Abstract
This study was designed to assess the molecular and cellular events involved in the up-regulation (and receptor supersensitivity) of brain α2-adrenoceptors as a result of chronic depletion of noradrenaline (and other monoamines) by reserpine.
Chronic reserpine (0.25 mg kg−1 s.c., every 48 h for 6 – 14 days) increased significantly the density (Bmax values) of cortical α2-adrenoceptor agonist sites (34 – 48% for [3H]-UK14304, 22 – 32% for [3H]-clonidine) but not that of antagonist sites (11 – 18% for [3H]-RX821002). Competition of [3H]-RX821002 binding by (−)-adrenaline further indicated that chronic reserpine was associated with up-regulation of the high-affinity state of α2-adrenoceptors.
In cortical membranes of reserpine-treated rats (0.25 mg kg−1 s.c., every 48 h for 20 days), the immunoreactivities of various G proteins (Gαi1/2, Gαi3, Gαo and Gαs) were increased (25 – 34%). Because the high-affinity conformation of the α2-adrenoceptor is most probably related to the complex with Gαi2 proteins, these results suggested an increase in signal transduction through α2-adrenoceptors (and other monoamine receptors) induced by chronic reserpine.
After α2-adrenoceptor alkylation, the analysis of receptor recovery (Bmax for [3H]-UK14304) indicated that the increased density of cortical α2-adrenoceptors in reserpine-treated rats was probably due to a higher appearance rate constant of the receptor (Δr=57%) and not to a decreased disappearance rate constant (Δk=7%).
Northern- and dot-blot analyses of RNA extracted from the cerebral cortex of saline- and reserpine-treated rats (0.25 mg kg−1, s.c., every 48 h for 20 days) revealed that reserpine markedly increased the expression of α2a-adrenoceptor mRNA in the brain (125%). This transcriptional activation of the receptor gene expression appears to be the cellular mechanism by which reserpine induces up-regulation in the density of brain α2-adrenoceptors.
Keywords: α2-Adrenoceptors, agonist and antagonist binding sites, G coupling proteins, receptor turnover, α2a-adrenoceptor mRNA, reserpine, monoamine depletion, rat brain
Introduction
Reserpine, a depleter of brain monoamines (noradrenaline, serotonin, dopamine), induces in rats a biochemical and behavioural syndrome that has been extensively studied as a potential animal model of depression (Leith & Barrett, 1980; Cooper et al., 1983; Vetulani et al., 1986; Ugedo et al., 1993). In the past, reserpine treatment was commonly associated with the induction of depressive symptoms in patients who had histories of major depression (Goodwin & Bunney, 1971). Moreover, depletion of noradrenaline or serotonin in the brain of depressed patients, who were in clinical remission, has been recently shown to cause a rapid return to depressive symptoms (Berman et al., 1999; Delgado & Moreno, 1999; Ressler & Nemeroff, 1999).
In major depression α2-adrenoceptors are of special interest because these receptors control, together with other mechanisms, the activity of noradrenergic and serotonergic neurones in the brain. Thus, a relevant proportion (20 – 40%) of α2-adrenoceptors in the rat brain appears to be presynaptic autoreceptors playing a crucial role in the regulation of noradrenergic neurones (Heal et al., 1993; García-Sevilla et al., 1994). When these presynaptic inhibitory α2A/C-adrenoceptors are stimulated (autoreceptors on noradrenergic neurones and a smaller proportion of heteroreceptors on serotonergic neurones), release and synthesis of noradrenaline and serotonin are inhibited (Trendelenburg et al., 1994; Esteban et al., 1996); therefore, increased α2A/C-adrenoceptor density and/or sensitivity could result in insufficient neuronal release of noradrenaline/serotonin and lead to depression. Supersensitivity of α2A/C-adrenoceptors in specific brain regions could represent a common feature of the reduced monoaminergic (noradrenergic and/or serotonergic) function postulated in major depression (see Delgado & Moreno, 1999), and this receptor up-regulation has been observed in some (e.g. De Paermentier et al., 1997; Callado et al., 1998; García-Sevilla et al., 1999) but not all (e.g. Ferrier et al., 1986; Arango et al., 1993) postmortem studies in brains of depressed suicides.
Supersensitivity of α2-adrenoceptors also appear to be associated with reserpine treatment. Thus, prolonged depletion of brain noradrenaline (and other monoamines) after treatment with low doses of reserpine is associated with marked increases in the density and/or affinity of agonist radioligands for brain α2A/C-adrenoceptors (U'Prichard & Snyder, 1978; Bylund & Martínez, 1980; Hong et al., 1988; Giralt & García-Sevilla, 1989; Ugedo et al., 1993). In line with these radioligand binding data, various functional in vivo responses mediated by central presynaptic and postsynaptic α2A/C-adrenoceptors were found to be potentiated after treatment with reserpine (Ugedo et al., 1993). Functional studies in peripheral tissues also have shown increases in α2A-adrenoceptor sensitivity after reserpine treatment (Estan et al., 1990; Ugedo et al., 1993) and have suggested that there is an increase in the receptor reserve at α2A-adrenoceptors (autoreceptors) in reserpine-treated rats (Pineda et al., 1997).
Therefore, prolonged depletion of brain noradrenaline (and other monoamines) in rats with reserpine can induce a neuronal presynaptic dysfunction (see Delgado & Moreno, 1999) and reproduce the relevant α2A/C-adrenoceptor supersensitivity detected in the postmortem brain of depressed suicide victims (e.g. García-Sevilla et al., 1999), suggesting that these receptor changes could indeed reflect some of the pathophysiology of major depression. As mentioned, however, other studies did not find up-regulation of α2-adrenoceptors in brains of suicide victims (Ferrier et al., 1986; Arango et al., 1993). The supersensitivity of brain α2-adrenoceptors induced by reserpine appears to be a homospecific adaptation of the noradrenergic pathway to the depletion of catecholamines. However, the cellular mechanisms underlying the reserpine-induced up-regulation of brain α2-adrenoceptors are unknown and a better understanding of these mechanisms would be relevant to the pathophysiology of depressive disorders. Against this background, the present study was designed to re-assess in detail the changes induced by chronic treatment with reserpine on the density and affinity of α2A/C-adrenoceptors (agonist and antagonist binding sites), as well as on the immunoreactive levels of associated regulatory G proteins, and to assess whether reserpine-induced receptor supersensitivity is associated with an accelerated turnover of α2A/C-adrenoceptors and an increased α2a-adrenoceptor gene expression in the rat brain. The α2A-adrenoceptor is the predominant α2-adrenoceptor subtype in the mammalian brain and it plays a crucial role as mediator of most of the inhibitory effects of α2-adrenoceptor agonists (MacDonald et al., 1997). The α2-adrenoceptor nomenclature recommended by the International Union of Pharmacology has been followed (Bylund et al., 1994).
