Abstract
The relative roles of endothelin (ET) and vasopressin (AVP) in the regulation of blood pressure (BP), cardiac output (CO) and total peripheral resistance (TPR) were investigated in the early stages (24 – 31 days) of development of hypertension in the conscious deoxycorticosterone acetate (DOCA)-salt hypertensive rat model.
BP was recorded with radiotelemetry devices and CO with ultrasonic transit-time probes. TPR was calculated from the BP and CO recordings. The contributions of endogenous ET and AVP were studied by infusing [d(CH2)51,O-Me_Tyr2,Arg8]-vasopressin, a V1-receptor antagonist, and bosentan, a mixed ETA/ETB receptor antagonist (Study 1). Vascular responsiveness was estimated from the changes in TPR evoked by i.v. infusions of ET-1 and AVP (Study 2).
In study 1, infusion of bosentan reduced TPR and BP dramatically in DOCA-salt hypertensive rats but not in SHAM control rats, and this effect was greater when the AVP system had been blocked. In contrast, the V1 receptor antagonist alone failed to change TPR and BP in DOCA-salt hypertensive rats. However, subsequent infusion of the V1 receptor antagonist during the plateau phase of the response in bosentan pretreated DOCA-salt hypertensive rats led to significant decreases in both BP and TPR.
In study 2, TPR and BP responses to ET-1, but not AVP, were greater in DOCA-salt rats than in control rats. CO responses to ET-1 or AVP were similar in the two groups.
The results suggest that both ET and AVP play a role in the maintenance of TPR and BP; when one system is blocked the other compensates. However, the magnitude of the contribution to the hypertensive state appears greater for ET than for AVP. Enhanced vascular responses to ET appear to contribute to this greater role.
Keywords: Endothelin, vasopressin, DOCA-salt hypertension, blood pressure, cardiac output, total peripheral resistance, vascular responsiveness
Introduction
Both endothelin (ET) and vasopressin (AVP) have been reported to play a role in the development and maintenance of high blood pressure (BP) in the deoxycorticosterone acetate (DOCA)-salt hypertensive rat (Berecek et al., 1980; Crofton et al., 1979; Larivière et al., 1993a; Li et al., 1994; Yu et al., 1999). ET-1 content and preproET-1 mRNA gene expression are elevated in the blood vessels of the DOCA-salt model but not the SHR model of hypertension (Larivière et al., 1993a,1993b). Brattleboro rats homozygous for diabetes insipidus, which lack AVP, fail to develop DOCA-salt hypertension unless AVP or desmopressin (dDAVP) replacement therapy is added to the treatment (Crofton et al., 1979; Saito & Yajima, 1982).
Studies on the effects of antagonists and studies on vascular/pressor responsiveness are two approaches used to implicate hormones in the maintenance of the hypertensive state. The effects of antagonists of ET and AVP provide direct evidence on the roles of these two peptides in the regulation of vascular tone. Administration of a mixed ETA/ETB receptor antagonist or a selective ETA receptor antagonist has been shown consistently to lower BP in DOCA-salt hypertensive rats (Bird et al., 1995; Yu et al., 1998; 1999). On the other hand, peripheral administration of a V1 receptor antagonist has produced conflicting results (Burrell et al., 1994; Crofton et al., 1979; Filep et al., 1987b; Hiwatari et al., 1986; Okada et al., 1995; Toba et al., 1994) . However, interpretation of the response to an antagonist requires caution when another contributing system is allowed to compensate. If both ET and AVP play a role in maintaining BP in the DOCA-salt model, then it is reasonable to postulate that inactivation of one system may evoke compensatory activation of the other system. Consequently, the magnitude of the response to either an ET antagonist or a V1 receptor antagonist may be underestimated unless the countervailing system is prevented from compensating.
While effects of antagonists provide direct evidence implicating hormones in the regulation of cardiovascular function, changes in vascular/pressor responsiveness provide at least indirect evidence on the roles of vasoactive agents in the hypertensive state. ‘Vascular responsiveness' in this study is defined as the overall changes in vascular resistance under the normal state of the animal, i.e. when the various regulatory systems of the circulation are intact. It should not be confused with the term ‘vascular reactivity' which refers to the direct vascular effects of an agonist, usually in the context of isolated tissue experiments. Studies in isolated tissue abound, but there appears to be a dearth of data on responses at the whole animal level. Recordings of BP alone are inadequate as changes in this variable may be due to changes in cardiac output (CO) or total peripheral resistance (TPR). Accordingly, data on total peripheral resistance is a more direct reflection of responses at the level of the resistance vessels of the circulation.
In order to address these issues in the early stages (24 – 31 days) of the development of hypertension in the DOCA-salt model, we studied the effect of bosentan, a non-peptide mixed ETA/ETB antagonist, both when the AVP system was functional and when it was prevented from compensating by a prolonged infusion of [d(CH2)51,O-Me_Tyr2,Arg8]-vasopressin, a V1 receptor antagonist. Conversely, we recorded the response to the V1 receptor antagonist both when ET system was functional and when it was prevented from compensating by an injection of bosentan. This crossover design was chosen to test the hypothesis that both the ET and AVP systems contribute to the hypertensive state, and that the role of either is underestimated when only one system is blocked. In order to determine if changes in vascular responsiveness contributed to a role for ET and AVP, we compared dose-response curves to these peptides in DOCA-salt and SHAM control rats. Finally, we sought to determine if the changes in BP evoked by these various interventions were due to changes in CO or TPR by recording BP with radiotelemetry devices and CO with ultrasonic transit-time flowprobes. TPR was calculated from the pressure and flow recordings.
