Abstract
We investigated the possible involvement of phospholipase A2 (PLA2) and its products in long-term potentiation (LTP) in the CA1 neurotransmission of rat hippocampal slices.
Inhibitors of Ca2+-independent PLA2 (iPLA2) prevented the induction of LTP without affecting the maintenance phase of LTP whereas Ca2+-dependent PLA2 inhibitors were virtually ineffective, which suggests a pivotal role of iPLA2 in the initiation of LTP.
We then investigated the effect of docosahexaenoic acid (DHA) and arachidonic acid (AA) on BEL (bromoenol lactone, an iPLA2-inhibitor) -impaired LTP, and found that either DHA or AA abolished the effect of BEL. However, DHA did not restore BEL-attenuated LTP when applied after the tetanus. DHA per se affected neither the induction nor maintenance of LTP. Linoleic acid had no effects, either.
These results suggest that DHA is crucial for the induction of LTP and that endogenously released DHA during tetanus is sufficient to trigger the formation of LTP.
Keywords: Phospholipase A2, docosahexaenoic acid, arachidonic acid, long-term potentiation, long-term depression, synaptic plasticity, learning and memory, hippocampus
Introduction
At hippocampal synapses, brief repetitive stimulation results in a long-lasting increase in synaptic strength, referred to as long-term potentiation (LTP). This form of synaptic plasticity, which in most cases requires the activation of N-methyl-D-aspartate (NMDA) receptors, remains one of the most compelling cellular models for learning and memory. Although it is universally accepted that a rise in postsynaptic Ca2+ is essential for triggering LTP, the subsequent mechanism of LTP remains unclear, and considerable debate exists as to what molecules are involved in LTP (Bliss & Collingridge, 1993; Kullmann & Siegelbaum, 1995; Larkman & Jack, 1995; Nicoll & Malenka, 1995).
NMDA receptor activation increases release of arachidonic acid (AA) as a result of calcium influx through the NMDA receptor channel and thereby stimulation of endogenous phospholipase A2 (PLA2) (Dumuis et al., 1988; 1993). Indeed, PLA2 inhibitors have been shown to block LTP expression in the CA1 area of hippocampal slices (Massicotte et al., 1990; Okada et al., 1989; Williams et al., 1988) and AA or its metabolites have been proposed as potential messenger signals released by postsynaptic cells to modify transmitter release in LTP (Bramham et al., 1994; Drapeau et al., 1990). For instance, exogenously applied AA facilitates LTP formation induced by high-frequency stimulation (Linden et al., 1987; Williams et al., 1989), and AA applied during low-frequency stimulation may result in slowly developing LTP (Williams et al., 1989). However, the observation that AA-induced LTP is blocked by an NMDA receptor antagonist (O'Dell et al., 1991) suggests that AA does not act as a retrograde messenger, but rather it likely serves to modify the functional properties of both NMDA and AMPA subtypes of glutamate receptor (Kovalchuk et al., 1994; Miller et al., 1992).
Of three subclasses of PLA2, i.e., Ca2+-dependent PLA2 (cPLA2), Ca2+-independent PLA2 (iPLA2) and secretory PLA2, Wolf et al. (1995) found that the induction of LTP was completely prevented by bromoenol lactone (BEL), a potent and selective inhibitor of iPLA2, suggests a pivotal role of iPLA2 in the induction of LTP. PLA2 serves as a key enzyme to produce free fatty acids including docosahexaenoic acid (DHA) and AA from membrane phospholipids. Although the content of DHA is slightly lower than AA in the central nervous system (Dhopeshwarkar & Subramanian, 1975), DHA is also one of major components of membrane phospholipids in the brain and is a good substrate of PLA2 partly because it is mainly localized in the 2-position of the phospholipids, particularly in phosphatidylethanolamine (Hadjiagapiou & Spector, 1987). Therefore, as a result of the activation of PLA2, DHA may be predominantly released and accumulated in extracellular spaces. Indeed, Kim et al. (1996) reported a novel PLA2 that primarily releases DHA. Thus, DHA may possibly contribute to the induction of LTP. On the other hand, recent behavioural studies have suggested a positive correlation between DHA and cognitive ability of mammals (Gordon, 1997; Horrocks & Yeo, 1999). For example, DHA deficiency is associated with loss of discriminant learning ability and visual activity (Neuringer et al., 1986). Patients with Zellwegner's syndrome (Martinez, 1990) or Alzheimer's disease (Soderberg et al. 1991) show extremely low levels of DHA in their brains. Also, electrophysiological studies indicate that DHA promotes the channel open probability of NMDA receptor without affecting the channel conductance (Nishikawa et al., 1994). These observations have driven us to investigations on the possible involvement of DHA in hippocampal LTP. Here we have found that DHA improves LTP that is attenuated by iPLA2 inhibitors in rat hippocampal slices.
