produce receptor-mediated vasoconstriction (Lundberg, 1996). Thus, as described for the rat CC, an arrangement of separate sympathetic adrenergic and parasympathetic cholinergic nerves seems likely for the innervation of the mouse CC. In both species, the observed close relation, with similar distribution patterns, between VAChT/NOS/VIP-IR and TH-IR nerves favours a suggested muscarinic modulation of the release of NA from adrenergic nerves (Hedlund et al., 1984). In further support of this mechanism in the mouse CC, scopolamine, an inhibitor of muscarinic receptor-mediated effects, significantly enhanced EFS-induced contractions, whereas the drug had no effect on base-line tension, NA-induced contractions, or inhibitory neurotransmission in isolated preparations.
Penile erection is dependent on cavernous smooth muscle relaxation, and it has been shown that NO, released from nerves, is the major relaxant agent in several mammals (Andersson & Wagner, 1995). This has also been proposed for mouse erectile tissues (Burnett et al., 1996; Gocmen et al., 1997; 1998; Hedlund et al, 2000a). With the presently developed isolated precontracted CC preparation, nerve-mediated, frequency-dependent, L-NOARG- and ODQ-sensitive relaxations were confirmed, and this inhibitory neurotransmission was found to be similar to responses described for other species, as well as humans (Holmquist et al., 1992; Hedlund et al., 1995; 1999).
During continuous monitoring of systemic blood pressure, subsystolic erectile responses in vivo were recorded as reproducible voltage- and frequency-dependent increases in ICP during stimulation of the cavernous nerve. These responses were also observed as penile engorgement and swelling of the glans penis. The presently observed values for BICP, filling and emptying rates, and the PICP, qualitatively correspond well with findings in rat, rabbit, dog and primates (Lue et al., 1983; Junemann et al., 1989; Holmquist et al., 1991; Martinez-Pineiro et al., 1994; Calabro et al., 1996). In mice, one study has demonstrated measurements of ICP before and during nerve-induced erectile responses (Sezen & Burnett, 2000). In this previous study, the mean BICP corresponded with the findings in our investigation, but the maximal PICP values, obtained in our experimental set-up, without activation of somatic nerve structures, were roughly 100% higher. This difference is noteworthy. Since the same anaesthetic agent was used during similar experimetal procedures, other methodological differences may exist between the studies. In the present study, erections were attenuated, and registered ICP changes were significantly counteracted by intraperitoneal administration of L-NAME. Systemically given sildenafil facilitated tumescence and prolonged detumescence. This was an expected effect of the drug, and is in accordance with findings in vivo by other investigations (Boolell et al., 1996; Carter et al., 1998; Andersson et al., 1999). Taken together, the present study verify that in the mouse, the NO/cyclic GMP pathway is of main importance for penile erectile responses in vitro and in vivo.
In functional experiments, VIP affected NA- or EFS-induced contractile activities to a minor extent, did not influence the base-line tension in isolated preparations. A direct smooth muscle regulatory function in the mouse penis for the peptide therefore seems unlikely. Still, a functioning adenylyl cyclase (AC)/cyclic AMP pathway in vitro has been suggested for mice (Hedlund et al., 2000a). This was confirmed in the present study, and was seen as pronounced relaxant and inhibitory effects of forskolin. When administered intracavernously, forskolin caused concentration-dependent and reproducible increases in the ICP in vivo, even in the presence of NO synthase inhibition. In mice with null mutations for the gene encoding for cyclic GKI, a preserved AC/cyclic AMP system did not compensate for the erectile dysfunction observed in these animals (Hedlund et al., 2000a), and at present, it is unclear if an endogenous transmitter, that may act with cyclic AMP as second messenger, can initiate penile erection.
