Abstract
This study investigates, whether in addition to the protease-activated receptor-1 (PAR-1), PAR-4 is present in vascular smooth muscle cells (SMC) of the human saphenous vein and whether this receptor is functionally active. PAR-1 and PAR-4 are stimulated by thrombin and by the synthetic peptides SFLLRN and GYPGQV, respectively.
mRNAs for both, PAR-1 and PAR-4, were detected in the SMC by using reverse transcriptase polymerase chain reaction (RT – PCR).
Treatment of the SMC with GYPGQV (200 μM) resulted in a transient increase in free intracellular calcium. This calcium signal was completely abolished after a preceding challenge with thrombin (10 nM), indicating homologous receptor desensitization.
Stimulation of the SMC with 10 nM thrombin or 200 μM SFLLRN caused a time-dependent activation of the extracellular signal-regulated kinases-1/2 (ERK-1/2) with a maximum at 5 min. In contrast, 100 nM thrombin as well as 200 μM of GYPGQV induced a prolonged phosphorylation of ERK-1/2 with a maximum at 60 min. These data suggest that PAR-1 and PAR-4 are activated by thrombin at distinct concentrations and with distinct kinetics.
GYPGQV stimulated [3H]-thymidine incorporation in SMC. At 500 μM, the peptide increased DNA synthesis 2.5 fold above controls. A comparable mitogenic effect was obtained after stimulation of the SMC by 10 nM thrombin or 100 μM SFLLRN, respectively.
These data indicate that a functionally active PAR-4 is present in SMC and, in addition to PAR-1, might contribute to thrombin-induced mitogenesis.
Keywords: PAR-4, vascular smooth muscle cells, [Ca2+]i mobilization, ERK-1/2, mitogenesis
Introduction
Protease-activated receptors (PARs) are a subfamily of seven transmembrane domain G protein-coupled receptors. Until now, four PARs, termed PAR-1, PAR-2, PAR-3 and PAR-4, have been cloned (Vu et al., 1991a; Nystedt et al., 1994; Ishihara et al., 1997; Xu et al., 1998). These receptors are activated by either thrombin or trypsin or both. In contrast to the known thrombin receptors PAR-1 and PAR-3, PAR-4 lacks the specialized thrombin-binding, hirudin-like domain downstream from the tethered ligand cleavage site (Vu et al., 1991b; Ishihara et al., 1997; Xu et al., 1998). Alignment of PAR-4 amino acid sequence with the three other protease-activated receptors indicated an about 33% amino acid sequence identity. Northern blot analysis of mRNA showed that the PAR-4 gene is expressed in many tissues with high levels in lung, pancreas, thyroid, testis, and small intestine. PAR-4 mRNA was also detected in human platelets (Xu et al., 1998).
Activation of PARs requires the proteolytic cleavage of the amino terminal exodomain of the receptor. A new amino terminus is exposed that binds as a tethered ligand to the body of the receptor to effect transmembrane signalling (Vu et al., 1991a; Chen et al., 1994). Synthetic peptides, corresponding to the first six amino acids of the new amino terminus, can activate their receptor directly, i.e. independent of protease and receptor cleavage (Vu et al., 1991a; Kahn et al., 1999; Faruqi et al., 2000). Such peptides are useful tools to study the functions of PARs in cells and tissues. While human PAR-1 is activated by the tethered ligand peptide sequence SFLLRN, human PAR-4 is activated by the peptide GYPGQV mimicking the tethered ligand of PAR-4 (Xu et al., 1998; Coughlin, 1999; Faruqi et al., 2000). The PAR-1-and PAR-4-activating peptides were shown to be specific for their respective receptors (Kahn et al., 1999; Faruqi et al., 2000).
The present study was designed to investigate whether a functionally active PAR-4 is present in human vascular smooth muscle cells (SMC). PAR-4 mRNA was detected by RT – PCR. To verify PAR-4-mediated signalling, mobilization of [Ca2+]i, phosphorylation of extracellular signal-regulated kinases (ERK-1/2) and stimulation of DNA synthesis were measured.
