Abstract
We have studied the effect of capsaicin, piperine and anandamide, drugs which activate vanilloid receptors and capsazepine, a vanilloid receptor antagonist, on upper gastrointestinal motility in mice.
Piperine (0.5 – 20 mg kg−1 i.p.) and anandamide (0.5 – 20 mg kg−1 i.p.), dose-dependently delayed gastrointestinal motility, while capsaicin (up to 3 mg kg−1 i.p.) was without effect. Capsazepine (15 mg kg−1 i.p.) neither per se affected gastrointestinal motility nor did it counteract the inhibitory effect of both piperine (10 mg kg−1) and anandamide (10 mg kg−1).
A per se non effective dose of SR141716A (0.3 mg kg−1 i.p.), a cannabinoid CB1 receptor antagonist, counteracted the inhibitory effect of anandamide (10 mg kg−1) but not of piperine (10 mg kg−1). By contrast, the inhibitory effect of piperine (10 mg kg−1) but not of anandamide (10 mg kg−1) was strongly attenuated in capsaicin (75 mg kg−1 in total, s.c.)-treated mice.
Pretreatment of mice with NG-nitro-L-arginine methyl ester (25 mg kg−1 i.p.), yohimbine (1 mg kg−1, i.p.), naloxone (2 mg kg−1 i.p.), or hexamethonium (1 mg kg−1 i.p.) did not modify the inhibitory effect of both piperine (10 mg kg−1) and anandamide (10 mg kg−1).
The present study indicates that the vanilloid ligands anandamide and piperine, but not capsaicin, can reduce upper gastrointestinal motility. The effect of piperine involves capsaicin-sensitive neurones, but not vanilloid receptors, while the effect of anandamide involves cannabinoid CB1, but not vanilloid receptors.
Keywords: Anandamide, piperine, capsazepine, primary afferent neurones, vanilloid receptors, cannabinoid receptors, capsaicin, intestinal motility
Introduction
A subpopulation of primary afferent neurones has been characterized by using the sensory neurotoxin capsaicin (Maggi & Meli, 1988), the active ingredient of chilli (from the Capsicum family). These neurones are small, ‘dark' and type ‘B', and give rise to unmyelinated afferent fibres (Torsoli et al., 1993). Capsaicin-sensitive sensory neurones can modulate intestinal motility as they convey signals coming from the gastrointestinal tract to the central nervous system and may simultaneously release transmitters (from the same terminal which is activated by an adequate stimulus) able to affect enteric neurotransmission (Holzer, 1991).
The action of capsaicin on afferent neurones is traditionally regarded as involving two phases: an acute excitatory effect which lead to transmitter release, followed by desensitization and damage after prolonged or repeated exposure (Holzer, 1991). In recent years it has been shown that the action of capsaicin on afferent neurones can be mediated through activation of specific receptors, namely vanilloid receptors (Caterina et al., 1997; Tominaga et al., 1998). Vanilloid receptors can be also activated by other irritant principles present in ‘hot' spices, such as piperine, the active ingredient of black pepper (Piper nigrum) and zingerone, isolated from ginger (Zingiber officinalis) (Liu & Simon, 1997; Sterner & Szallasi, 1999). Capsaicin, piperine and gingerone are structurally similar, as they share a vanillyl moiety essential for bioactivity (Sterner & Szallasi, 1999). A functional vanilloid receptor (VR1) has been cloned (Caterina et al., 1997) and a vanilloid receptor antagonist, namely capsazepine, is available for pharmacological characterization (Sterner & Szallasi, 1999). VR1 is a cation channel that is expressed in a major sub-group of small ‘dark' neurones of the dorsal root, trigeminal and vagal sensory ganglia (Caterina et al., 1997; Helliwell et al., 1998; Sterner & Szallasi, 1999; Szolcsanyi, 2000) and in several brain areas (Sasamura et al., 1998). The discovery of vanilloid receptors suggests the existence of endogenous vanilloid receptor ligands; in fact, the first of such vanilloids has identified as anandamide (arachidonylethanolamide) (Zygmunt et al., 1999; Smart et al., 2000), originally isolated as an endogenous cannabinoid receptor ligand (Devane et al., 1992). These findings suggest the existence of endogenous vanilloid receptor modulators lacking a vanillyl motif (Sterner & Szallasi, 1999).
