Abstract
The effects of tonabersat (SB-220453) were evaluated on trigeminal nerve ganglion stimulation-induced sensory-autonomic neurovascular reflexes in the anaesthetized cat. Comparisons were made to intravenous administration of carabersat (SB-204269), and to valproate, gabapentin and lamotrigine following intraduodenal administration.
There were no effects on resting blood pressure, heart rate, carotid blood flow or carotid vascular resistance for any compound evaluated.
Trigeminal nerve ganglion stimulation increased carotid blood flow by 65% and reduced vascular resistance by 41% with minimal effect on blood pressure (<10%) and no effect on heart rate. Intravenous infusion of tonabersat or carabersat (both 3.4 μmol h−1) produced time related reductions in stimulation-induced responses with a maximal inhibition (relative to control) of 30±7% (n=4), at 240 min for tonabersat and 33±4% (n=3) at 180 min for carabersat. Tonabersat (11.5 μmol h−1) produced a similar inhibitory effect (32±9%, n=4) after 120 min of infusion.
Following intraduodenal administration of tonabersat, the maximal inhibition of nerve stimulation-induced responses was 55±4% at 120 min (n=4) for tonabersat 10 mg kg−1, and 24±2% after 180 min for 1 mg kg−1 (n=4).
Intraduodenal administration of sodium valproate (10 or 100 mg kg−1 n=4/group) had no effect on neurovascular reflexes. Maximal inhibition of nerve ganglion-stimulated reductions in carotid vascular resistance were observed at 150 min for lamotrigine (50 mg kg−1, 52±12%, n=4) and gabapentin (100 mg kg−1, 17±13%, n=3). Lamotrigine 10 mg kg−1 produced 22±11% (n=3) inhibition after 180 min.
These data demonstrate blockade of trigeminal parasympathetic reflexes with tonabersat, carabersat and other anticonvulsants. These agents may therefore have therapeutic benefit in conditions where this type of reflex is evident.
Keywords: Tonabersat, carabersat, lamotrigine, trigeminal nerve, neurovascular reflex, migraine, cluster headache
Introduction
Tonabersat (SB-220453) is a member of a family of novel benzoylamino-benzopyran compounds, typified by carabersat (SB-204269), which have potent anticonvulsant activity in a number of seizure models with potential for a good therapeutic ratio compared to other anticonvulsants (Chan et al., 1996; 1998; 1999; Herdon et al., 1997; Upton et al., 1997; Upton & Thompson, 2000). The pharmacology of this class of compound has been extensively investigated to demonstrate that they bind selectively to their own unique specific CNS binding site and have little effect on a variety of known anticonvulsant mechanisms (Herdon et al., 1996; 1997; Upton et al., 1997; 1999; Upton & Thompson, 2000). We have also recently demonstrated that tonabersat, abolishes trigeminal nerve ganglion-induced plasma protein extravasation in rats (Chan et al., 1999). In addition, intraperitoneal administration of tonabersat (10 mg kg−1) has been shown to inhibit the generation of spreading depression events and associated nitric oxide release following a cortical KCl stimulus in anaesthetized cats (Read et al., 2000). The pharmacological profile of tonabersat suggests a potential therapeutic role for this compound in the treatment of migraine headache.
The aim of the present series of experiments was to further understand the biological profile of this compound by evaluating its activity in a model of trigeminal nerve – VII (parasympathetic) neurovascular reflexes in the anaesthetized cat (Lambert et al., 1984). Stimulation of the trigeminal nerve ganglion produces a frequency related increase in carotid blood flow and concomitant reduction in carotid vascular resistance with minimal effects on arterial blood pressure in cats (Lambert et al., 1984; Raval et al., 1999), guinea-pigs (Beattie & Connor, 1994) and rats (Spokes & Middlefell, 1995). The trigeminal ganglion sends collateral fibres containing Substance P and Calcitonin gene-related peptide (CGRP) to parasympathetic nerve ganglia (Suzuki et al., 1989) indicating an axon reflex mechanism with the potential to modulate parasympathetic activity (May & Goadsby, 1999). This appears to be the case with the trigeminal nerve stimulation-induced reduction in carotid vascular resistance, this response is greatly reduced by section of the VII cranial nerve (Lambert et al., 1984) and blocked by a vasoactive intestinal polypeptide (VIP) antagonists (Goadsby & Macdonald, 1985; Beattie & Connor, 1994) and endothelinB receptor antagonists (Raval et al., 1999). However, sumatriptan (Spokes & Middlefell, 1995; Raval et al., 1999), NK1 antagonist (Beattie & Connor, 1994) or CGRP receptor antagonists (Raval et al., 1999) have no effect. Therefore, the data regarding sensory ganglion – parasympathetic reflex vasodilatation demonstrates marked differences from trigeminal ganglion-induced antidromic c-fibre mediated responses such as plasma protein extravasation or vasodilatation. For example, trigeminal ganglion-induced extravasation is blocked by sumatriptan (Buzzi & Moskowitz, 1990), NK-1 antagonists (Shepheard et al., 1993) or, in guinea-pigs, CGRP receptor blockade (O'Shaughnessy & Connor, 1994). Furthermore, sensory nerve-induced dilatation is also blocked by CGRP receptor antagonists (Escott et al., 1995; Goadsby, 1993).
In this study, we investigated the effects of tonabersat and carabersat on trigeminal ganglion stimulation-induced reductions in carotid vascular resistance in cats (Raval et al., 1999). We also compared the effects of tonabersat with the other anticonvulsants lamotrigine, sodium valproate and gabapentin following bolus intraduodenal administration.
Methods
Animals
This work was conducted in compliance with the Home Office Guidance on the operation of the Animals (Scientific Procedures) Act 1986, and was reviewed and approved by the SmithKline Beecham Procedures Review Panel.
Male cats weighing 2 – 8 kg were used in this study. Animals were housed in groups and allowed free access to food and water.