Methods
Male Sprague-Dawley (SD) rats were purchased from Charles River (St. Constant, Quebec, Canada) at 6 weeks of age and raised in our animal quarters under standardized conditions. Some SD rats were bred and raised in our animal quarters. At 8 – 10 weeks of age, the right kidney was removed through a dorsal flank incision under ether anaesthetization. One week later, these rats were divided randomly into two groups, DOCA- and SHAM-control groups. In the DOCA-group, a Silastic strip impregnated with 100 mg kg−1 body wt of DOCA (ICN Biomedicals Inc., Aurora, Ohio, U.S.A.) was implanted subcutaneously in the midscapular region. From this point on, these rats were given a 0.9% NaCl-0.2% KCl solution for drinking ad libitum over 24 days (in study 2) or 31 days (in study 1). Under this regimen, this group of rats developed benign, not malignant hypertension without losing their body weight. In the SHAM-group, a DOCA-free Silastic strip was implanted subcutaneously. Tap water was provided as a drinking solution to the SHAM-group.
After 14 or 21 days, the animals were anaesthetized with Somnotol (sodium pentobarbitone, 60 mg kg−1) intraperitoneally. Thoracotomy was performed under aseptic conditions. A 2.5 SB series ultrasonic flowprobe (Transonic Systems Inc., NY, U.S.A.) was implanted on the ascending aorta of each rat for the recording of CO. The flowprobe cable was tunneled subcutaneously and the connector was exited at the back of the neck. The catheter of a radiotelemetry device (TA11PA-C40, Data Sciences, Minneapolis, MN, U.S.A.) was inserted into the left femoral artery and pushed so that its tip reached the abdominal aorta above the iliac bifurcation for monitoring BP. The capsule containing the transducer and radiotransmitter was positioned in the left flank region subcutaneously. The right and left femoral veins were cannulated with MRE040 tubing (Braintree Scientific Inc., Braintree, MA, U.S.A.) for drug infusions.
Ten days later, these rats regained their body weights from the loss of surgery. One rat did not recover from the anaesthetic and surgery. BP and CO were recorded in conscious and unrestrained rats. Pressure data were collected with a computer driven data acquisition system (Data Sciences). The pressure waveform was sampled every 30 s with a 5-s sample duration. CO was recorded by feeding the signal from the flowmeter to a pen recorder (Grass Instrument Co. Quincy, MA, U.S.A.). The BP and CO data were synchronized from timing devices on the instrumentation. TPR was calculated as the quotient of BP and CO, assuming right atrial pressure is close to zero. The rats were placed in the recording room for 2 days before any experimental interventions. On the day of an experiment, BP and CO were recorded in the basal state for 2 h before any experimental interventions. Four rats did not enter the intervention phase (antagonist and agonist studies), in each case because the flow signals failed to meet pre-set criteria.
In the one study (antagonist study), the effects of a V1 antagonist and an ET antagonist were recorded separately, and also in the presence of one another. In all experiments a 2 h control period was recorded before any interventions. In one series of experiments (study 1b), the effects of the V1 receptor antagonist were tested during blockade of ET receptors in both DOCA-salt hypertensive (n=8) and SHAM control (n=7) rats. After the 2-h control period, a single dose of bosentan (30 mg kg−1) was injected intravenously. One hundred minutes later, a bolus dose of 8 μg kg−1 of the V1 receptor antagonist was injected, and this loading dose was followed by a maintenance dose of 0.05 μg kg−1 min−1 for the remainder of the experiment (30 min). In another series of experiments (study 1a), we recorded the effects of bosentan after blockade of the V1 receptors in both DOCA-salt (n=8) and SHAM- (n=6) rats. The protocol adopted here was similar to the previous one, except that the V1 antagonist was administered first. Thirty minutes after the start of the V1 receptor antagonist (8 μg kg−1 followed by 0.05 μg kg−1 min−1), a bolus dose of bosentan (30 mg kg−1) was injected through another venous catheter. The response to bosentan was recorded over the following 100 min. The 100-min period after bosentan was chosen because we had shown previously that the response to bosentan in DOCA-rats had reached the maximum by this time and was sustained for at least an additional 80 min. Moreover, in our laboratory, this dose of bosentan effectively antagonized responses to exogenously administered ET-1. The dosage regimen for the V1 antagonist was chosen on the basis of previous work published from this laboratory and from preliminary experiments. The maximum fall in BP occurs within the first 5 – 10 min of the infusion of the V1 antagonist with no further decreases over 2 h. This regimen virtually abolished responses to infusions of 1, 3, 10 and 30 pmoles kg−1 min−1 of AVP (data not shown).
In a second study (agonist study), responses to various doses of ET and AVP were recorded. In one series of experiments (study 2a) consisting of six DOCA-salt hypertensive rats and six SHAM normotensive control rats, increasing doses of ET-1 (0.03, 0.06, 0.1 and 0.3 nmol kg−1) were injected intravenously every 10 min in a cumulative fashion. In a second series of experiments (study 2b) consisting of six DOCA-salt hypertensive rats and five SHAM normotensive control rats, increasing doses of AVP (1, 3, 10, 30 pmoles kg−1 min−1) were infused through a femoral vein catheter at 10 min intervals in a cumulative fashion.
