Abstract
We have determined the binding characteristics of [125I]-(Pyr1)Apelin-13, a putative ligand for the APJ orphan receptor in human cardiovascular and rat tissue and investigated the functional properties of (Pyr1)Apelin-13 in human saphenous vein.
The binding of [125I]-(Pyr1)Apelin-13 to sections of human heart tissue was time dependent and rapid at 23°C. Data were fitted to a single site model with an association rate constant (kobs) of 0.115 min−1. [125I]-(Pyr1)Apelin-13 also dissociated from a single site with a dissociation rate constant of 0.0105 min−1.
In saturation binding experiments [125I]-(Pyr1)Apelin-13 bound to human left ventricle with a KD value of 0.35±0.08 nM, Bmax of 4.3±0.9 fmol mg−1 protein with a Hill slope of 0.97±0.04 and to the right atria with a KD of 0.33±0.09 nM, Bmax of 3.1±0.6 fmol mg−1 protein and a Hill slope of 0.93±0.05.
[125I]-(Pyr1)Apelin-13 binding sites were localized using autoradiography to human cardiovascular tissue, including coronary artery, aorta and saphenous vein grafts. In rat tissue a high density of receptors were localized to the molecular layer of the rat cerebellum, rat lung, rat heart and low levels in the rat kidney cortex.
(Pyr1)Apelin-13 potently contracted human saphenous vein with a pD2 value of 8.4±0.2 (n=8). The maximum response elicited by the peptide was 22.6±6% of 100 mM KCl.
We provide the first evidence of APJ receptor expression, relative densities and functional properties of (Pyr1)Apelin-13 in human cardiovascular tissue.
Keywords: Orphan receptor, APJ, apelin, human heart, human vascular tissue, autoradiography, human coronary artery, rat tissue, [125I]-(Pyr1)Apelin-13, in-vitro pharmacology
Introduction
Almost all of the known endogenous peptides exert their biological activity by binding to G protein-coupled receptors (GPCR). Progress in cDNA analysis has lead to the identification of numerous GPCR where the natural ligand has yet to be identified. These ‘orphan' receptors are potential targets for drug discovery. A human gene has been identified with significant homology to the gene encoding the angiotensin receptor. This novel receptor encoded by this gene has been named APJ (O'Dowd et al., 1993). APJ shares 40 to 50% identity with the angiotensin type 1 (AT1) receptor in the hydrophobic transmembrane regions, but did not bind angiotensin II (O'Dowd et al., 1993).
The proposed endogenous ligand for the APJ receptor, apelin-36, was isolated from bovine stomach extracts by monitoring extracellular acidification rate changes in a Chinese hamster ovary cell line expressing the human APJ receptor (Tatemoto et al., 1998). Subsequently in this assay, shorter sequences of the C-terminus of apelin-36 were found to be two orders of magnitude more potent than apelin-36, with an N-terminal pyroglutamylate form, ([Pyr1] apelin-13) being most effective (Tatemoto et al., 1998).
In homogenates of peripheral rat tissues, Northern analysis revealed highest levels of preproapelin mRNA in the heart (Lee et al., 2000). The highest levels of APJ mRNA were found in both adult and neonate rat heart together with the lung (Hosoya et al., 2000). The precise function of the apelin peptides has not yet been established. However, apelin-13 administered systemically to rats immediately lowered blood pressure, with a small increase in heart rate (Lee et al., 2000), although plasma concentrations to date have not been measured. It is not yet clear if apelin functions as a circulating hormone or as a locally derived mediator.
Messenger RNA encoding the predicted sequence of the putative APJ receptor has been detected, but it is not yet known whether or where the receptor protein is expressed. Therefore, our aim was to determine whether the APJ protein is present in human tissue, its anatomical distribution and the functional properties of (Pyr1)Apelin-13. We synthesized [125I]-(Pyr1)Apelin-13, since the unlabelled peptide was the most potent agonist at the cloned APJ receptor (Tatemoto et al., 1998). We concentrated on characterizing this novel ligand in human cardiac and vascular tissues since high levels of both preporapelin and APJ receptor mRNA were detected in the rat heart and in vivo studies suggested a vascular function. High levels of APJ mRNA have been shown by in situ hybridization to be present in the rat cerebellum (Lee et al., 2000) and lung (Hosoya et al., 2000). Therefore, we also examined these tissues to seek evidence for expression of the receptor protein. Preliminary data were presented to the British Pharmacological Society (Katugampola et al., 2000).
