Abstract
We investigated the effect of KB-R7943, a Na+/Ca2+ exchange inhibitor, on the aggregation response induced by adrenaline and 5-hydroxytryptamine (5-HT), alone or in combination in human and rabbit platelets in the presence or absence of ouabain.
KB-R7943 inhibited aggregation induced by the combination of adrenaline and 5-HT in a concentration-dependent manner. The IC50 values of KB-R7943 were 4.2±2.0 or 3.0±0.7 μM with washed rabbit platelets with or without ouabain pretreatment, respectively.
In platelet-rich human plasma, the aggregation was biphasic. The IC50 value of KB-R7943 was 17.2±4.4 μM for the first phase aggregation.
KB-R7943 did not inhibit the first phase of aggregation induced by adrenaline alone, or the monophasic aggregation induced by 5-HT alone.
The aggregation of rabbit platelets depended on the presence of K+ in the medium, and K+-dependent and K+-independent Ca2+ influx were observed in resting platelets. Ouabain treatment increased only the K+-dependent but not the K+-independent Ca2+ influx.
KB-R7943 inhibited K+-dependent Ca2+ influx with or without ouabain pretreatment, but not K+-independent Ca2+ influx.
From these results, we conclude that KB-R7943 inhibits the adrenaline plus 5-HT induced aggregation of rabbit and human platelets by inhibiting K+-dependent Na+/Ca2+ exchange (NCKX). Our results suggest that NCKX plays an important role in platelet aggregation.
Keywords: KB-R7943, K+-dependent Na+/Ca2+ exchange, rabbit platelets, human platelets, K+-dependent aggregation, K+-dependent Ca2+-influx, adrenaline and 5-HT
Introduction
Blood platelets play a role in haemostasis through their aggregation in response to various endogenous agonists such as thrombin, collagen, thromboxane A2, platelet-activating factor, adrenaline and 5-HT. Although adrenaline and 5-HT are weak activators of platelets (Holmsen, 1987), their combined administration produces marked aggregation of human and rabbit platelets (Takano et al., 1980; Roevens et al., 1993). The cooperative effects of these two ligands may be relevant in vivo for the activation of platelets, since a supraphysiological concentration of either adrenaline or 5-HT alone is required to induce aggregation. A low level of adrenaline in the blood may potentiate the effects of 5-HT and/or ADP released endogenously from the platelets via α2-adrenoceptors (Vanags et al., 1998). 5-HT induces shape change or a slight aggregation of platelets via 5-HT2A receptors (Hoyer et al., 1994). However, the signal-processing mechanism, which mediates the cooperative effect, has not been clarified.
K+-dependent Na+/Ca2+ exchange (NCKX) activity has been demonstrated in human platelets by Kimura et al. (1993). Na+/Ca2+ exchange (NCX) through the plasma membrane plays an important role in controlling cytosolic Ca2+ concentrations ([Ca2+]i) in a variety of tissues. Two families of Na+/Ca2+ exchange proteins have been reported in mammalian tissues. One is the NCX present in cardiac muscle, brain and skeletal muscle, which has a stoichiometry of 3 Na+: 1Ca2+ (review by Blaustein & Lederer, 1999) or 4 Na+: 1Ca2+ (Fujioka et al., 2000). The other is the NCKX, found initially in retinal rods, which has a stoichiometry of 4 Na+: (1Ca2++1K+) (Schnetkamp et al., 1989; Cervetto et al., 1989). The NCKX in human platelets was found to be identical to that in the human retinal rods (Kim et al., 1998; Kimura et al., 1999). Ca2+ influx via the exchanger in human platelets is dependent on the presence of extracellular K+ (Kimura et al., 1993).
KB-R7943 has been reported to be a potent and relatively selective inhibitor of NCX in guinea-pig cardiac ventricular cells (Watano et al., 1996; Iwamoto et al., 1996). In the present study, we observed that KB-R7943 inhibited the aggregation of human and rabbit platelets induced by simultaneous administration of adrenaline and 5-HT. Our experiments had three major objectives: to see if the inhibition of the synergistic aggregation by KB-R7943 is at the level of the adrenaline or 5-HT receptor, to determine if the drug inhibits K+-dependent Ca2+ influx through the Na+/Ca2+ exchanger in resting platelets, and to see if K+-dependent Na+/Ca2+ exchange is involved in the aggregation induced by the combined administration of adrenaline and 5-HT.