All mean values are expressed as means±s.e.mean. Graphs of the residuals were plotted to detect heterogeneity of variances. If needed, homogeneity of variance was achieved by log transformation of the data. Control values were compared by one factor ANOVA. Interactions between Group and Time (study 1a and 1b), between Pretreatment and Time (study 1a vs 1b), and between Group and Dose (study 2) were determined by ANOVA for repeated measures.
Result
Study 1: Antagonist study
Control values
During the pre-infusion control periods (Table 1), BP was significantly higher (P<0.05) in DOCA-salt hypertensive rats than in age matched SHAM-rats. This elevated BP was due to the elevated TPR (P<0.05) observed in the DOCA-salt hypertensive groups compared to the SHAM groups. CO was similar in the DOCA-salt hypertensive and SHAM groups.
Table 1.
Control values for Study 1 (Antagonist study)
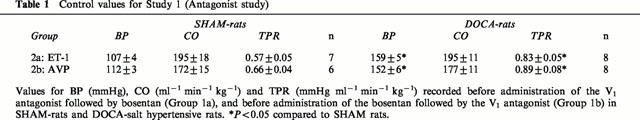
Responses to bosentan after blockade of V1 receptors (Study 1a)
The time-course of the changes in BP, CO and TPR in DOCA-salt hypertensive and SHAM-rats treated with the V1 receptor antagonist followed by bosentan are depicted in Figure 1. The V1 antagonist alone failed to elicit significant changes in BP, CO and TPR in the two groups. There were no interactions between group and time for BP (F=1.213, df=3, P=0.318), CO (F=1.271, df=3, P=0.298) and TPR (F=2.310, df=3, P=0.091) for the 0 – 30 min period. After the blockade of V1 receptors, bosentan evoked a dramatic fall in TPR and BP in DOCA-salt hypertensive rats but not SHAM rats: there were significant interactions between group and time for BP (F=20.767, df=4, P<0.001) and TPR (F=7.998, df=4, P<0.001) for the 30 – 130 min period. In contrast to BP and TPR, there was no interaction between group and time for CO (F=0.241, df=4, P=0.914) for this period.
Figure 1.
Changes in BP, CO and TPR of DOCA-salt hypertensive and SHAM control rats following treatment with the V1 receptor antagonist (V1-ant.) and then bosentan (Bos). After a 2 h control period, 8 μg kg−1 of the V1 receptor antagonist was injected followed by 0.05 μg kg−1 min−1 for the remainder of the experiment. Thirty minutes after the start of the V1 antagonist, a bolus dose of bosentan (30 mg kg−1) was injected through another venous catheter and the responses to bosentan were followed for another 100 min. Group-Time Interactions (ANOVA for repeated measures): 0 – 30 min – BP (F=1.213, df=3, P=0.318), CO (F=1.271, df=3, P=0.298), TPR (F=2.310, df=3, P=0.091); 30 – 130 min – BP (F=20.767, df=4, P<0.001), CO (F=0.241, df=4, P=0.914), TPR (F=7.998, df=4, P<0.001).
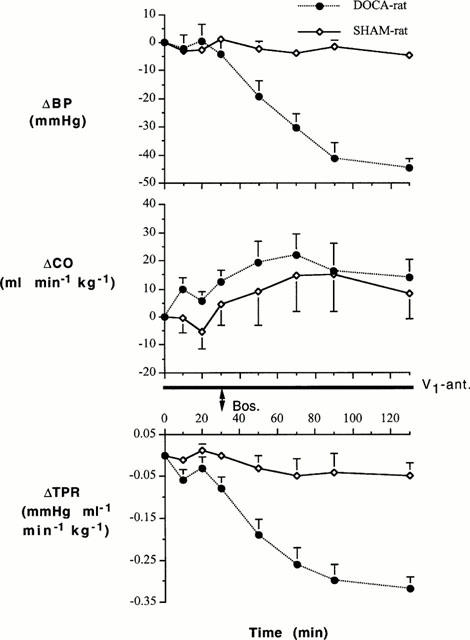
Responses to the V1 receptor antagonist after blockade of ET receptors (Study 1b)
The time-course of the changes in BP, CO and TPR in DOCA-salt hypertensive and SHAM-control rats treated with bosentan followed by the V1 receptor antagonist are shown in Figure 2. Bosentan alone decreased BP and TPR in DOCA-salt hypertensive rats but not in SHAM rats: there were significant interactions between group and time for BP (F=6.363, df=4, P<0.001) and TPR (F=3.527, df=4, P=0.013), but not for CO (F=1.397, df=4, P=0.249), for the 0 – 100 min period. After blockade of ET system, the V1 antagonist lowered BP and TPR: however, there were no interactions between group and time for BP (F=1.613, df=3, P=0.203), CO (F=0.145, df=3, P=0.932), and TPR (F=1.143, df=3, P=0.345) for the 100 – 130 min period.
Figure 2.
Changes in BP, CO and TPR of DOCA-salt hypertensive and SHAM control rats following treatment with bosentan (Bos.) and then the V1 receptor antagonist (V1-ant.). A bolus dose of bosentan (30 mg kg−1) was injected through a venous catheter and the responses to bosentan were followed for 100 min. At this time, the V1 antagonist was administered through another venous catheter (8 μg kg−1 followed by 0.05 μg kg−1 min−1). Group-Time Interactions (ANOVA for repeated measures): 0 – 100 min – BP (F=6.363, df=4, P<0.001), CO (F=1.397, df=4, P=0.249), TPR (F=3.527, df=4, P=0.013); 100 – 130 min – BP (F=1.613, df=3, P=0.203), CO (F=0.145, df=3, P=0.932), TPR (F=1.143, df=3, P=0.345).