Methods
Tissue collection
With local ethical approval, human left ventricle and right atrial tissues were obtained from patients with histologically normal hearts not required for further transplantation (21 – 35 years). Other cardiovascular and pulmonary tissue were obtained from individuals (21 – 52 years) undergoing heart transplants for cardiomyopathy or heart lung transplants. Samples of epicardial coronary artery containing advanced atheromatous lesions and occluded saphenous vein bypass grafts were from individuals undergoing cardiac transplantation for ischaemic heart disease (48 – 69 years). Saphenous veins were from patients receiving coronary artery by-pass grafts (54 – 71 years). Experiments described below were carried out using tissue from at least four individuals. Tissues were from female Sprague-Dawley rats (300 – 350 g). Animals were killed by an overdose of sodium pentobarbitone followed by exsanguination.
Association studies
Sections of human left ventricle were incubated for 0 – 150 min with 0.15 nM [125I]-(Pyr1)Apelin-13. Unlabelled (Pyr1)Apelin-13 (1 μM) defined non-specific binding.
Dissociation studies
Sections of human left ventricle were incubated with 0.15 nM [125I]-(Pyr1)Apelin-13 for 30 min before sections were washed in an excess of buffer at room temperature for increasing time periods (0 – 240 min).
Saturation binding assays
Following optimization of binding conditions, 30 μm cryostat sections of human and rat tissue were pre-incubated in 20 mM HEPES buffer containing 1 mM EDTA, 0.3% BSA and a cocktail of protease inhibitors (pH 7.4) for 20 min at room temperature. Saturation binding studies were carried out using 12 increasing concentrations (0.01 – 1.25 nM) of [125I]-(Pyr1)Apelin-13 in buffer containing (mM) HEPES 50, EDTA 1, NaCl 100, MgCl2 5 and KCl 10 (pH 7.4). Non-specific binding was defined using 1 μM (Pyr1)Apelin-13. Following a 30 min incubation at room temperature the sections were rapidly washed in 50 mM Tris (4°C, pH 7.4). Protein concentration was determined using the Biorad DC 96-well microtiter plate system (Biorad Laboratories, Hertfordshire, U.K.).
In vitro receptor autoradiography
Cryostat tissue sections were incubated with 0.15 nM [125I]-(Pyr1)Apelin-13 in the absence or presence of unlabelled (Pyr1)Apelin-13 (1 μM). Washed sections were apposed to radiation sensitive film (Hyperfilm βmax, Amersham Pharmacia Biotech, Bucks, U.K.) for 4 days. Data were analysed by computer assisted densitometry as previously described (Davenport et al., 1995).
In vitro pharmacology
Human saphenous veins were cleaned of connective tissue, cut into 4-mm rings and their luminal surface rubbed gently with a metal seeker to remove the endothelium (confirmed by the absence of staining with rabbit antiserum to human von Willebrand factor as previously described (Davenport et al., 1996)). Vessels were set up for isometric force recordings in 5 ml organ baths containing oxygenated Kreb's solution (37°C) and allowed to equilibrate for 1 h. Contractile responses were then recorded to 100 mM KCl at incrementally increasing levels of basal tension until no further increase in KCl response was obtained. This determined the optimum resting tension for each preparation and was followed by a further 30 min equilibration period. Cumulative concentration response curves were then constructed to (Pyr1)Apelin-13 (1×10−10 – 3×10−7 M). Experiments were terminated by addition of 100 mM KCl to determine the maximum possible response for each preparation. Agonist responses were subsequently expressed as a percentage of this KCl maximum.
Modified Kreb's solution had the following composition (mM): NaCl 90, KCl 5, MgSO4.7H2O 0.5, Na2HPO4 1, NaHCO3 45, CaCl2 2.25, glucose 10, Na pyruvate 5, fumaric acid 5 and L-glutamic acid 5.