Methods
All animal experiments were carried out in accordance with the Japanese Pharmacological Society guide for the care and use of laboratory animals
Slice preparation
Hippocampal slices were prepared from young adult male Wistar rats (3 – 5 weeks old) using a vibratome in ice-cold artificial cerebrospinal fluid (ACSF) aerated with 95% O2/5% CO2 and then incubated in the same ACSF at 32°C for at least 1 h. For electrophysiological recordings, slices were submerged in a stream of ACSF at 32°C. The remaining slices were stored in an interface chamber at 32°C. ACSF was composed of (mM): NaCl 119, KCl 2.5, MgSO4 1.3, CaCl2 2.5, NaH2PO4 1.0, NaHCO3 26.2, and glucose 11.0.
Electrophysiology
Field excitatory postsynaptic potentials (fEPSP) evoked by test stimulation of the Schaffer collateral/commissural afferents were measured in the CA1 stratum radiatum. Extracellular recording electrodes were filled with 0.16 M NaCl. A single test stimulation (50-μs duration) was applied at an interval of 30 s. In each experiment, after the responses were stabilized, the stimulus intensity was adjusted to produce fEPSP slope of about 50% of the maximum responses. To induce LTP and LTD, tetanic stimulation (100 Hz for 1 s) and low-frequency stimulation (1 Hz for 15 min) were delivered at the same stimulus intensity through the same electrode as used for test stimulation, respectively.
Data analysis
Electrophysiological signals were digitized at 20 kHz using commercially available 12-bit analogue-to-digital boards (PCI-20428K-1, Intelligent Instrumentation Offices, Bay Colony Drive, MS, U.S.A.) and stored on disk for off-line analysis of fEPSP by the software Wave-kun developed by Y. Ikegaya. Two cursors, separated by 1 ms, were placed in the digitalized negative field potentials to obtain slope magnitude. fEPSP slope was determined from measuring the maximal slope in the initial phase of the fEPSP. Ensemble averages were constructed using all data points, aligned with respect to the time of LTP induction or drug application. Error bars indicate standard error of the mean (s.e.mean) calculated for the entire data set for a given time point. To assess statistical significance, Tukey's multiple range tests, comparing the average slope size for 10 min prior to tetanic stimulation to 40 – 50 min after the tetanic stimulation, were performed without normalization.
Drugs
The drugs used in the present experiment were as follows: DHA, AA (Sigma, St. Louis, MO, U.S.A.), linoleic acid (Sigma), BEL (Cayman Chem., Ann Arbor, MI, U.S.A.), palmitoyl trifluoromethyl ketone (PACOCF3) (Biomol Res. Lab., Inc. Plymouth Meeting, PA, U.S.A.), arachidonyl trifluoromethyl ketone (AACOCF3) (Biomol Res. Lab.), and methyl arachidonyl fluorophosphonate (MAFP) (Calbiochem, San Diego, CA, U.S.A.). DHA was kindly provided by Maruha Co., Ltd. (Tokyo, Japan). They were dissolved in DMSO at a concentration of between 10 and 100 mM, and diluted with ACSF through sonication in the presence of 100% N2 immediately before use. The final concentration of DMSO was 0.1 – 0.2%. In control experiment associated with each drug, slices were exposed to an equivalent final DMSO concentration.
Results
Effects of PLA2 inhibitors on LTP
First, we investigated the effect of the iPLA2 inhibitor BEL on the induction of LTP in the Schaffer collateral-CA1 pyramidal cell synapses. Ten μM BEL was applied from −10 min to 5 min after tetanic stimulation (100 Hz for 1 s) (Figure 1a). Although basal synaptic responses were unaffected by application of BEL, the tetanic stimulation failed to induce LTP whereas post-tetanic potentiation was normally evoked. Although this result confirms the previous findings reported by Wolf et al. (1995), these authors did not perform further characterization on the effect of BEL. In the following experiments, therefore, we analyse it in detail. The inhibitory effect of BEL on the LTP induction displayed a concentration dependency and was statistically significant at the concentration of 10 μM (Figure 1b). Similar effects were found with more selective inhibitor of iPLA2 PACOCF3 (100 μM) or the dual inhibitor of cPLA2 and iPLA2 MAFP (10 μM), but not with the selective inhibitor of cPLA2 AACOCF3 (10 μM) (Figure 1b). De Petrocellis et al. (1997) indicated that MAFP also inhibits anandamide aminohydrolase, an enzyme that catalyzes the hydrolysis of endogenous cannabimimetic compounds, and that a lower concentration of MAFP (100 nM) specifically blocks the activity of anandamide aminohydrolase. Therefore, we tested the effect of 100 nM MAFP to investigate the involvement of anandamide aminohydrolase in the MAFP-attenuated LTP. However, LTP was unaffected by 100 nM MAFP. Taken together, the results suggest that iPLA2 serves as a key enzyme in the formation of LTP. We next determined whether iPLA2 is involved in the maintenance phase of LTP. Bath application of 10 μM BEL (15 min after tetanic stimulation) did not affect the preexisting LTP (Figure 1c). Therefore, these data indicate that iPLA2 is required only for the induction of LTP.