Methods
Washed rabbit platelets
Rabbits of both sexes, weighing 3.0 – 3.5 kg, were anaesthetized by intramuscular injection of sodium pentobarbital (30 mg kg−1). Blood was collected from the carotid artery into an acid-citrate-dextrose (ACD) solution (v v−1: 1/6), which was composed of 85 mM sodium citrate, 65 mM citric acid and 2% dextrose. Platelet-rich plasma was prepared from whole blood by centrifugation at 140×g for 12 min at room temperature. Centrifuging the platelet-rich plasma at 900×g for 10 min then pelleted platelets. The platelets were washed first with Tyrode-HEPES albumin buffer (pH 6.35) containing apyrase (0.05 u ml−1) without Ca2+, and then with NaCl buffer (pH 6.35) containing apyrase (0.05 U ml−1) without Ca2+ and K+. The platelets were finally suspended in the NaCl buffer (pH 7.35) containing 1.8 mM CaCl2 and 5 mM KCl without apyrase. Apyrase, an adenosine nucleotidase, was necessary to minimize the desensitization of purinoceptors by ATP and ADP spontaneously from the platelets during the washing procedure. The Tyrode-HEPES albumin buffer consisted of (mM) NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1, NaHCO3 4, NaH2PO4 0.4, glucose 5.6, HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid) 10 and 0.35% albumin. The NaCl buffer contained (mM): NaCl 140, KCl 5, CaCl2 1.8, MgCl2 1, glucose 10, HEPES 10 and 0.35% albumin.
Ouabain-pretreated platelets
To load Na+ intracellularly, the platelets were incubated for 40 – 120 min with 0.1 mM ouabain in 5 ml of pH 7.35 Ca2+-free Tyrode-HEPES albumin buffer and then diluted with the same volume of pH 6.35 buffer. The platelet concentration was adjusted to 3 – 5×105 platelets μl−1. For the controls, normal platelets were incubated with 5% glucose (solvent of ouabain) under the same conditions.
Platelet-rich human plasma
Blood from informed healthy human volunteers, who were free of any medication for 10 days, was collected into a 3.8% trisodium citrate solution (v v−1: 1/9). Platelet-rich plasma (PRP) was obtained by centrifugation at 140×g for 12 min at room temperature and platelet-poor plasma (PPP) by centrifugation at 2000×g for 10 min. Concentration of the platelets in PRP was adjusted to 3 – 5×105 platelets μl−1 by diluting the PRP with PPP. The experiments using platelet-rich human plasma were performed within 3 h.
Platelet aggregation
Platelet aggregation was measured by turbidimetry using an aggregometer (NBC Hematracer 601; Nikou Bioscience Co., Tokyo, Japan) as described previously (Takano, 1995). The platelets were finally suspended in the pH 7.35 NaCl buffer, which did not contain apyrase. CaCl2 (1.8 mM) was added slowly to the platelet suspension (5×108 platelets ml−1). After a 2-min preincubation, various concentrations of K+ and KB-R7943, and various stimuli, were added to the platelet suspension.
45Ca2+ influx measurement
Platelets were preincubated for 40 min or more at 37°C with or without ouabain, and then centrifuged and suspended in the medium described above. The platelet concentration was adjusted to 1 – 5×105 platelets μl−1. An aliquot of the platelet suspension containing 0.1 mM cold CaCl2 was incubated with 45Ca2+ (0.2 – 0.4 μCi) in a final volume of 200 μl in the presence or absence of 5 mM K+. After incubation at 37°C, the mixture was diluted with 4 ml of ice-cold buffer containing (mM): KCl 200, EGTA 0.1 and Tris 5 (pH 7.4) and harvested by filtration (Advantec Toyo, GC-50, Toyo Roshi Kaisha Ltd., Tokyo). The filter was rinsed twice with the same buffer, and radioactivity was measured in a liquid scintillation counter.