Surgical procedures
Cats were prepared for stimulation of the trigeminal nerve ganglion as previously described (Raval et al., 1999). Briefly, animals were anaesthetized with halothane (4%) in oxygen and maintained with intravenous administration of α-chloralose (100 – 120 mg kg−1) dissolved in borax 33 mg ml−1. The trachea was cannulated and cats were artificially ventilated with room air to maintain blood oxygen and carbon dioxide tensions within normal limits. The right femoral artery was cannulated for measurement of blood pressure and derived heart rate and the left femoral vein for the administration of drugs.
Bipolar electrodes (Rhodes NEX-100, Clark Electromedical, Reading U.K.) were placed through a burr hole craniotomy (relative to Bregma at −9.5 mm in the anterior posterior direction and +6.0 in the lateral direction). An electromagnetic flow probe (Statham Gould diameter 1.5 – 2.0 mm) was placed around the right carotid artery and calibrated to a standard flow and recorded on a Gould flow-meter. Using the above coordinates, a bipolar electrode was advanced into the right trigeminal nerve ganglion which was stimulated briefly (10 Hz, 2 mA) using opposite polarity square wave pulses from 2 Grass constant current units (CCU 1A) driven by Grass stimulator isolation units (SIU5A) and a Grass stimulator (S11). Correct placement of the electrode was verified by lower jaw movements, salivation and an increase in carotid blood flow. Animals were treated with guanethidine sulphate (3 mg kg−1 i.v) to minimize the effects of sympathetic stimulation (Raval et al., 1999).
Changes in arterial blood pressure, heart rate, carotid flow and carotid vascular resistance were assessed following control responses to trigeminal nerve stimulation (Raval et al., 1999).
Intravenous administration
Following control stimulations, tonabersat (3.4 μmol h−1), carabersat (3.4 μmol h−1) or vehicle (5% polyethylene glycol (PEG) in 5% glucose) was infused (25 ml h−1) via a femoral vein and trigeminal nerve stimulation was conducted at 30 – 60 min intervals for up to 240 min. In a second group of studies, vehicle (5% PEG in 5% glucose (30 ml h−1) or tonabersat (11.5 μmol h−1) was infused for 240 min.
Intraduodenal administration
In order to investigate the effects of intraduodenal administration of test substances, a mid-line incision was made to open the abdomen. A cannula was then inserted into the duodenum and secured by a purse string suture and positioned so that the tip was pointing towards the stomach but did not enter. Following control nerve ganglion stimulations, vehicle (1% w v−1 methylcellulose in water), tonabersat (1 or 10 mg kg−1), sodium valproate (10 or 100 mg kg−1), gabapentin (100 mg kg−1) or lamotrigine (10 or 50 mg kg−1) were administered intraduodenally as a bolus. Trigeminal nerve ganglion stimulations were then repeated at 15 – 30 min intervals for up to 3 h.
Materials
Tonabersat (SB-220453) (cis-(−)-6-acetyl-4S-(3-chloro-4-fluorobenzoylamino) -3,4-dihydro-2,2-dimethyl-2H-1-benzopyran-3S-ol) and Carabersat (SB 204269-EO) (trans-(+)-6-acetyl-4S-(4-fluorobenzoylamino) -3,4-dihydro-2,2-dimethyl-2H-1-benzopyran-3R-ol hemihydrate), gabapentin, lamotrigine, sodium valproate were synthesized by SmithKline Beecham Pharmaceuticals.
Data analysis
Carotid vascular resistance was calculated as mean arterial blood pressure divided by carotid blood flow. The following formula was used to calculate mean arterial blood pressure:((systolic−diastolic blood pressure)×1/3)+diastolic blood pressure.Baseline mean arterial blood pressure, carotid blood flow and heart rate were measured before and after each nerve ganglion stimulation. Trigeminal nerve ganglion stimulation-induced changes in mean arterial blood pressure, heart rate, carotid blood flow and calculated carotid vascular resistance were determined before and at the end of the 2-min stimulation period and the percentage change from control stimulation calculated. Control changes in mean arterial blood pressure, heart rate and carotid vascular resistance after nerve stimulation were derived from the mean values for the two responses obtained before administration of any drug.
Effects of drugs on nerve ganglion stimulation were expressed as per cent inhibition of the control response and their effects on resting carotid vascular resistance, heart rate and blood pressure were expressed as per cent change from initial resting values prior to drug administration.
To rule out the small effects of trigeminal nerve ganglion stimulation induced changes in arterial blood pressure on carotid blood flow, the effects of drugs were investigated on changes in carotid vascular resistance alone.
Data is presented as mean±s.e.mean. Effects of nerve stimulation on haemodynamic parameters was made by Student's t-test. Following intravenous or intraduodenal administration of drugs, comparisons were made between treatment groups at each time point using analysis of variance (ANOVA) followed by Duncan's multiple range test with a significance level of P<0.05.
Results
Effects of drugs on resting haemodynamic parameters in guanethidine pre-treated anaesthetized cats
Resting haemodynamic parameters are shown in Table 1 prior to control trigeminal ganglion stimulation in guanethidine pre-treated anaesthetized cats. Mean arterial blood pressure, heart rate, carotid blood flow and carotid vascular resistance were all in the physiological range and not significantly different between any of the treatment groups (ANOVA, P>0.05).
Table 1.