Methods
Animals and treatments
Adult male Sprague-Dawley rats (250 – 300 g) were used. The animals received a standard diet with water freely available and were housed at 20±2°C with a 12 h light/dark cycle. The animals received s.c. every 48 h either 0.9% saline vehicle or reserpine (0.25 mg kg−1) from day 1 to day 6 – 36 of treatment. The rats were killed by decapitation 48 h after the last injection of the corresponding treatment. The dose of reserpine (0.25 mg kg−1) chosen did not induce weight loss (see also Fortin & Sundaresan, 1989). Chronic treatment with this dose of reserpine (0.25 mg kg−1, s.c., every 48 h, for 20 days) resulted in prolonged depletion of noradrenaline (>80%), serotonin (>75%) and dopamine (>85%) in various rat brain regions (data not shown). EEDQ (1.6 mg kg−1) was dissolved in ethanol and then diluted sequentially with propilenglycol and purified water (final ratio 1:1:2, v/v/v) and it was administered i.p. in a single dose. To study the modulation of α2-adrenoceptors turnover by reserpine, EEDQ was injected only once in saline-treated rats and in rats treated with the monoamine depleter drug for 6 days, and treatment with saline or reserpine was then continued for various periods of time. Thus, rats were killed 6 h, and 1, 2, 4, 7, 9, 14, 18, 21, 26 and 30 days after EEDQ administration to evaluate the recovery of brain α2-adrenoceptor density, which allowed estimation of receptor turnover parameters. The brains were rapidly removed and the frontal and parieto-occipital cortex dissected on ice and stored at −70°C until assay. These experiments on rats were performed according to the guidelines of the University of the Balearic Islands.
[3H]-UK14304, [3H]-clonidine and [3H]-RX821002 binding assays
The specific binding of [3H]-UK14304 (full agonist), [3H]-clonidine (partial agonist) and that of [3H]-RX821002 (antagonist) to parieto-occipital cortical membranes were used as biochemical indexes to quantify the density of α2-adrenoceptor binding sites (α2A/C-subtypes). Preparation of cortical membranes (P2 membrane fraction) and [3H]-radioligand binding assays were carried out as described previously (Olmos et al., 1993; Ribas et al., 1993). Briefly, total [3H]-UK14304, [3H]-clonidine and [3H]-RX821002 binding was measured with eight concentrations of radioligand (6×10−11 M to 8×10−9 M, for [3H]-UK14304 and [3H]-RX821002; 1.25×10−10 M to 1.6×10−8 M, for [3H]-clonidine). For all radioligands, nonspecific binding was determined in the presence of 10−5 M (−)-adrenaline. Agonist competition studies also were performed in cortical membranes, which were incubated with [3H]-RX821002 (1×10−9 M), in the absence or presence of various concentrations of (−)-adrenaline (3.3×10−11 M to 10−3 M, 22 concentrations). Membrane-bound [3H]-radioligands were quantified as described previously (Ribas et al., 1993). Protein was determined by the method of Lowry et al. (1951) with bovine serum albumin as the standard. Analyses of saturation isotherms (Kd, dissociation constant; Bmax, maximum density of binding sites) and competition experiments (Ki, inhibition constant) as well as the fitting of data to the appropriate binding model were performed by computer-assisted non-linear analysis from untransformed data, using the EBDA-LIGAND programs (McPherson, 1985; Munson & Rodbard, 1980).
Analyses of the recovery of α2A/C-adrenoceptor binding sites after EEDQ
For analysis of α2-adrenoceptor turnover, data from the recovery (all individual experiments together) of brain α2-adrenoceptor density (Bmax for the full agonist [3H]-UK14304 binding) after irreversible inactivation by EEDQ, during saline and reserpine treatments, were analysed according to the standard mono-exponential model (Mauger et al., 1982; Barturen & García-Sevilla, 1992; Ribas et al., 1993). Exponential recovery data were fitted, using the simple nonlinear least-squares fitting program GraFit (Leatherbarrow, 1990), to the equation:
where Rt is expressed as fmol mg−1 protein and represents the receptor number at a given discrete time t; r is the rate constant of receptor appearance expressed as fmol mg−1 protein day−1, and k is the rate constant of receptor disappearance (in units of day−1) which allows estimation of the apparent half-life of the receptor (t1/2= ln 2/k). The ratio r/k represents the density of receptors at steady state.
In saline-treated rats, but not in reserpine-treated rats, the recovery of [3H]-UK14304 binding after EEDQ also fitted well to a newly proposed biphasic model for the recovery of α2-adrenoceptor agonist binding sites (Ribas et al., 1998) (data not shown). For this reason α2-adrenoceptor turnover parameters in saline- and reserpine-treated rats were calculated and compared only according to the monoexponential model (Equation 1).
Immunoblot analysis of G protein subunits
Groups of rats were treated s.c. with saline (n=4) or reserpine (0.25 mg kg−1) (n=4), every 48 h for 20 days. The rats were killed 48 h after the last injection. Preparation of cortical membranes (P2 membrane fraction), immunoblot analysis of specific G protein subunits and quantitation of specific immunoreactivity were performed as described previously (Escribá et al., 1994; Ribas et al., 1998). Briefly, solubilized G proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS – PAGE), transferred to nitrocellulose membranes (Western blotting) and then labelled with specific antibodies: anti-Gαi1/2 (AS/7) at a dilution of 1 : 7000, anti-Gαi3 (EC/2) at a dilution of 1 : 3000, anti-Gαo (GC/2) at a dilution of 1 : 4000 and anti-Gαs (RM/1) at a dilution of 1 : 3000. The secondary antibody, horseradish peroxidase-labelled donkey anti-rabbit immunoglobulin G, was incubated at a dilution of 1 : 5000. Immunoreactivity was detected with the Enhanced Chemiluminescence Western Blot Detection system (Amersham International), followed by exposure to Hyperfilm ECL film for 1 – 10 min. The film was scanned in the image analyser Bio Image (Millipore, Ann Arbor, MI, U.S.A.). The quantitation of specific immunoreactivity was done as described previously (Escribá et al., 1994), using appropriate standard curves (i.e., total protein loaded versus Integrated Optical Density, IOD), which consisted of at least four different protein contents (the protein was from naïve control rats), all loaded on the same gel, resulting in linear relationships in the range of protein content used (for further details see Escribá et al., 1994).
Northern and dot-blot analyses of α2a-adrenoceptor mRNA
Total RNA was extracted from rat brain specimens by use of a single-step RNA isolation system (TRIzol reagent, GIBCO – BRL, Berlin, Germany) which is based on the method of Chomczynski & Sacchi (1987) and quantitated spectrophotometrically by measuring the absorbance at 260 nm. Total cerebral cortex RNA yields were routinely 0.5 – 1 μg mg−1 tissue. The Northern and dot-blot procedures for the quantitation of α2a-adrenoceptor mRNA have been described elsewhere (Busquets et al., 1997). The plasmid containing the cDNA encoding the human platelet α2A-adrenoceptor was kindly provided by Dr Robert J. Lefkowitz (Department of Medicine, Duke University, Durham, NC, U.S.A.). In the rat cerebral cortex, however, the α2a-adrenoceptor probe also was shown to cross-hybridize weakly with the α2c-adrenoceptor mRNA (Lorenz et al., 1990). This was confirmed in preliminary experiments which demonstrated that the α2a-adrenoceptor probe cross-hybridized with the α2c- but not with the α2b-adrenoceptor cDNA (cDNAs provided by R.J. Lefkowitz) in dot-blot analyses (see Busquets et al., 1997). The weak cross hybridization of the probe used suggests that also α2c-mRNA can be quantitated, but this contamination appears to be of minor relevance. In the rat cerebral cortex, both α2a- and α2c-adrenoceptor mRNAs are expressed (Nicholas et al., 1993). For the routine quantitation of α2a-adrenoceptor mRNA levels dot-blot analyses were performed (see Busquets et al., 1997 for details). Quantitative analysis of dot-blot densities was performed by scanning densitometry in the image analyser Bio Image (Millipore, Ann Arbor, MI, U.S.A.). The relative levels of α2a-adrenoceptor mRNA signals in dot-blots (IOD) were normalized to correct for any loading discrepancies of RNA by scanning the ethidium bromide fluorescence of total RNA signals from the same dot blots after u.v. light irradiation of the nylon membranes (Sambrook et al., 1989). The IOD of the α2a-adrenoceptor probe hybridization signal (α2a mRNA signal) and the IOD of the ethidium bromide fluorescence (total RNA signal) were concentration-dependent in a linear fashion with respect to the total RNA content. In saline- and reserpine-treated rats, the results are expressed as the ratio α2a mRNA/total RNA (IODs).