Materials
KB-R7943 (2-[2{4-(4-nitrobenzyloxy)phenyl}ethyl]isothiourea methanesulphonate) was a kind gift from Kanebo Co. Ltd, Osaka (now Nippon Organon, Japan), Adrenaline bitartrate, ouabain, apyrase (grade 1) and bovine albumin (type V) were from Sigma Chemical Co., St. Louis MS, U.S.A. 5-hydroxytryptamine creatinine sulfate monohydrate and HEPES were from Wako Pure Chemicals, Osaka, Japan. 45CaCl2 (specific activity; 20 mCi mg−1 calcium) was from Amersham Pharmacia Biotech UK Ltd. (Buckinghamshire, U.K.).
KB-R7943 was dissolved in dimethylsulfoxide (DMSO) and diluted with 5% glucose. The final concentration of DMSO was less than 0.3%. For control experiments 0.3% DMSO or 5% glucose alone was applied and found to have no direct effect. Other drugs were dissolved in and diluted with 5% glucose.
Statistical analyses
Statistical results were expressed as the mean±s.e.mean. The statistical significance was analysed by Student's t-test. Differences with a value of P<0.05 were considered significant.
Results
Inhibition of aggregation by KB-R7943
At first we tested the effect of KB-R7943 on the aggregation of rabbit platelets induced by adrenaline plus 5-HT. Although adrenaline and 5-HT alone at 10 μM did not induce aggregation of washed rabbit platelets (data not shown), the combined administration of two agonists caused a dose-dependent aggregation of washed rabbit platelets (Figure 1A inset). Adrenaline and 5-HT both at 10 μM caused a maximum aggregation of 45.3±4.6% (n=8). KB-R7943 inhibited this aggregation in a concentration-dependent manner (Figure 1A). The IC50 value of KB-R7943 was 3.0±0.7 μM (n=8) (Figure 1A). Pretreatment of human platelets with ouabain increases [Na+]i and consequently Ca2+ influx (Kimura et al., 1993) Ouabain-pretreatment augmented the maximum aggregation to 69.1±2.5% (n=6) (Figure 1B). KB-R7943 also inhibited this response with an IC50 value of 4.2±2.0 μM (n=6) (Figure 1C). KB-R7943 at 30 μM completely inhibited the response of platelets both treated and not treated with ouabain.
Figure 1.
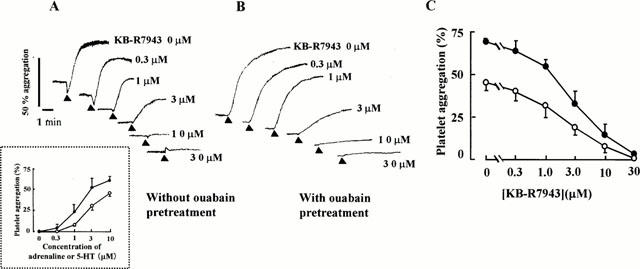
Effect of KB-R7943 on aggregation of washed rabbit platelets induced by adrenaline and 5-HT. (A) without ouabain pretreatment. Inset: Dose-response curves produced by combined administration of adrenalin and 5-HT in the presence or absence of ouabain (n=6). (B) with ouabain pretreatment. KB-R7943 was added 2 min before adding agonists. K+ (5 mM) was present in the external media. Solid triangles indicate where 10 μM adrenaline and 10 μM 5-HT were added simultaneously. (C) Concentration-inhibition curves of KB-R 7943 on the aggregation of rabbit platelets representatively shown in (A) and (B). Each point represents the mean±s.e.mean •; without ouabain pretreatment (n=8). ○; with ouabain pretreatment (n=6).
We next performed the same protocol using human platelets. In human platelet-rich plasma adrenaline and 5-HT alone at 1 μM did not induce aggregation (data not shown). As shown in Figure 2A, the combination of the two agonists both at 1 μM, induced aggregation in a biphasic manner in five out of six samples while one showed only a single-phased aggregation (data not shown). The first phase of the synergistic aggregation was small and less sensitive to KB-R7943 with an IC50 value of 17.2±4.4 μM (n=6). The second phase was much larger and more sensitive to KB-R7943 (IC50=5.4±2.6 μM, n=5) (Figure 2A,B).
Figure 2.