In the flaccid state, it is generally accepted that CC smooth muscle is kept contracted mainly by NA acting on postjunctional α-adrenoceptors (Andersson & Wagner, 1995). This also seems to be the case in the mouse. Confirming findings in cyclic GKI-deficient mice (Hedlund et al., 2000a), large amounts of TH-IR nerve structures were observed in the erectile tissue from normal mice. In non-activated isolated mouse CC preparations, frequency-dependent and α-adrenoceptor-mediated contractions were produced in response to transmural stimulation of nerves. The presence of moderate numbers of NPY-IR nerve fibres, and a 30% potentiation of the EFS-induced contractile activity, suggests a possible function for the peptide in the CC, preferably during detumescence, as previously proposed (Kirkeby et al., 1991, Giuliano et al., 1993).
Cholinergic agents are shown to release NO from vascular endothelium (Furchgott & Zawadzki, 1980), a finding also described for erectile tissue (Ignarro et al., 1987; Palmer et al., 1987). It is, however, disputed to what extent the endothelium contributes with NO-mediated effects during erection, and differences between species appears to be present. In the rat, often used for the study of penile erection in vivo, NADPH diaphorase staining has been observed in parts of the sinusoidal endothelium (Keast, 1992). Using immunohistochemistry, later investigators found eNOS-IR endothelial cells to be confined to the penile arteries (Schirar et al., 1994; Dail et al., 1995; Hedlund et al., 1999), and functional in vitro correlates suggested that endothelial NO was of minor importance for erectile responses in the rat (Hedlund et al., 1999). In the presently isolated mouse CC, carbachol produced a 50% reduction in the NA-induced contraction, and this relaxation was almost abolished by L-NOARG or ODQ. These findings correspond well with functional results obtained in cavernous tissue from humans and other mammals (Andersson & Wagner, 1995), and suggest that the endothelium in the mouse CC may provide NO during penile erection. However, it cannot be excluded that an additional release of NO may occur, due to carbachol acting on nicotinic receptors on cholinergic ganglion cells in the preparation.
Endothelial NOS has previously been located to the mouse erectile tissue, and an up-regulation of eNOS has been suggested to compensate for loss of the neuronal isoform in nNOS transgenic mice with normal erectile responses (Burnett et al., 1996). In the present study, immunoreactivity for eNOS was found in endothelial cells lining arteries and veins of the erectile tissue, but no distinct eNOS-IR cells were observed in sinusoidal endothelium.
Binding sites and immunoreactivity for CGRP have been described in CC tissue, and when given intracavernously, the peptide can induce erectile responses. Functional effects in vitro are previously reported to be small (Andersson & Wagner, 1995). Similar to effects seen in the rat (Hedlund et al., 1999). CGRP attenuated α-adrenoceptor mediated contractions in the mouse isolated CC. Based on the present functional results, although only few CGRP-IR nerve fibres were observed, a prejunctional action of CGRP cannot be excluded.
Conclusion
In comparison with results from other mammals, including humans, the mouse corpus cavernosum exhibit similar functions of nerves and endothelium, as well as similar intrinsic systems, i.e the sGC/cyclic GMP and AC/cyclic AMP pathways, for activation of smooth muscle functions. In addition, reproducible pharmaco- and nerve-induced erectile responses were obtained in vivo. The mouse is a suitable model for studies of penile erection.
Acknowledgments
This work was supported by the Swedish Medical Research Council (grants no 6837 and 11205), the Royal Physiographic Society, the Foundation of Crafoord, Magnus Bergvall, Åke Wiberg, Thelma Zoéga, and the Medical Faculty, University of Lund, Sweden.