Statistics
Radioligand binding, immunoblot and dot-blot data are expressed as means±s.e.mean. Two-way analysis of variance (ANOVA) followed by Fisher's LSD multiple comparison test, and Student's two-tailed t-test were used for the statistical evaluations. The level of significance was chosen as P=0.05. Radioligand binding experiments were analysed initially assuming a one-site model of radioligand binding and then assuming two or three-site binding models. Receptor turnover parameters are expressed as the best fit values±s.e. determined by the matrix inversion method, using the non-linear regression program GraFit (Leatherbarrow, 1990). Comparisons of experimental data sets for the recovery of α2-adrenoceptor binding sites were performed by comparing the goodness of fit of a model with and without a set of constraints by means of an F-test. The selection between the different binding and recovery models was made statistically using the extra sum of squares principle (F-test) as outlined by Munson & Rodbard (1980). The more complex model was accepted if the P-value resulting from the F-test was less than 0.05.
Drugs and chemicals
[3H]-UK14304 (bromoxidine or brimonidine, specific activity, 60 – 87 Ci mmol−1) and [3H]-clonidine (specific activity, 66 – 68 Ci mmol−1) were purchased from New England Nuclear Du Pont (U.S.A.). [3H]-RX821002 (2-methoxy-idazoxan, specific activity, 48 – 56 Ci mmol−1) was purchased from Amersham International (U.K.). Rabbit anti-G protein subunits polyclonal antisera raised against specific C-terminal peptides or the N-terminus (Gαo) were purchased from New England Nuclear Du Pont: anti-Gαi1/2 (AS/7), anti-Gαi3 (EC/2), anti-Gαo (GC/2) and anti-Gαis (RM/1). Horseradish peroxidase-labelled donkey anti-rabbit immunoglobulin G, Enhanced chemiluminescence reagents and Hyperfilm ECL film for Western blot autoradiography were obtained from Amersham International. Single-step RNA isolation system, TRIzol reagent, and restriction endonucleases were purchased from GIBCO – BRL (Germany). Redivue [α-32P]-dCTP (deoxycytidine triphosphate, 3000 Ci mmol−1), Megaprime DNA labelling systems, Rapid-hibridization buffer and Hyperfilm-MP film for Northern and dot-blot autoradiography were obtained from Amersham International. Reserpine, EEDQ (N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline), (−)-adrenaline bitartrate and other reagents were obtained from Sigma Chemical Co. (U.S.A.).
Results
Effect of chronic treatment with reserpine on brain α2A/C-adrenoceptors
Saturation experiments with the α2-adrenoceptor agonists [3H]-UK14304 and [3H]-clonidine and the antagonist [3H]-RX821002 were performed in order to accurately quantitate the density of rat brain α2-adrenoceptors after chronic treatment with reserpine. Non-linear analysis of the saturation isotherms indicated in all cases the existence of single populations of high-affinity sites for [3H]-UK14304, [3H]-clonidine and [3H]-RX821002. In cortical membranes of saline-treated rats the density of binding sites (Bmax) for the three radioligands differed. The lowest density was for the full agonist [3H]-UK14304 and the greatest density for the antagonist [3H]-RX821002 (Figure 1 and Table 1).
Figure 1.
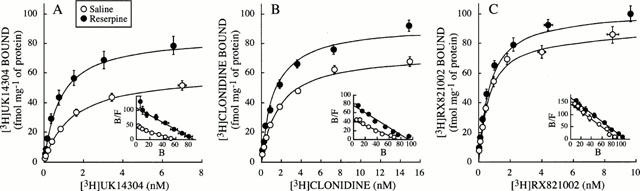
Specific binding of [3H]-UK14304 (A), [3H]-clonidine (B), and [3H]-RX821002 (C) to brain cortical membranes of saline- and reserpine (0.25 mg kg−1, s.c., every 48 h for 6 – 14 days)-treated rats as a function of increasing concentrations of the radioligands. Data shown are mean±s.e.mean derived from 7 – 11 experiments per group. Binding parameters (Kd in nM and Bmax in fmol mg−1 of protein) for pooled saline- and reserpine-treated rats (6 and 14 days) were as follows: [3H]-UK14304, saline: Kd=1.4±0.2, Bmax=60±4, n=10; reserpine: Kd=0.8±0.1, Bmax=85±6, n=10; [3H]-clonidine, saline: Kd=1.7±0.2, Bmax=60±4, n=7; reserpine: Kd=1.2±0.1, Bmax=92±2, n=10; [3H]-RX821002, saline: Kd=0.62±0.05, Bmax=88±5, n=10; reserpine: Kd=0.64±0.04, Bmax =101±4, n=11. Insets: Scatchard plots of the same data.
Table 1.
Effect of chronic treatments with reserpine on the specific binding of [3H]-UK14304, [3H]-clonidine and [3H]-RX821002 to rat brain cortical membranes
Analysis of saturation curves for the full agonist [3H]-UK14304 binding to cortical membranes from saline and reserpine-treated rats (0.25 mg kg−1, i.p., every 48 h for 6 – 14 days) revealed the induction of up-regulation of α2-adrenoceptor density induced by the monoamine depleter (Bmax increased by 34 – 48%, P<0.001) (Table 1 and Figure 1). Moreover, chronic treatment (6 – 14 days) with reserpine also led to marked reductions (34 – 47%, P<0.001) of the dissociation constants (Kd) of [3H]-UK14304 (increased affinity) for the α2-adrenoceptor (Table 1). More prolonged treatments with reserpine (28 and 36 days) were also effective in increasing both the density (Bmax increased by 40 and 44%, n=2 for each treatment) and the affinity (Kd reduced by 30 and 37%, n=2 for each treatment) of [3H]-UK14304 binding to cortical membranes. Analysis of saturation curves for the partial agonist [3H]-clonidine also showed a significant increase in receptor density (Bmax increased by 22 – 31%, P<0.001) and receptor affinity (Kd reduced by 24 – 29%, P<0.05) induced by chronic reserpine (6 – 14 days) (Table 1 and Figure 1). By contrast, analysis of saturation curves for the antagonist [3H]-RX821002 did not reveal significant changes in binding parameters after chronic treatment with reserpine (6 – 14 days) (Table 1 and Figure 1)
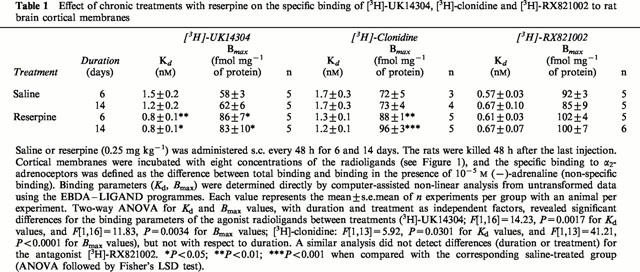
Competition experiments with (−)-adrenaline against [3H]-RX821002 binding in both saline- and reserpine-treated (14 days) rats resulted in shallow curves, and non linear analysis indicated that a three-site binding model for the agonist fitted the data best (Figure 2). The estimated Ki values for each site were assumed to correspond to the high-affinity (Ki=4.5 – 7 nM) and low-affinity (Ki=168 – 183 nM) states of the α2-adrenoceptor, and to a third binding site (Ki=3006 – 4050 nM) previously identified as binding to the 5-HT1A receptor (Vauquelin et al., 1990; Grijalba et al., 1996) for which (−)-adrenaline has very low affinity (Figure 2). The simultaneous analysis of these competition curves (i.e. the two mean curves together) according to the more complex model (i.e. the three site binding model) yielded significant differences compared to the analysis without constraints (i.e. the mean curves separately) (F [7,32]=46.6, P<0.0001). The main difference was the estimated density for the high-affinity state of the α2-adrenoceptor (Bmax: 49 and 62 fmol mg−1 of protein for saline- and reserpine-treated rats, respectively), because the estimated Bmax values for the second (α2-adrenoceptor low affinity) and third (5-HT1A receptor) binding sites were not affected by reserpine treatment (31 versus 34, and 10 versus 14, fmol mg−1 of protein, respectively).