Methods
Cell culture
Specimens of saphenous vein were obtained from patients undergoing aortocoronary bypass surgery. The SMC were obtained by outgrowth from explants of the vessel media and cultured as described previously (Bretschneider et al., 2000). The cells were identified as SMC by their typical ‘hill and valley' growth pattern and by immunostaining with a specific monoclonal α-actin antibody. Cells of passages 3 – 8 were used for the experiments.
Detection of PAR-1 and PAR-4 mRNA
The mRNA of PAR-1 and PAR-4 was detected by RT – PCR and agarose gel electrophoresis. Total mRNA was extracted from 1×107 cells (Oligotex Direct mRNA Mini Kit, Qiagen GmbH, Hilden, Germany) and first-strand cDNA was synthesized from 0.5 μg of mRNA by oligo (dt) primed reverse transcription using a commercial cDNA Cycle kit (Invitrogen, Leek, The Netherlands). Aliquots of cDNA were taken for PCR amplification using the following primers: PAR-1: forward CTCGAATTCTGAAGGTCAAGAAGCCGG; reverse CTCGAATTCAGCTTTTTGTATATGCTG. This resulted in a PCR product of 895 bp. PAR-4: forward AACCTCTATGGTGCCTACGTGC; reverse CCAAGCCCAGCTAATTTTTG (Kahn et al., 1999). This resulted in a PCR product of 541 bp. PCR was performed in a 50 μl volume containing a final concentration of 1 μM primers, 200 μM dNTPs and 1.25 u Taq polymerase (Perkin Elmer, Foster City, U.S.A.), following the manufacturer's instructions. Cycling parameters were as follows: 3 min at 95°C, followed by 33 cycles of 30 s at 95°C, 30 s at 55°C (PAR-1) and 61.5°C (PAR-4), 20 s at 72°C, and finally a 5-min extension step at 72°C. After thermocycling, 10 μl of each PCR product were electrophoresed on a 1% agarose gel, containing 10 μg ml−1 ethidiumbromide and visualized under UV transillumination.
Calcium measurements
Mobilization of [Ca2+]i was measured as described elsewhere with minor modifications (Kaufmann et al., 2000). Briefly, SMC were grown on Lab Tek chambered borosilicate coverglass (Nunc GmbH&Co.KG, Wiesbaden, Germany). After washing twice with HEPES buffer (mM) HEPES 10 (pH 7.4), NaCl2 145, Na2HPO4 0.5, glucose 6, Mg2SO4 1, and CaCl2 1.5, cells were incubated for 15 min at 37°C in the same buffer, supplemented with 0.5 μM fluo-4 acetoxymethyl ester. After fluo-4 loading, the cells were washed twice, reincubated with HEPES buffer and stimulated by the agents indicated. For Ca2+ measurement in single cells, an inverted confocal laser scanning microscope (LSM 410, Carl Zeiss, Göttingen, Germany) was used. Fluorescence images were collected by using the 488 nm argon ion laser line. [Ca2+]i was calculated according to Grynkiewicz et al. (1985). Fmax was obtained by the addition of 10 μM ionomycin (+6 mM CaCl2), Fmin by the addition of 20 mM EGTA.
Activation of ERK-1/2
Phosphorylation of ERK-1/2 was detected in cell lysates by immunoblotting. Briefly, cell extracts were prepared in sodium dodecyl sulphate (SDS) lysis buffer (2% w v−1 SDS, 10% glycerol, 0.0625 M phosphate buffer, pH 7.0, 50 mM dithiothreitol, 0.001% bromophenol blue). Proteins were separated by SDS polyacrylamide gel electrophoresis (10%) and blotted onto polyvinylidene difluoride membranes (Immobilon-P, Millipore, Bedford, U.S.A.). Membranes were blocked in blocking buffer containing TBS-T (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween-20) and 5% w v−1 non-fat dry milk and were then incubated with a phosphospecific antibody against ERK-1/2 (1 : 1000) for 60 min. After washing three times (10 min each) in TBS-T, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1 : 3000) for 60 min. After washing three times, immunoreactive bands were visualized by chemiluminescence (Roche Diagnostics, Mannheim, Germany).