Given the importance of primary afferent neurones in the control of intestinal motility in vivo and since vanilloid receptors are highly expressed on these neurones (Szallasi & Blumberg, 1999), we have evaluated the effect vanilloid drugs on upper gastrointestinal transit in mice. We have used anandamide, capsaicin and piperine, which activate vanilloid receptors (Liu & Simon, 1997; Sterner & Szallasi, 1999; Zygmunt et al., 1999) and capsazepine, a vanilloid receptor antagonist (Bevan et al., 1992).
Methods
Animals
Male ICR mice (Harlan Italy, Corezzana, MI, U.S.A.) (20 – 22 g) were used after 1 week of acclimation (temperature 23±2°C; humidity 60%). Food was withheld 3 h before experiments but there was free access to drinking water.
Upper gastrointestinal transit
Gastrointestinal transit was measured as previously described (Izzo et al., 1999; 2000b). Mice received orally a black marker (10% charcoal suspension in 5% gum arabic, 0.1 ml 10 g mouse−1) and 20 min later the mice were killed by asphyxiation with CO2 and the gastrointestinal tract removed. The distance travelled by the marker was measured and expressed as a percentage of the total length of the small intestine from pylorus to caecum.
Capsaicin (0.1 – 3 mg kg−1), piperine (0.5 – 20 mg kg−1), anandamide (0.5 – 20 mg kg−1), were given (i.p.) 20 min before charcoal administration. In some experiments capsaicin (3 mg kg−1 i.p.) was given immediately (t=0) or 10 min (t=10) before charcoal administration.
In some experiments capsazepine (15 mg kg−1), SR141716A (0.3 mg kg−1), yohimbine (1 mg kg−1), naloxone (2 mg kg−1), hexamethonium (1 mg kg−1) or NG-nitro-L-arginine methyl ester (L-NAME 25 mg kg−1) were given (i.p.) 30 min before piperine (10 mg kg−1) or anandamide (10 mg kg−1). The dose of capsazepine was selected on the basis of preliminary experiments: capsazepine (15 mg kg−1 i.p.) prevented the antinociceptive effect of capsaicin (3 mg kg−1 i.p.) in the hot plate test model (Perkins & Campbell, 1992). The other doses were selected on the basis of previous published work (Santos & Rao, 1999; Izzo et al., 1994).
In another set of experiments, the effect of anandamide (10 mg kg−1 i.p.) and piperine (10 mg kg−1 i.p.) was evaluated in capsaicin-treated animals: mice were anaesthetized with sodium pentobarbital (30 mg kg−1) and treated with increasing doses of capsaicin for 2 consecutive days (25 and 50 mg kg−1) to deplete neuropeptides in primary afferent neurones (Matsuda et al., 1999). To counteract any respiratory impairment associated with administration of capsaicin, the mice were pretreated with aminophylline (10 mg kg−1) 30 min before capsaicin injection. After 14 days, the efficacy of capsaicin treatment was assessed by the eye-wiping test (Holzer et al., 1990): impaired chemosensitivity of corneal afferents which are no longer sensitive to a solution of 1% NH4OH.
Drugs
Drugs used were: anandamide (soya oil/water emulsion), capsaicin, capsazepine (Tocris Cookson, Bristol, U.K.), aminophylline, hexamethonium bromide, piperine, naloxone hydrochloride, NG-nitro-methyl arginine methyl ester (L-NAME) hydrochloride, yohimbine hydrochloride, (SIGMA, Milan, Italy), sodium pentobarbital (Carlo Sessa), SR141716A [(N-piperidin-1-yl)-5-(4-chlorophenyl)-1-2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride was a gift from Dr Madaleine Mossè (SANOFI-Recherche, Montpellier, France). Capsaicin, capsazepine and piperine were dissolved in dimethyl sulphoxide (DMSO), while the other drugs were dissolved in saline.