Figure 2.
Competition curves for (−)-adrenaline against [3H]-RX821002 (1 nM) binding to brain cortical membranes from saline- and reserpine (0.25 mg kg−1, s.c., every 48 h for 14 days)-treated rats. Data shown are mean±s.e.mean derived from 5 – 6 experiments per group. Computer-assisted curve fitting demonstrated that a three-site fit was significantly better than a two-site binding model for both saline- and reserpine-treated rats (P<0.001, F-test). Binding parameters (Ki values) were determined directly by simultaneous analysis of 5 – 6 independent experiments for each group using the EBDA – LIGAND programs. Simultaneous analysis of both set of data yielded significant differences between the two curves (F[7,32]=46.6, P<0.0001), indicating that reserpine increased the density of the high-affinity state of the α2-adrenoceptor.
Together the radioligand binding data indicated that chronic treatment with reserpine was associated with up-regulation of high affinity α2-adrenoceptor agonist binding sites.
Effect of chronic treatment with reserpine on brain regulatory G protein subunits
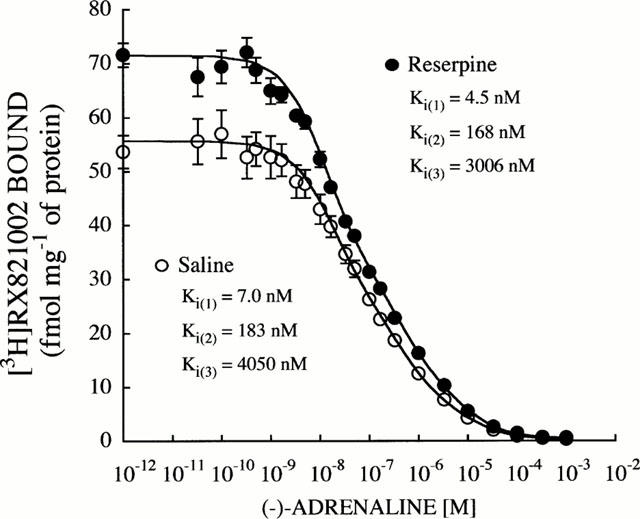
To assess for a possible relation between the increased agonist binding sites and parallel increases of specific G proteins in brain, the densities of various regulatory Gα protein subunits were assessed after chronic treatment with reserpine. In cortical membranes of reserpine-treated rats (0.25 mg kg−1, every 48 h for 20 days), the immunoreactivities of the inhibitory subunits Gαi1/2, Gαi3 and Gαo were increased (25 – 34%, 0.5>P>0.01) compared with those in saline-treated rats (Figure 3). Also the immunoreactivity of the stimulatory Gαs protein subunit was increased (29%, P<0.01) in cortical membranes of reserpine-treated rats (Figure 3). These results suggested an increase in signal transduction through α2-adrenoceptors (and other monoamine receptors) induced by chronic reserpine.
Figure 3.
Effect of chronic treatment with reserpine on the immunoreactivity of regulatory Gα protein subunits in the rat frontal cortex. Groups of rats (n=4) were treated s.c. with saline or reserpine (0.25 mg kg−1) every 48 h for 20 days. The rats were killed 48 h after the last injection. The levels of Gα protein subunits were quantified by immunoblotting (Western blotting) of cortical membranes as described in Methods. Upper panel: data are the mean±s.e.mean of four experiments per group performed in triplicate with an animal per experiment, and expressed as percentage of control (standard curves from naïve rats). Lower panel: representative immunoblots for brain Gα protein subunits from the saline (S) and reserpine (R) groups. The amount of total protein loaded on the gels was 3.2 – 3.4 μg for both groups of rats. Note that the immunoreactive densities of Gα protein subunits were significantly increased after chronic reserpine treatment. *P<0.05, **P<0.01 when compared with the corresponding saline-treated group (two-tailed Student's t-test).
Effect of chronic treatment with reserpine on the turnover of brain α2A/C-adrenoceptors
In saline- and reserpine-treated rats (0.25 mg kg−1, s.c., every 48 h, for 6 days), EEDQ (1.6 mg kg−1, i.p., for 6 h) induced an almost complete reduction in the density of α2-adrenoceptors in the cerebral cortex (Bmax for [3H]-UK14304 reduced more than 95%) (Figure 4). The initial loss of receptor density was followed by a progressive and time-dependent recovery towards control values (i.e., saline receptor density or receptor density after 36 days of reserpine treatment). These experiments provided the Bmax values for the analysis of the exponential recovery function (Figure 4), whose parameters are summarized in Table 2.
Figure 4.
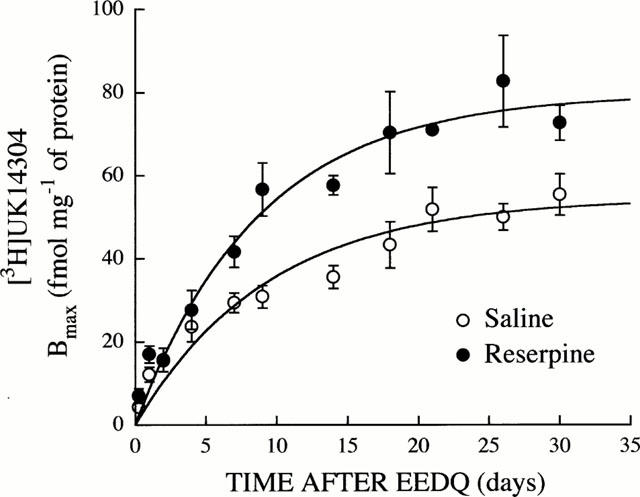
Recovery of α2-adrenoceptor density (Bmax for [3H]-UK14304) in the cerebral cortex after EEDQ-induced receptor inactivation in saline-treated rats and during reserpine treatment (0.25 mg kg−1, s.c., every 48 h for 6 to 36 days). Rats were killed 6 h and 1, 2, 4, 7, 9, 14, 18, 21, 26, and 30 days after the administration of EEDQ (1.6 mg kg−1, i.p.). Reserpine treated rats were injected with EEDQ at day 6 and treatment with reserpine was continued until 48 h before killing. The Bmax values were determined from complete saturation experiments for the agonist [3H]-UK14304 using the non-linear regression programme LIGAND. Other details as for Tables 1 and 2. Data shown are mean±s.e.mean derived from 3 – 6 experiments. The solid lines represent the computer-assisted curve fitting of experimental data to the monoexponential model described by the equation Rt=r/k (1-e−kt). See Table 2 for reserpine-induced changes in α2-adrenoceptor turnover parameters.
Table 2.