Measurement of [3H]-thymidine incorporation
Cells were seeded into 24-well plates (2×104 cells per well) and allowed to attach for 24 h. The SMC were growth arrested in serum-free culture medium for 24 h, and thereafter stimulated by the indicated agents for the following 24 h. Four hours prior to the end of the stimulation period, SMC were pulse-labelled with [3H]-thymidine (2 μCi ml−1). The labelling period was terminated by washing the SMC twice with ice-cold phosphate-buffered saline. After fixing the cells with HClO4 (0.3 M), the precipitated material was solubilized with NaOH (0.1 M) for 1 h at 37°C. Aliquots of 0.2 ml were added to 3 ml of scintillant. [3H]-thymidine incorporation into the cellular DNA was determined by liquid scintillation spectrometry using a beta-scintillation counter (Wallac 1410, EG&G Wallac, Freiburg, Germany).
Drugs and solutions
Synthetic human PAR-1-activating peptide (SFLLRN-NH2), synthetic human PAR-4-activating peptide (GYPGQV-NH2) (Biogenes, Berlin, Germany); phospho-p44/42 MAP kinase (ERK-1/2) monoclonal antibody (New England Biolabs, Beverly, MA, U.S.A.); [3H]-thymidine (NEN Life Science Products, Boston, MA, U.S.A.); fluo-4 acetoxymethyl ester (Molecular Probes Europe B.V., Leiden, The Netherlands); purified α-thrombin was kindly provided by Dr J. Stürzebecher, Zentrum für Vaskuläre Biologie und Medizin Erfurt, Friedrich-Schiller-Universität Jena, Germany. Media and supplements for the cell culture were from Life Technologies (Eggenstein, Germany).
Statistics
The data on [3H]-thymidine incorporation are mean (s.e.mean) of n independent measurements performed in triplicate. Statistical analysis was performed by two-tailed t-test. Differences were considered significant at P⩽0.05.
Results
Expression of PAR mRNAs
RT – PCR and subsequent agarose gel electrophoresis of human SMC resulted in the detection of a 895- and 541-bp PCR product, indicating the mRNA for PAR-1 and PAR-4, respectively (Figure 1).
Figure 1.
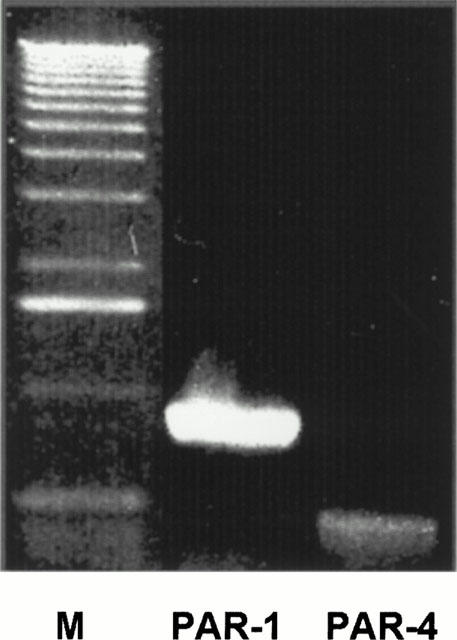
Expression of mRNAs encoding PAR-1 and PAR-4 in SMC. Extraction of mRNA and synthesis of cDNA was performed as described in Methods. Lane M, molecular-weight marker (1-kb ladder, Sigma Chemie, Deisenhofen, Germany). Each sample was analysed at least twice. The presented experiment is representative of six independent experiments with similar results.
