Abstract
Several lines of evidence suggest a crucial involvement of glutamate in the mechanism of action of anxiolytic and/or antidepressant drugs. The involvement of group I mGlu receptors in anxiety and depression has also been proposed. Given the recent discovery of a selective and brain penetrable mGlu5 receptor antagonists, the effect of 2-methyl-6-(phenylethynyl)-pyridine (MPEP), i.e. the most potent compound described, was evaluated in established models of anxiety and depression.
Experiments were performed on male Wistar rats or male Albino Swiss or C57BL/6J mice. The anxiolytic-like effects of MPEP was tested in the conflict drinking test and the elevated plus-maze test in rats as well as in the four-plate test in mice. The antidepressant-like effect was estimated using the tail suspension test in mice and the behavioural despair test in rats.
MPEP (1 – 30 mg kg−1) induced anxiolytic-like effects in the conflict drinking test and the elevated plus-maze test in rats as well as in the four-plate test in mice. MPEP had no effect on locomotor activity or motor coordination. MPEP (1 – 20 mg kg−1) did shorten the immobility time in a tail suspension test in mice, however it was inactive in the behavioural despair test in rats.
These data suggest that selective mGlu5 receptor antagonists may play a role in the therapy of anxiety and/or depression, further studies are required to identify the sites and the mechanism of action of MPEP.
Keywords: mGlu5 receptors, MPEP, conflict drinking test, four-plate test, plus-maze test, tail suspension test, anxiety, depression
Introduction
Glutamate is the major excitatory neurotransmitter in the brain, and as such involved in several physiological and pathological conditions (Wroblewski & Danysz, 1989; Danysz et al., 1996). Glutamate acts at two classes of receptors, the ionotropic and the metabotropic glutamate receptors (mGlu receptors) (Monaghan et al., 1989; Conn & Pin, 1997). Metabotropic glutamate receptors are a family of eight G-protein coupled receptors which are classified into three groups according to their sequence homology, effector coupling and pharmacology. Group I mGlu receptors (mGlu1 and mGlu5) are positively coupled to phospholipase C; group II mGlu receptors (mGlu2 and mGlu3) and group III mGlu receptors (mGlu4, mGlu6, mGlu7 and mGlu8) are negatively coupled to adenylate cyclase (Conn & Pin, 1997). Activation of group I mGlu receptors leads to a transient increase in intracellular calcium via the production of inositol-trisphosphates (Conn & Pin, 1997). Generally, it has been shown that activation of group I receptors enhances or facilitates the excitatory effects of glutamate by modulation of ion channel activity (Conn & Pin, 1997). Antagonists of group I mGlu receptors have been proposed to exhibit potential positive therapeutic effects (Bruno et al., 1994; Conn & Pin, 1997) in CNS disorders related to excessive excitatory neurotransmission such as epilepsy, ischaemia and pain (Nicoletti et al., 1996; Conn & Pin, 1997).
Several lines of evidence suggest an important role for glutamate in anxiety and depression (Wiley et al., 1995; Skolnick et al., 1996; Danysz & Parsons, 1998; Skolnick, 1999). Involvement of group I mGlu receptors in psychiatric conditions such as depression and anxiety has also been proposed. It has been shown that antagonists of group I mGlu receptors exert anxiolytic-like effects after intrahippocampal injection in rats (Chojnacka-Wójcik et al., 1997); and that antidepressant treatment influences group I mGlu receptors in the hippocampus (Bajkowska et al., 1999; Pilc et al., 1998).
Up to now studies concerning involvement of mGlu5 receptors in CNS functions were largely based on compounds which have only limited selectivity between mGlu1 and mGlu5 receptor subtypes (Nicoletti et al., 1996; Conn & Pin, 1997) and which do not penetrate into the brain. Only recently, novel, selective and systemically active compounds have been described (Varney et al., 1999; Gasparini et al., 1999). The most potent of this series is 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a noncompetitive antagonist with an IC50 of 36 nM at the human mGlu5a receptor in the PI hydrolysis assay but no significant effect at other metabotropic or ionotropic glutamate receptors (Gasparini et al., 1999). To evaluate whether MPEP has anxiolytic-or antidepressant-like effects, we studied its effects in several models of anxiety or depression in rats and mice.
Methods
Animals and housing
The experiments were performed on male Wistar rats (200 – 250 g) and male Albino Swiss or male C57BL/6J mice (22 – 26 g). The animals were kept on a natural day – night cycle at a room temperature of 19 – 21°C, with free access to food and water. Each experimental group consisted of 6 – 10 naïve animals/dose. In rats, all injections were given in a volume of 2 ml kg−1, and in mice in a volume of 10 ml kg−1. Experiments were performed by an observer blind to the treatment. All experimental procedures were approved by Animal Care and Use Committee at the Institute of Pharmacology, Polish Academy of Sciences in Kraków.