Figure 1.
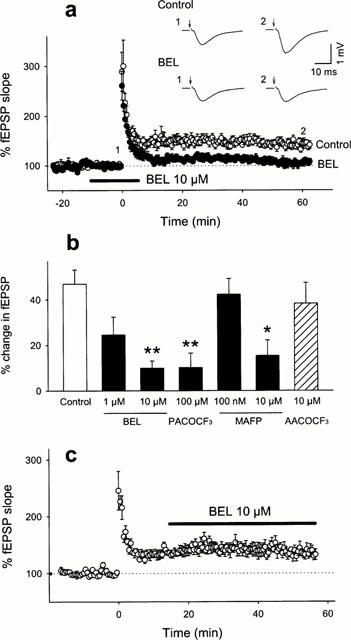
Inhibitory effect of iPLA2 inhibitor on the induction of LTP. (a) Time course of the fEPSP slope following the tetanic stimulation in the absence or the presence of 10 μM BEL. The drug was perfused from −10 min to 5 min, and the tetanic stimulation was applied at time 0. The fEPSP slope is expressed as a percentage of the baseline value immediately before the tetanic stimulation. Recording traces represent typical field potentials immediately before (1) and 60 min after (2) tetanic stimulation (100 Hz for 1 s) in the absence (Control) or the presence (BEL) of 10 μM BEL. The Schaffer collaterals were stimulated at time indicated by arrows. (b) Summary of the effects of various inhibitors of PLA2. The ordinate shows an average change in fEPSP slope 40 – 50 min after the tetanic stimulation. (c) Lack of the effect of BEL on the maintenance phase of LTP. Ten μM BEL was continuously applied from 15 min after the tetanic stimulation. All data are expressed as the means±s.e.mean of five cases. *P<0.05, **P<0.01 vs Control: Tukey's test following one-way ANOVA.
We next investigated the effect of BEL on other types of hippocampal synaptic plasticity, i.e., long-term depression (LTD) and paired-pulse facilitation (PPF). LTD was induced by low-frequency stimulation protocol (1 Hz for 15 min). Ten μM BEL was applied from 10 min before to 5 min after the low-frequency stimulation, but did not affect LTD (Figure 2a), which suggests that iPLA2 does not participate in the induction of LTD. Next, we evaluated the profile of PPF before and after BEL perfusion. The CA1 synapses demonstrated a significant PPF ratio at paired-pulse intervals of <200 ms, but this characteristic profile of PPF was unchanged in the presence of 10 μM BEL (Figure 2b). Considering that BEL did not change post-tetanic potentiation (Figure 1a), iPLA2 was unlikely involved in short-term synaptic plasticity. Taken together, these detailed investigations reinforced the view that, of diverse forms of hippocampal synaptic plasticity, iPLA2 may contribute specifically to mechanisms of initial phase of LTP.
Figure 2.
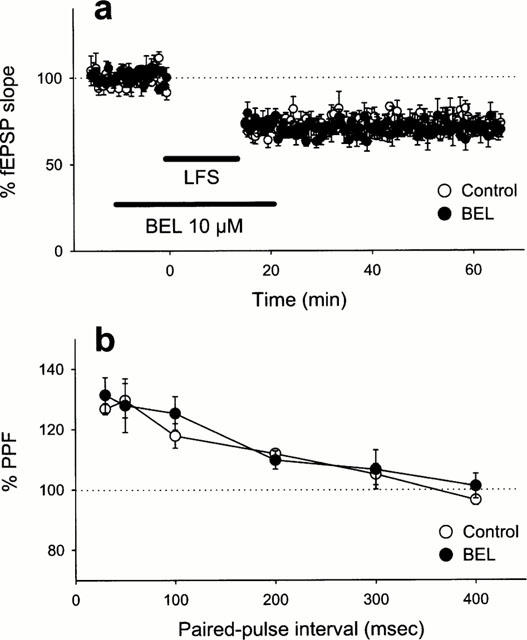
(a) Lack of the effect of BEL on the induction of LTD. Time course of the fEPSP slope following low-frequency stimulation (LFS: 1 Hz for 15 min) in the absence or the presence of 10 μM BEL. (b) Lack of the effect of BEL on PPF. The PPF profiles were examined immediately before and 10 min after application of 10 μM BEL. PPF ratio is expressed as a percentage of the second fEPSP slope to the first fEPSP evoked by paired-pulse stimulation. Data are expressed as the means±s.e.mean of five cases.