Mobilization of [Ca2+]i
Stimulation of SMC with thrombin (10 nM) or the PAR-4-activating peptide GYPGQV (200 μM) resulted in a transient rise in [Ca2+]i (Figure 2A,B). When the cells were stimulated twice with thrombin or GYPGQV, no second calcium signal was observed after the first application (data not shown). A further Ca2+ response to thrombin was seen when the cells were stimulated with thrombin after a prior application of GYPGQV (Figure 2A). However, after a preceding challenge with thrombin, the Ca2+ signal to GYPGQV was completely abolished (Figure 2B). In order to verify the functional integrity of the SMC the cells were also stimulated with angiotensin II (200 nM). In each experimental setup angiotensin II elicited a Ca2+ signal (data not shown).
Figure 2.
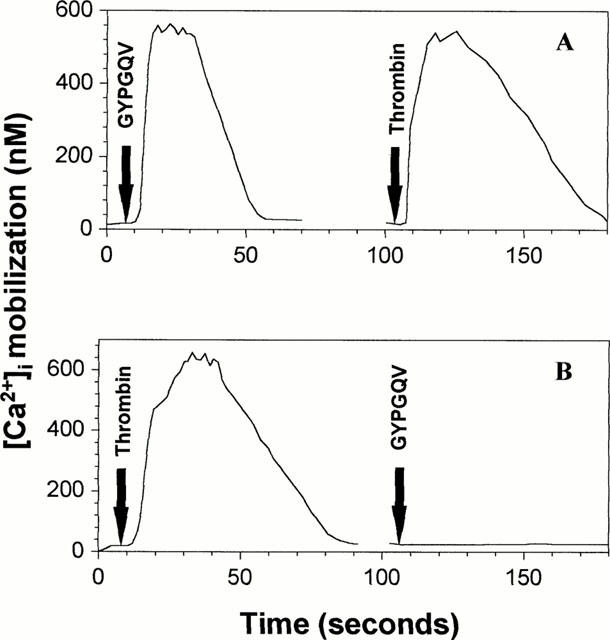
(A,B) [Ca2+]i mobilization in SMC after stimulation by thrombin and GYPGQV. SMC were loaded with fluo-4 acetoxymethyl ester and treated with thrombin (10 nM) and GYPGQV (200 μM) for the times indicated. The next stimulant was applied after a short washout of the cells. Similar data were obtained in three independent experiments.
Activation of ERK-1/2
GYPGQV (200 μM) activated ERK-1/2 time-dependently. A maximum effect was obtained after 60 min. SFLLRN (200 μM) also activated ERK-1/2, however, the maximum was detected already 5 min after stimulation of the cells (Figure 3A). Figure 3B shows ERK-1/2 phosphorylation induced by low and high thrombin concentrations. At 10 nM thrombin, maximum activation of ERK-1/2 was detected after 5 min. Stimulation of the SMC with 100 nM thrombin also resulted in a first peak response at 5 min which was followed by a more prolonged ERK-1/2 activation with a further peak at 60 min.
Figure 3.
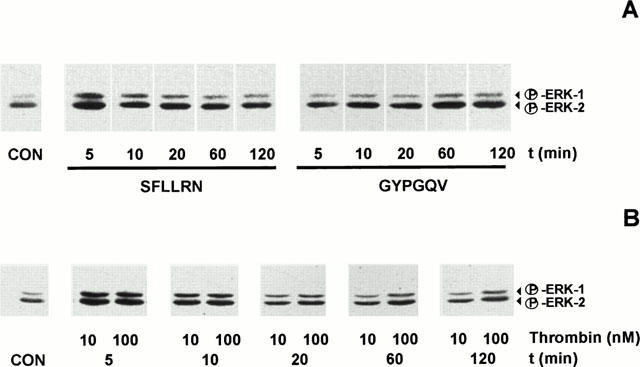
(A) Time-dependent activation of ERK-1/2 by the PAR-1-activating peptide SFLLRN (200 μM) and the PAR-4-activating peptide GYPGQV (200 μM). Activation of ERK-1/2 was detected in cell lysates by Western blotting. The presented experiment is representative of three independent experiments with similar results. (B) Time-dependent phosphorylation of ERK-1/2 by 10 and 100 nM thrombin. Activation of ERK-1/2 was detected in cell lysates by Western blotting. The presented experiment is representative of three independent experiments with similar results.