Effects of trigeminal nerve ganglion stimulation prior to drug administration
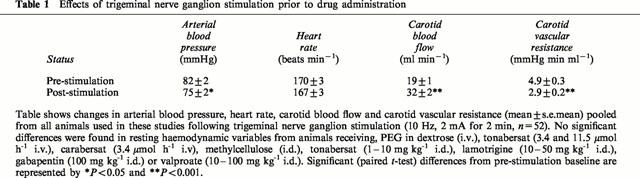
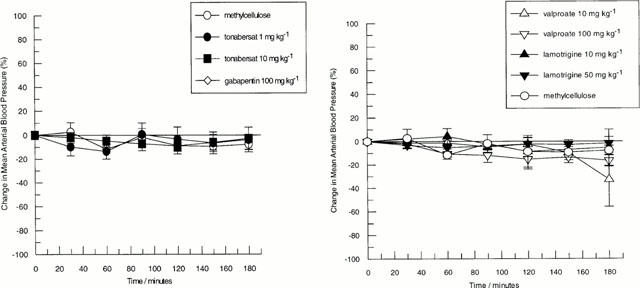
Following intraduodenal administration of tonabersat (1 or 10 mg kg−1), sodium valproate (10 or 100 mg kg−1), lamotrigine (10 or 50 mg kg−1) or gabapentin (100 mg kg−1) there was no significant effects on mean arterial blood pressure (Figure 1) or carotid vascular resistance (Figure 2), carotid blood flow or heart rate (not shown). Similarly following intravenous infusion of tonabersat (3.4 – 11.5 μmol h−1), carabersat (3.4 μmol h−1) or 5% PEG in 5% glucose (25 or 30 ml h−1) there were no significant effects on any recorded haemodynamic parameter (not shown).
Figure 1.
Effects of intraduodenal administration of anti-convulsants on changes in mean arterial blood pressure in the guanethidine pre-treated anaesthetized cat. Left panel shows the effects of tonabersat 1 mg kg−1 (n=4) and 10 mg kg−1 (n=4), gabapentin 100 mg kg−1 (n=3) and methylcellulose (n=3). Right panel shows the effects of lamotrigine 10 mg kg−1 (n=4) and 50 mg kg−1 (n=3), sodium valproate 10 mg kg−1 (n=4) and 100 mg kg−1 (n=4). For comparison, the effects of methylcellulose are also shown. Data points represent mean±s.e.mean of the per cent change from basal values.
Figure 2.
Effects of intraduodenal administration of anti-convulsants on changes in carotid vascular resistance in the guanethidine pre-treated anaesthetized cat. Left panel shows the effects of tonabersat 1 mg kg−1 (n=4) and 10 mg kg−1 (n=4), gabapentin 100 mg kg−1 (n=3) and methylcellulose (n=3). Right panel shows the effects of lamotrigine 10 mg kg−1 (n=4) and 50 mg kg−1 (n=3), sodium valproate 10 mg kg−1 (n=4) and 100 mg kg−1 (n=4). For comparison, the effects of methylcellulose are also shown. Data points represent mean±s.e.mean of the per cent change from basal values.
Effects of trigeminal nerve ganglion stimulation in guanethidine pre-treated anaesthetized cats
Trigeminal nerve stimulation (10 Hz, 2 mA) for 2 min produced a small depressor response (<10%) with no effect on heart rate (Table 1). In contrast to the systemic circulation, trigeminal nerve ganglion stimulation produced marked increases (65%) in carotid blood flow from 19±1 to 32±2 ml min−1 (Student's t-test, P<0.0001) with concomitant reductions (41%) in carotid vascular resistance from 4.9±0.3 to 2.9±0.2 mmHg min−1 ml−1 (Student's t-test, P<0.0001) (Table 1). Changes in carotid blood flow and carotid vascular resistance were similar in all experimental groups prior to drug intravenous or intraduodenal drug administration (ANOVA, P>0.05).
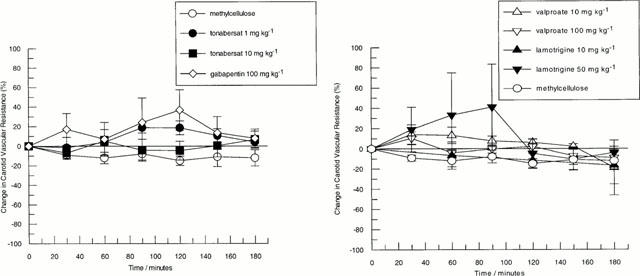
Intravenous administration of tonabersat or carabersat
Repeated trigeminal nerve ganglion stimulation induced reproducible reductions in carotid vascular resistance in the guanethidine pre-treated anaesthetized cat. Intravenous infusion of vehicle (5% PEG in 5% glucose) at rates of 25 or 30 ml h−1 had no significant effect on trigeminal nerve ganglion stimulation induced responses (n=3 – 4/group, Figure 3). Tonabersat (3.4 – 11.5 μmol h−1) and carabersat (3.4 μmol h−1) produced time related inhibition of trigeminal nerve stimulation-induced reductions in carotid vascular resistance. Comparison of each treatment group showed significant differences between groups at 120 min (F value 4.4), 180 min (F value 4.5) and 240 min (F value 5.0) post infusion (n=3 – 4). Post hoc analyses revealed that significant differences were observed for tonabersat (11.5 μmol h−1) at 120 min, 180 min and 240 min, whereas carabersat (3.4 μmol h−1) produced significant reductions in trigeminal stimulation-induced reflexes beginning at 180 min. Both carabersat and tonabersat (3.4 μmol h−1) significantly reduced trigeminal nerve stimulation-induced responses at 240 min (Figure 3). Similar peak inhibition of trigeminal nerve stimulation-induced carotid dilatation was observed in all groups but at different times post commencement of infusion (Figure 3). Thus, maximal observed inhibition of carotid vascular reflex was 32±9% at 120 min for tonabersat (11.5 μmol h−1), 33±4% at 180 min for carabersat (3.4 μmol h−1) and 30±7% at 240 min for tonabersat (3.4 μmol h−1).
Figure 3.
Effects of intravenous administration of vehicle (n=3 – 4, 5% PEG, 5% glucose) at 25 or 30 ml h−1 and carabersat (n=3, 3.4 μmol h−1) or tonabersat (n=4, 3.4 or 11.5 μmol h−1) on changes in trigeminal nerve ganglion stimulation-induced reductions in carotid vascular resistance in the guanethidine pre-treated anaesthetized cat. Data points represent mean±s.e.mean of the per cent inhibition of trigeminal nerve ganglion stimulation (TGN)-induced response from individual animals (*P<0.05 and **P<0.01, ANOVA with post hoc Duncans multiple range test at each time point).