Data analysis
The results from binding experiments were analysed using the iterative, non-linear curve fitting programmes EBDA and LIGAND in the KELL package (Biosoft, Cambridge, U.K.). All values are expressed as mean±s.e.mean. Kinetic analysis of [125I]-(Pyr1)Apelin-13 binding provided estimates for the observed association (Kobs) and dissociation rate constants. These were in turn used to derive values for the association rate constant and the kinetically determined KD. Individual saturation binding experiments were analysed with EBDA (McPherson, 1983) to obtain initial estimates. The resulting data files were co-analysed with LIGAND (Munson & Rodbard, 1980) to obtain values of ligand affinity (KD) and receptor density (Bmax expressed as fmol mg−1 protein). The presence of one or two sites was determined using the F-ratio test in LIGAND. The model adopted was that which provided the significantly best fit (P<0.05).
Data from in vitro pharmacology experiments were analysed using the iterative curve-fitting programme Fig P (Biosoft, Cambridge, U.K.) to give values of pD2 (negative log10 of the molar concentration producing 50% of the maximum response) and EMax (maximum agonist response as a percentage of the terminal KCl response). The response to (Pyr1)Apelin-13 was unaffected by the inclusion of protease inhibitors in the Kreb's medium, therefore data obtained in the presence or absence of this inhibitor was pooled and expressed as mean±s.e.mean.
Materials
[125I]-(Pyr1)Apelin-13 (2000 Ci mmol−1) (Figure 1) was prepared (Amersham Pharmacia Biotech Bucks, U.K) from the unlabelled material (Pyr1)Apelin-13 (pGlu-Arg-Pro-Arg-Leu-Ser-His-Lys-Gly-Pro-Met-Pro-Phe) (Peptide Institute, Osaka, Japan) by conjugation with monoiodinated (125I) Bolton & Hunter reagent (Amersham Pharmacia Biotech Bucks, U.K.) which labelled the Lys8 residue and was purified by reverse phase HPLC to be carrier-free. (Pyr1)Apelin-13 used in all other experiments was from Bachem (Essex, U.K.) and other peptides from Peptide Institute (Osaka, Japan). All other chemicals were obtained from Sigma-Aldrich (Poole, Dorset, U.K.).
Figure 1.
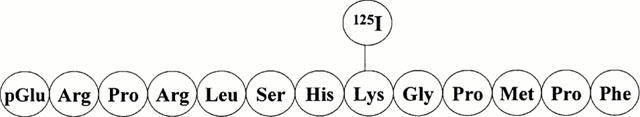
Structure of [125I]-(Pyr1)Apelin-13. [125I]-(Pyr1)Apelin-13 was prepared from the unlabelled material (Pyr1)Apelin-13 by conjugation with monoiodinated (125I) Bolton & Hunter reagent, which labelled the Lys8 residue.
Results
Association studies
At a concentration of 0.15 nM, the binding of [125I]-(Pyr1)Apelin-13 was time-dependent to sections of human left ventricle with an association rate constant (kobs) of 0.115±0.004 min−1 and a half time for association (t1/2) of 6 min (Figure 2).
Figure 2.
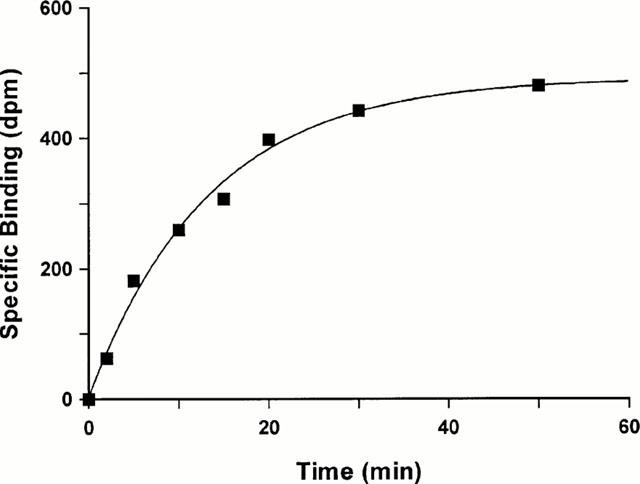
Time-dependent association of [125I]-(Pyr1)Apelin-13 binding to sections of human left ventricle. Tissue sections were incubated for 150 min at room temperature. Data represents a single experiment, with an association rate constant (kobs) of 0.109 min−1 for this particular experiment.
Dissociation studies
The binding of [125I]-(Pyr1)Apelin-13 was reversible at room temperature and analysis of the data indicated dissociation from a single site with a dissociation rate constant of 0.013±0.0003 min−1. The half time for dissociation (t1/2) was 53 min (Figure 3). The KD derived from this data was 0.01 nM.