Ameliorative effect of DHA on impaired LTP
We next explored what molecules produced by iPLA2 are involved in the induction of LTP. First, we examined the effect of DHA, one of the end products of the iPLA2-mediated reaction, on BEL-inhibited LTP. When 30 μM DHA was co-applied with 10 μM BEL from −10 min to 5 min after tetanic stimulation, robust LTP was normally induced (Figure 3a). The same dose of DHA also restored the LTP abolished by 100 μM PACOCF3 (Figure 3b). The improving effect of DHA was concentration-dependent (Figure 3b), which suggests that the level of DHA released during tetanus is a determining factor to invoke signals for the initiation of LTP. Interestingly, the effect of DHA was mimicked by 30 μM AA but not by 30 μM linoleic acid, both of which are products of PLA2. These results suggest a specificity of fatty acids that can contribute to LTP. To examine whether DHA recovered the maintenance phase of the impaired LTP, 30 μM DHA was added 15 min after tetanic stimulation in the presence of 10 μM BEL. However, this application of DHA did not recuperate the abortive LTP (Figure 4). These results indicate the existence of a temporal window in the restorative effect of DHA, and the critical period is plausibly the induction phase of LTP. This phase is widely accepted to involve the activation of postsynaptic NMDA receptor as the essential step to trigger LTP (Bliss & Collingridge, 1993; Kullmann & Siegelbaum, 1995; Larkman & Jack, 1995; Nicoll & Malenka, 1995). Indeed, Nishikawa et al. (1994) indicated that DHA enhanced physiological function of NMDA receptor. Furthermore, the result that BEL inhibited LTP induction without changing PPF profile suggests that DHA probably acts on a postsynaptic target. Thus, the effect of DHA on LTP possibly results from modulating NMDA receptor. To address this possibility, NMDA responses were pharmacologically isolated using Mg2+-free ACSF containing 10 μM 6-cyano-7-nitroquinoxaline-2,3-dion, a non-NMDA receptor antagonist, and we examined the effect of DHA on the NMDA responses. However, 100 μM DHA did not enhance the evoked NMDA responses (Figure 5). Incidentally, BEL 10 μM did not reduce the NMDA responses (the mean per cent of baseline 10 min after BEL treatment was 97.1±10.9% (n=4)).
Figure 3.
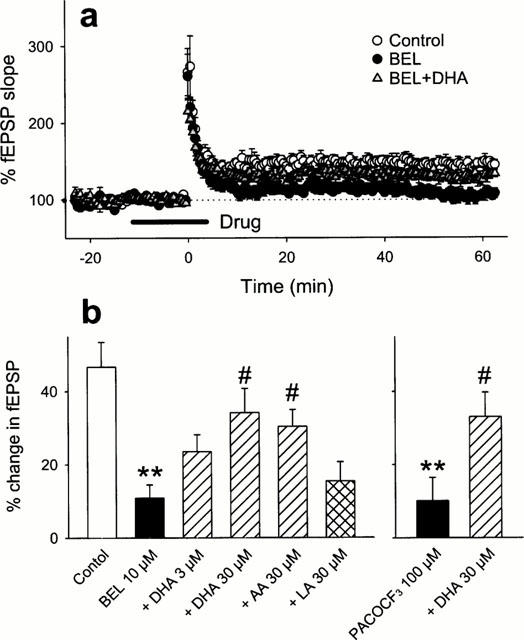
Ameliorative effect of unsaturated fatty acids on BEL-impaired LTP. (a) Time course of fEPSP slope following the tetanic stimulation in control slices, or the slices treated with 10 μM BEL or co-treated with 10 μM BEL and 30 μM DHA. (b) Summary of the effects of DHA, AA or linoleic acid (LA) on LTP impaired by BEL or PAFCOCF3. The ordinate shows an average change in fEPSP slope 40 – 50 min after the tetanic stimulation. All data are expressed as the means±s.e.mean of 5 – 6 cases. **P<0.01 vs Control,#P<0.05 vs BEL or PACOCF3: Tukey's test following one-way ANOVA.
Figure 4.