The low solubility of these compounds limited additional investigation following intravenous administration. Tonabersat was selected for comparison against sodium valproate, lamotrigine and gabapentin following intraduodenal administration in the guanethidine pre-treated anaesthetized cat.
Comparison of effects of intraduodenal tonabersat and standard anticonvulsants on trigeminal nerve ganglion stimulation-induced reflex
Trigeminal nerve ganglion stimulation-induced reproducible reductions in carotid vascular resistance at 30-min intervals following bolus administration of vehicle (1% methylcellulose) (Figure 4). No significant inhibition of trigeminal nerve ganglion-induced responses was observed and the results were similar to those obtained following intravenous infusion of 5% PEG in 5% glucose vehicle (Figure 4).
Figure 4.
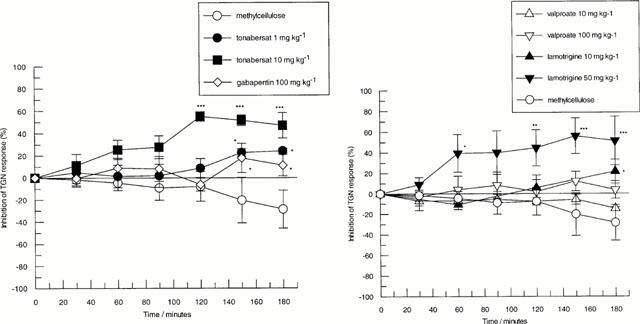
Effects of intraduodenal administration of anti-convulsants on changes in trigeminal nerve ganglion stimulation-induced reductions in carotid vascular resistance in the guanethidine pre-treated anaesthetized cat. Left panel shows the effects of tonabersat 1 mg kg−1 (n=4) and 10 mg kg−1 (n=4), gabapentin 100 mg kg−1 (n=3) and methylcellulose (n=3). Right panel shows the effects of lamotrigine 10 mg kg−1 (n=4) and 50 mg kg−1 (n=3), sodium valproate 10 mg kg−1 (n=4) and 100 mg kg−1 (n=4). For comparison, the effects of methylcellulose are also shown. Data points represent mean±s.e.mean of the per cent inhibition of trigeminal nerve ganglion stimulation (TGN)-induced response from individual animals (*P<0.05, **P<0.01 and ***P<0.001, ANOVA with post hoc Duncans multiple range test at each time point).
Comparisons of all treatment groups (n=3 – 4) showed significant differences between groups at 60 min (F value 2.7), 90 min (F value 2.1), 120 min (F value 6.1), 150 min (F value 6.5) and 180 min (F value 5.2). Post hoc analysis (Duncans multiple range test) revealed that tonabersat 1 mg kg−1 (n=4) produced a significant inhibition of response relative to vehicle at 150 and 180 min post administration (n=4). A higher dose of tonabersat (10 mg kg−1) produced significant inhibition of responses beginning at 120 min post administration (n=4). At an earlier time point (60 min), the effects of tonabersat (10 mg kg−1) showed a trend towards blockade of trigeminal nerve mediated responses (P=0.08). The maximal degree of inhibition of trigeminal nerve-induced carotid dilatation was 55±4 and 24±2% following tonabersat 10 and 1 mg kg−1 respectively (n=4).
Sodium valproate (10 or 100 mg kg−1) showed no significant differences from vehicle treated animals at any time point whereas gabapentin (100 mg kg−1) and lamotrigine (10 mg kg−1) produced a moderate but significant (<25%) degree of inhibition at 150 and 180 min respectively (Figure 4). A higher dose of lamotrigine (50 mg kg−1) produced significant inhibition of trigeminal nerve-induced responses from 60 min onwards with a peak inhibition of 52±12% (n=3) at 150 min (Figure 4).
Comparison of the minimum effective dose for inhibition of trigeminal nerve ganglion-induced vasodilatation show the following rank order of potency over the time course of the experiment: tonabersat>lamotrigine>gabapentin>sodium valproate (no effect). Tonabersat (10 mg kg−1) and lamotrigine (50 mg kg−1) produced a similar degree of maximal inhibition in this model whereas lower doses of tonabersat (1 mg kg−1) and lamotrigine (50 mg kg−1) had comparable efficacy to gabapentin (100 mg kg−1).
Discussion
This study is the first to document inhibition of sensory ganglion-parasympathetic neurovascular reflexes produced by the novel halogenated benzopyran anticonvulsants, tonabersat and carabersat. These agents lack direct cardiovascular activity in the guanethidine anaesthetized cat which is consistent with previous observations in rats (Chan et al., 1999; Upton et al., 1997; Upton & Thompson, 2000). The present study also demonstrates that these agents significantly attenuate trigeminal nerve ganglion stimulation-induced reductions in carotid vascular resistance. Carabersat was the lead agent identified in this family of compounds and has undergone extensive evaluation. Carabersat binds selectively with moderately high affinity (pKi 7.3 – 7.5) at a novel binding site found in the brain of rats and a range of other species, including man (Chan et al., 1999; Herdon et al., 1996; 1997), which has low affinity for a number of other standard ligands for a variety of channels, enzymes, G-protein coupled transmembrane receptors and transporters. Tonabersat also has high affinity for this site with a pKi value of 7. (Chan et al., 1999), and low affinity for a range of other molecular targets.
Effects of benzopyran anticonvulsants on sensory – autonomic reflexes
Following intravenous infusion, both tonabersat and carabersat produced time related inhibition of trigeminal ganglion stimulation-induced responses. Equimolar (3.4 μmol h−1) infusion of carabersat or tonabersat produced inhibition of trigeminal stimulation-induced responses with peak inhibition occurring at 3 h for carabersat and similar response after 4 h of tonabersat infusion. Increasing the rate of infusion of tonabersat (11.5 μmol h−1) produced a more rapid inhibition of trigeminal nerve ganglion-induced responses with an observed peak inhibition at 2 h. Although tonabersat is 2 – 3 fold more potent than carabersat at their novel binding site in vitro (Chan et al., 1999) our results are consistent with a longer half life for tonabersat in the guanethidine pre-treated anaesthetized cat. In preliminary experiments, infusion of either carabersat or tonabersat (3.4 μmol h−1) produced similar blood concentrations only after 3 or 4 h of infusion respectively (n=2 – 3, data not shown). Thus, these novel benzopyran anticonvulsants produce marked inhibition of trigeminal – autonomic neurovascular reflexes without any effect on systemic arterial blood pressure, heart rate, or carotid blood flow.