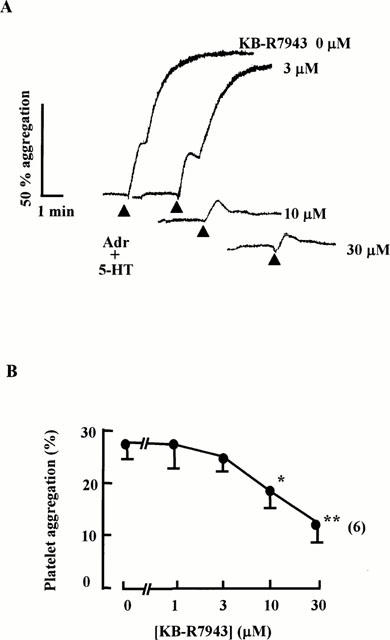
Effect of KB-R7943 on combined aggregation by adrenaline (1 μM) and 5-HT (1 μM) in platelet-rich human plasma. (A) Typical traces of inhibition of biphasic aggregation by KB-R7943. KB-R7943 was added 2 min before agonist application indicated by solid triangles. (B) Concentration-inhibition curves of KB-R7943 on the first phase of human platelet aggregation produced by the combined administration shown in (A). Each point represents the mean±s.e.mean with the number of preparations in parenthesis. *P<0.05, **P<0.01. Significant difference compared to the value in the absence of KB-R7943 using Student's t-test.
Adrenaline alone at 10 μM also produced biphasic aggregation in platelet-rich human plasma (Figure 3A). Unlike the combined aggregation, KB-R7943 at 30 μM did not inhibit the first phase and inhibited the second phase by about 30% (Figure 3A,B). 5-HT alone at 10 μM induced a slight aggregation of 12% (n=3) (data not shown) and KB-R7943 had no effect on this (Figure 3B).
Figure 3.
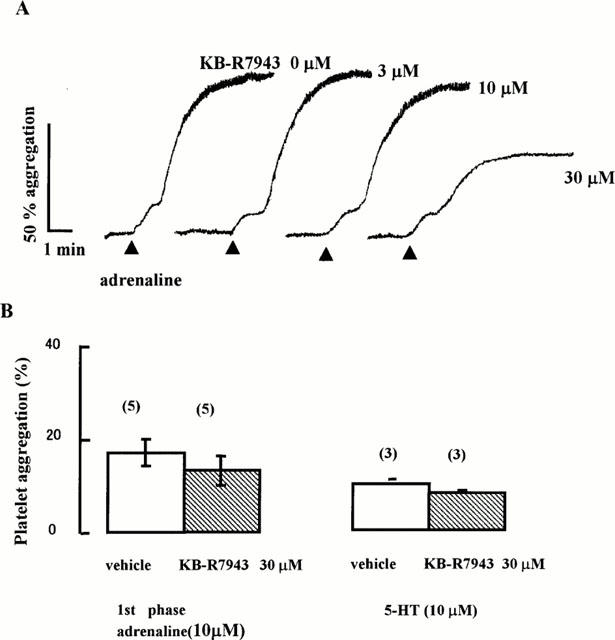
Effect of KB-R7943 on aggregation by adrenaline alone or 5-HT alone in platelet-rich human plasma. (A) Adrenaline (10 μM) was added (solid triangles) 2 min after KB-R7943. (B) Summary of effects of KB-R7943 30 μM on the platelet aggregation induced by adrenaline (10 μM) or 5-HT (10 μM). Each column represents the mean±s.e.mean with the number of preparations in parenthesis. Open columns, vehicle; Shaded columns, 30 μM KB-R7943.
Thus, inhibition by KB-R7943 is most potent against synergistic aggregation, and less potent against the second phase of adrenaline-induced aggregation with little effect on the first phase induced by adrenaline or 5-HT. These results indicate that the inhibitory effect of KB-R7943 is not due to inhibition at the levels of adrenaline and 5-HT receptors.
Effect of K+ on the aggregation of rabbit platelets
Since KB-R7943 inhibits NCX, it is possible that it also inhibits NCKX. We examined the possible involvement of NCKX in aggregation. We employed rabbit washed platelets, because their combined aggregation consists of a single phase.
First we examined the effect of external K+ concentration ([K+]o) on the aggregation with and without ouabain pretreatment. Aggregation was induced at various [K+]o between 0 – 5 mM, with rabbit platelets in the presence or absence of ouabain. Ouabain at 0.1 mM was used to elevate [Na+]i by inhibiting the Na+/K+ pump (Kimura et al., 1993). As shown in Figure 4, aggregation was [K+]o-dependent. In the absence of [K+]o, aggregation was completely suppressed, indicating that [K+]o was essential for aggregation. In addition, the aggregation was more pronounced with ouabain-pretreated platelets. These results indicate the involvement of NCKX in aggregation, the requirement of external K+ and the acceleration of aggregation by increased [Na+]i.