Comparison of the two series
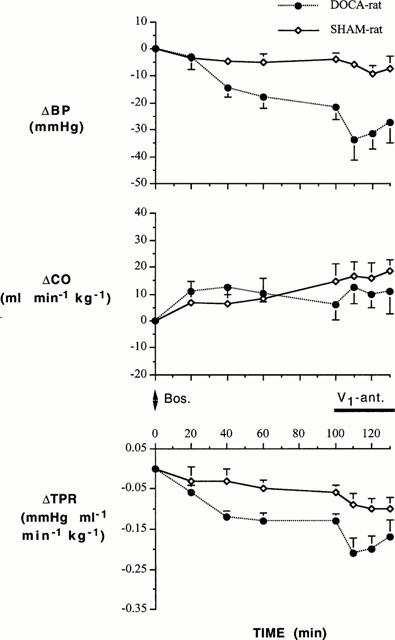
Comparison of the changes in BP and TPR elicited by the two antagonists when administered alone and in the presence of one another in the DOCA-salt group is important (Figures 1, 2 and 3). While bosentan alone (V1 receptors functional) evoked a large fall in BP and TPR in the DOCA-salt hypertensive group (Figure 2, 0 – 100 min), the changes in these two variables were much greater when the V1 receptors had been blocked (Figure 1, 30 – 130 min): there were significant interactions between pretreatment and time for both BP (F=4.478, df=4, P=0.003) and TPR (F=3.002, df=4, P=0.026). Conversely, the V1 receptor antagonist alone (ET receptors functional) failed to induce significant changes in BP and TPR in the DOCA-salt hypertensive group (Figure 1, 0 – 30 min). However, when ET receptors were blocked by bosentan, the V1 receptor antagonist did evoke small but statistically significant changes in these two variables (Figure 2, 100 – 130 min): there were significant interactions between pretreatment and time for BP (F=3.076, df=3, P=0.038) and TPR (F=2.945, df=3, P=0.044). In contrast to BP and TPR, there were no interactions between pretreatment and time for CO for bosentan (F=0.234, df=4, P=0.918) or the V1 antagonist (F=0.631, df=3, P=0.599). Comparison of the responses to the two antagonists when administered alone and in combination is facilitated by comparing the maximum responses attained during the infusions (Figure 3).
Figure 3.
Changes in BP and TPR to the V1 receptor antagonist and bosentan in DOCA-salt hypertensive rats (DOCA) and SHAM control rats (SHAM). *P<0.05 compared to the antagonist pretreatment value and †P<0.05 compared to response when V1 receptors were functional.
Study 2: Agonist study
Control values
The values for BP, CO, and TPR recorded during the pre-infusion control periods are shown in Table 2. BP was elevated in the DOCA-salt hypertensive groups compared to the SHAM groups. The elevated pressure was due to the elevated TPR, as CO was similar in the two groups.
Table 2.
Control values for Study 2 (Agonist study)

Responses to ET-1 and AVP
Changes in BP, CO and TPR in DOCA-salt hypertensive and SHAM-control rats to ET-1 are shown in Figure 4, while changes in these variables to AVP are shown in Figure 5. ET-1 evoked dose-related increases in TPR and BP and decreases in CO. These changes were greater in the DOCA-salt hypertensive group than in the SHAM normotensive group: there were significant interactions between group and dose for BP (F=3.560, df=3, P=0.027) and TPR (F=3.001, df=3, P=0.048), but not for CO (F=0.534, df=3, P=0.663). In contrast to ET-1, the dose-related increases in TPR and BP evoked by AVP were similar in the DOCA-salt and SHAM groups: there were no interactions between group and dose for BP (F=0.099, df=3, P=0.959), CO (F=1.154, df=3, P=0.348), and TPR (F=0.0898, df=3, P=0.965).
Figure 4.
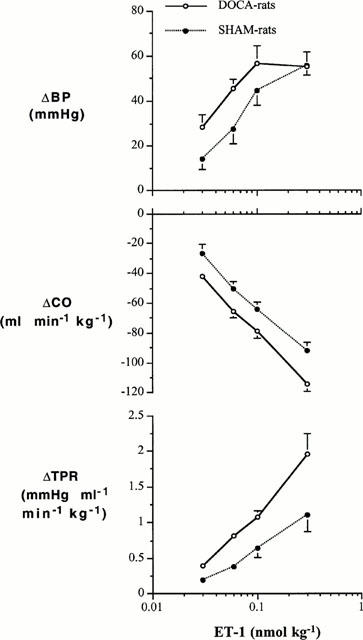
Changes in BP, CO and TPR to cumulative i.v. injections of ET-1 in DOCA-salt hypertensive rats and SHAM control rats. Group-Dose Interactions (ANOVA for repeated measures): BP (F=3.560, df=3, P=0.027), CO (F=0.534, df=3, P=0.663), TPR (F=3.001, df=3, P=0.048).
Figure 5.