Stimulation of [3H]-thymidine incorporation
Stimulation of the SMC with 100 or 500 μM GYPGQV, respectively, caused a concentration-dependent increase in [3H]-thymidine incorporation. At 500 μM, the peptide increased DNA synthesis to about 250% of unstimulated controls. Thrombin and the PAR-1-activating peptide SFLLRN elicited a comparable mitogenic effect at 10 nM and 100 μM, respectively (Figure 4).
Figure 4.
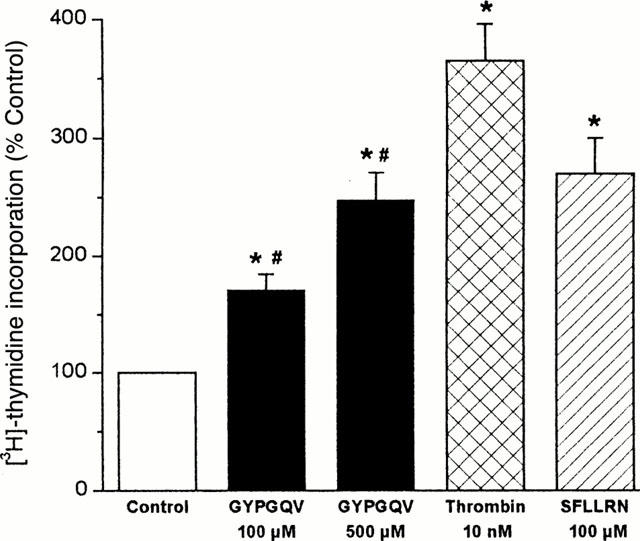
[3H]-thymidine incorporation in SMC stimulated by GYPGQV, SFLLRN or thrombin. Quiescent SMC were incubated with the agonists at the concentrations indicated for 24 h. After pulse-labelling with [3H]-thymidine (2 μCi ml−1) during the last 4 h of the incubation period, [3H]-thymidine incorporation was determined. Means±s.e.mean from 5 – 9 separate experiments; *P<0.05 (treatment vs control); #P<0.05 (100 vs 500 μM GYPGQV).
Discussion
It is well established that thrombin elicits its cellular effects via activation of PAR-1 (McNamara et al., 1993; Hung et al., 1992). Recently, two additional thrombin receptors, PAR-3 and PAR-4, were identified (Ishihara et al., 1997; Xu et al., 1998). This raised the question whether these receptors are also involved in thrombin-induced cellular responses. PAR-4, in addition to PAR-1, appears to be involved in thrombin signalling in human platelets (Kahn et al., 1999; Shapiro et al., 2000). A signalling role of PAR-4 was also demonstrated in human astrocytoma cells (Kaufmann et al., 2000) and in airway smooth muscle of the murine trachea (Lan et al., 2000). The PAR-4 activating peptide was found to elicit an endothelium-dependent relaxation of precontracted rat aorta and a contractile response in rat gastric longitudinal muscle preparations (Hollenberg et al., 1999).
This study demonstrates for the first time that PAR-4 is present in SMC of the human saphenous vein and mediates cellular signalling. In addition to PAR-1 mRNA, PAR-4 mRNA was detected in all analysed SMC preparations by RT – PCR. Interestingly, PAR-4 mRNA (Andrade-Gordon et al., 1999) or Ca2+-signalling by PAR-4 activating peptide (Ahn et al., 2000) were not detected in SMC of human aorta and coronary artery, respectively. Whether this might be explained by a different thrombin receptor expression in SMC of arteries and veins remains to be determined.