Drugs
2-Methyl-6-(phenylethynyl)-pyridine (MPEP) was synthesized as described previously (Gasparini et al., 1999). MPEP and diazepam (Polfa-Poznań, Poland) were suspended in a 1% aqueous solution of Tween 80. Imipramine hydrochloride (Polfa-Starogard Gdański, Poland) and L-5-hydroxytryptophan (L-5-HTP; Sigma, St. Louis, MO, U.S.A.) were dissolved in sterile saline. MPEP was administered intraperitoneally (i.p.) or perorally (p.o.), diazepam, imipramine and L-5-HTP were administered i.p. All compounds were given at 60 min before the tests.
Conflict drinking test (Vogel test)
A modification of the method of (Vogel et al., 1971) described below was used. On the first day of the experiment, the rats were adapted to the test chamber for 10 min. It was a plexiglass box (27×27×50 cm), equipped with a grid floor of stainless steel bars and a drinking bottle containing tap water. After the adaptation period, the animals were deprived of water for 24 h, and were then placed in the test chamber for another 10 min adaptation period, during which they had a free access to the drinking bottle. Afterwards, they were allowed a 30 min free-drinking session in their home cage. After another 24 h water deprivation period, the rats were again placed in the test chamber and were allowed to drink for 30 s. Immediately afterwards, drinking attempts were punished with an electric shock (0.5 mA). The impulses were released every 2 s (timed from the moment when a preceding shock was delivered), between the grid floor and the spout of the drinking bottle. Each shock lasted for 1 s and if the rat was drinking when an impulse was released, it received a shock. The number of shocks accepted throughout a 5 min experimental session was recorded. MPEP (0.3, 1 and 10 mg kg−1, i.p.) and diazepam (10 mg kg−1, i.p.) were administered 60 min before the test.
Shock threshold and free-drinking tests
To control the possibility of drug-induced changes in the perception of a stimulus or in the thirst drive, which might have contributed to the activity in the conflict drinking test, stimulus threshold measurements and a free-drinking experiment were also carried out. In both cases, the rats were treated before the experiment in the same manner as described in the conflict drinking test, including two 24 h water deprivation periods separated by 30 min of water availability. In the shock threshold test, the rats were placed individually in the box, and electric shocks were delivered through the grid floor. The shock threshold was determined stepwise with 15 s shock free intervals by manually increasing the current (0.1, 0.2, 0.3, 0.4, 0.5 mA). The shock lasted for 1 s and was delivered through the grid-floor until a rat showed an avoidance reaction (jump or jerk) to the electric stimulus.
In the free-drinking test, each animal was allowed to drink from the water spout. Licking was not punished. The total amount of water (ml), consumed in 5 min, was recorded for each rat. MPEP (1 and 10 mg kg−1, i.p.) was administered 60 min before the test.
Water intake test
The rats were housed and tested in individual cages (40×27×15 cm), with free access to food and water at all times. On the day of the test, water bottles were weighed at the time of drug administration. Water was presented immediately after drug injection. Water intake (ml) was recorded at 1, 2, 4, 6 and 24 h time points. L-5-hydroxytryptophan (L-5-HTP) was used as a reference drug (Rowland et al., 1987). MPEP (1 and 10 mg kg−1, i.p.) and L-5-HTP (20 mg kg−1, i.p.) were administered immediately before the test.
Elevated plus-maze test
The construction and the testing procedure of the elevated plus-maze were based on a method described by Pellow & File (1986). Each rat was placed in the centre of the plus-maze, facing one of the enclosed arms immediately after a 5 min adaptation in a wooden box (60×60×35 cm). During a 5 min test period, two experimenters, who were sitting in the same room approximately 1 m from the end of the open arms, recorded the number of entries into the closed or the open arm, as well as the time spent in each type of arms. The entry with all four feet put onto one arm was defined as an arm entry. At the end of each trial the maze was wiped clean. MPEP (1, 3 and 10 mg kg−1, i.p. or 10 and 30 mg kg−1, p.o.) and diazepam (1.25, 2.5 and 5 mg kg−1, i.p.) were administered 60 min before the test.
Four-plate test
The box is made of an opaque plastic and has the shape of a rectangle (25×18×16 cm). The floor is covered with four rectangular metal plates (11.3×7.7 cm) separated by a gap of 4 mm. The plates are connected to a source of direct current and the 180 V difference of potential between two adjacent plates occurs for 0.5 s when the experimenter presses a switch. Single mice were placed gently onto the plate, and allowed to explore for 15 s. Afterwards, each time a mouse passed from one plate to another, the experimenter electrified the whole floor, which evoked a visible flight reaction of the animal. If the animal continued running, it received no new shocks for the following 3 s. The number of punished crossings was counted for 60 s (Aron et al., 1971). MPEP (3, 10 and 30 mg kg−1, i.p.) and diazepam (2 mg kg−1, i.p) were administered 60 min before the test.
Rota-rod test
Mice were preselected 1 h before the test on the rotating rod (3 cm in diameter, 6 r.p.m). Those staying on the rotating road for 2 min (approximately 95% of animals) were placed again on the same rotating rod after drug administration and were observed for 2 min. The number of animals falling from the rota-rod within 2 min was recorded. MPEP (30 mg kg−1, i.p.) was administered 60 min before the test.