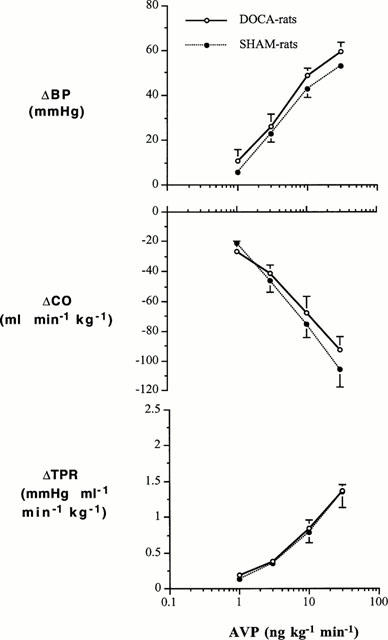
Changes in BP, CO and TPR to cumulative i.v. infusions of AVP in DOCA-salt hypertensive rats and SHAM control rats. Group-Dose Interactions (ANOVA for repeated measures): BP (F=0.099, df=3, P=0.959), CO (F=1.154, df=3, P=0.348), and TPR (F=0.0898, df=3, P=0.965).
Discussion
A feature of the studies reported in this article is that BP was recorded with radiotelemetry devices and CO was recorded with ultrasonic transit-time flowmeters. The devices can be implanted well in advance of any experimental interventions permitting longer recovery times from the surgery and conditioning of the animals to the recording room. As well, pressure and flow can be recorded for several weeks. BP tends to be lower in hypertensive animals when pressure has been recorded by radiotelemetry, an observation which has been attributed to the longer recovery periods from surgery and the reduced level of stress associated with this technique (Balakrishnan et al., 1998; Bazil et al., 1993). By recording CO and BP, we were able to calculate TPR, a true measure of the resistance function of the circulation and a better index of vascular responsiveness than BP. In this study we focused on the early developmental phase of hypertension before the onset of the late malignant phase. The observation that blood pressure was higher in the 31 day treated rats (Table 1) than in the 24 day treated rats (Table 2) is consistent with the development phase.
The data reported in this article suggest that both the ET system and the AVP system contribute to the maintenance of BP in the DOCA-salt hypertensive rat and that the two systems appear to operate in a redundant overlapping fashion. Others have studied the effects of either an ET antagonist or an AVP antagonist in DOCA-salt hypertensive rats (see Introduction), but this study appears to be the first report in which changes in BP, CO, and TPR were recorded when the two antagonists were administered together. While bosentan alone reduced BP in the DOCA-salt group, even when the AVP system was fully functional, the response to bosentan was much greater when the AVP system was prevented from compensating. Conversely, the V1 antagonist alone failed to change BP when the ET system was functional, but the antagonist did reduce BP when the ET system was blocked and prevented from compensating. Thus, in the absence of one system, the other compensated. It follows that the magnitude of the contribution of the two systems to the support of BP may be underestimated when studies are limited to one of these two systems. The maximum changes in BP and TPR recorded with combined blockade were somewhat different depending on the order of administration of the two antagonists. When the infusion of the V1 antagonist followed bosentan, the maximum fall in BP evoked by combined blockade was approximately 35 mmHg (Figure 2), whereas when the V1 antagonist preceded the administration of bosentan, the maximum fall in pressure was approximately 45 mmHg (Figure 1). The reason for this difference is not apparent. One difference between the two protocols is that the V1 antagonist was infused for a much longer period in the second instance (Figure 1). However, based on our current understanding of V1 receptor function and the pharmacology of the V1 antagonist, there is no reason to suspect this could account for the differences as the maximum effect of the antagonist is achieved in the first 5 – 10 min following its administration. We have infused the V1 antagonist alone to hypertensive animals for over 2 h without effect. Thus it appears more related to the order of administration. It is possible that other counterregulatory mechanisms respond more robustly with the second protocol. However, such possibilities are highly speculative. In the final analysis, while the data from Figures 1 and 2 show some unexplained quantitative differences (35 mmHg vs 45 mm Hg), the effects are qualitatively similar and support the overall hypothesis, i.e. the effect of either antagonist is underestimated when the countervailing system is functional.
The decreases in BP evoked by the two antagonists were due to changes in TPR, and not on factors that regulate CO. Indeed, while the changes in CO evoked by the two antagonists were not statistically significant, the trend was towards increases in CO, which would tend towards attenuating the antihypertensive effects of the antagonists.