Figure 4.
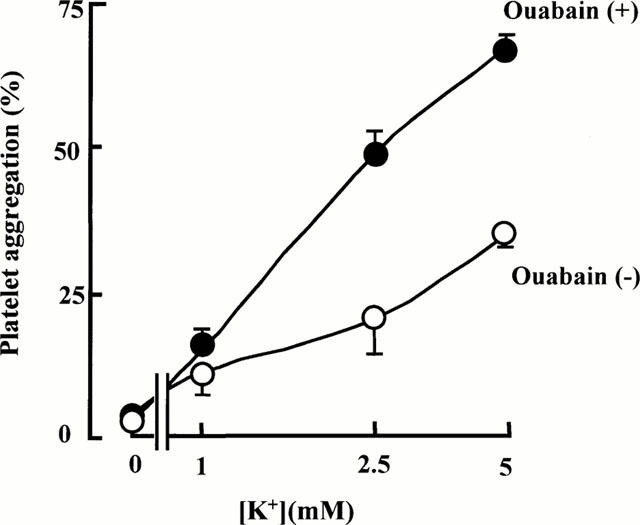
K+-dependence of the aggregation of rabbit platelets induced by adrenaline (10 μM) and 5-HT (10 μM) with (•) or without (○) ouabain pretreatment. Each point represents the mean±s.e.mean of four experiments.
Effect of K+ on 45Ca2+ influx in rabbit platelets
We next measured [K+]o-dependent and -independent 45Ca2+ influx in rabbit platelets using 45Ca2+ in the presence or absence of 5 mM [K+]o. As shown in Figure 5, 45Ca2+ influx was significantly greater in the presence than in the absence of external K+ and also in ouabain-pretreated than in non-treated platelets. In the presence of external K+, 45Ca2+ influx attained a level about twice that in its absence after 10 min in platelets not treated with ouabain. Ouabain-pretreatment markedly accelerated 45Ca2+ influx only in the presence of external K+. These results suggest that 45Ca2+ influx was dependent on external K+ and also internal Na+, suggesting that Ca2+o and K+o are cotransported in exchange for Na+i via NCKX in resting rabbit platelets.
Figure 5.
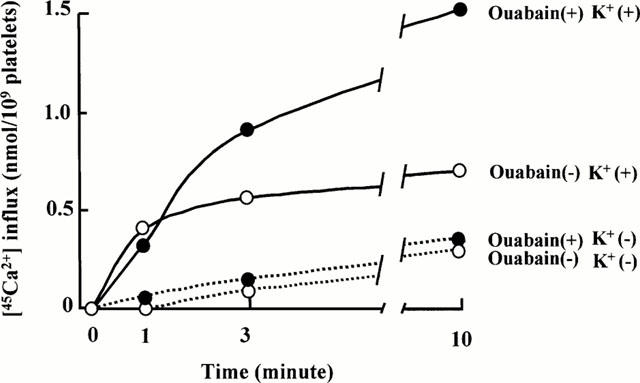
Time course of Ca2+ influx in rabbit platelets in the presence (solid line) and absence (dotted line) of external 5 mM K+ with (•) and without (○) ouabain. Platelets were incubated for 40 min at 37°C in Ca-free Tyrode-HEPES albumin buffer with or without 0.1 mM ouabain. The graph shows a typical experiment from four experiments with duplicate or triplicate determinations.
Effect of KB-R7943 on K+-dependent 45Ca2+ influx
Since K+- and ouabain-dependent 45Ca2+ influx was most likely via NCKX, we investigated whether KB-R7943 inhibited 45Ca2+ influx. Figure 6 shows the effect of KB-R7943 on 45Ca2+ influx in rabbit platelets treated and not treated with ouabain in the presence of 5 mM external K+. KB-R7943 at 30 μM inhibited 45Ca2+ influx significantly in both. In the absence of [K+]o, 30 μM KB-R7943 had no effect on 45Ca2+ influx (Figure 6). Thus, KB-R7943 inhibits 45Ca2+ influx in platelets treated and not treated with ouabain in the presence of external K+, but not in its absence, supporting the view that KB-R7943 inhibits NCKX.