Abbreviations
- AC
adenylyl cyclase
- BICP
basal intracavernous pressure
- cyclic AMP
cyclic adenosine 3′,5′monophosphate
- CC
corpus cavernosum
- cyclic GKI
cyclic GMP-dependent protein kinase I
- cyclic GMP
cyclic guanosine 3′,5′monophosphate
- CGRP
calcitonin gene-related peptide
- EFS
electrical field stimulation
- eNOS
endothelial nitric oxide synthase
- FITC
fluorescein isothiocyanate
- ICP
intracavernous pressure
- IR
immunoreactive
- L-NAME
L-NG-nitro-L-arginine methyl ester
- L-NOARG
NG-nitro-L-arginine
- NA
noradrenaline
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- NPY
neuropeptide Y
- ODQ
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one
- 6-OHDA
6-hydroxydopamine
- PBS
phosphate buffered saline
- PGP
protein gene product 9.5
- PICP
peak intracavernous pressure
- sGC
soluble guanylyl cyclase
- TR
Texas Red
- TH
tyrosine hydroxylase
- TTX
tetrodotoxin
- VAChT
vesicular acetylcholine transporter protein
- VIP
vasoactive intestinal polypeptide
References
- ANDERSSON K.-E., GEMALMAZ H., WALDECK K., CHAPMAN T.N., TUTTLE J.B., STEERS WD. The effect of sildenafil on apomorphine-evoked increases in intracavernous pressure in the awake rat. J. Urol. 1999;161:1707–1712. [PubMed] [Google Scholar]
- ANDERSSON K.-E., WAGNER G. Physiology of penile erection. Physiol. Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- BOOLELL M., ALLEN M.J., BALLARD S.A., GEPI-ATTEE S., MUIRHEAD G.J., NAYLOR A.M., OSTERLOH I.H., GINGELL C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996;8:47–52. [PubMed] [Google Scholar]
- BURNETT A. L., NELSON R.J., CALVIN D.C., LIU J.-X., DEMAS G.E., KLEIN S.L., KRIEGSFELD L.J., DAWSON V.L., DAWSON T.M., SNYDER S.H. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol. Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- CALABRO A., ITALIANO G., PESCATORI E.S., MARIN A., GAETANO O., ABATANGELO G., ABATANGELO G., PAGANO F. Physiological aging and penile erectile function: a study in the rat. Eur. Urol. 1996;29:240–244. [PubMed] [Google Scholar]
- CARTER A.J., BALLARD S.A., NAYLOR A.M. Effect of the selective phosphodiesterase type 5 inhibitor sildenafil on erectile dysfunction in the anesthetized dog. J. Urol. 1998;160:242–246. [PubMed] [Google Scholar]
- DAIL W. G., BARBA V., LEYBA L., GALINDO R. Neural and endothelial nitric oxide synthase activity in rat penile erectile tissue. Cell Tissue Res. 1995;282:109–116. doi: 10.1007/BF00319137. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R. F., & ZAWADZKI J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GIULIANO F., BERNABE J., JARDIN A., ROUSSEAU J.P. Antierectile role of the sympathetic nervous system in rats. J. Urol. 1993;150:519–524. doi: 10.1016/s0022-5347(17)35539-8. [DOI] [PubMed] [Google Scholar]
- GOCMEN C., SECILMIS A., UCAR P., KARATAS Y., ONDUR S., DIKMEN A., BAYSAL F. A possible role for S-nitrosothiols at the nitrergic relaxations in the mouse CC. Eur. J. Pharmacol. 1998;361:85–92. doi: 10.1016/s0014-2999(98)00703-1. [DOI] [PubMed] [Google Scholar]
- GOCMEN C., UCAR P., SINGIRIK E., DIKMEN A., BAYSAL F. An in vitro study of nonadrenergic-noncholinergic activity on the cavernous tissue of mouse. Urol. Res. 1997;25:269–275. doi: 10.1007/BF00942097. [DOI] [PubMed] [Google Scholar]
- HEDLUND P., ALM P., ANDERSSON K.-E. NO synthase in cholinergic nerves and NO-induced relaxation in the rat isolated corpus cavernosum. Br. J. Pharmacol. 1999;126:349–360. doi: 10.1038/sj.bjp.0702556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K.-E., MATTIASSON A. Pre- and postjunctional adreno- and muscarinic receptor functions in the isolated human corpus spongiosum urethrae. J. Auton. Pharmacol. 1984;4:241–249. doi: 10.1111/j.1474-8673.1984.tb00101.x. [DOI] [PubMed] [Google Scholar]