Responses to ET-1 were enhanced in DOCA-salt hypertensive rats compared to their SHAM controls (Figure 4): the dose-response curve relating the dose of ET-1 to changes in TPR in DOCA-salt hypertensive rats was positioned to the left of the curve for SHAM rats. A change of 1 mmHg ml−1 min−1 kg−1 required a dose of 0.1 nmol kg−1 of ET-1 in the DOCA-salt group and 0.3 nmol kg−1 in the SHAM group, a 3 fold difference. This increased vascular responsiveness was expressed as a parallel increase in pressor responsiveness in the DOCA-salt group. In contrast to TPR and BP, the dose-related decreases in CO in the DOCA-salt and SHAM groups were not significantly different. ET-1 has been associated with both negative and positive inotropic effects in the rabbit heart (Zhu et al., 1997), but in the rat heart, ET-1 evokes positive inotropic effects (Garcia et al., 1990). Thus, the decreases of CO were unlikely due to direct negative inotropic effects of ET-1 and were more likely the consequence of the increases in TPR, i.e. afterload. In contrast to our findings at the haemodynamic level, others have reported that contractile responses of aorta and mesenteric artery rings are decreased, and that ET receptors are downregulated (Larivière et al., 1988; Nguyen et al., 1992) in DOCA-salt hypertensive rats. It is unlikely that the differences between the our findings on TPR and those in ring preparations can be explained by structural changes in the vasculature reported to occur in various hypertensive rat models i.e. the decrease in the lumen to wall thickness ratio of resistance vessels (Folkow, 1982; Folkow et al., 1973). Admittedly, vascular hypertrophy has been reported in resistance arteries of DOCA-salt hypertensive rats with a prominent thickening of the media (Deng & Schiffrin, 1992b). The remodelled resistance arteries have a reduced lumen size, which could act as an amplifier to vasoconstrictor stimuli. However, structural remodelling would result in a non-specific increase in vascular responsiveness. We found that responsiveness to ET-1, but not AVP, was increased. Thus, remodelling does not appear to explain the differences between the studies in isolated ring preparations and our data in the whole animal. Moreover, our experiments were performed early in the development of hypertension. While some remodelling may have occurred during this time, the dramatic changes reported to occur develop later in the course of hypertension. Another possible explanation for these discrepancies is that the in vitro studies were performed in endothelium-denuded ring preparations. On the basis of these findings, it is tempting to suggest that the increased vascular responsiveness (TPR) found in our studies may be more related to endothelial dysfunction than changes at the level of the vascular smooth muscle. Alternatively, the in vitro studies in ring preparations were performed on larger conducting vessels upstream from the resistance vessels, while TPR recorded in our studies is a measure of tone at the level of the resistance vessels. In the final analysis, the increased vascular responsivenss (TPR) of DOCA-salt hypertensive rats to ET-1 appears to contribute to the hypertensive state.
In contrast to ET-1, vascular responsiveness to AVP was not increased in the DOCA-salt hypertensive rat. Pressor responsiveness to AVP has been reported to be increased (Mimura et al., 1995), unchanged (Burnier et al., 1983), or decreased (Filep et al., 1985) in DOCA-salt hypertensive rats compared to normotensive controls. However, none of these studies employed the radiotelemetry system for recording blood pressure, a system more suitable for chronic recordings. More importantly, none of the studies reported on the changes in TPR as an index of vascular responsiveness. In view of the lack of enhanced vascular responsiveness to AVP observed in our study, another explanation must be sought to explain the fall in blood pressure evoked by the V1 antagonist in DOCA-salt hypertensive rats. Plasma levels of AVP have been reported to be increased in this model of hypertension. Elevated levels of the peptide are the most likely explanation accounting for the modest contribution of AVP to maintaining vascular tone.
Other regulatory systems may contribute to the DOCA-salt model. A role for the sympathetic nervous system cannot be excluded as both pre- and postsynaptic adrenergic dysfunctions have been reported in this model (de Champlain, 1990). On the other hand, the DOCA-salt model appears to be a renin-independent model. In any case, it is possible that these systems may interact with the ET and AVP systems and contribute indirectly to the hypertensive state. However, their direct contribution would appear to be small because the combined blockade with the ET and AVP antagonist caused decreases in BP of 35 – 45 mmHg leaving little room for additional BP reductions towards normotensive levels.
The relative importance of ET and AVP subtypes in this model is undoubtedly complex. ETA receptors appear to account for much of the vascular effects of the mixed ETA/ETB antagonist, bosentan, because the effects of a selective ETA antagonist were very similar to the effects of bosentan (Yu et al., 1998). Moreover, earlier studies have shown increased ET generation and reduced vascular reactivity to exogenous ET-1 in aortic and mesenteric blood vessels of DOCA-salt hypertensive rats. In this study, endothelium removal did not affect the decreased responsiveness to ET-1 suggesting that ETB receptor function, at least in the vascular endothelium, is unaltered in DOCA-salt hypertension (Deng & Schiffrin, 1992a). On the other hand, the development and maintenance of hypertension was found to be exaggerated in ET(B) receptor-deficient DOCA-salt hypertensive rats (Matsumura et al., 2000), and increased ETB expression in kidneys of DOCA-salt hypertensive rats has been reported (Pollock et al., 2000). Thus, ETB receptor function in the kidney may act as a compensatory mechanism to attenuate the development of hypertension. In the case of AVP, V2 receptors in the kidney may contribute to the volume retention observed in this model of hypertension (Filep et al., 1987a; Hofbauer et al., 1984). Thus, receptors in the kidney for both ET and for AVP may well participate in the overall response associated with this model of hypertension. However, most of the vascular effects appear to be accounted for by ETA and V1 receptors.
In summary, both ET and AVP appear to play a role during the early stages in the development (24 – 31 days) of hypertension in the DOCA-salt model through an elevation in TPR. The contribution of either the ET system or the AVP system is underestimated when the countervailing system is allowed to compensate. The magnitude of the contribution was greater for ET than for AVP. An increase in vascular responsiveness to ET appears to contribute to its greater role.
Acknowledgments
We thank Dr Martine Clozel (Actelion, Allschwil, Switzerland) for the supply of bosentan and D. Brown for excellent care of the animals. The research was supported by grants from the Medical Research Council of Canada and the Heart and Stroke Foundations of Saskatchewan.