Treatment of SMC with either thrombin or the PAR-4-activating peptide GYPGQV resulted in a transient mobilization of free intracellular calcium. Thrombin-induced responses can result from activation of both PAR-1 and PAR-4 (Vu et al., 1991a; Xu et al., 1998). After stimulation of the SMC by GYPGQV (200 μM), the response to thrombin was unchanged, suggesting that thrombin is still able to elicit a calcium signal via activation of PAR-1. In contrast, when the cells were first exposed to thrombin, subsequent stimulation with GYPGQV failed to evoke a calcium signal, indicating homologous receptor desensitization.
Like PAR-1, PAR-4 is also coupled to Gq (Kanthou et al., 1996; Faruqi et al., 2000) which implies activation of ERK-1/2. GYPGQV (200 μM) was found to phosphorylate ERK-1/2 time-dependently with a maximum effect at 60 min. Activation of PAR-1 by the peptide SFLLRN also caused a time-dependent phosphorylation of ERK-1/2, however, a maximum was already detected 5 min after stimulation of the SMC. This observation agrees with findings in platelets and fibroblasts, showing that PAR-4 is shut off less rapidly than PAR-1, probably due to differences in the rate and/or extent of agonist-dependent receptor phosphorylation (Shapiro et al., 2000).
In human platelets, PAR-1 appears to mediate responses to low concentrations of thrombin, while PAR-4 mediates signalling at higher concentrations of the enzyme (Coughlin, 1999). Our findings in SMC support the utilization of a dual receptor system by thrombin in dependence on its concentration. At 10 nM, thrombin activated ERK-1/2 with a similar time course as the PAR-1-activating peptide SFLLRN. However, at a 10 fold higher concentration, thrombin induced a prolonged activation of ERK-1/2 which was comparable to that of GYPGQV. The two receptors might allow thrombin to activate concentration-dependently distinct signalling pathways or to trigger signalling at different levels of activation or shut off (Coughlin, 1999; Shapiro et al., 2000).
Thrombin has been shown to induce mitogenesis in rabbit and rat aortic SMC (Herbért et al., 1992; McNamara et al., 1993) as well as in SMC of human aorta, internal mammary artery and saphenous vein (Kanthou et al., 1992; Yang et al., 1997). These mitogenic effects are thought to be mediated via activation of PAR-1. Interestingly, thrombin-induced mitogenesis in SMC of human saphenous vein was much stronger than that in SMC of mammary artery. From these data it was suggested that additional thrombin receptors exist in the venous SMC (Yang et al., 1997). We show that, in addition to PAR-1, PAR-4 is present in SMC of human saphenous vein and might contribute to the potency of thrombin to induce mitogenesis in these cells. Stimulation of SMC with 500 μM of the PAR-4-activating peptide GYPGQV resulted in a significant increase in DNA synthesis which was comparable to that of 10 nM thrombin and 100 μM SFLLRN, respectively. The requirement of rather high concentrations of GYPGQV to activate its receptor is in agreement with findings in other cells and tissues (Xu et al., 1998; Hollenberg et al., 1999; Faruqi et al., 2000). Unlike PAR-1, PAR-4 appears not to be coupled to Gi (Faruqi et al., 2000). Elevated cyclic AMP levels inhibit SMC proliferation (Kanthou et al., 1996; Zucker et al., 1998). Thus, coupling to Gi and subsequent inhibition of adenylyl cyclase may contribute to the efficacy of PAR-1 to mediate the mitogenic response to thrombin. Conversely, the lack of PAR-4 to inhibit adenylyl cyclase may be one possible explanation for its lower potency as compared to PAR-1 (Faruqi et al., 2000).
In summary, the present study indicates that, in addition to PAR-1, a functionally active PAR-4 is present in SMC of the human saphenous vein. The receptors become activated by thrombin at distinct concentrations and with distinct kinetics. Thus, depending on the local thrombin concentration, both receptors might mediate mitogenic responses to thrombin.