- HEDLUND P., ASZODI A., PFEIFER A., ALM P., HOFMANN F., AHMAD M., FÄSSLER R., & ANDERSSON K.-E. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2000a;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND P., LARSSON B., ALM P., ANDERSSON K.-E. Distribution and function of nitric oxide containing nerves in canine corpus cavernosum and spongiosum. Acta Physiol. Scand. 1995;155:445–455. doi: 10.1111/j.1748-1716.1995.tb09994.x. [DOI] [PubMed] [Google Scholar]
- HEDLUND P., NY L., ALM P., ANDERSSON K.-E. Cholinergic nerves in human corpus cavernosum and spongiosum contain nitric oxide synthase and heme oxygenase. J. Urol. 2000b;164:868–875. doi: 10.1097/00005392-200009010-00064. [DOI] [PubMed] [Google Scholar]
- HOLMQUIST F., HEDLUND H., ANDERSSON K.-E. Characterisation of inhibitory neurotransmission in the isolated corpus cavernosum from rabbit and man. J. Physiol. (Lond.) 1992;449:295–311. doi: 10.1113/jphysiol.1992.sp019087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMQUIST F., STIEF C.G., JONAS U., ANDERSSON K.-E. Effects of the nitric oxide synthase inhibitor NG-nitro-L-arginine on the erectile response to cavernous nerve stimulation in the rabbit. Acta Physiol. Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- IGNARRO L. J., BUGA G.M., WOOD K.S., BYRNS R.E., CHAUDHURI G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNEMANN K.P., PERSSON-JUNEMANN C., LUE T.F., TANAGHO E.A., ALKEN P. Neurophysiological aspects of penile erection: the role of the sympathetic nervous system. Br. J. Urol. 1989;64:84–92. doi: 10.1111/j.1464-410x.1989.tb05528.x. [DOI] [PubMed] [Google Scholar]
- KEAST J. R. A possible neural source of nitric oxide in the rat penis. Neurosci. Lett. 1992;143:69–73. doi: 10.1016/0304-3940(92)90235-y. [DOI] [PubMed] [Google Scholar]
- KIRKEBY H.J., JORGENSEN J.C., OTTESEN B. Neuropeptide Y (NPY) in human penile corpus cavernosum tissue and circumflex veins – occurrence and in vitro effects. J. Urol. 1991;145:605–609. doi: 10.1016/s0022-5347(17)38404-5. [DOI] [PubMed] [Google Scholar]
- LINCOLN T.M., CORNWELLT L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- LUE T.F., TAKAMURA T., SCHMIDT R.A., PALUBINSKAS A.J., TANAGHO E.A. Hemodynamics of erection in the monkey. J. Urol. 1983;130:1237–1241. doi: 10.1016/s0022-5347(17)51768-1. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J. M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- MARTINEZ-PINEIRO L., BROCK G., TRIGO-ROCHA F., HSU G.L., LUE T.F., TANAGHO E.A. Rat model for the study of penile erection: pharmacologic and electrical-stimulation parameters. Eur. Urol. 1994;25:62–70. doi: 10.1159/000475249. [DOI] [PubMed] [Google Scholar]
- PALMER R. M. J, FERRIGE A. G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- SCHIRAR A., GIULIANO F., RAMPIN O., ROUSSEAU J.-P. A large proportion of pelvic neurons innervating the corpora cavernosa of the rat penis exhibit NADPH-diaphorase activity. Cell Tissue Res. 1994;278:517–525. doi: 10.1007/BF00331369. [DOI] [PubMed] [Google Scholar]
- SEZEN S. F., BURNETT A. L. Intracavernosal pressure monitoring in mice: responses to electrical field stimulation of the cavernous nerve and to intracavernousal drug administration. J. Androl. 2000;21:311–315. [PubMed] [Google Scholar]