Abbreviations
- ANOVA
analysis of variance
- AVP
arginine vasopressin
- BP
blood pressure
- CO
cardiac output
- DOCA
deoxycorticosterone acetate
- ET
endothelin
- i.v.
intravenous
- TPR
total peripheral resistance
References
- BALAKRISHNAN S., TATCHUM-TALOM R., MCNEILL J.R. Radiotelemetric versus externalized catheter monitoring of blood pressure: Effect of vasopressin in spontaneous hypertension. J. Pharmacol. Toxicol. Methods. 1998;40:87–93. doi: 10.1016/s1056-8719(98)00042-2. [DOI] [PubMed] [Google Scholar]
- BAZIL M.K., KRULAN C., WEBB R.L. Telemetric monitoring of cardiovascular parameters in conscious spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1993;22:897–905. doi: 10.1097/00005344-199312000-00019. [DOI] [PubMed] [Google Scholar]
- BERECEK K.H., MURRAY R.D., BRODY M.J., GROSS F. Vasopressin and vascular reactivity in the development of deoxycorticosterone hypertension in rats with hereditary diabetes insipidus. Circulation. 1980;III:838. doi: 10.1161/01.hyp.4.1.3. [DOI] [PubMed] [Google Scholar]
- BIRD J.E., MORELAND S., WALDRON T.L., POWELL J.R. Antihypertensive effects of a novel endothelin-A receptor antagonist in rats. Hypertension. 1995;25:1191–1195. doi: 10.1161/01.hyp.25.6.1191. [DOI] [PubMed] [Google Scholar]
- BURNIER H., BIOLLAZ J., BRUNNER D.B., GAVRAS H., BRUNNER H.R. Alpha and beta adrenoceptor blockade in normotensive and deoxycorticosterone (DOC)-hypertensive rats; plasma vasopressin and vasopressin pressor effect. J. Pharmacol. Exp. Ther. 1983;224:222–227. [PubMed] [Google Scholar]
- BURRELL L.M., PHILLIPS P.A., STEPHENSON J.M., RISVANIS J., ROLLS K.A., JOHNSTON C.I. Blood pressure-lowering effect of an orally active vasopressin V1 receptor antagonist in mineralocorticoid hypertension in the rat. Hypertension. 1994;23:737–743. doi: 10.1161/01.hyp.23.6.737. [DOI] [PubMed] [Google Scholar]
- CROFTON J.T., SHARE L., SHADE R.E., LEE-KWON W.J., MANNING M., SAWYER W.H. The importance of vasopressin in the development and maintenance of DOCA-salt hypertension in the rat. Hypertension. 1979;1:31–38. doi: 10.1161/01.hyp.1.1.31. [DOI] [PubMed] [Google Scholar]
- DE CHAMPLAIN J. Pre- and postsynaptic adrenergic dysfunctions in hypertension. J. Hypertens. 1990;8 Suppl:S77–S85. [PubMed] [Google Scholar]
- DENG L.Y., SCHIFFRIN E.L. Effects of endothelin on resistance arteries of DOCA-salt hypertensive rats. Am. J. Physiol. 1992a;262:H1782–H1787. doi: 10.1152/ajpheart.1992.262.6.H1782. [DOI] [PubMed] [Google Scholar]
- DENG L.Y., SCHIFFRIN E.L. Effects of endothelin on resistance arteries of DOCA-salt hypertensive rats. Am. J. Physiol. 1992b;262:H1782–H1787. doi: 10.1152/ajpheart.1992.262.6.H1782. [DOI] [PubMed] [Google Scholar]
- FILEP J., FROLICH J.C., FEJES-TOTH G. Evidence against a vasopressor role of ADH in malignant DOC-salt Hypertension. Clin. Exper.-Theory and Practise. 1985;A7(10):1457–1470. doi: 10.3109/10641968509073603. [DOI] [PubMed] [Google Scholar]
- FILEP J., FROLICH J.C., FOLDES-FILEP E. Role of AVP in malignant DOC-salt hypertension: studies using vascular and antidiuretic antagonists. Am. J. Physiol. 1987a;253:F952–F958. doi: 10.1152/ajprenal.1987.253.5.F952. [DOI] [PubMed] [Google Scholar]
- FILEP J., FROLICH J.C., FOLDES-FILEP E. Role of AVP in malignant DOC-salt hypertension: studies using vascular and antidiuretic antagonists. Am. J. Physiol. 1987b;253:F952–F958. doi: 10.1152/ajprenal.1987.253.5.F952. [DOI] [PubMed] [Google Scholar]
- FOLKOW B. Physiological aspects of primary hypertension. Physiol. Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- FOLKOW B., HALLBACK M., LUNDGREN Y., SIVERSTSSON R., WEILL L. Importance of adaptive changes in vascular design for establishment of primary hypertension studied in man and in spontaneously hypertensive rats. Circ. Res. 1973;32/33 suppl. I:I-2–I-16. doi: 10.1007/978-3-642-65441-1_23. [DOI] [PubMed] [Google Scholar]
- GARCIA R., LACHANCE D., THIBAULT G. Positive inotropic action, natriuresis and atrial natriuretic factor release induced by endothelin in the conscious rat. J. Hypertens. 1990;8:725–731. doi: 10.1097/00004872-199008000-00006. [DOI] [PubMed] [Google Scholar]