Figure 6.
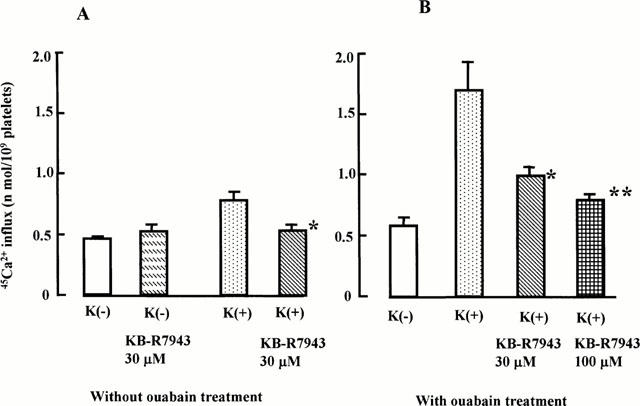
Effect of KB-R7943 on 45Ca2+ influx in rabbit platelets without (A) or with ouabain treatment (B). The graph shows mean±s.e.mean from three rabbits with triplicate determinations. *P<0.05, **P<0.01. Significant difference compared to the value in the presence of 5 mM K+ in the external medium using Student t-test.
Discussion
This is the first report to our knowledge that KB-R7943 is a potent inhibitor of the aggregation of the rabbit and human platelets induced by adrenaline and 5-HT. In rabbit and human platelets, a low concentration of adrenaline and 5-HT alone do not induce aggregation, but their simultaneous addition produces a significant aggregation. The combined aggregation of citrated human PRP was biphasic and adrenaline alone also induced biphasic aggregation. The following mechanism of biphasic aggregation has been suggested. The first phase is induced by stimulation of α2 adrenoceptors which activate Gi protein and inhibit adenylate cyclase. The secondary aggregation is included by thromboxane A2 generation from arachidonic acid via cyclo-oxygenase (Colman & Walsh, 1987; Hourani & Cusack, 1991). There is also a possibility of an artifact of low calcium effect in citrated human PRP, as discussed by Jarvis et al. (2000) for ADP. 5-HT stimulates 5-HT2A receptors which are coupled to Gq protein to activate phospholipase C (PLC) (Hoyer et al., 1994).
The combined administration of adrenaline and 5-HT should simultaneously activate α2-adrenoceptors and 5-HT2A receptors. However, the mechanism of the synergism of adrenaline and 5-HT has not been clarified. In the present study KB-R7943 hardly inhibited the first phase of aggregation induced by adrenaline alone or the monophasic aggregation induced by 5-HT alone, while it apparently inhibited the first phase of the aggregation induced by the simultaneous addition of the two ligands. Therefore, the inhibitory effects of KB-R7943 were not initiated at the levels of the adrenaline or 5-HT receptors.
ADP is known as another weak agonist, and it alone induces platelet aggregation in rabbits. A combination of low concentrations of ADP and adrenaline or ADP and 5-HT also produces marked aggregation. KB-R7943 at 30 μM, however, does not inhibit those combined aggregation (Takano, unpublished observation). These results indicate that KB-R7943-induced inhibition is specific for combined aggregation produced by adrenaline and 5-HT.
External K+ accelerated the aggregation in a concentration-dependent manner (Figure 4). Aggregation was more marked in rabbit platelets treated with ouabain than in those not treated. Since ouabain inhibits the Na+/K+ pump and increases [Na+]i, an increase in [K+]o and [Na+]i accelerates synergistic aggregation. These results may coincide with the effects of external K+ and internal Na+ on 45Ca2+ influx in resting platelets (Figures 5 and 6). The synergistic aggregation likely depends on K+-dependent but not K+-independent Ca2+ influx, because the aggregation did not occur in the absence of external K+. Furthermore, acceleration of the aggregation by ouabain is likely due to increases in Na+i-dependent Ca2+ influx. Taken together, these results strongly suggest that increased [K+]o and [Na+]i of the platelets facilitated Na+i/K+o+Ca2+o (NCKX) exchange and that NCKX is involved in the aggregation.