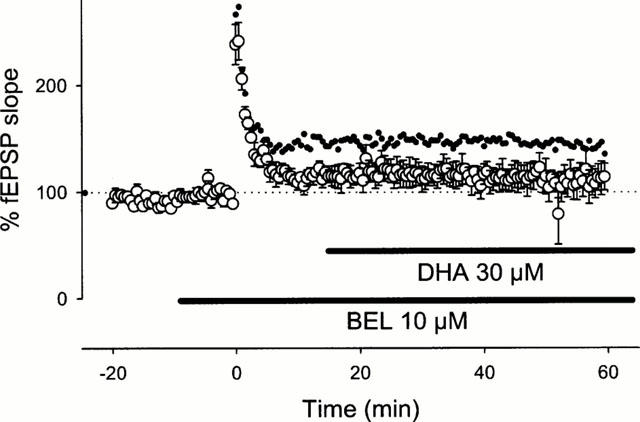
Lack of the effect of DHA on the maintenance phase of impaired LTP. Ten μM BEL was continuously applied from 10 min prior to the tetanic stimulation, and 30 μM DHA was added from 15 min after the tetanic stimulation. To promote a comparison, the time course of intact LTP (the same data as Figure 1a) is superimposed as small black dots. Data are expressed as the means±s.e.mean of five cases.
Figure 5.
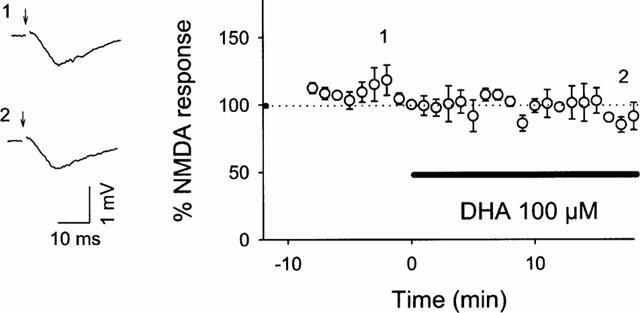
Effect of DHA on NMDA responses. Two traces represent pharmacologically isolated NMDA components immediately before (1) and 18 min after application of 100 μM DHA (2). The Schaffer collaterals were stimulated at the time indicated by arrows. The graph indicates the time course of NMDA responses following DHA treatment. Data are expressed as the means±s.e.mean of five cases.
We finally determined whether DHA per se alters the natures of synaptic plasticity. However, when 100 μM DHA was added 15 min before tetanic stimulation or low-frequency stimulation, it showed no effects on the formation of LTP or LTD (Figure 6). These data indicate that exogenous DHA alone did not contribute to neurotransmission or synaptic plasticity, and thus suggest that endogenously released DHA during tetanic stimulation is sufficient to induce LTP.
Figure 6.
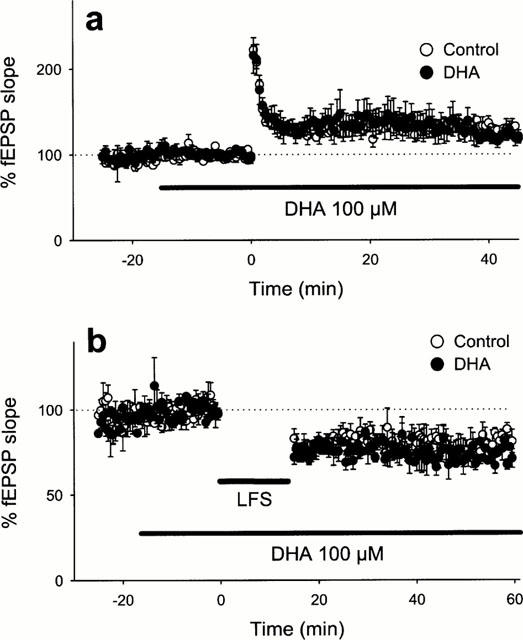
Lack of the effect of DHA on LTP (a) or LTD (b). DHA (100 μM) was continuously applied from 15 min before high-frequency stimulation (100 Hz for 1 s) (a) or low-frequency stimulation (1 Hz for 15 min) (b). Data are expressed as the means±s.e.mean of five cases.
Discussion
One of main observations in the present study is that the iPLA2 inhibitor BEL prevented the induction of LTP. In addition, we have shown for the first time that the iPLA2 inhibitor did not affect the maintenance phase of LTP or other forms of synaptic plasticity including LTD and PPF. The induction of LTP was also abolished by more selective iPLA2 inhibitor PACOCF3 but not by the cPLA2 inhibitor AACOCF3. These results suggested that iPLA2 plays a crucial role on the initiation of LTP. Interestingly, the attenuated LTP by BEL was restored by exogenous supplement with either DHA or AA. These results again substantiated the specific blockade of PLA2 activities by BEL. Furthermore, DHA did not restore LTP when added 15 min after the application of tetanus in the presence of BEL. Also, DHA alone did not affect LTP. These results indicate that endogenous release of DHA through iPLA2 activities during the tetanus may be important and sufficient to produce LTP.