Open field test
The studies were carried out with rats according to a slightly modified method of Janssen et al. (1960). The centre of the open arena (1 m in diameter), divided into six symmetrical sectors without walls, was illuminated with a 75 W electric bulb hung directly 75 cm above it. During all the experiments the laboratory room was dark. Individual control or drug-injected animals were placed gently in the centre of the arena and were allowed to explore freely. The time of walking, ambulation (the number of crossing of sector lines) and the number of rearing and peeping episodes (looking under the edge of the arena) were recorded for 3 min. MPEP (3 and 10 mg kg−1, i.p.) was administered 60 min before the test.
Behavioural despair test
The studies were carried out on rats according to the method of Porsolt et al. (1978). Briefly, the rats were placed individually into a glass cylinder (height 40 cm; diameter 18 cm) containing 15 cm of water, maintained at 25°C. After 15 min they were removed to a drying room (30°C) for 30 min. They were replaced in the cylinder 24 h later and the total duration of immobility was measured during a 5 min test. MPEP (0.1, 1 and 10 mg kg−1, i.p.) and imipramine (30 mg kg−1, i.p.) were administered 60 min before the test.
Tail suspension test
Immobility was induced by tail suspension according to the procedure of Steru et al. (1985). C57BL/6J mice were hung individually on a plastic string, 75 cm above the table top with an adhesive tape placed ca. 1 cm from the tip of the tail. Duration of immobility was recorded for 8 min. Mice were considered immobile only when they hung passively and completely motionless. MPEP (0.1, 1, 10 and 20 mg kg−1, i.p.) and imipramine (20 mg kg−1, i.p.) were administered 60 min before the test.
Analysis of the data
The data obtained were presented as means±s.e.mean and evaluated using one-way ANOVA, followed by Dunnett's post hoc determination, using GraphPad Prism version 3.00 for Windows 97 (Graph Pad Software, San Diego CA, U.S.A.).
Results
Conflict drinking test in rats
MPEP, which at a dose of 0.3 mg kg−1 was not effective, at doses of 1 and 10 mg kg−1 i.p. significantly (F (3,30)=11.193, P<0.001), increased the number of shocks (by 330 and 507%, respectively) accepted during the experimental session in the Vogel test (Figure 1). The maximal effect of MPEP at a dose of 10 mg kg−1 was comparable to that seen with diazepam at a dose of 10 mg kg−1. At the effective doses in the conflict drinking test, neither the threshold current (0.4±0.04 mA) nor the water intake (10.6±0.6 ml) were changed compared to vehicle treatment (Table 1). As a control water intake in non-deprived rats was also evaluated. MPEP tested at doses effective in the conflict drinking test (1 or 10 mg kg−1) had no significant effect on water consumption, while L-5-HTP (20 mg kg−1) used as a standard drug (Rowland et al., 1987) significantly increased the water intake (Table 2).
Figure 1.
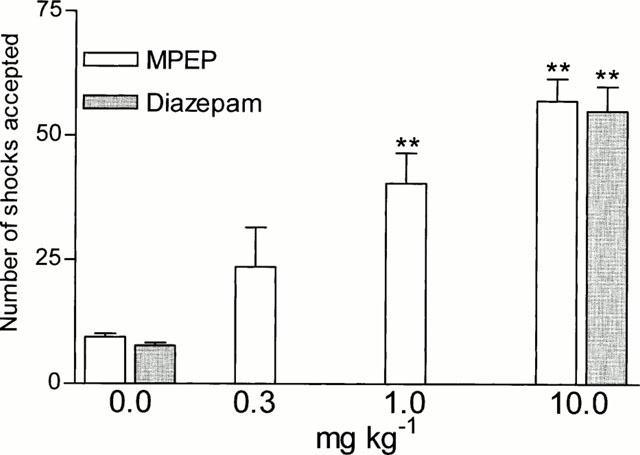
Effects of MPEP and diazepam in the conflict drinking test in rats. MPEP and diazepam were administered i.p. at 60 min before the test. The given values represent the mean±s.e.mean of the number of shocks accepted during a 5 min experimental session, n=7 – 9, ** P<0.01 vs control group.
Table 1.
The effect of MPEP on the shock threshold and the amount of water consumed in water deprived rats

Table 2.