Figure 3.
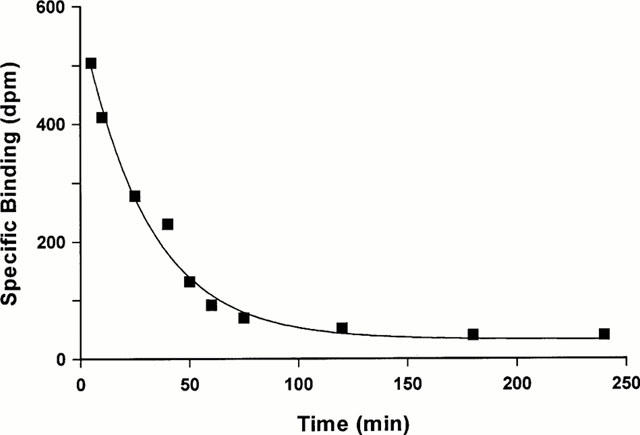
Time course for dissociation of 0.15 nM [125I]-(Pyr1)Apelin-13 from sections of human left ventricle. Tissue sections were incubated for 30 min and washed in excess of Tris buffer for differing time points up to 4 h. A representative curve is shown, with a dissociation rate constant of 0.016 min−1 for this particular experiment.
Saturation studies
Over the concentration range tested (0.01 – 1.25 nM) [125I]-(Pyr1)Apelin-13 bound with sub-nanomolar affinity to both human cardiac tissue (Figure 4) and rat tissues. There was no significant difference in ligand affinity or receptor density comparing the atria and ventricle of the human heart. For each tissue examined, a one site fit was preferred to a two site model and the Hill coefficients were close to unity (Table 1).
Figure 4.
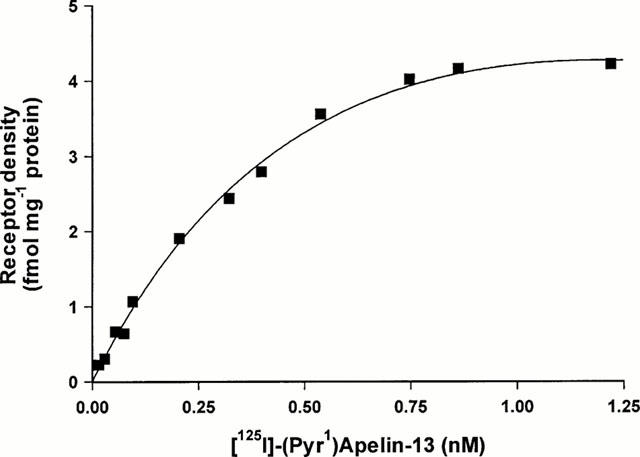
Saturation binding curve for [125I]-(Pyr1)Apelin-13. Increasing concentrations of radioligand (0.01 – 1.25 nM) were incubated with sections of human left ventricle for 30 min at room temperature. Curve represents a typical result, with a dissociation constant (KD) of 0.3 nM and maximal receptor density (Bmax) of 4.2 fmol mg−1 protein.
Table 1.