Turnover parameters of α2-adrenoceptors labelled with the agonist [3H]-UK14304 in the cerebral cortex of saline- and reserpine-treated rats
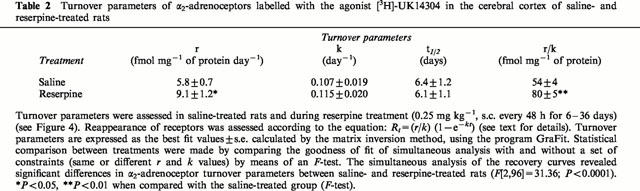
The simultaneous analysis of the monoexponential recovery functions (Equation 1) in saline and reserpine-treated rats, revealed the existence of a marked modulation by chronic reserpine of brain α2-adrenoceptor turnover function (F[2,96]=31.36; P<0.0001). This analysis also indicated that the increased density of cortical α2-adrenoceptor binding sites in reserpine-treated rats (Bmax or r/k values, see Tables 1 and 2) was probably due to a higher appearance (synthesis) rate constant of the receptor (Δk=57%, P<0.05) and not to a decreased disappearance (degradation) rate constant (Δk= 7%, P>0.05) (Table 2 and Figure 4). Thus, chronic reserpine treatment did not modify the t1/2 of brain α2-adrenoceptors (Table 2). These results suggested that an increased receptor synthesis might explain the up-regulation of α2-adrenoceptors during reserpine treatment.
Effect of chronic treatment with reserpine on the expression of brain α2a-adrenoceptor mRNA
In the rat cerebral cortex, Northern blot analysis demonstrated the presence of ∼3.8-kb band corresponding to the α2a-adrenoceptor mRNA transcript (Figure 5A), which was in agreement with previous studies (see Lorenz et al., 1990).
Figure 5.
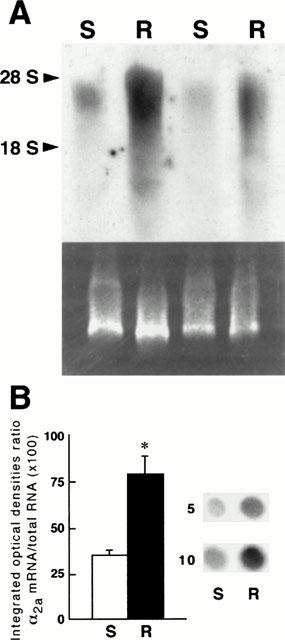
(A) Autoradiogram of a representative Northern blot analysis of brain RNA examining the expression of α2a-adrenoceptor mRNA in saline-treated rats (S) and in reserpine-treated (0.25 mg kg−1, s.c., every 48 h for 20 days) rats (R). Northern blot analysis was performed as described in Methods. Each lane was loaded with 20 μg of total RNA extracted from the cerebral cortex of one animal (two different S and R are shown). The size of the α2a-adrenoceptor mRNA (approximately 3.8 kb) was estimated by comparison with 28S (5.0 kb) and 18S (2.0 kb) RNAs (arrow heads). Bottom panel: ethidium bromide-stained RNA agarose gel showing the 28S and 18S ribosomal bands for size marker comparison. (B) Effect of chronic reserpine treatment on the relative amount of α2a-adrenoceptor mRNA in the cerebral cortex from saline (S) and 20-day reserpine-treated (R) rats. Receptor mRNA levels were assessed by dot-blot analysis as described in Methods. Left panel: The relative levels of α2a-adrenoceptor mRNA signal in dot-blots (integrated optical density) were normalized to correct any loading discrepancies of RNA by scanning the acridine orange fluorescence signal from the same dot-blots after u.v. light irradiation of the nylon membranes (see Methods). Ratios of α2amRNA/total RNA (×100); S: 35±3, R: 79±10. Each value represents the mean±s.e.mean of six experiments per group. *P<0.01 when compared with the saline group (two-tailed Student's t-test). Right panel: A representative experiment (dot-blots) is shown for saline (S) and reserpine (R) groups. Duplicate dot-blots of total RNA (5 and 10 μg) were performed for each treatment.
Northern blot analysis of RNA extracted from the cerebral cortex of saline- and reserpine-treated rats (0.25 mg kg−1, s.c., every 48 h for 20 days) clearly revealed that chronic treatment with reserpine markedly increased the expression of α2a-adrenoceptor mRNA in the brain (Figure 5A). This up-regulation in the levels of α2a-adrenoceptor mRNA was confirmed and quantitated by dot-blot experiments. In these experiments, chronic treatment with reserpine, compared with saline administration, was also associated with a marked up-regulation in the expression of α2a-adrenoceptor mRNA in the cerebral cortex (125%, P<0.01) (Figure 5B). Therefore, chronic treatment with reserpine is associated with an increased α2a-adrenoceptor gene expression in the rat brain.
Discussion
The main finding of this study is the demonstration that chronic treatment with reserpine is associated with an accelerated turnover of α2-adrenoceptors (increased appearance rate of agonist binding sites) and with a transcriptional activation of the α2a-adrenoceptor mRNA (and probably also of the α2c-mRNA because of cross hybridization of the probe used) in the rat cerebral cortex. This increased receptor synthesis appears to be the cellular mechanism by which chronic reserpine treatment induces up-regulation in the density of α2-adrenoceptors (probably of the α2A/C-subtypes) in the rat brain (U'Prichard & Snyder, 1978; current results). Moreover, chronic reserpine treatment also induced an increase in the abundance of α2-adrenoceptor-coupled inhibitory G proteins, which would lead to an improvement in the efficiency of the stimulus-response coupling (Kenakin, 1997) resulting in receptor supersensitivity (e.g. Ugedo et al., 1993; current results). In reserpine-treated rats the discrepancy between α2a/c-adrenoceptor mRNA levels (125% increase after 20 days of treatment) and α2-adrenoceptor density (Bmax for [3H]-RX821002: 18% increase after 14 days of treatment; r/k value: 48% increase after 36 days of treatment) suggests that the membrane content of α2-adrenoceptors is post-transcriptionally regulated. A similar discrepancy between the increases in α2a/c-adrenoceptor mRNA levels and α2A/C-adrenoceptor densities was previously reported in morphine-withdrawn rats (Busquets et al., 1997).
The increased density of α2-adrenoceptor agonist binding sites in reserpine-treated rats appears to be related to a selective enhancement in the high-affinity conformation of the receptor rather than to an increase of the total α2-adrenoceptor population. Previous studies have shown that chronic treatments with low doses of reserpine increase the affinity of agonist radioligands ([3H]-clonidine, [3H]-p-aminoclonidine, [3H]-UK14304) for the brain α2-adrenoceptor, with no change in receptor density (Hong et al., 1988; Giralt & García-Sevilla, 1989; Ugedo et al., 1993), whereas acute treatments with high doses of reserpine have been associated with decreases in brain α2-adrenoceptor density, with no change in receptor affinity (Kuno et al., 1990). It is currently accepted that agonist radioligands label preferentially the high-affinity state of α2-adrenoceptors, whereas antagonist radioligands label both the high- and low-affinity states of this group of receptors (Asakura et al., 1985). Therefore, the increase in α2-adrenoceptor density observed in reserpine-treated rats with the agonist radioligands [3H]-clonidine and [3H]-UK14304 as compared with the antagonist [3H]-RX821002 could be related to an enhanced expression of the high-affinity conformation of the receptor. In this sense, the competition experiments with the agonist (−)-adrenaline against the binding of [3H]-RX821002 to α2-adrenoceptors demonstrated that the enhanced receptor density in reserpine-treated rats was related to a selective increase in the density of the high-affinity state of the receptor. Moreover, these results agree well with the increased immunodensity of inhibitory G proteins (Gαi/o) detected in the brain of reserpine-treated rats. Because the high-affinity conformation of the α2-adrenoceptor is most probably related to the complex with Gαi2 proteins (Sastre & García-Sevilla, 1994; Ribas et al., 1998), an enhanced expression of Gαi1/2 proteins linked to a higher abundance of α2-adrenoceptor agonist binding sites was expected in brains of reserpine-treated rats. Similarly, the increased immunodensity of stimulatory Gαs proteins could be related to the well-known supersensitivity of β-adrenoceptors induced by chronic reserpine (U'Prichard & Snyder, 1978; Grassby & Broadley, 1986).