Effects of MPEP and L-5-HTP on the amount of water intake in water non-deprivated rats

Plus-maze test in rats
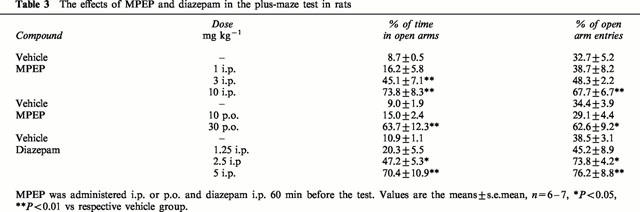
The total number of entries (open+closed arm entries) observed with control rats during the 5 min test session was about six in the present set of experiments and was taken as 100%. In control rats 32.7, 34,4 and 38.5% of the entries were made into the open arms (Table 3), and 8.7, 9.0 and 10.9% of the total time (255 s) spent in the arms (either type) was spent in the open arms. MPEP administered at a dose of 1 mg kg−1 i.p. did not change the entries into and time spent in the open arms. When given at doses of 3 and 10 mg kg−1 i.p. it significantly (F (3,24)=22.978, P<0.001) dose-dependently increased the time spent in the open arms (up to 45 and 74%, respectively), and the percentage of entries into the open arms (up to 48 and 68%, respectively, F (3,24)=5.678, P<0.01) (Table 3). MPEP at doses of 3 and 10 mg kg−1 i.p. significantly increased (by 64%) the total number of entries and reduced (by about 25%) the total time spent (data not shown) in the arms (either type). After p.o. administration higher doses of MPEP were required to induce significant behavioural changes: at the dose of 30 mg kg−1 (but not 10 mg kg−1) MPEP significantly (up to 64%, F (2,16)=14.249, P<0.001) increased the percentage of the time spent in the open arms and the percentage of entries into the open arms (up to 63%, F (2,16)=7.295, P<0.01). MPEP given p.o. in both doses used did not change the total number of entries nor the total time spent in the arms (either type). Diazepam, i.e. the positive standard, administered in a dose of 1.25 mg kg−1 i.p. was ineffective in that test, however when given at doses of 2.5 and 5 mg kg−1 i.p. it significantly (F (3,22)=14.52, P<0.001) increased the percentage of the time spent in the open arms (up to 47 and 70%, respectively), as well as the percentage of entries into the open arms (up to 74 and 76%, respectively (F (3,22)=5.871, P<0.01) (Table 3). Diazepam at a dose of 5 mg kg−1 (but not lower) significantly reduced (by 52%) the total number of entries (data not shown).
Table 3.
The effects of MPEP and diazepam in the plus-maze test in rats
Open field test in rats and rota-rod test in mice
MPEP at doses of 3 and 10 mg kg−1 i.p. did not change exploratory locomotor activity in rats (F (2,18)=2; 0.273; 0.011, n.s.), as evaluated by the open field test (Table 4). MPEP at a dose of 30 mg kg−1 i.p. did not disturb endurance performance on the rotating rod in mice (data not shown).
Table 4.
The effect of MPEP on the exploratory activity in the open field test in rats

Four-plate test in mice
MPEP administered at 30 mg kg−1 i.p. slightly but significantly increased (by 39%) the number of punished crossings in the four-plate test (Figure 2), lower doses of the compound (3 and 10 mg kg−1) did not affect the number of punished crossings in that test (F (3,36)=3.240, P<0.05). Diazepam, i.e. the positive standard, in a dose of 2 mg kg−1 increased the number of crossings by 70%.
Figure 2.
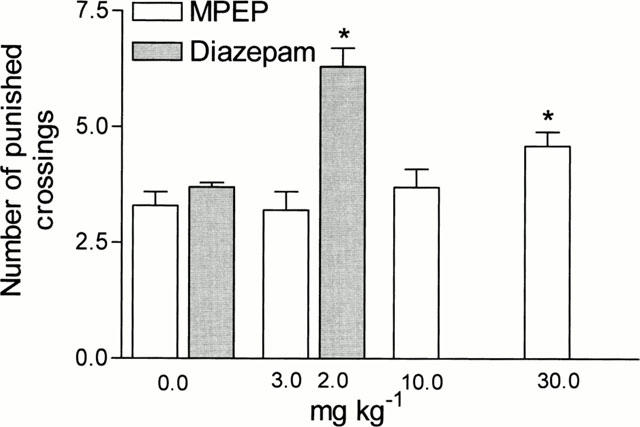
Effects of MPEP and diazepam in the four-plate test in mice. MPEP and diazepam were administered i.p. 60 min before the test. The given values represent the mean±s.e.mean of the number of shocks accepted during a 1 min experimental session, n=10. *P<0.05 vs control group.
Behavioural despair test in rats and tail suspension test in mice
MPEP in doses of 0.1, 1 and 10 mg kg−1 i.p. did not change the behaviour of rats in the behavioural despair test, while imipramine, 30 mg kg−1, used as standard drug, significantly (F (6,49)=25.02, P<0.001) decreased the immobility time in that test (Figure 3).
Figure 3.