Dissociation constant (KD), maximal density or receptors (Bmax) and Hill coefficients (nH) for [125I]-(Pyr1)Apelin-13 binding to human cardiac and rat tissues
In vitro receptor autoradiography
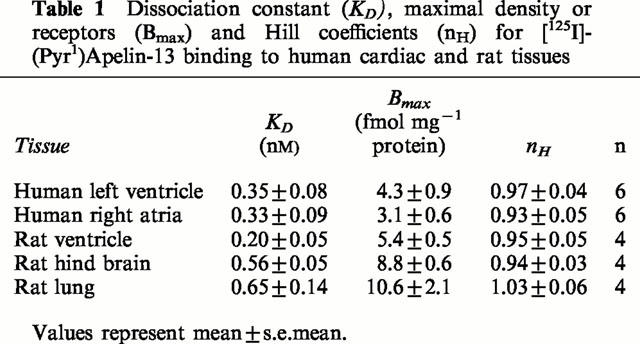
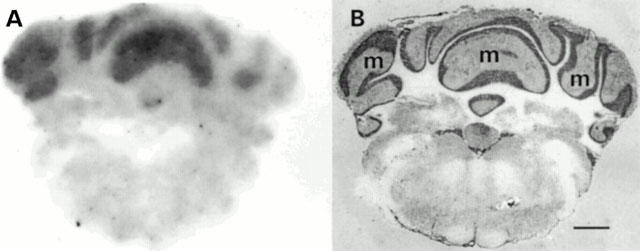
In the rat tissues examined, the highest density of binding sites measured by quantitative autoradiography using a fixed concentration of [125I]-(Pyr1)Apelin-13 was in the rat cerebellum (Figure 5). Binding sites were localized to the molecular layer of the cerebellum with no binding detected above non-specific levels in the granular layer. Binding was also localized to the rat lung and rat heart with lower densities in the rat kidney cortex. Autoradiography revealed [125I]-(Pyr1)Apelin-13 bound to all human blood vessels examined (Figure 6). Normal epicardial coronary artery 3.5±0.4 amol mm−2 (n=6), atherosclerotic coronary artery 2.8±0.9 amol mm−2 (n=6), aorta 2.8±0.5 amol mm−2 (n=5), internal mammary artery 3.1±0.2 amol mm−2 (n=6), pulmonary artery 2.4±0.2 amol mm−2 (n=4), saphenous vein grafts 2.6±0.9 amol mm−2 (n=5). Binding was below the level for detection in non-vascular tissue of human lung.
Figure 5.
(A) Autoradiographical localization of APJ receptor to rat brain with high density in the molecular layer (m) of the rat cerebellum. (B) Section stained with heamatoxylin and eosin. Scale bar=2 mm.
Figure 6.
Autoradiographical localization of APJ receptors to (A) human left ventricle section. (B) Transverse section of a human non-diseased coronary artery. (C) Longitudinal section of a pulmonary artery. (D) Transverse section of a saphenous vein graft with binding in both the media (m) as well as the intimal (i) smooth muscle layers. No image was detected in the presence of 1 μM cold ligand. Scale bars=5 mm (A and C), 2 mm (B and D).
Other cardiovascular peptides (endothelin-1, angiotensin II, atrial natriuretic peptide, calcitonin gene related peptide, adrenomedullin, and ghrelin) at 1 μM concentration did not compete for [125I]-(Pyr1)Apelin-13 binding site in human left ventricle (data not shown).
In vitro pharmacology
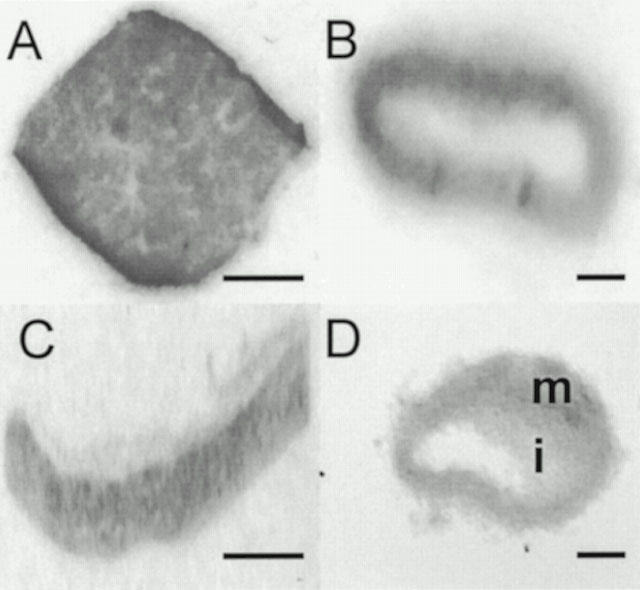
(Pyr1)Apelin-13 potently contracted human saphenous vein (Figure 7) with a pD2 value of 8.4±0.2 (n=8). The maximum response elicited by the peptide was 22.6±6% of the terminal response to 100 mM KCl (Figure 8).
Figure 7.
Cumulative concentration response curves to (Pyr1)Apelin-13 (0.1 – 300 nM) in human saphenous vein. This trace is representative of eight experiments.
Figure 8.