Also in line with these results, the accelerated turnover of α2-adrenoceptors during reserpine treatment indicated that the increased density of [3H]-UK14304 binding sites was due to an apparent higher rate of receptor appearance and not to a decreased receptor disappearance. A similar α2-adrenoceptor turnover study in morphine-dependent rats also demonstrated that the up-regulation of cortical α2-adrenoceptors ([3H]-clonidine agonist binding sites) observed during morphine withdrawal (Ulibarri et al., 1987) was probably due to an enhanced rate of receptor appearance (Gabilondo & García-Sevilla, 1995). These results suggested that an increased receptor synthesis may explain the up-regulation of α2-adrenoceptors. In fact, chronic reserpine treatment (current results) and morphine withdrawal (Busquets et al., 1997) are associated with a transcriptional activation of the α2a-adrenoceptor mRNA in the rat brain. The molecular mechanism by which chronic reserpine induces a higher α2a-adrenoceptor gene expression in the brain is not known, but it could be mediated through modulation of the adenylyl cyclase/cyclic AMP/protein kinase A system and cyclic AMP-responsive enhancer elements which are associated with changes in gene expression for these receptors (see Busquets et al., 1997 and other references therein).
In other receptor systems, such as the inhibitory D2 dopamine receptor, similar cellular mechanisms seem to be involved in reserpine-induced receptor supersensitivity. Thus, in the striatal D2 dopamine receptor system, reserpine-induced supersensitivity appears to be accompanied by increases of D2 dopamine receptor density and mRNA (Rubinstein et al., 1990; Jaber et al., 1992; Butkerait & Friedman, 1993). Moreover, turnover studies also indicated that the up-regulation of D2 dopamine receptor is an active process resulting from an increase in the rate of receptor appearance (Norman et al., 1987). Furthermore, the receptor up-regulation was paralleled by a significant increase of the high-affinity population of the D2 dopamine receptor (Rubinstein et al., 1990), that could be associated with the increases of specific G proteins (Gαi2 and Gαo) mRNAs (Butkerait & Friedman, 1993) and of the dopamine-stimulated guanine nucleotide binding to Gαi/o (Butkerait et al., 1994). In contrast to the current results, reserpine treatment did not change the striatal membrane content of Gαs, Gαi1/2 or Gαo as assessed by immunoblotting or by toxin-catalysed ADP ribosylation (Butkerait et al., 1994).
The current findings further support the concept that depletion of brain monoamines in rats with reserpine is a relevant biochemical model of major depression, as far as the induction of up-regulation of α2A-adrenoceptors and associated regulatory proteins and mechanisms is concerned. Postmortem studies in human brain have shown an enhancement of α2-adrenoceptor density in depressed suicide victims when labelled with agonist (Meana et al., 1992; González et al., 1994; Ordway et al., 1994) or antagonist (De Paermentier et al., 1997) radioligands. In other studies with agonist or antagonist radioligands, however, this receptor up-regulation was not observed (Ferrier et al., 1986; Arango et al., 1993; Ordway et al., 1994). In a recent study, the simultaneous analysis of agonist and antagonist binding sites indicated that a selective increase in the high-affinity conformation of the α2A-adrenoceptor was present in the prefrontal cortex of depressed suicide victims (Callado et al., 1998). Moreover, recent data have shown that the immunodensities of α2A-adrenoceptors, Gαi1/2 proteins, and G protein-coupled receptor kinase 2 are up-regulated in brains of suicides and depressed suicides (García-Sevilla et al., 1999). Clinical studies in depressed patients also indicate that depletion of brain noradrenaline or serotonin induces a presynaptic dysfuntion in the brain, which is relevant for the understanding of the pathophysiology of major depression and the therapeutic effects of antidepressant drugs (for a review see Delgado & Moreno, 1999).
In summary, the results of this study indicate that chronic reserpine treatment in rats induces up-regulation of brain α2A/C-adrenoceptors (agonist binding sites), which is associated with an increase in the abundance of inhibitory G coupling proteins (Gαi1/2 subunits), an acceleration of receptor turnover (increase in the rate of agonist binding sites appearance) and a transcriptional activation of the α2a/c-adrenoceptor mRNA. Together, the results provide a better understanding of the cellular events associated with reserpine-induced supersensitivity of α2-adrenoceptors in the brain.
Acknowledgments
This study was funded by Grant BFI2000-0306 (Fondo Nacional para el Desarrollo de la Investigación Científica y Técnica) from MCT (Madrid, Spain). C. Ribas was supported by a predoctoral fellowship from MEC. The α2-adrenoceptor cDNAs were generous gifts from Dr Robert J. Lefkowitz (Department of Medicine, Duke University, Durham, NC, U.S.A.). J.A. García-Sevilla is a member of the Institut d'Estudis Catalans (Barcelona, Spain). This paper on the neuropharmacology of reserpine is dedicated to Arvid Carlsson on the occasion of his Nobel Prize in Medicine (2000).
Abbreviations
- ANOVA
analysis of variance
- EEDQ
N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline
- G protein
guanine nucleotide-binding regulatory protein
- IOD
integrated optical density
- RX821002
2-methoxy idazoxan or 2-[2-(2-methoxy-1,4-benzodioxanyl)] imidazoline
- SDS – PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- UK14304
bromoxidine or 5-bromo-6-(2-imidazolin-2-yl-amino) quinoxaline
References
- ARANGO V., ERNSBERGER P., SVED A.F., MAN J.J. Quantitative autoradiography of α1-. and α2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Res. 1993;630:271–282. doi: 10.1016/0006-8993(93)90666-b. [DOI] [PubMed] [Google Scholar]
- ASAKURA M., TSUKAMOTO T., IMAFURU J., MATSUI H., INO M., HASEGAWA K. Quantitative analysis of rat brain α2-receptors discriminated by [3H]-clonidine and [3H]-rauwolscine. Eur. J. Pharmacol. 1985;106:141–147. doi: 10.1016/0014-2999(84)90687-3. [DOI] [PubMed] [Google Scholar]
- BARTUREN F., GARCÍA-SEVILLA J.A. Long-term treatment with desipramine increases the turnover of α2-adrenoceptors in the rat brain. Mol. Pharmacol. 1992;42:846–855. [PubMed] [Google Scholar]
- BERMAN R.M., NARASIMHAN M., MILLER H.L., ANAND A., CAPPIELLO A., OREN D.A., HENINGER G.R., CHARNEY D.S. Transient depressive relapse induced by catecholamine depletion. Arch. Gen. Psychiatry. 1999;56:395–403. doi: 10.1001/archpsyc.56.5.395. [DOI] [PubMed] [Google Scholar]