It has remained inconsistent whether PLA2 plays a role in LTD. Fitzpatrick & Baudry (1994) reported that the inhibitor of PLA2 bromophenacylbromide blocked LTD in Schaffer collateral-CA1 synapses of rat hippocampal slices. On the other hand, Stanton (1995) indicated that another inhibitor of PLA2 3-(4-octadecyl)-benzoylacrylic acid did not prevented LTD at the same synapses. However, the specificity of these inhibitors is relatively low; particularly, bromophenacylbromide is a completely nonselective PLA2 inhibitor. Here we have shown that the selective inhibitor of iPLA2 BEL did not block the induction of LTD while the same concentration of BEL inhibited LTP. Although our results could not exclude the possibility that the other types of phospholipase A2 play some role in synaptic modifications causing LTD, we concluded that iPLA2, at least, is not required for the formation of LTD.
Young et al. (1998) indicated that application of 50 μM DHA reversibly suppressed the baseline of synaptic transmission and occluded LTD in Schaffer collateral-CA1 synapses. However, we were not able to confirm these phenomena even at a higher concentration of DHA (100 μM). Rather, our present study indicates that DHA did not affect basal neurotransmission, LTP nor LTD. Although explanation for this discrepancy apparently needs further investigations, it is our impression that sufficient DHA was endogenously released in our preparations so that exogenous application of DHA has no additional effect. In our experiments, indeed, DHA could exert its effect only if endogenous PLA2 activities were pharmacologically blocked. The level of releasable DHA is determined by the DHA content in membrane phospholipids, which is controlled by dietary intake of DHA and relevant fatty acids. Further analyses of the effect of PLA2 inhibitors and DHA in DHA-deficient rats would explain these discrepancies.
The mechanism by which DHA and AA contribute to LTP is unclear. Although NMDA receptor was assumed as the primary target of these fatty acids, our results show that DHA or BEL failed to affect NMDA responses. Therefore, we consider that exogenous or endogenous DHA has no influences on NMDA receptor in hippocampal slices. In addition, our preliminary study revealed that neither BEL nor DHA affected the induction of LTP at the mossy-fibre-CA3 synapses, where LTP is generated purely through presynaptic and NMDA-receptor independent mechanisms (Nicoll & Malenka, 1995). Thus, DHA and AA may be involved in the downstream mechanism of NMDA receptor in the induction of CA1 LTP. On the other hand, several studies indicate that DHA and AA inhibit A-type K+ channel (Villarroel & Schwarz, 1996; Keros & McBain, 1997; Colbert & Pan, 1999), which regulates back-propagation of action potentials into dendritic arborization (Hoffman et al., 1997; Johnston et al., 1999). Because the back-propagation is known to regulate long-lasting synaptic plasticity (Linden, 1999), DHA and AA may contribute to LTP by changing membrane excitability, such as back-propagation property of action potentials.
Numerous previous studies on DHA have focused their attention on learning and memory in mammals (Gordon, 1997; Horrocks, 1999). Particularly, behavioural analyses have suggested a significant correlation between DHA and cognitive ability in mammals. Nonetheless, the present study is the first evidence that DHA affects LTP. Very recently, we indicated that intracerebroventricular injection of iPLA2 inhibitor impaired spatial memory of mice (Fujita et al., 2000). Therefore, the iPLA2 activation and thereby DHA release in the hippocampus may be an important factor for both synaptic plasticity and learning and memory. To address this possibility, the measurement of changes in PLA2 activity and the concentrations of DHA following tetanic stimulation or task leaning would provide useful evidence.