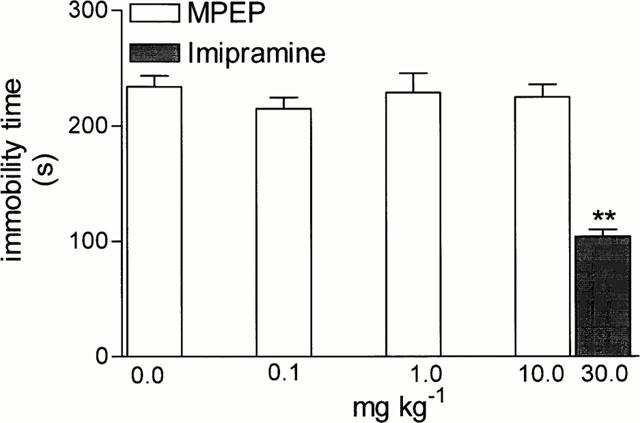
The effects of MPEP and imipramine on the total duration of immobility in the forced swimming test in rats. MPEP and imipramine were administered i.p. at 60 min before the test. Values represent the mean±s.e.mean of the immobility time during a 5 min experimental session, n=9 – 10. ** P<0.01 vs control group.
MPEP used in doses of 1, 10 and 20 mg kg−1 significantly (by 55% after the highest dose), (F (3,28)=15.47, P<0.001) decreased the immobility time of mice in the tail suspension test. Its efficacy was similar to that of imipramine (20 mg kg−1), used as the positive standard (Figure 4).
Figure 4.
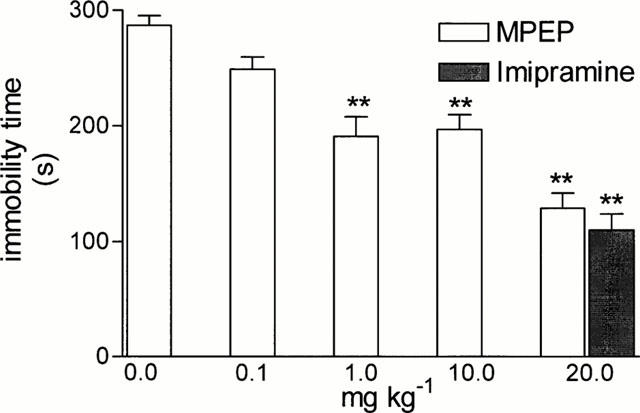
The effects of MPEP and imipramine on the total duration of immobility in the tail suspension test in mice. MPEP and imipramine were administered i.p. at 60 min before the test. Values represent the mean±s.e.mean of the immobility time during a 8 min experimental session, n=9 – 10. ** P<0.01 vs control group.
Discussion
Anxiolytic-like effects of MPEP
Benzodiazepines which are the most commonly used anxiolytic drugs, act via facilitation of the inhibitory GABA-ergic transmission. Benzodiazepines are effective agents, but disadvantageous side effects such as sedation, ataxia and abuse liability are associated with their administration. Decreased glutamatergic transmission, which leads to overall inhibitory effects in the central nervous system may have consequences similar to the effect of increased GABA-ergic transmission. Hence substances which inhibit stimulatory glutamatergic neurotransmission may possess anxiolytic effects. Indeed, antagonists of ionotropic glutamate receptors exhibit an anxiolytic-like activity in animal models (Stephens et al., 1986; Bennett et al., 1989; Jessa et al., 1996), however the potential clinical utility of competitive and noncompetitive NMDA antagonists is strictly limited by their undesirable side effects (Danysz & Parsons, 1998).
Substances which influence mGlu receptors, including agonists of group II mGluR and antagonists of group I mGluR, can also exert an inhibitory function in the brain (Conn & Pin 1997). Our earlier data have shown that (S)-4-carboxy-3-hydroxyphenylglycine (S-4C3H-PG), an antagonist of group I mGluR, exhibits anxiolytic-like activity in animals (Chojnacka-Wójcik et al., 1997). However, S-4C3H-PG is also an agonist of group II mGluR (Sekiyama et al., 1996) and agonists of group II mGluR exert anxiolytic-like effects in animals (Helton et al., 1998; Kłodzińska et al., 1999).
In order to further investigate the involvement of group I mGlu receptors in anxiety, we decided to evaluate the action of the selective antagonist of the mGlu5 receptor MPEP, which is devoid of agonist activity on group II mGlu receptors and which penetrates into the brain (Gasparini et al., 1999). An anxiolytic-like effect of MPEP was evaluated in three behavioural tests: the rat Vogel test (Vogel et al., 1971), the elevated plus-maze test (Pellow & File, 1986), and the four-plate test in mice (Aron et al., 1971). In the elevated plus-maze test the total number of entries (open+closed arm entries/test session) is taken as an index of drug effect on the locomotor activity, but this is a relatively insensitive measure (Dawson & Tricklebank, 1995). MPEP caused a small but significant increase in the total number of entries into the arms of the maze, but did not change the exploratory activity of rats in the open field test. Therefore, the increase in the percentage of the open arm entries/time spent on the open arms induced by MPEP is likely to reflect a specific anti-anxiety effect and can not be explained by competing behaviour such as exploration. This is further supported by the anxiolytic-like effects of MPEP in two conditioned response paradigms, i.e. the Vogel test and the four-plate test. In the Vogel test in rats the action of MPEP was not related to reduced perception of the stimulus or to an increased thirst drive. Preliminary findings of anxiolytic-like effects of MPEP in unconditioned response tests (social exploration test, stress-induced hyperthermia and marble burying test (Spooren et al., 2000), suggest, that MPEP exhibits anxiolytic effects in various rodent models of anxiety. Taken together, all the data suggests that MPEP produces potential anti-anxiety effects and indicate an involvement of mGlu5 receptors in anxiety.