These findings might be clinically relevant in so far as thrombin might contribute to venous graft disease by activating its dual receptor system. Harvesting and implantation of aortocoronary saphenous vein grafts is associated with loss of endothelium and medial damage (Angelini et al., 1987; Roubos et al., 1995). As a consequence of vessel injury, blood coagulation is initiated leading to the generation of thrombin and thrombus formation. Clot-bound thrombin exerts a procoagulant activity (Weitz et al., 1990) and may further enhance local thrombin concentration. Under these conditions, PAR-4, in addition to PAR-1, might become activated and augment thrombin-induced proliferation of SMC and intimal hyperplasia which is an essential component of venous graft disease (Motwani & Topol 1998).
Acknowledgments
This study was supported by the Verbund für Klinische Forschung of the Friedrich-Schiller-Universität Jena (FS 5/4). The authors are grateful to Marlies Laube, Svetlana Tausch, Michael Zieger and Christine Machunsky for competent technical assistance and to Erika Lohmann for secretarial help.
Abbreviations
- ERK-1/2
extracellular signal-regulated kinases
- FCS
foetal calf serum
- MAP kinases
mitogen activated protein kinases
- PAR
protease-activated receptor
- RT – PCR
reverse transcriptase polymerase chain reaction
- SMC
vascular smooth muscle cells
References
- AHN H.-S., FOSTER C., BOYKOW G., STAMFORD A., MANNA M., GRAZIANO M. Inhibition of cellular action of thrombin by N3-cyclopropyl-7-{[4-(1-methylethyl)phenyl]methyl}-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochem. Pharmacol. 2000;60:1425–1434. doi: 10.1016/s0006-2952(00)00460-3. [DOI] [PubMed] [Google Scholar]
- ANDRADE-GORDON P., MARYANOFF B.E., DERIAN C.K., ZHANG H.-C., ADDO M.F., DARROW A.L., ECKARDT A.J., HOEKSTRA W.J., MCCOMSEY D.F., OKSENBREG D., REYNOLDS E.E., SANTULLI R.J., SCARBOROUGH R.M., SMITH C.E., WHITE K.B. Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tethered-ligand receptor. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12257–12262. doi: 10.1073/pnas.96.22.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANGELINI G.D., PASSANI S.L., BRECKENRIDGE I.M., NEWBY A.C. Nature and pressure dependence of damage induced by distension of human saphenous vein coronary artery bypass grafts. Cardiovasc. Res. 1987;21:902–907. doi: 10.1093/cvr/21.12.902. [DOI] [PubMed] [Google Scholar]
- BRETSCHNEIDER E., BRAUN M., FISCHER A., WITTPOTH M., GLUSA E., SCHRÖR K. Factor Xa acts as a PDGF-independent mitogen in human vascular smooth muscle cells. Thromb. Haemost. 2000;84:499–505. [PubMed] [Google Scholar]
- CHEN J., ISHII M., WANG L., ISHII K., COUGHLIN S.R. Thrombin receptor activation. Confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intramolecular liganding mode. J. Biol. Chem. 1994;269:16041–16045. [PubMed] [Google Scholar]
- COUGHLIN S.R. Protease-activated receptors and platelet function. Thromb. Haemost. 1999;82:353–356. [PubMed] [Google Scholar]
- FARUQI T.R., WEISS E.J., SHAPIRO M.J., HUANG W., COUGHLIN S.R. Structure-function analysis of protease-activated receptor 4 tethered ligand peptides. Determinants of specificity and utility in assays of receptor function. J. Biol. Chem. 2000;275:19728–19734. doi: 10.1074/jbc.M909960199. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HERBÉRT J.M., LAMARCHE I., DOL F. Induction of vascular smooth muscle cell growth by selective activation of the thrombin receptor. Effect of heparin. FEBS Lett. 1992;301:155–158. doi: 10.1016/0014-5793(92)81237-g. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., GUI Y. Proteinase-activated receptor 4 (PAR4): action of PAR4-activating peptides in vascular and gastric tissue and lack of cross-reactivity with PAR1 and PAR2. Can. J. Physiol. Pharmacol. 1999;77:458–464. [PubMed] [Google Scholar]