Activation of the sensory – autonomic reflex can be elicited by stimulation of the trigeminal nerve afferents on the sagittal sinus as electrical stimulation of this structure results in release of VIP into the circulation (Zagami et al., 1990) by a reflex activation of the parasympathetic nervous system (see May & Goadsby, 1999). However, sensory nerve stimulation also results in activation of central pain processing structures (May & Goadsby, 1999) and it is possible that carabersat and tonabersat could act by moduating excitatory inputs to parasympathetic ganglia by modulation of afferent or efferent parts of this pathway.
The tonabersat and carbersat specific CNS binding site shows high binding density in cerebral cortex, hypothalamus, striatum and other brain areas with no detectable binding in rat liver or heart (Herdon et al., 1997). Binding to these specific CNS sites may result in the modulation of sensory-autonomic reflexes presently observed but, the precise site of action of these novel antinconvulsant benzopyrans will require further elucidation. However, it is unlikely to involve a direct action on release of transmitters from the afferent or efferent nervous systems. Both tonabersat and carbersat are highly selective compounds with little affinity at a range of transmembrane receptors (including cholinoceptors and receptors for VIP, endothelin and CGRP), channels, enzymes, or transporters and lack effects on aterial blood pressure or heart rate (Chan et al., 1999; Herdon et al., 1997; Upton et al., 1997; 1999; Upton & Thompson, 2000). In addition, carbersat has no effect on field stimulation-evoked contraction of rabbit isolated iris sphincter muscle or non-adrenergic, non-cholinergic relaxation of rat isolated stomach fundus (Medhurst, Parsons, Upton; unpublished).
Comparison of tonabersat with other anti-convulsants
Bolus intraduodenal administration of tonabersat produced dose related inhibition of trigeminal nerve stimulated reductions in carotid vascular resistance. Tonabersat (1 mg kg−1) produced a slowly developing blockade of nerve stimulation-induced reductions in carotid vascular resistance while higher doses (10 mg kg−1) produced a more rapid and greater degree of blockade over the time course of the experiments. The effective dose range for tonabersat in this experiment is therefore similar to the rat maximal electroshock threshold test following oral administration, and trigeminal nerve stimulation-induced plasma protein extravasation in rats following intraperitoneal administration (Chan et al, 1999). This dose range is consistent across species and between rat models of seizure and trigeminal nerve stimulation.
Sodium valproate is an effective preventative therapy for migraine (Silberstein, 1996) but lack significant effects on trigeminal nerve stimulation-induced reductions in carotid vascular resistance (this study). This is in contrast to the potent inhibitory effects of this compound on trigeminal nerve mediated increases in plasma protein extravasation (Cutrer et al., 1997) and c-fos accumulation (Cutrer & Moskowitz, 1996) which appear to be mediated by facilitation of GABA receptor activity (Lee et al., 1995). In the present study, we utilized intraduodenal doses of 10 and 100 mg kg−1, which is comparable to the intraperitoneal doses used by other workers in rats (Cutrer et al., 1997) and guinea-pigs (Cutrer & Moskowitz, 1996), to show that this classical anticonvulsant has no effect in this model. It is therefore unlikely that elevation or facilitation of GABAergic pathways will have a major pharmacological response under these conditions.
In contrast, lamotrigine (50 mg kg−1) produced marked inhibition of trigeminal nerve mediated responses, with a more rapid (⩾60 min) inhibition and maximal effect similar to tonabersat (10 mg kg−1). Lamotrigine is a second generation anti-epileptic drug which acts to stabilize Na+ channels (Xie et al., 1995) and inhibit glutamate release, thereby producing anticonvulsant activity. Glutamate acts at a variety of G-protein coupled receptors and ion channels and a number of these receptor systems may be involved in mediation of this reflex. Agents which modify glutamatergic neurotransmission possess inhibitory properties in sensory systems and produce antinociceptive effects (Dickenson et al., 1997). For example, NMDA- and AMPA-antagonists or type III mGluR agonists inhibit intracisternal capsaicin-induced activation of trigeminal nucleus caudalis (Mitsikostas et al., 1998). However, it is unlikely that NMDA receptors play a role in this reflex as MK-801 had no effect in this model of trigeminal nerve stimulation-induced reductions in carotid vascular resistance (Smith et al., 1997). However, we cannot exclude a role for other glutamatergic receptors in this response.
Interestingly, lower doses of lamotrigine (10 mg kg−1) had only minimal effects. Lamotrigine is a highly effective anticonvulsant agent with similar potency to carabersat in rat and mouse MEST models (Upton et al., 1997; Upton & Thompson, 2000). Based on the relative anticonvulsant potency of carbersat and tonabersat, one may have expected lamotrigine (10 mg kg−1) to show a similar efficacy to tonabersat (10 mg kg−1). Although therapeutic anticonvulsant doses of lamotrigine (200 mg day−1) have no benefit as a migraine prophylactic (Steiner et al., 1997), higher doses (400 mg day−1) have shown therapeutic benefit in clinical trials with trigeminal neuralgia (Zakrzewska et al., 1997). The significance of the different doses of lamotrigine in these conditions is unknown, however, markedly higher doses of lamotrigine are required to produce anti-hyperalgesic activity compared to its effects as an anticonvulsant (Nakamuracraig & Follenfant, 1995). This data would suggest that high doses of lamotrigine could have efficacy in migraine or other headaches, although the tolerability of the compound may limit its clinical usefulness.