The hippocampus is involved in anxiety (Gray, 1982) and effects of different anxiolytics, including a variety of agents acting upon the glutamatergic system (e.g. Przegalinski et al., 1997). In the hippocampus, a high expression of mRNA for group I mGlu receptors (see Testa et al., 1998), as well as the high immunoreactivity of group I mGlu receptors (Shigemoto et al., 1997; Blumcke et al., 1996) were found. That structure is also intensely immunolabelled by mGluR5 antibody (Lujan et al., 1996). The ability of S-4C3H-PG, an antagonist of group I mGlu receptors to produce anxiolytic responses in the Vogel test after intrahippocampal administration (Chojnacka-Wójcik et al., 1997), further suggests that this structure might be related to anxiolytic effects of group I mGlu receptor antagonists including MPEP. To verify that hypothesis experiments with intra-hippocampal injections of the MPEP are in progress.
Antidepressant-like effects of MPEP
The antidepressant-like effects of MPEP were evaluated in two behavioural tests, the tail suspension test in mice and the Porsolt test in rats. While MPEP did shorten the immobility time in one model of depression in mice, a tail suspension test, it was inactive in the behavioural despair test. The tail suspension test shows a higher predictive validity for identifying potentially useful pharmacotherapies for depression, compared to the behavioural despair test, e.g. it detects the antidepressant effects of specific serotonin reuptake inhibitors (Ali-Kodja et al., 1986), and as shown by the above results, an action of the mGlu5 receptor antagonist. Earlier data indicate that the excitatory effect of an agonist of the group I mGlu receptor system is influenced by prolonged treatment with an antidepressant drug imipramine or by chronic electroconvulsive (ECS) treatment (Palucha et al., 1997; Pilc et al., 1998). In those experiments performed in the CA1 area of the hippocampus, the (R,S)-3,5-dihydroxyphenylglycine-mediated increase of the population spike, was attenuated both by chronic imipramine and ECS (Palucha et al., 1997; Pilc et al., 1998). It can be speculated therefore, that it is the inhibition of group I mGlu receptor mediated neurotransmission which can contribute to the antidepressant-like effects of both MPEP and imipramine or ECS.
The preclinical data indicate that compounds which reduce transmission at NMDA receptors behave like antidepressants (Skolnick, 1999). Glutamatergic transmission via stimulation of group I mGlu receptors has also been shown to potentiate the ionotropic glutamate responses in various preparations (Glaum & Miller, 1994), including potentiation of NMDA currents (Fitzjohn et al., 1996; Ugolini et al., 1999). The blockade of group I mGlu receptors by MPEP may therefore lead to a decrease in NMDA-receptor-mediated neurotransmission and might contribute to the antidepressant-like effect of MPEP. It can be speculated that MPEP, which neither causes sedation nor disturbs the rota-rod performance, might be free of side effects produced by antagonists of NMDA receptors.
In conclusion, MPEP is a selective, systematically active antagonist of mGlu5 receptors. It produced anxiolytic-like effects in several tests such as the Vogel test in rats, the elevated plus-maze test in rats as well as the four-plate test in mice. MPEP also exerted antidepressant-like effects in the tail suspension test in mice. It was also found that MPEP did not induce sedation nor disturb motor coordination in animals. The above results indicate that antagonists of mGlu5 receptors may play a role in the therapy of anxiety and/or depression. Identification of the sites of action of MPEP and of the mechanism of these effects still requires further studies.
Acknowledgments
The study was supported by the Institute of Pharmacology, Polish Acad. Sci., and by the KBN grants No 4.P05A.091.17 to A. Pilc.