Abbreviations
- AA
arachidonic acid
- AACOCF3
arachidonyl trifluoromethyl ketone
- ACSF
artificial cerebrospinal fluid
- BEL
bromoenol lactone
- cPLA2
Ca2+-dependent PLA2
- DHA
docosahexaenoic acid
- fEPSP
field excitatory postsynaptic potential
- iPLA2
Ca2+-independent PLA2
- LTD
long-term depression
- LTP
long-term potentiation
- MAFP
methyl arachidonyl fluorophosphonate
- NMDA
N-methyl-D-aspartate
- PACOCF3
palmitoyl trifluoromethyl ketone
- PLA2
phospholipase A2
- PPF
paired-pulse facilitation
References
- BLISS T.V., COLLINGRIDGE G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- BRAMHAM C.R., ALKON D.L., LESTER D.S. Arachidonic acid and diacylglycerol act synergistically through protein kinase C to persistently enhance synaptic transmission in the hippocampus. Neuroscience. 1994;3:737–743. doi: 10.1016/0306-4522(94)90501-0. [DOI] [PubMed] [Google Scholar]
- COLBERT C.M., PAN E. Arachidonic acid reciprocally alters the availability of transient and sustained dendritic K+ channels in hippocampal CA1 pyramidal neurons. J. Neurosci. 1999;19:8163–8171. doi: 10.1523/JNEUROSCI.19-19-08163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETROCELLIS L., MELCK D., UEDA N., MAURELLI S., KURAHASHI Y., YAMAMOTO S., MARINO G., DI MARZO V. Novel inhibitors of brain, neuronal, and basophilic anandamide amidohydrolase. Biochem. Biophys. Res. Commun. 1997;231:82–88. doi: 10.1006/bbrc.1997.6000. [DOI] [PubMed] [Google Scholar]
- DHOPESHWARKAR G.A., SUBRAMANIAN C. Metabolism of linoleic acid in developing brain. Lipids. 1975;10:238–241. doi: 10.1007/BF02532486. [DOI] [PubMed] [Google Scholar]
- DRAPEAU C., PELLERIN L., WOLFE L.S., AVOLI M. Long-term changes in synaptic transmission induced by arachidonic acid in the CA1 subfield of the rat hippocampus. Neurosci. Lett. 1990;115:286–292. doi: 10.1016/0304-3940(90)90470-t. [DOI] [PubMed] [Google Scholar]
- DUMUIS A., SEBBEN M., FAGNI L., PREZEAU L., MANZONI O., CRAGOE E.J., BOCKAERT J. Stimulation by glutamate receptors of arachidonic acid release depends on the Na+/Ca2+ exchanger in neuronal cells. Mol. Pharmacol. 1993;43:976–981. [PubMed] [Google Scholar]
- DUMUIS A., SEBBEN M., HAYNES L., PIN J.P., BOCKAERT J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988;336:69–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- FITZPATRICK J.S., BAUDRY M. Blockade of long-term depression in neonatal hippocampal slices by a phospholipase A2 inhibitor. Brain Research. Dev. Brain Res. 1994;78:81–86. doi: 10.1016/0165-3806(94)90012-4. [DOI] [PubMed] [Google Scholar]
- FUJITA S., IKEGAYA Y., NISHIKAWA M., MATSUKI N. Ca2+-independent Phospholipase A2 Inhibitor Impairs Spatial Memory of Mice. Jpn. J. Pharmacol. 2000;83:277–278. doi: 10.1254/jjp.83.277. [DOI] [PubMed] [Google Scholar]
- GORDON N. Nutrition and cognitive function. Brain. Dev. 1997;19:165–170. doi: 10.1016/s0387-7604(96)00560-8. [DOI] [PubMed] [Google Scholar]
- HADJIAGAPIOU C., SPECTOR A.A. Docosahexaenoic acid metabolism and effect on prostacyclin production in endothelial cells. Arch. Biochem Biophys. 1987;253:1–12. doi: 10.1016/0003-9861(87)90631-x. [DOI] [PubMed] [Google Scholar]
- HOFFMAN D.A., MAGEE J.C., COLBERT C.M., JOHNSTON D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- HORROCKS L.A., YEO Y.K. Health benefits of docosahexaenoic acid (DHA) Pharmacol. Res. 1999;40:211–225. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- JOHNSTON D., HOFFMAN D.A., COLBERT C.M., MAGEE J.C. Regulation of back-propagating action potentials in hippocampal neurons. Curr. Opin. Neurobiol. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- KEROS S., MCBAIN C.J. Arachidonic acid inhibits transient potassium currents and broadens action potentials during electrographic seizures in hippocampal pyramidal and inhibitory interneurons. J. Neurosci. 1997;17:3476–3487. doi: 10.1523/JNEUROSCI.17-10-03476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM H.Y., EDSALL L., MA Y.C. Specificity of polyunsaturated fatty acid release from rat brain synaptosomes. Lipids. 1996;31:S229–S233. doi: 10.1007/BF02637081. [DOI] [PubMed] [Google Scholar]
- KOVALCHUK Y., MILLER B., SARANTIS M., ATTWELL D. Arachidonic acid depresses non-NMDA receptor currents. Brain Res. 1994;643:287–295. doi: 10.1016/0006-8993(94)90035-3. [DOI] [PubMed] [Google Scholar]