Gabapentin is another new anti-epileptic agent which has been suggested to provide a therapeutic benefit in patients with chronic pain and migraine (Magnus, 1999). Indeed, gabapentin shows marked anti-nociceptive activity in a range of models of chronic pain and hyperalgesia (Field et al., 1999; Hunter et al., 1997). An effective anti-convulsant- and anti-nociceptive dose of gabapentin was therefore evaluated on trigeminal nerve stimulation-induced reductions in carotid vascular resistance. Gabapentin (100 mg kg−1) produced a significant inhibition of trigeminal – autonomic reflex-induced responses, and was similar to tonabersat (1 mg kg−1). In preliminary experiments (n=2) we evaluated higher doses of intraduodenal gabapentin (500 mg kg−1) but failed to see any additional inhibition (unpublished observations).
Therefore, although other anticonvulsant agents do appear to modulate sensory-autonomic reflexes, these agents act via a variety of mechanisms to reduce neuronal excitability. Differences in the relative potency and/or efficacy of lamotrigine, tonabersat, gabapentin and sodium valproate were evident in these studies, therefore suggesting that specific mechanisms of inhibition are involved. The exact nature and site of action of tonabersat and carabersat will require further study and it would be of interest to investigate the effects of these agents on stimulation of the paraysmpathetic ganglia and brain stem nuclei.
Role of trigeminal – autonomic neurovascular reflexes
There is increasing evidence for a role of the parasympathetic nervous system in headaches. Blockade of the sphenopalatine (pterygopalatine) ganglion with intra-nasal lidocaine has been shown to produce a rapid, but short-lived, relief of migraine headache and associated symptoms, such as nausea and vomiting (Maizels et al., 1996). A recent case report highlights that intra-nasal lidocaine can prevent headache following migraine aura (Maizels, 1999) and appears to have some benefit for patients with chronic daily headaches (Hand & Stark, 2000).
CGRP and VIP plasma concentrations are elevated in primary headaches (Edvinsson & Goadsby, 1995; Goadsby & Edvinsson, 1994a; Goadsby et al., 1990) indicating activation of sensory and parasympathetic nervous systems (Edvinsson & Goadsby, 1998). Similarly, elevations in external jugular blood concentrations of CGRP and VIP have been demonstrated following stimulation of the trigeminal nerve ganglion in anaesthetized cats (Goadsby & Edvinsson, 1994b). Clearly there is stimulation of both sensory and parasympathetic pathways during primary headache which has led to the concept of a neurovascular event involving reflex activation of the parasympathetic nervous system (May & Goadsby, 1999). This strengthens the importance of identification and classification of trigeminal – autonomic cephalalgias (Goadsby & Lipton, 1997) which may be a prominent symptom in migraine and other headache patients.
Effects of anti-convulsants on sensory pathways
Sodium valproate inhibits substance P- or trigeminal nerve ganglion stimulation-induced meningeal plasma protein extravasation via GABA-A receptors in the rat with ED50 values of 6.6 and 3.2 mg kg−1 i.p. respectively (Lee et al., 1995). These effects are thought to be mediated by enhancement of GABA synthesis. Sodium valproate and neurosteroids such as allopregnanolone can inhibit c-fos accumulation following intracisternal administration of capsaicin (Cutrer & Moskowitz, 1996). We are unaware of any studies investigating the effects of gababpentin or lamotrigine on trigeminal ganglion stimulation-induced plamsa protein extravasation. However, both gabapentin and lamotrigine possess marked anti-hyperalgesic properties in animal models.
Lamotrigine (10 – 100 mg kg−1 s.c) and gabapentin (30 – 300 mg kg−1 i.p) reversed cold allodynia following nerve injury (Hunter et al., 1997). Similar doses of lamotrigine (Nakamuracraig & Follenfant, 1995) and gabapentin (Field et al., 1999) following oral administration blocked streptozocin-induced mechanical hyperalgesia. Some differences in the pharmacological profile of these agents are also apparent as gabapentin (ED50 34 mg kg−1 i.p.) reversed tactile allodynia following spinal nerve ligation, whereas lamotrigine had no effect in this model.
In conclusion, tonabersat and carabersat show marked inhibitory effects of trigeminal nerve-autonomic reflex vasodilatation of the carotid vascular bed. These effects are independent of effects on the systemic circulation and are compatible with the potent binding properties of these ligands to their specific CNS binding site. Comparison of tonabersat with other anticonvulsant agents following intraduodenal administration demonstrated differential effects of tonabersat, lamotrigine, gabapentin and sodium valproate. These studies therefore extend the evaluation of tonabersat and carbersat. Together with their unique mechanism of action, the data suggests that they may provide an additional therapeutic approach to primary headaches and other diseases where reflex activation of sensory-autonomic reflexes may occur.
Acknowledgments
The authors would like to thank Jeffrey Legos, PhD for critical discussions concerning the manuscript.