Abbreviations
- S-4C3H-PG
(S)-4-Carboxy-3-hydroxyphenylglycine
- CNS
central nervous system
- GABA
γ-aminobutyric acid
- L-5-HTP
L-5-hydroxytryptophan
- mGluR
metabotropic glutamate receptors
- MPEP
2-methyl-6-(phenylethynyl)-pyridine
- NMDA
N-methyl-D-aspartic acid
References
- ALI-KODJA F., BOUGARD M., PERRAULT G., ZIVKOVIC B. Effect of serotonin uptake inhibitors on the immobility of mice in the tail suspension test. Br. J. Pharmacol. 1986;87:130P. [Google Scholar]
- ARON C., SIMON P., LAROUSSE C., BOISSIER J.R. Evaluation of a rapid technique for detecting minor tranquilizers. Neuropharmacology. 1971;10:459–469. doi: 10.1016/0028-3908(71)90074-8. [DOI] [PubMed] [Google Scholar]
- BAJKOWSKA M., BRAŃSKI P., ŚMIAŁOWSKA M., PILC A. Effect of chronic antidepressant or electroconvulsive shock treatment on mGLuR1a immunoreactivity expression in the rat hippocampus. Pol. J. Pharmacol. 1999;51:539–541. [PubMed] [Google Scholar]
- BENNETT D.A., BERNARD P.S., AMRICK C.L., WILSON D.E., LIEBMAN J.M., HUTCHISON A.J. Behavioral pharmacological profile of CGS 19755, a competitive antagonist at N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 1989;250:454–460. [PubMed] [Google Scholar]
- BLUMCKE I., BEHLE K., MALITSCHEK B., KUHN R., KNOPFEL T., WOLF H.K., WIESTLER O.D. Immunohistochemical distribution of metabotropic glutamate receptor subtypes mGluR1b, mGluR2/3, mGluR4a and mGluR5 in human hippocampus. Brain Res. 1996;736:217–226. doi: 10.1016/0006-8993(96)00697-x. [DOI] [PubMed] [Google Scholar]
- BRUNO V., COPANI A., BATTAGLIA G., RAFFAELE R., SHINOZAKI H., NICOLETTI F. Protective effect of the metabotropic glutamate receptor agonist, DCG-IV, against excitotoxic neuronal death. Eur. J. Pharmacol. 1994;256:109–112. doi: 10.1016/0014-2999(94)90624-6. [DOI] [PubMed] [Google Scholar]
- CHOJNACKA-WÓJCIK E., TATARCZYŃSKA E., PILC A. The anxiolytic-like effect of metabotropic glutamate receptor antagonists after intrahippocampal injection in rats. Eur. J. Pharmacol. 1997;319:153–156. doi: 10.1016/s0014-2999(96)00941-7. [DOI] [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Ann. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- DANYSZ W., PARSONS C.G. Glycine and N-methyl-D-aspartate receptors: Physiological significance and possible therapeutic applications. Pharmacol. Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- DANYSZ W., PARSONS C.G., BRESNIK I., QUACK G. Glutamate in CNS Disorders. Drug News & Perspectives. 1996;8:261–277. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- DAWSON G.R., TRICKLEBANK M.D. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends. Pharmacol. Sci. 1995;16:33–36. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- FITZJOHN S.M., IRVING A.J., PALMER M.J., HARVEY J., LODGE D., COLLINGRIDGE G.L. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci. Lett. 1996;203:211–213. doi: 10.1016/0304-3940(96)12301-6. [DOI] [PubMed] [Google Scholar]
- GASPARINI F., LINGENHOHL K., STOEHR N., FLOR P.J., HEINRICH M., VRANESIC I., BIOLLAZ M., ALLGEIER H., HECKENDORN R., URWYLER S., VARNEY M.A., JOHNSON E.C., HESS S.D., RAO S.P., SACAAN A.I., SANTORI E.M., VELICELEBI G., KUHN R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- GLAUM S.R., MILLER R.J.Acute Regulation of Synaptic Transmission by Metabotropic Glutamate Receptors The Metabotropic Glutamate Receptors 1994Totowa, NJ: Humana Press; 147–172.ed. Conn, P.J. and Patel, J. pp [Google Scholar]
- GRAY J.A. Precis of the neuropsychology of anxiety:an enquiry into the functions of the septo-hippocampal system. Behav. Brain Sci. 1982;5:469–534. [Google Scholar]
- HELTON D.R., TIZZANO J.P., MONN J.A., SCHOEPP D.D., KALLMAN M.J. Anxiolytic and side-effect profile of LY354740: a potent, highly selective, orally active agonist for group II metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 1998;284:651–660. [PubMed] [Google Scholar]
- JANSSEN P.A., JAGENEAU A.H., SCHELLEKENS K.H. Chemistry and pharmacology of compounds related to 4-(4-hydroxy-4-phenyl-piperidino)-butyrophenone. IV. Influence of haloperidol (R 1625) and of chlorpromazine on the behaviour of rats in an unfamiliar ‘open field' situation. Psychopharmacologia. 1960;1:389–392. doi: 10.1007/BF00441186. [DOI] [PubMed] [Google Scholar]
- JESSA M., NAZAR M., BIDZINSKI A., PŁAŹNIK A. The effects of repeated administration of diazepam, MK-801 and CGP 37849 on rat behavior in two models of anxiety. Eur. Neuropsychopharmacol. 1996;6:55–61. doi: 10.1016/0924-977x(95)00068-z. [DOI] [PubMed] [Google Scholar]
- KŁODZIŃSKA A., CHOJNACKA-WÓJCIK E., PAŁUCHA A., BRAŃSKI P., POPIK P., PILC A. Potential anti-anxiety, anti-addictive effects of LY 354740, a selective group II glutamate metabotropic receptors agonist in animal models. Neuropharmacology. 1999;38:1831–1839. doi: 10.1016/s0028-3908(99)00066-0. [DOI] [PubMed] [Google Scholar]