KB-R7943 inhibited the aggregation produced by adrenaline and 5-HT. In resting platelets, KB-R7943 at the high concentrations of 30 or 100 μM markedly decreased K+-dependent Ca2+ influx in platelets treated and not treated with ouabain. In contrast to K+-dependent Ca2+ influx, K+-independent Ca2+ influx was not inhibited by KB-R7943 (Figure 6). This result is consistent with the finding that 30 μM KB-R7943 inhibits the aggregation almost completely in the presence of external K+ (Figure 1) with or without ouabain pretreatment. Therefore, K+-dependent Ca2+ influx, which was inhibited by KB-R7943 may contribute to the synergistic aggregation. These results lead us to speculate that inhibition of aggregation by KB-R7943 was due to inhibition of NCKX in the platelets.
KB-R7943 inhibits the Na+/Ca2+ exchanger in guinea-pig cardiac ventricular cells, which exchanges 3 Na+ for 1 Ca2+ (Kimura et al., 1987; Blaustein & Lederer, 1999) or as reported more recently, 4 Na+ for 1 Ca2+ (Fujioka et al., 2000) with an IC50 value of 0.3 to 3 μM (Watano et al., 1996; Iwamoto et al., 1996; Kimura & Watano, 1998). Blood platelets have a distinct type of Na+/Ca2+ exchanger, called the Na+/Ca2++K+ exchanger (NCKX), because it transports 1 K+ ion in addition to 1 Ca2+ ion in exchange for 4 Na+ ions (Kimura et al., 1993; 1999). The topology of the two types of exchangers, platelet NCKX and cardiac NCX, are reported to be similar, although very little similarity was found in their amino acid sequences (Kimura et al., 1999; Iwamoto et al., 1999)
In vivo, the aggregation induced by the combination of adrenaline and 5-HT is important for microcirculation. Acutely injured endothelial cells in vascular beds are exposed to collagen fibrils. Collagen fibrils activate circulating platelets and release 5-HT into a localized area (Weksler, 1987). The injured cells may bring about a local rise in extracellular [K+]o. Thereby even a low blood level of adrenaline may be able to induce platelet aggregation in cooperation with 5-HT in the presence of elevated [K+]o. This condition might actually promote Ca2+ entry via NCKX and contribute to platelet activation. Further work is necessary to elucidate the function of NCKX in platelets.
Acknowledgments
We are grateful to Dr Isao Matsuoka for helpful advice and discussion throughout the study. This work was supported by Grants-in-Aids from the Ministry of Education, Science, Sports and Culture of Japan (11670096, 11357020).
Abbreviations
- 5-HT
5-hydroxytryptamine
- KB-R7943
(2-[2{4-(4-nitrobenzyloxy)phenyl}ethyl]isothiourea methanesulphonate
- NCKX
K+-dependent Na+/Ca2+ exchange
- NCX
Na+/Ca2+ exchange
- NCX1
a cardiac type Na+/Ca2+ exchange
- PPP
platelet-poor plasma
- PRP
platelet rich plasma
References
- BLAUSTEIN M.P., LEDERER W.J. Sodium/Calcium exchange: Its physiological implications. Physiol. Rev. 1999;47:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- CERVETTO L., LAGNADO L., PERRY R.J., ROBINSON D.W., MCNAUGHTON P.A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989;337:740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- COLMAN R.W., WALSH P.N.Mechanisms of platelet aggregation Hemostasis and Thrombosis 1987Lippincott Co.: Philadelphia; 594–605.ed. Colman, R.W., Hirsh, J., Marder, V.J. & Salzman, E.W. pp [Google Scholar]
- FUJIOKA Y., KOMEDA M., MATSUOKA S. Stoichiometry of Na+-Ca2+ exchange in inside-out patches excised from guinea-pig ventricular myocytes. J. Physiol. 2000;523:339–351. doi: 10.1111/j.1469-7793.2000.t01-2-00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMSEN H.Platelet secretion Hemostasis and Thrombosis 1987Lippincott Co.: Philadelphia; 606–617.ed. Colman, R.W., Hirsh, J., Marder, V.J. & Salzman, E.W. pp [Google Scholar]