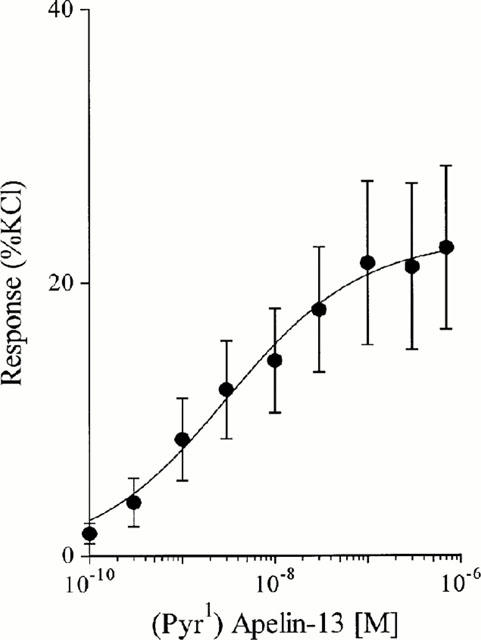
Concentration response curve to (Pyr1)Apelin-13 in human saphenous vein. Data points are mean±s.e.mean, n=8.
Discussion
We report for the first time the binding characteristics of [125I]-(Pyr1)Apelin-13, a putative ligand for the APJ orphan receptor, in human cardiac tissue and used these initial findings to localize binding sites in human and rat tissues by autoradiography. [125I]-(Pyr1)Apelin-13 binding in human heart was saturable, reversible and specific, characteristic of a receptor. Within the concentration range used in saturation experiments, the radioligand bound with high affinity and a one site fit was preferred. Hill slopes were close to unity indicating that [125I]-(Pyr1)Apelin-13 bound with a single affinity, with no evidence for receptor subtypes. In agreement, in kinetic studies the radioligand bound rapidly at room temperature to a single site. Binding was reversible with the ligand also dissociating from a single site. The density of receptors in the human myocardium was comparable to that of the important cardiac vasoactive peptide, angiotensin II (Asano et al., 1997; Katugampola & Davenport, 2000) where Bmax values of 6 – 12 fmol mg−1 protein has been reported. However, this density is much lower than that for endothelin receptors in the human left ventricle (65 fmol mg−1 protein; Molenaar et al., 1993). The functional importance of the low density of [125I]-(Pyr1)Apelin-13 binding sites in mediating chronotropic and inotropic effects in the human heart remains to be determined. Saturation studies performed in rat heart, rat lung and rat hind brain identified a single binding site. The binding density and ligand affinity in the rat heart being comparable to rat lung and the rat hind brain.
Quantitative autoradiography revealed levels of [125I]-(Pyr1)Apelin-13 binding to be similar in the media and intimal layers of human muscular arteries, large elastic arteries and veins. There was no evidence for a change in density comparing normal versus atherosclerotic coronary arteries with advanced intimal thickening. [125I]-(Pyr1)Apelin-13 bound to pulmonary arteries, but the lack of binding sites in nonvascular tissue of human lung suggests that most of the receptors in this organ are localized to the vasculature.
Autoradiography also revealed the presence of APJ binding sites in discrete regions of rat brain with greatest levels in the molecular layer of the cerebellum, in agreement with the reported high levels of APJ mRNA (Lee et al., 2000). Since there are no previous studies characterizing apelin binding, we used selected tissues where high levels of APJ mRNA have been reported as a positive control. Expression of APJ mRNA has been detected in the rat lung, rat heart and rat kidney cortex (Hosoya et al., 2000; O'Carroll et al., 2000). In agreement with the mRNA levels, we were able to detect high receptor protein expression in the rat lung and rat heart, but in comparison lower densities in the rat kidney cortex.
(Pyr1)Apelin-13 contracts human saphenous vein with a potency comparable to endothelin-1 (pD2 8.49±0.40; Maguire et al., 2000) and approximately 10 fold less than the recently described human urotensin II (pD2 9.43±0.57; Maguire et al., 2000). Whilst the maximum contractile response to (Pyr1)Apelin-13 was equivalent to that of urotensin II (31.48±9.54% KCl; Maguire et al., 2000) it was significantly lower than for endothelin-1 (94.83±3.42% KCl; Maguire et al., 2000). Therefore, in human saphenous vein we find that (Pyr1)Apelin-13 is potent vasoconstrictor like endothelin-1 and urotensin II, but has low efficacy which is in agreement with the low density of high affinity receptors localized to the vascular smooth muscles.