Abbreviations
- CdVR
carotid vascular resistance
- CGRP
calcitonin gene-related peptide
- TGN
trigeminal nerve ganglion stimulation
- VIP
vasoactive intestinal polypeptide
References
- BEATTIE D.T., CONNOR H.E. The influence of the trigeminal ganglion on carotid blood flow in anaesthetized guinea-pigs. Br. J. Pharmacol. 1994;112:262–266. doi: 10.1111/j.1476-5381.1994.tb13061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUZZI M.G., MOSKOWITZ M.A. The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br. J. Pharmacol. 1990;99:202–206. doi: 10.1111/j.1476-5381.1990.tb14679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN W.N., EVANS J.M., HADLEY M.S., HERDON H.J., JERMAN J.C., MORGAN H.K., STEAN T.O., THOMPSON M., UPTON N., VONG A.K. Synthesis of novel trans-4-(substituted-benzamido)-3,4-dihydro-2H-benzo[b]-pyran-3-ol derivatives as potential anticonvulsant agents with a distinctive binding profile. J. Med. Chem. 1996;39:4537–4539. doi: 10.1021/jm960535w. [DOI] [PubMed] [Google Scholar]
- CHAN W.N., EVANS J.M., HADLEY M.S., HERDON H.J., JERMAN J.C., PARSONS A.A., READ S.J., STEAN T.O., THOMPSON M., UPTON N. Identification of (−)-cis-6-acetyl-4S-(3-chloro-4-fluoro-benzoylamino)-3,4-dihydro-2,2-dimethyl-2H-benzo[b]pyran-3S-ol as a potential antimigraine agent. Bioorg. Med. Chem. Lett. 1999;9:285–290. doi: 10.1016/s0960-894x(98)00728-8. [DOI] [PubMed] [Google Scholar]
- CHAN W.N., HADLEY M.S., HARLING J.D., HERDON H.J., JERMAN J.C., ORLEK B.S., STEAN T.O., THOMPSON M., UPTON N., WARD R.W. Identification of a series of 1,2,3,4-tetrahydroisoquinolinyl-benzamides with potential anticonvulsant activity. Bioorg. Med. Chem. Lett. 1998;8:2903–2906. doi: 10.1016/s0960-894x(98)00523-x. [DOI] [PubMed] [Google Scholar]
- CUTRER F.M., LIMMROTH V., MOSKOWITZ M.A. Possible mechanisms of valproate in migraine prophylaxis. Cephalalgia. 1997;17:93–100. doi: 10.1046/j.1468-2982.1997.1702093.x. [DOI] [PubMed] [Google Scholar]
- CUTRER F.M., MOSKOWITZ M.A. The actions of valproate and neurosteroids in a model of trigeminal pain. Headache. 1996;36:579–585. doi: 10.1046/j.1526-4610.1996.3610579.x. [DOI] [PubMed] [Google Scholar]
- DICKENSON A.H., CHAPMAN V., GREEN G.M. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen. Pharmacol. 1997;28:633–638. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., GOADSBY P.J. Neuropeptides in the cerebral circulation: relevance to headache. Cephalalgia. 1995;15:272–276. doi: 10.1046/j.1468-2982.1995.1504272.x. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., GOADSBY P.J. Neuropeptides in headache. Eur. J. Neurol. 1998;5:329–341. [Google Scholar]
- ESCOTT K.J., BEATTIE D.T., CONNOR H.E., BRAIN S.D. Trigeminal ganglion stimulation increases facial skin blood flow in the rat: a major role for calcitonin gene-related peptide. Brain Res. 1995;669:93–99. doi: 10.1016/0006-8993(94)01247-f. [DOI] [PubMed] [Google Scholar]
- FIELD M.J., MCCLEARY S., HUGHES J., SINGH L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J. Inhibition of calcitonin gene-related peptide by h-CGRP(8-37) antagonizes the cerebral dilator response from nasociliary nerve stimulation in the cat. Neurosci. Lett. 1993;151:13–16. doi: 10.1016/0304-3940(93)90033-h. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994a;117:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. Joint 1994 Wolff Award Presentation. Peripheral and central trigeminovascular activation in cat is blocked by the serotonin (5HT)-1D receptor agonist 311C90. Headache. 1994b;34:394–399. doi: 10.1111/j.1526-4610.1994.hed3407394.x. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., LIPTON R.B. A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with autonomic feature, including new cases. Brain. 1997;120:193–209. doi: 10.1093/brain/120.1.193. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., MACDONALD G.J. Extracranial vasodilation mediated by vasoactive intestinal polypeptide (VIP) Brain Res. 1985;329:285–288. doi: 10.1016/0006-8993(85)90535-9. [DOI] [PubMed] [Google Scholar]
- HAND P.J., STARK R.J. Intravenous lignocaine infusions for severe chronic daily headache. Med. J. Australia. 2000;172:157–159. doi: 10.5694/j.1326-5377.2000.tb125538.x. [DOI] [PubMed] [Google Scholar]
- HERDON H., JERMAN J., STEAN T., CHAN W., MIDDLEMISS D., UPTON N. The novel anticonvulsant SB 204269 binds to a stereospecific site in the mouse brain. Eur. J. Pharmacol. 1996;314:R7–R8. doi: 10.1016/s0014-2999(96)00737-6. [DOI] [PubMed] [Google Scholar]
- HERDON H.J., JERMAN J.C., STEAN T.O., MIDDLEMISS D.N., CHAN W.N., VONG A.K., EVANS J.M., THOMPSON M., UPTON N. Characterization of the binding of [3H]-SB-204269, a radiolabelled form of the new anticonvulsant SB-204269, to a novel binding site in rat brain membranes. Br. J. Pharmacol. 1997;121:1687–1691. doi: 10.1038/sj.bjp.0701331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER J.C., GOGAS K.R., HEDLEY L.R., JACOBSON L.O., KASSOTAKIS L., THOMPSON J., FONTANA D.J. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur. J. Pharmacol. 1997;324:153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- LAMBERT G.A., BOGDUK N., GOADSBY P.J., DUCKWORTH J.W., LANCE J.W. Decreased carotid arterial resistance in cats in response to trigeminal stimulation. J. Neurosurg. 1984;61:307–315. doi: 10.3171/jns.1984.61.2.0307. [DOI] [PubMed] [Google Scholar]