- KULLMANN D.M., SIEGELBAUM S.A. The site of expression of NMDA receptor-dependent LTP: new fuel for an old fire. Neuron. 1995;15:997–1002. doi: 10.1016/0896-6273(95)90089-6. [DOI] [PubMed] [Google Scholar]
- LARKMAN A.U., JACK J.J.B. Synaptic plasticity: hippocampal LTP. Curr. Opin. Neurobiol. 1995;5:324–334. doi: 10.1016/0959-4388(95)80045-x. [DOI] [PubMed] [Google Scholar]
- LINDEN D.J. The return of the spike: postsynaptic action potentials and the induction of LTP and LTD. Neuron. 1999;22:661–656. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]
- LINDEN D.J., SHEU F.S., MURAKAMI K., ROUTTENBERG A. Enhancement of long-term potentiation by cis-unsaturated fatty acid; relation to protein kinase C and phospholipase A2. J. Neurosci. 1987;7:3783–3792. doi: 10.1523/JNEUROSCI.07-11-03783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ M. Severe deficiency of docosahexaenoic acid in peroxisomal disorders: a defect of delta 4 desaturation. Neurology. 1990;40:1292–1298. doi: 10.1212/wnl.40.8.1292. [DOI] [PubMed] [Google Scholar]
- MASSICOTTE G., BAUDRY M. Modulation of AMPA/Quisqualate receptors by phospholipase A2 treatment. Neurosci. Lett. 1990;118:245–248. doi: 10.1016/0304-3940(90)90638-p. [DOI] [PubMed] [Google Scholar]
- MASSICOTTE G., OLIVER M.W., LYNCH G., BAUDRY M. Effect of Bromophenacylbromide, a phospholipase A2 inhibitor, on the induction and maintenance of LTP in hippocampal slices. Brain Res. 1990;537:49–53. doi: 10.1016/0006-8993(90)90338-c. [DOI] [PubMed] [Google Scholar]
- MILLER B., SARANTIS M., TAYNELIS S.F., ATTWELL D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355:722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- NEURINGER M., CONNOR W.E., LIN D.S., BARSTAD L., LUCK S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4021–4025. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOLL R.A., MALENKA R.C. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- NISHIKAWA M., KIMURA S., AKAIKE N. Facilitatory effect of docosahexaenoic acid on N-methyl-D-aspartate response in pyramidal neurones of rat cerebral cortex. J. Physiol. (Lond.) 1994;475:83–93. doi: 10.1113/jphysiol.1994.sp020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'DELL T.J., HAWKINS R.D., KANDEL E.R., ARANCIO O. Tests for the role of two diffusible substances in long-term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proc. Natl. Acad. Sci. U.S.A. 1991;88:11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA D., YAMAGISHI S., SUGIYAMA H. Differential effects of phospholipase inhibitors in long-term potentiation in the rat hippocampal mossy fiber synapses and Schaffer/commissural synapses. Neurosci. Lett. 1989;100:141–146. doi: 10.1016/0304-3940(89)90674-5. [DOI] [PubMed] [Google Scholar]
- SODERBERG M., EDLUNK C., KRISTENSSON K., DALLNER G. Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease. Lipids. 1991;26:421–425. doi: 10.1007/BF02536067. [DOI] [PubMed] [Google Scholar]
- STANTON P.K. Phospholipase A2 activation is not required for long-term synaptic depression. Eur. J. Pharmacol. 1995;273:R7–R9. doi: 10.1016/0014-2999(95)00022-d. [DOI] [PubMed] [Google Scholar]
- VILLARROEL A., SCHWARZ T.L. Inhibition of the Kv 4 (Shal) family of transient K+ currents by arachidonic acid. J. Neurosci. 1996;16:2522–2532. doi: 10.1523/JNEUROSCI.16-08-02522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS J.H., BLISS T.V. Induction but not maintenance of calcium-induced long-term potentiation in dentate gyrus and area CA1 of the hippocampal slice is blocked by nordihydroguaiaretic acid. Neurosci. Lett. 1988;88:81–95. doi: 10.1016/0304-3940(88)90319-9. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.H., ERRINGTON M.L., LYNCH M.A., BLISS T.V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989;341:739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- WOLF M.J., IZUMI Y., ZORUMSKI C.F., GROSS R.W. Long-term potentiation requires activation of calcium-independent phospholipase A2. FEBS Lett. 1995;377:358–362. doi: 10.1016/0014-5793(95)01371-7. [DOI] [PubMed] [Google Scholar]
- YOUNG C., GEAN P.W., WU S.P., LIN C.H., SHEN Y.Z. Cancellation of low-frequency stimulation-induced long-term depression by docosahexaenoic acid in the rat hippocampus. Neurosci. Lett. 1998;247:198–200. doi: 10.1016/s0304-3940(98)00272-9. [DOI] [PubMed] [Google Scholar]