- HUNG D.T., WONG Y.H., VU T.-K.H., COUGHLIN S.R. The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J. Biol. Chem. 1992;267:20831–20834. [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- KAHN M.L., NAKANISHI-MATSUI M., SHAPIRO M.J., ISHIHARA H., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANTHOU C., KANSE S.M., KAKKAR V.V., BENZAKOUR O. Involvement of pertussis toxin-sensitive and -insensitive G proteins in α-thrombin signalling on cultered human vascular smooth muscle cells. Cell. Signal. 1996;8:59–66. doi: 10.1016/0898-6568(95)02018-7. [DOI] [PubMed] [Google Scholar]
- KANTHOU C., PARRY G., WIJELATH E., KAKKAR V.V., DEMOLIOU-MASON C. Thrombin-induced proliferation and expression of platelet-derived growth factor-A chain gene in human vascular smooth muscle cells. FEBS Lett. 1992;314:143–148. doi: 10.1016/0014-5793(92)80961-f. [DOI] [PubMed] [Google Scholar]
- KAUFMANN R., PATT S., ZIEGER M., KRAFT R., TAUSCH S., HENKLEIN P., NOWAK G. The two-receptor system PAR-1/PAR-4 mediates α-thrombin-induced [Ca2+]i mobilization in human astrocytoma cells. J. Cancer Res. Clin. Oncol. 2000;126:91–94. doi: 10.1007/pl00008481. [DOI] [PubMed] [Google Scholar]
- LAN R.S., STEWART G.A., HENRY P.J. Modulation of airway smooth muscle tone by protease activated receptor-1, -2, -3 and -4 in trachea isolated from influenza A virus-infected mice. Br. J. Pharmacol. 2000;129:63–70. doi: 10.1038/sj.bjp.0703007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAMARA C.A., SAREMBOCK I.J., GIMPLE L.W., FENTON J.W., II, COUGHLIN S.R., OWENS G.K. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J. Clin. Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTWANI J.G., TOPOL E.J. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUBOS N., ROSENFELDT F.L., RICHARDS S.M., CONYERS R.A.J., DAVIS B.B. Improved preservation of saphenous vein grafts by the use of glyceryl trinitrate-verapamil solution during harvesting. Circulation. 1995;92 Suppl. II:II-31–II-6. doi: 10.1161/01.cir.92.9.31. [DOI] [PubMed] [Google Scholar]
- SHAPIRO M.J., WEISS E.J., FARUQI T.R., COUGHLIN S.R. Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J. Biol. Chem. 2000;275:25216–25221. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- VU T.K., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991a;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- VU T.K., WHEATON V.I., HUNG D.T., CHARO I., COUGHLIN S.R. Domains specifying thrombin-receptor interaction. Nature. 1991b;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- WEITZ J.I., HUDOBA M., MASSEL D., MARAGANORE J., HIRSH J. Clot-bound thrombin is protected from inhibition by heparin-antithrombin III but is susceptible to inactivation by antithrombin III-independent inhibitors. J. Clin. Invest. 1990;86:385–391. doi: 10.1172/JCI114723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU W.F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., FOSTER D.C. Cloning and characterisation of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Z., RUSCHITZKA F., RABELINK T.J., NOLL G., JULMY F., JOCH H., GAFNER V., ALEKSIC I., ALTHAUS U., LÜSCHER T.F. Different effects of thrombin receptor activation on endothelium and smooth muscle cells of human coronary bypass vessels. Implications for venous bypass graft failure. Circulation. 1997;95:1870–1876. doi: 10.1161/01.cir.95.7.1870. [DOI] [PubMed] [Google Scholar]
- ZUCKER T.-P., BÖNISCH D., HASSE A., GROSSER T., WEBER A.-A., SCHRÖR K. Tolerance development to antimitogenic actions of prostacyclin but not of prostaglandin E1 in coronary artery smooth muscle cells. Eur. J. Pharmacol. 1998;345:213–220. doi: 10.1016/s0014-2999(98)00022-3. [DOI] [PubMed] [Google Scholar]