- BUSQUETS X., VENTAYOL P., GARCIA-SEVILLA J.A. Naloxone-precipitated withdrawal in morphine-dependent rats increases the expression of α2a–adrenoceptor mRNA in brain. Mol. Brain Res. 1997;45:154–158. doi: 10.1016/s0169-328x(96)00307-5. [DOI] [PubMed] [Google Scholar]
- BUTKERAIT P., FRIEDMAN E. Repeated reserpine increases striatal dopamine receptor and guanine nucleotide binding protein RNA. J. Neurochem. 1993;60:566–571. doi: 10.1111/j.1471-4159.1993.tb03186.x. [DOI] [PubMed] [Google Scholar]
- BUTKERAIT P., WANG H.Y., FRIEDMAN E. Increases in guanine nucleotide binding to striatal G proteins is associated with dopamine receptor supersensitivity. J. Pharmacol. Exp. Ther. 1994;271:422–428. [PubMed] [Google Scholar]
- BYLUND D., EIKENBERG D., HIEBLE J., LANGER S., LEFKOWITZ R., MINNEMAN K., MOLINOFF P., RUFFOLO R., TRENDELENBURG U. IV International Union of Pharmacology Nomenclature of Adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- BYLUND D.B., MARTINEZ R. α2-Adrenergic receptors appears in rat salivary glands after reserpine treatment. Nature. 1980;285:229–230. doi: 10.1038/285229a0. [DOI] [PubMed] [Google Scholar]
- CALLADO L.F., MEANA J.J., GRIJALBA B., PAZOS A., SASTRE M., GARCIA-SEVILLA J.A. Selective increase of α2A–adrenoceptor agonist binding sites in brains of depressed suicide victims. J. Neurochem. 1998;70:1114–1123. doi: 10.1046/j.1471-4159.1998.70031114.x. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- COOPER B.R., HOWARD J.L., SOROKO F.E. Animal models used in prediction of antidepressant effects in man. J. Clin. Psychiatry. 1983;44:63–66. [PubMed] [Google Scholar]
- DE PAERMENTIER F.D., MAUGER J.M., LOWTHER S., CROMPTON M.R., KATONA C.L.E., HORTON R.W. Brain α-adrenoceptors in depressed suicides. Brain Res. 1997;757:60–68. doi: 10.1016/s0006-8993(97)00138-8. [DOI] [PubMed] [Google Scholar]
- DELGADO P., MORENO F. Antidepressants and the brain. Inter. Clin. Psychopharmacol. 1999;14 suppl. 1:S9–S16. doi: 10.1097/00004850-199905001-00003. [DOI] [PubMed] [Google Scholar]
- ESCRIBA P.V., SASTRE M., GARCIA-SEVILLA J.A. Increased density of guanine nucleotide-binding proteins in the postmortem brain of heroin addicts. Arch. Gen. Psychiatry. 1994;51:494–501. doi: 10.1001/archpsyc.1994.03950060058006. [DOI] [PubMed] [Google Scholar]
- ESTAN L., SENARD J.-M., TRAN M.-A., MONTASTRUC J.-L., BERLAN M. Reserpine induces vascular α2-adrenergic supersensitivity and platelet α2-adrenoceptor up-regulation in dog. Br. J. Pharmacol. 1990;101:329–336. doi: 10.1111/j.1476-5381.1990.tb12710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTEBAN S., LLADO J., GARCIA-SEVILLA J.A. α2-Autoreceptors and α2-heteroreceptors modulating tyrosine and tryptophan hydroxylase activity in the rat brain in vivo: an investigation into the α2-adrenoceptor subtypes. Naunyn Schmiedeberg's Arch. Pharmacol. 1996;353:391–399. doi: 10.1007/BF00261435. [DOI] [PubMed] [Google Scholar]
- FERRIER I.N., MCKEITH I.G., CROSS A.J., PERRY E.K., CANDY J.M., PERRY R.H. Postmortem neurochemical studies in depression. Ann. N.Y. Acad. Sci. 1986;487:128–142. doi: 10.1111/j.1749-6632.1986.tb27893.x. [DOI] [PubMed] [Google Scholar]
- FORTIN T.L., SUNDARESAN P.R. Reserpine but not surgical denervation regulates rat renal beta-adrenergic receptors. Am. J. Physiol. 1989;256:F532–F539. doi: 10.1152/ajprenal.1989.256.4.F532. [DOI] [PubMed] [Google Scholar]
- GABILONDO A.M., GARCIA-SEVILLA J.A. Spontaneous withdrawal from long-term treatment with morphine accelerates the turnover of α2-adrenoceptors in the rat brain: up-regulation of receptors associated with increased receptor appearance. J. Neurochem. 1995;64:2590–2597. doi: 10.1046/j.1471-4159.1995.64062590.x. [DOI] [PubMed] [Google Scholar]
- GARCIA-SEVILLA J.A., BARTUREN F., PI F. Decreased [3H]-agonist binding to presynaptic α2-adrenoceptors and altered α2-autoreceptor function in the rat brain revealed by short-term 6-hydroxydopamine lesioning. Br. J. Pharmacol. 1994;112:155P. [Google Scholar]
- GARCIA-SEVILLA J.A., ESCRIBA P.V., OZAITA A., LA HARPE R., WALZER C., EYTAN A., GUIMON J. Up-regulation of immunolabeled α2A-adrenoceptors, Gi coupling proteins, and regulatory receptor kinases in the prefrontal cortex of depressed suicides. J. Neurochem. 1999;72:282–291. doi: 10.1046/j.1471-4159.1999.0720282.x. [DOI] [PubMed] [Google Scholar]
- GIRALT M.T., GARCIA-SEVILLA J.A. Acute and long-term regulation of brain α2-adrenoceptors after manipulation of noradrenergic transmission in the rat. Eur. J. Pharmacol. 1989;164:455–466. doi: 10.1016/0014-2999(89)90253-7. [DOI] [PubMed] [Google Scholar]
- GONZALEZ A.M., PASCUAL J., MEANNA J.J., BARTUREN F., DEL ARCO C., PAZOS A., GARCIA-SEVILLA J.A. Autoradiographic demonstration of increased α2-adrenoceptor agonist binding sites in the hippocampus and frontal cortex of depressed suicide victims. J. Neurochem. 1994;63:256–265. doi: 10.1046/j.1471-4159.1994.63010256.x. [DOI] [PubMed] [Google Scholar]
- GOODWIN F., BUNNEY W. Depressions following reserpine: a reevaluation. Semin. Psychiatry. 1971;3:435–448. [PubMed] [Google Scholar]
- GRASSBY P.F., BROADLEY K.J. Responses mediated via beta-1 adrenoceptors but not beta-2 adrenoceptors exhibit supersensitivity after chronic reserpine pretreatment. J. Pharmacol. Exp. Ther. 1986;237:950–957. [PubMed] [Google Scholar]
- GRIJALBA B., CALLADO L.F., MEANA J.J., GARCIA-SEVILLA J.A., PAZOS A. α2-Adrenoceptor subtypes in the human brain: a pharmacological delineation of [3H]-RX821002 binding to membranes and tissue sections. Eur. J. Pharmacol. 1996;310:83–93. doi: 10.1016/0014-2999(96)00381-0. [DOI] [PubMed] [Google Scholar]
- HEAL D.J., BUTLER S.A., PROW M.R., BUCKETT W.R. Quantification of presynaptic alpha 2-adrenoceptors in rat brain after short-term DSP-4 lesioning. Eur. J. Pharmacol. 1993;249:37–41. doi: 10.1016/0014-2999(93)90659-6. [DOI] [PubMed] [Google Scholar]
- HONG M., ROOTS E.J., JENNER P., MARSDEN C.D. The effect of long-term treatment with amine-depleting drugs or chlorpromazine on α-adrenoreceptors and 5-HT2 receptors in the brain of the rat. Neuropharmacology. 1988;27:519–527. doi: 10.1016/0028-3908(88)90135-9. [DOI] [PubMed] [Google Scholar]
- JABER M., FOURNIER M., BLOCH B. Reserpine treatment stimulates enkephalin and D2 dopamine receptor gene expression in the rat striatum. Mol. Brain Res. 1992;15:189–194. doi: 10.1016/0169-328x(92)90108-n. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Differences between natural and recombinant G protein-coupled receptor systems with varying receptor/G protein stoichiometry. Trends Pharmacol. Sci. 1997;18:456–464. doi: 10.1016/s0165-6147(97)01136-x. [DOI] [PubMed] [Google Scholar]