- LEE W.S., LIMMROTH V., AYATA C., CUTRER F.M., WAEBER C., YU X.J., MOSKOWITZ M.A. Peripheral gaba(a) receptor-mediated effects of sodium valproate on dural plasma protein extravasation to substance p and trigeminal stimulation. Br. J. Pharmacol. 1995;116:1661–1667. doi: 10.1111/j.1476-5381.1995.tb16388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGNUS L. Nonepileptic uses of gabapentin. Epilepsia. 1999;40 Suppl 6:S73–S74. doi: 10.1111/j.1528-1157.1999.tb00936.x. [DOI] [PubMed] [Google Scholar]
- MAIZELS M. Intranasal lidocaine to prevent headache following migraine aura. Headache. 1999;39:439–442. doi: 10.1046/j.1526-4610.1999.3906439.x. [DOI] [PubMed] [Google Scholar]
- MAIZELS M., SCOTT B., COHEN W., CHEN W. Intranasal lidocaine for treatment of migraine–a randomized, double-blind, controlled trial. JAMA. 1996;276:319–321. [PubMed] [Google Scholar]
- MAY A., GOADSBY P.J. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J. Cereb. Blood Flow Metab. 1999;19:115–127. doi: 10.1097/00004647-199902000-00001. [DOI] [PubMed] [Google Scholar]
- MITSIKOSTAS D.D., DELRIO M.S., WAEBER C., MOSKOWITZ M.A., CUTRER F.M. The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain. 1998;76:239–248. doi: 10.1016/s0304-3959(98)00051-7. [DOI] [PubMed] [Google Scholar]
- NAKAMURACRAIG M., FOLLENFANT R.L. Effect of lamotrigine in the acute and chronic hyperalgesia induced by PGE(2) and in the chronic hyperalgesia in rats with streptozotocin-induced diabetes. Pain. 1995;63:33–37. doi: 10.1016/0304-3959(95)00016-L. [DOI] [PubMed] [Google Scholar]
- O'SHAUGHNESSY C.T., CONNOR H.E. Investigation of the role of tachykinin NK1, NK2 receptors and CGRP receptors in neurogenic plasma protein extravasation in dura mater. Eur. J. Pharmacol. 1994;263:193–198. doi: 10.1016/0014-2999(94)90541-x. [DOI] [PubMed] [Google Scholar]
- RAVAL P., BINGHAM S., AIYAR N., ELLIOTT J.D., HUNTER A.J., OHLSTEIN E.H., PARSONS A.A. Trigeminal nerve ganglion stimulation-induced neurovascular reflexes in the anaesthetized cat: role of endothelin(B) receptors in carotid vasodilatation. Br. J. Pharmacol. 1999;126:485–493. doi: 10.1038/sj.bjp.0702306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- READ S.J., SMITH M.I., HUNTER A.J., UPTON N., PARSONS A.A. SB-220453, a potential novel antimigraine agent, inhibits nitric oxide release following induction of cortical sprading depression in the anaesthetised cat. Cephalalgia. 2000;20:92–99. doi: 10.1046/j.1468-2982.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- SHEPHEARD S.L., WILLIAMSON D.J., HILL R.G., HARGREAVES R.J. The non-peptide neurokinin1 receptor antagonist, RP 67580, blocks neurogenic plasma extravasation in the dura mater of rats. Br. J. Pharmacol. 1993;108:11–12. doi: 10.1111/j.1476-5381.1993.tb13432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILBERSTEIN S.D. Divalproex sodium in headache–literature review and clinical guidelines. Headache. 1996;36:547–555. doi: 10.1046/j.1526-4610.1996.3609547.x. [DOI] [PubMed] [Google Scholar]
- SMITH M.I., RAVAL P., BINGHAM S., PARSONS A.A.Effects of MK-801 on cortical spreading depression and trigeminal nerve stimulation in anaesthetised cats Cephalalgia 199717396(abstract) [Google Scholar]
- SPOKES R.A., MIDDLEFELL V.C. Simultaneous measurement of plasma protein extravasation and carotid vascular resistance in the rat. Eur. J. Pharmacol. 1995;281:75–79. doi: 10.1016/0014-2999(95)00231-9. [DOI] [PubMed] [Google Scholar]
- STEINER T.J., FINDLEY L.J., YUEN A.W.C. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia. 1997;17:109–112. doi: 10.1046/j.1468-2982.1997.1702109.x. [DOI] [PubMed] [Google Scholar]
- SUZUKI N., HARDEBO J.E., OWMAN C. Trigeminal fibre collaterals storing substance P and calcitonin gene-related peptide associate with ganglion cells containing choline acetyltransferase and vasoactive intestinal polypeptide in the sphenopalatine ganglion of the rat. An axon reflex modulating parasympathetic ganglionic activity. Neuroscience. 1989;30:595–604. doi: 10.1016/0306-4522(89)90154-1. [DOI] [PubMed] [Google Scholar]
- UPTON N., BLACKBURN T.P., CAMPBELL C.A., COOPER D., EVANS M.L., HERDON H.J., KING P.D., RAY A.M., STEAN T.O., CHAN W.N., EVANS J.M., THOMPSON M. Profile of SB-204269, a mechanistically novel anticonvulsant drug, in rat models of focal and generalized epileptic seizures. Br. J. Pharmacol. 1997;121:1679–1686. doi: 10.1038/sj.bjp.0701330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UPTON N., RAVAL P., HERDON H., JERMAN J., PARSONS A.A., CHAN W., THOMPSON M. SB-220453, a mechanistically novel benzopyran compound, inhibits trigeminal nerve mediated (TGN) stimulation-induced carotid vasodilatation. Cephalalgia. 1999;19:351. [Google Scholar]
- UPTON N., THOMPSON M. Benzo[b]pyranols and related novel antiepileptic agents. Prog. Med. Chem. 2000;37:177–200. doi: 10.1016/s0079-6468(08)70060-2. [DOI] [PubMed] [Google Scholar]
- XIE X., LANCASTER V., PEAKMAN T., GARTHWAITE J. Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type II Na+ channels and with native Na channels in rat hippocampal neurones. Pflügers Arch. Eur. J. Physiol. 1995;430:437–446. doi: 10.1007/BF00373920. [DOI] [PubMed] [Google Scholar]
- ZAGAMI A.S., GOADSBY P.J., EDVINSSON L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990;16:69–75. doi: 10.1016/0143-4179(90)90114-e. [DOI] [PubMed] [Google Scholar]
- ZAKRZEWSKA J.M., CHAUDHRY Z., NURMIKKO T.J., PATTON D.W., MULLENS E.L. Lamotrigine (Lamictal) in refractory trigeminal neuralgia–results from a double-blind placebo controlled crossover trial. Pain. 1997;73:223–230. doi: 10.1016/S0304-3959(97)00104-8. [DOI] [PubMed] [Google Scholar]