This data suggests that [125I]-(Pyr1)Apelin-13 will be of use for further characterization of the APJ receptors and their anatomical distribution in other human and animal tissue in order to elucidate the other important physiological and/or pathophysiological roles in the central nervous system and periphery. These results show for the first time that [125I]-(Pyr1)Apelin-13 binds with high affinity and selectivity to human cardiac tissue with evidence for a wide distribution of the APJ receptor in the human vasculature and highlights the properties of a novel potent vasoconstrictor in the human vasculature.
Acknowledgments
This work was supported by grants from the British Heart Foundation and The Royal Society.
Abbreviations
- BSA
bovine serum albumin
- EDTA
ethylenediaminetetracetic acid
References
- ASANO K., DUTCHER D.L., PORT J.D., MINOBE W.A., TREMMEL K.D., RODEN R.L., BOHLMEYER T.J., BUSH E.W., JENKIN M.J., ABRAHAM W.T., RAYNOLDS M.V., ZISMAN L.S., PERRYMAN M.B., BRISTOW M.R. Selective downregulation of the angiotensin II AT1-receptor subtype in failing human ventricular myocardium. Circulation. 1997;95:1193–1200. doi: 10.1161/01.cir.95.5.1193. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P., O'REILLY G., KUC R.E. Endothelin ETA and ETB mRNA and receptors expressed by smooth muscle in the human vasculature: majority of the ETA sub-type. Br. J. Pharmacol. 1995;114:1110–1116. doi: 10.1111/j.1476-5381.1995.tb13322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT A.P., HOSKINS S.L., KUC R.E., PLUMPTON C. Differential distribution of endothelin peptides and receptors in human adrenal gland. Histochem. J. 1996;28:779–789. doi: 10.1007/BF02272151. [DOI] [PubMed] [Google Scholar]
- HOSOYA M., KAWAMATA Y., FUKUSUMI S., FUJII R., HABATA Y., HINUMA S., KITADA C., HONDA S., KUROKAWA T., ONDA H., NISHIMURA O., FUJINO M. Molecular and functional characteristics of APJ: tissue distribution of mRNA and interaction with endogenous ligand, Apelin. J. Biol. Chem. 2000;275:21061–21067. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- KATUGAMPOLA S.D., MATHEWSON S.R., DAVENPORT A.P. Characterisation of 125I-(Pyr1) Apelin-13, a putative ligand for the APJ orphan receptor in human tissue. Br. J. Pharmacol. 2000;131:85P. [Google Scholar]
- KATUGAMPOLA S.D., DAVENPORT A.P. Angiotensin receptors and alternative pathways for the local generation of angiotensin II in the human left ventricle. Br. J. Pharmacol. 2000;129:205P. [Google Scholar]
- LEE D.K., CHENG R., NGUYEN T., FAN T., KARIYAWASAM A.P., LIU Y., OSMOND D.H., GEORGE S.R., O'DOWD B.F. Characterization of Apelin, the Ligand for the APJ Receptor. J. Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., DAVENPORT A.P. Orphan-receptor ligand human urotensin II: receptor localisation in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br. J. Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHERSON G. A practical computer-based approach to the analysis of radioligand binding experiments. Comput. Prog. Biomed. 1983;17:107–114. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- MOLENAAR P., O'REILLY G., SHARKEY A., KUC R., HARDING D., PLUMTON C., GRESHAM A., DAVENPORT A.P. Characterisation and localisation of endothelin receptor subtypes in the human atrioventricular conducting system and myocardium. Circ. Res. 1993;72:526–538. doi: 10.1161/01.res.72.3.526. [DOI] [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. LIGAND: a versatile computerised approach for the characterisation of ligand binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- O'CARROLL A.M., SELBY T.L., PALKOVITS M., LOLAIT S.J. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochemica. et. Biophysica. Acta. 2000;1492:72–80. doi: 10.1016/s0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- O'DOWD B.F., HEIBER M., CHAN A., HENG H.H., TSUI L.C., KENNEDY J.L., SHI X., PETRONIS A., GEORGE S.R., NGUYEN T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- TATEMOTO K., HOSOYA M., HABATA Y., FUJII R., KAKEGAWA T., ZOU M.X., KAWAMATA Y., FUKUSUMI S., HINUMA S., KITADA C., KUROKAWA T., ONDA H., FUJINO M. Isolation and characterisation of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]