- HOURANI S.M.O., CUSACK N.J. Pharmacological receptors on blood platelets. Pharmacol. Rev. 1991;43:243–298. [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.A. International Union of pharmacology classification of receptors for 5-hydroxytryotamine (serotonin) Pharmacol. Rev. 1994;46:158–203. [PubMed] [Google Scholar]
- IWAMOTO T., WATANO T., SHIGEKAWA M. A novel isothiourea derivative selectivity inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J. Biol. Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- IWAMOTO T., NAKAMURA T.Y., PAN Y., UEHARA A., IMANAGA I., SHIGEKAWA M. Unique topology of the internal repeats in the cardiac Na+/Ca2 exchanger. FEBS Lett. 1999;446:264–268. doi: 10.1016/s0014-5793(99)00218-5. [DOI] [PubMed] [Google Scholar]
- JARVIS G.E., HUMPHRIES R.G., ROBERTSON M.J., LEFF P. ADP can induce aggregation of human platelets via both P2Y1 and P2T receptors. Br. J. Pharmacol. 2000;129:275–282. doi: 10.1038/sj.bjp.0703046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM T.S.Y., REID D.M., MOLDAY R.S. Structure-function relationships and localization of the Na/Ca-K exchange in rod photoreceptors. J. Biol. Chem. 1998;273:16561–16567. doi: 10.1074/jbc.273.26.16561. [DOI] [PubMed] [Google Scholar]
- KIMURA J., MIYAMAE S., NOMA A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J. Physiol. (Lond.) 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA J., WATANO T. Calcium-dependent inhibition of the sodium-calcium exchange current by KB-R7943. Can. J. Cardiol. 1998;14:259–262. [PubMed] [Google Scholar]
- KIMURA M., AVIV A., REEVES J.P. K+-dependent Na+/Ca2+ exchange in human platelets. J. Biol. Chem. 1993;268:6874–6877. [PubMed] [Google Scholar]
- KIMURA M., JEANCLOS E.M., DONNELLY R.J., LYTTON J., REEVES J.P., AVIV A. Physiological and molecular characterization of the Na+/Ca2+ exchanger in human platelets. Am. J. Physiol. Heart Circ. Physiol. 1999;27746:H911–H917. doi: 10.1152/ajpheart.1999.277.3.H911. [DOI] [PubMed] [Google Scholar]
- ROEVENS P., DE CLERCK F., DE CHAFFOY DE COURCELLES D. The synergistic effect of 5-hydroxytryptamine and epinephrine on the human platelet is related to the activation of phospholipase C. Eur. J. Pharmacol. 1993;245:273–280. doi: 10.1016/0922-4106(93)90107-k. [DOI] [PubMed] [Google Scholar]
- SCHNETKAMP P.P.M., BASU D.K., SZERENCSEI R.T. Na+-Ca2+ exchange in bovine rod outer segments requires and transports K+ Am. J. Physiol. Cell Physiol. 1989;25726:C153–C157. doi: 10.1152/ajpcell.1989.257.1.C153. [DOI] [PubMed] [Google Scholar]
- TAKANO S., SATO M., ROKKAKU Y., SUZUKI T. Influence of amoxapine, a new anti-depressant, on platelet aggregation in rabbit. Res. Commum. Chem. Pathol. Pharmacol. 1980;28:185–188. [Google Scholar]
- TAKANO S. Role of 5-hydroxytryptamine in platelet thrombus formation and mechanisms of inhibition of thrombus formation by 5-hydroxytryptamine2A antagonists in rabbits. Arch. Int. Pharmacodyn. 1995;330:297–307. [PubMed] [Google Scholar]
- VANAGS D.M., LIOYD J.V., RODGERS S.E., BOCHER F. ADP, adrenaline and Serotonin stimulate inositol 1,4,5-trisphosphate production in human platelets. Eur. J. Pharmacol. 1998;358:93–100. doi: 10.1016/s0014-2999(98)00595-0. [DOI] [PubMed] [Google Scholar]
- WATANO T., KIMURA J., MORITA T., NAKANISHI H. A novel antagonist, No.7945, of the Na+/Ca2+ exchange current in guinea-pig cardiac ventricular cells. Br. J. Pharmacol. 1996;119:555–563. doi: 10.1111/j.1476-5381.1996.tb15708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEKSLER B.B.Platelet interactions with the blood vessel wall Hemostasis and Thrombosis 1987Lippincott Co.: Philadelphia; 804–815.ed. Colman, R.W., Hirsh, J., Marder, V.J. & Salzman, E.W. pp [Google Scholar]