- LUJAN R., NUSSER Z., ROBERTS J.D.B., SHIGEMOTO R., SOMOGYI P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- MONAGHAN D.T., BRIDGES R.J., COTMAN C.W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- NICOLETTI F., BRUNO V., COPANI A., CASABONA G., KNOPFEL T. Metabotropic glutamate receptors: A new target for the therapy of neurodegenerative disorders. Trends Neurosci. 1996;19:267–271. doi: 10.1016/S0166-2236(96)20019-0. [DOI] [PubMed] [Google Scholar]
- PAŁUCHA A., BRAŃSKI P., TOKARSKI K., BIJAK M., PILC A. Influence of imipramine treatment on the group I of metabotropic glutamate receptors in CA1 region of hippocampus. Pol. J. Pharmacol. 1997;49:495–497. [PubMed] [Google Scholar]
- PELLOW S., FILE S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmcol. Biochem. Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- PILC A., BRANSKI P., PALUCHA A., TOKARSKI K., BIJAK M. Antidepressant treatment influences group I of glutamate metabotropic receptors in slices from hippocampal CA1 region. Eur. J. Pharmacol. 1998;349:83–87. doi: 10.1016/s0014-2999(98)00169-1. [DOI] [PubMed] [Google Scholar]
- PORSOLT R.D., ANTON G., BLAVET N., JALFRE M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- PRZEGALIŃSKI E., TATARCZYŃSKA E., DEREŃ-WESOŁEK A., CHOJNACKA-WÓJCIK E. Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist. Neuropharmacology. 1997;36:31–37. doi: 10.1016/s0028-3908(96)00157-8. [DOI] [PubMed] [Google Scholar]
- ROWLAND N.E., CAPUTO F.A., FREGLY M.J. Water intake induced in rats by serotonin and 5-hydroxytryptophan: different mechanisms. Brain Res. Bull. 1987;18:501–508. doi: 10.1016/0361-9230(87)90115-8. [DOI] [PubMed] [Google Scholar]
- SEKIYAMA N., HAYASHI Y., NAKANISHI S., JANE D.E., TSE H.W., BIRSE E.F., WATKINS J.C. Structure-activity relationships of new agonists and antagonists of different metabotropic glutamate receptor subtypes. Br. J. Pharmacol. 1996;117:1493–1503. doi: 10.1111/j.1476-5381.1996.tb15312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIGEMOTO R., KINOSHITA A., WADA E., NOMURA S., OHISHI H., TAKADA M., FLOR P.J., NEKI A., ABE T., NAKANISHI S., MIZUNO N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOLNICK P., LAYER R.T., POPIK P., NOWAK G., PAUL I.A., TRULLAS R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: Implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- SKOLNICK P. Antidepressants for the new millennium. Eur. J. Pharmacol. 1999;375:31–40. doi: 10.1016/s0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- SPOOREN W.P., VASSOUT A., NEIJT H.C., KUHN R., GASPARINI F., ROUX S., PORSOLT R.D., GENTSCH C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J. Pharmacol. Exp. Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- STEPHENS D.N., MELDRUM B.S., WEIDMANN R., SCHNEIDER C., GRUTZNER M. Does the excitatory amino acid receptor antagonist 2-APH exhibit anxiolytic activity. Psychopharmacology (Berl.) 1986;90:166–169. doi: 10.1007/BF00181234. [DOI] [PubMed] [Google Scholar]
- STERU L., CHERMAT R., THIERRY B., SIMON P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- TESTA C.M., FRIBERG I.K., WEISS S.W., STANDAERT D.G. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J. Comp. Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- UGOLINI A., CORSI M., BORDI F. Potentiation of NMDA and AMPA responses by the specific mGluR(5) agonist CHPG in spinal cord motoneurons. Neuropharmacology. 1999;38:1569–1576. doi: 10.1016/s0028-3908(99)00095-7. [DOI] [PubMed] [Google Scholar]
- VARNEY M.A., COSFORD N.D.P., JACHEC C., RAO S.P., SACAAN A., LIN F.-F., BLEICHER L., SANTORI E.M., FLOR P.J., ALLGEIER H., GASPARINI F., KUHN R., HESS S.D., VELIÇELEBI G., JOHNSON E.C. SIB-1757 and SIB-1893: Selective, non-competitive antagonists of metabotropic glutamate receptor type 5 (mGluR5) Mol. Pharmacol. 1999;290:170–181. [PubMed] [Google Scholar]
- VOGEL J.R., BEER B., CLODY D.E. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971;21:1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- WILEY J.L., CRISTELLO A.F., BALSTER R.L. Effects of site-selective NMDA receptor antagonists in an elevated plus-maze model of anxiety in mice. Eur. J. Pharmacol. 1995;294:101–107. doi: 10.1016/0014-2999(95)00506-4. [DOI] [PubMed] [Google Scholar]
- WRÓBLEWSKI J.T., DANYSZ W. Modulation of glutamate receptors: molecular mechanisms and functional implications. Modulation of glutamate receptors: molecular mechanisms and functional implications. Ann. Rev. Pharmacol. Toxicol. 1989;29:441–474. doi: 10.1146/annurev.pa.29.040189.002301. [DOI] [PubMed] [Google Scholar]