- HIWATARI M., ABRAHAMS J.M., SAITO T., JOHNSTON C.I. Contribution of vasopressin to the maintenance of blood pressure in deoxycorticosterone-salt induced malignant hypertention in spontaneously hypertensive rats. Clinical Science. 1986;70:191–198. doi: 10.1042/cs0700191. [DOI] [PubMed] [Google Scholar]
- HOFBAUER K.G., MAH S.C., BAUM H.P., HANNI H., WOOD J.M., KRAETZ J. Endocrine control of salt and water excretion: the role of vasopressin in DOCA-salt hypertension. J. Cardiovasc. Pharmacol. 1984;6:S184–S191. doi: 10.1097/00005344-198400061-00029. [DOI] [PubMed] [Google Scholar]
- LARIVIÈRE R., DAY R., SCHIFFRIN E.L. Increased expression of endothelin-1 gene in blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1993a;21:916–920. doi: 10.1161/01.hyp.21.6.916. [DOI] [PubMed] [Google Scholar]
- LARIVIÈRE R., ST-LOUIS J., SCHIFFRIN E.L. Vascular binding sites and biological activity of vasopressin in DOCA-salt hypertensive rats. J. Hypertens. 1988;6:211–217. doi: 10.1097/00004872-198803000-00005. [DOI] [PubMed] [Google Scholar]
- LARIVIÈRE R., THIBAULT G., SCHIFFRIN E.L. Increased Endothelin-1 Content in Blood Vessels of Deoxycorticosterone Acetate-Salt Hypertensive but not in Spontaneously Hypertensive Rats. Hypertension. 1993b;21:294–300. doi: 10.1161/01.hyp.21.3.294. [DOI] [PubMed] [Google Scholar]
- LI J.S., LARIVIÈRE R., SCHIFFRIN E.L. Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats: Evidence for a role of endothelin in vascular hypertrophy. Hypertension. 1994;24:183–188. doi: 10.1161/01.hyp.24.2.183. [DOI] [PubMed] [Google Scholar]
- MATSUMURA Y., KURO T., KONISHI F., TAKAOKA M., GARIEPY C.E., YANAGISAWA M. Enhanced blood pressure sensitivity to DOCA-salt treatment in endothelin ET(B) receptor-deficient rats. Br. J. Pharmacol. 2000;129:1060–1062. doi: 10.1038/sj.bjp.0703157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMURA Y., OGURA T., YAMAUCHI T., OTSUKA F., OISHI T., HARADA K., HASHIMOTO M., OTA Z. Effect of Vasopressin V1- and V2-Receptor Stimulation on Blood Pressure in DOCA-Salt hypertensive Rats. Acta Med. Okayama. 1995;49:187–194. doi: 10.18926/AMO/30375. [DOI] [PubMed] [Google Scholar]
- NGUYEN P.V., PARENT A., DENG L.Y., FLÜCKIGER J.-P., THIBAULT G., SCHIFFRIN E.L. Endothelin vascular receptors and responses in DOCA-salt hypertensive rats. Hypertension. 1992;19 suppl II:II-98–II-104. doi: 10.1161/01.hyp.19.2_suppl.ii98. [DOI] [PubMed] [Google Scholar]
- OKADA H., KANNO Y.S., SARUTA T. Effect of nonpeptide vasopressin receptor antagonists on developing, and established DOCA-salt hypertension in rats. Clin. Exper. Hypertension. 1995;17:469–483. doi: 10.3109/10641969509037419. [DOI] [PubMed] [Google Scholar]
- POLLOCK D.M., ALLCOCK G.H., KRISHNAN A., DAYTON B.D., POLLOCK J.S. Upregulation of endothelin B receptors in kidneys of DOCA-salt hypertensive rats. Am. J. Physiol. Renal. Physiol. 2000;278:F279–F286. doi: 10.1152/ajprenal.2000.278.2.F279. [DOI] [PubMed] [Google Scholar]
- SAITO T., YAJIMA Y. Development of DOCA-salt hypertension in the brattleboro rat. NYAS. 1982;394:309–318. doi: 10.1111/j.1749-6632.1982.tb37442.x. [DOI] [PubMed] [Google Scholar]
- TOBA K., OUCHI Y., LIANG J., AKISHITA M., ORIMO H. Effect of an intracerebroventricularly administered vasopressin V1 antagonist on blood pressure and heart rate in deoxycorticosterone-salt hypertensive rats. J. Auton. Nerv. Syst. 1994;50:123–129. doi: 10.1016/0165-1838(94)90002-7. [DOI] [PubMed] [Google Scholar]
- YU M., GOPALAKRISHNAN V., MCNEILL J.R. Hemodynamic Effect of a selective endothelin-A receptor antagonist in deoxycorticosterone acetate-salt hypertensive rats. J. Cardiovasc. Pharmacol. 1998;31 Suppl. 1:S262–S264. doi: 10.1097/00005344-199800001-00074. [DOI] [PubMed] [Google Scholar]
- YU M., GOPALAKRISHNAN V., MCNEILL J.R. Total peripheral conductance mediates antihypertensive effect of nonpeptide deoxycorticosterone acetate-salt hypertensive rats. Am. J. Hypertens. 1999;12:845–848. doi: 10.1016/s0895-7061(99)00055-2. [DOI] [PubMed] [Google Scholar]
- ZHU Y., YANG H.T., ENDOH M. Negative chronotropic and inotropic effects of endothelin isopeptides in mammalian cardiac muscle. Am. J. Physiol. 1997;273:H119–H127. doi: 10.1152/ajpheart.1997.273.1.H119. [DOI] [PubMed] [Google Scholar]