- KUNO N., KAMISAKI Y., ITOH T. Inhibition of cyclic AMP accumulation by alpha 2-adrenoceptors in the rat cerebral cortex. Eur. J. Pharmacol. 1990;176:281–287. doi: 10.1016/0014-2999(90)90021-w. [DOI] [PubMed] [Google Scholar]
- LEATHERBARROW . GraFit, version 2.0. Erithacus Software Ltd., Staines: U.K.; 1990. [Google Scholar]
- LEITH N.J., BARRETT R.J. Effects of chronic amphetamine or reserpine on self-stimulation responding: animal model of depression. Psychopharmacology. 1980;72:9–15. doi: 10.1007/BF00433801. [DOI] [PubMed] [Google Scholar]
- LORENZ W., LOMASNEY J.W., COLLINS S., REGAN J.W., CARON M.G., LEFKOWITZ R.J. Expression of three α2-adrenergic receptor subtypes in rat tissues: implications for α2 receptor classification. Mol. Pharmacol. 1990;38:599–603. [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.F., FARR A.G., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MACDONALD E., KOBILKA B.K., SCHEININ M. Gene targeting-homing in on α2-adrenoceptor subtype function. Trends Pharmacol. Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- MAUGER J.P., SLADECZEK F., BOCKAERT J. Characteristics and metabolism of α1-adrenergic receptors in a nonfusing muscle cell line. J. Biol. Chem. 1982;257:875–879. [PubMed] [Google Scholar]
- MCPHERSON G.A. Analysis of radioligand binding experiments: a collection of computer programs for IBM PC. J. Pharmacol. Methods. 1985;14:213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- MEANA J.J., BARTUREN F., GARCIA-SEVILLA J.A. α2-Adrenoceptors in the brain of suicide victims: increased receptor density associated with major depression. Biol. Psychiatry. 1992;31:471–490. doi: 10.1016/0006-3223(92)90259-3. [DOI] [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. Ligand: a versatile computerized approach for the characterization of ligand binding system. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- NICHOLAS A.P., PIERIBONE V., HOKFELT T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J. Comp. Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- NORMAN A.B., BATTAGLIA G., CREESE I. Differential recovery rates of rat D2 dopamine receptors as a function of aging and chronic reserpine treatment following irreversible modification: a key to receptor regulatory mechanisms. J. Neurosci. 1987;7:1484–1491. doi: 10.1523/JNEUROSCI.07-05-01484.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLMOS G., MIRALLES A., GARCIA-SEVILLA J.A., GIRALT M.T., PINEDA J., GARRO M.A., UGEDO L., MENARGUES A., OBACH R. Acute and chronic effects of cholinesterase inhibitors and pilocarpine on the density and sensitivity of central and peripheral α2-adrenoceptors. Eur. J. Pharmacol. 1993;236:467–476. doi: 10.1016/0014-2999(93)90486-2. [DOI] [PubMed] [Google Scholar]
- ORDWAY G.A., WIDDOWSON P.S., SMITH K.S., HALARIS A. Agonist binding to α2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J. Neurochem. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- PINEDA J., UGEDO L., GARCIA-SEVILLA J.A. Enhanced α2A-autoreceptor reserve for clonidine induced by reserpine and cholinomimetic agents in the rat vas deferens. Br. J. Pharmacol. 1997;122:833–840. doi: 10.1038/sj.bjp.0701455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESSLER K.J., NEMEROFF C.B. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol. Psychiatry. 1999;46:1219–1233. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- RIBAS C., MIRALLES A., GARCIA-SEVILLA J.A. Acceleration by chronic treatment with clorgyline of the turnover of brain α2-adrenoceptors in normotensive but not in spontaneously hypertensive rats. Br. J. Pharmacol. 1998;110:99–106. doi: 10.1111/j.1476-5381.1993.tb13777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBAS C., MIRALLES A., ESCRIBA P.V., GARCIA-SEVILLA J.A. Effects of the alkylating agent EEDQ on regulatory G proteins and recovery of agonist and antagonist α2-adrenoceptor binding sites in rat brain. Eur. J. Pharmacol. 1993;351:145–154. doi: 10.1016/s0014-2999(98)00295-7. [DOI] [PubMed] [Google Scholar]
- RUBINSTEIN M., MUSCHIETTI J.P., GERSHANIK O., FLAWIA M.M., STEFANO F.J. Adaptative mechanisms of striatal D1 and D2 dopamine receptors in response to a prolonged reserpine treatment in mice. J. Pharmacol. Exp. Ther. 1990;252:810–816. [PubMed] [Google Scholar]
- SAMBROOK J., FRITSCH E.F., MANIATIS T. Molecular cloning, 2nd edition. New York; 1994. [Google Scholar]
- SASTRE M., GARCIA-SEVILLA J.A. Density of alpha-2A adrenoceptors and Gi proteins in the human brain: ratio of high-affinity agonist sites to antagonist sites and effect of age. J. Pharmacol. Exp. Ther. 1989;269:1062–1072. [PubMed] [Google Scholar]
- TRENDELENBURG A.-U., TRENDELENBURG M., STARKE K., LIMBERGERN N. Release-inhibiting α2-adrenoceptors at serotonergic axons in rat and rabbit brain: evidence for pharmacological identity with α2-autoreceptors. Naunyn Schmiedeberg's Arch. Pharmacol. 1994;349:25–33. doi: 10.1007/BF00178202. [DOI] [PubMed] [Google Scholar]
- UGEDO L., GARRO M.A., PINEDA J., GIRALT M.T., MIRALLES A., OLMOS G., GARCIA-SEVILLA J.A., MENARGUES A., OBACH R. Acute and chronic effects of reserpine on biochemical and functional parameters of central and peripheral α2-adrenoceptors. Eur. J. Pharmacol. 1993;239:149–157. doi: 10.1016/0014-2999(93)90988-t. [DOI] [PubMed] [Google Scholar]
- ULIBARRI I., GARCIA-SEVILLA J.A., UGEDO L. Modulation of brain α2-adrenoceptor and μ-opioid receptor densities during morphine dependence and spontaneous withdrawal in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;336:530–537. doi: 10.1007/BF00169310. [DOI] [PubMed] [Google Scholar]
- U'PRICHARD D.C., SNYDER S.H. 3H-Catecholamine binding to α-receptors in rat brain: enhancement by reserpine. Eur. J. Pharmacol. 1978;51:145–155. doi: 10.1016/0014-2999(78)90339-4. [DOI] [PubMed] [Google Scholar]
- VAUQUELIN G., DE VOS H., DE BACKER J.-P., EBINGER G. Identification of α2 adrenergic receptors in human frontal cortex membranes by binding of [3H]-RX821002, the 2-methoxy analog of [3H]-idazoxan. Neurochem. Int. 1990;17:537–546. doi: 10.1016/0197-0186(90)90041-q. [DOI] [PubMed] [Google Scholar]
- VETULANI J., ANTKIEWICZ-MICHALUK L., ROKOSZ-PELC A., MICHALUK J. Effects of chronically administered antidepressants and electroconvulsive treatment on cerebral neurotransmitter receptors in rodents with ‘model depression'. Ciba Found Symp. 1986;123:234–245. doi: 10.1002/9780470513361.ch13. [DOI] [PubMed] [Google Scholar]