Statistics
Data are means±s.e.mean. To determine statistical significance, Student's t-test for unpaired data or one-way analysis of variance followed by Tukey-Kramer multiple comparisons test was used. A P value less than 0.05 was considered significant.
Results
Administration of piperine (0.5 – 20 mg kg−1) or anandamide (0.5 – 20 mg kg−1) produced a dose-dependent delay in upper gastrointestinal transit (Figure 1). This effect was significant (from both compounds) starting from the dose of 10 mg kg−1. By contrast capsaicin (up to 3 mg kg−1 i.p.) did not modify significantly intestinal motility (transit: control 48±5%, capsaicin 0.1 mg kg−1 45±5%, capsaicin 0.3 mg kg−1 40±3%, capsaicin 1 mg kg−1 39±8, capsaicin 3 mg kg−1 45±4%, n=7 – 8 for each experimental group, P>0.2). When capsaicin (3 mg kg−1 i.p.) was given immediately (t=0) or 10 min (t=10) before charcoal administration, it did not modify significantly intestinal motility (transit at t=0: control 46±4%; capsaicin 41±3%; transit at t=10: control 49±5, capsaicin 46±4, n=7 – 8 for each experimental group, P>0.2)
Figure 1.
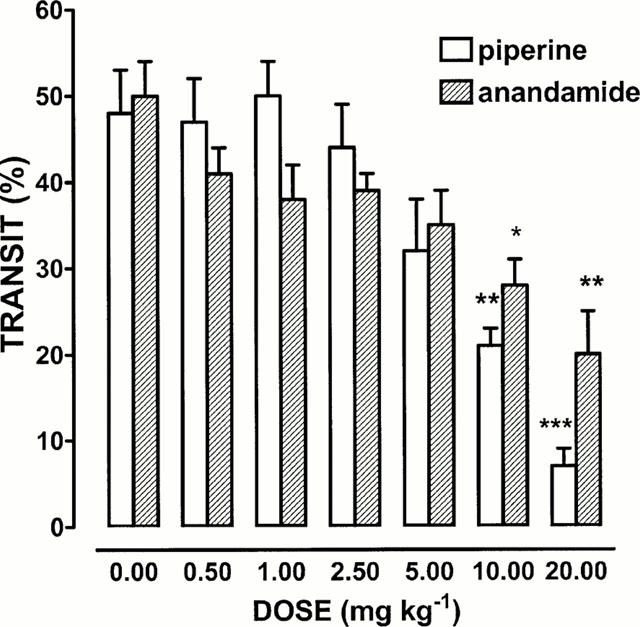
Effect of i.p.-injected anandamide and piperine on upper gastrointestinal transit. Each point represents the mean±s.e.mean of six animals for each experimental group. *P<0.05, **P<0.01 and ***P<0.001 vs corresponding control.
Upper gastrointestinal transit was not significantly modified by pretreatment (15 mg kg−1 i.p.) with the vanilloid receptor antagonist capsazepine (transit: control 52±4%, capsazepine 42±5%, n=8 for each experimental group, P>0.2). Lower doses of capsazepine (1, 3 and 10 mg kg−1) were also ineffective (data not shown).
The cannabinoid CB1 receptor antagonist SR141716A (0.3 mg kg−1), but not the vanilloid receptor antagonist capsazepine (15 mg kg−1) counteracted the inhibitory effect of anandamide (10 mg kg−1) (Figure 2). However, both capsazepine or SR141716A did not modify the inhibitory effect of piperine (10 mg kg−1) (Figure 2). Vehicle (DMSO 5 μl) for SR141716A or capsazepine did not modify significantly anandamide (10 mg kg−1 i.p.)- or piperine (10 mg kg−1 i.p.)-induced changes in motility (per cent transit: control 50±4, anandamide 27±6, anandamide+DMSO 25±5, piperine 23±3, piperine+DMSO 21±5, n=7 – 8 for each experimental group).
Figure 2.
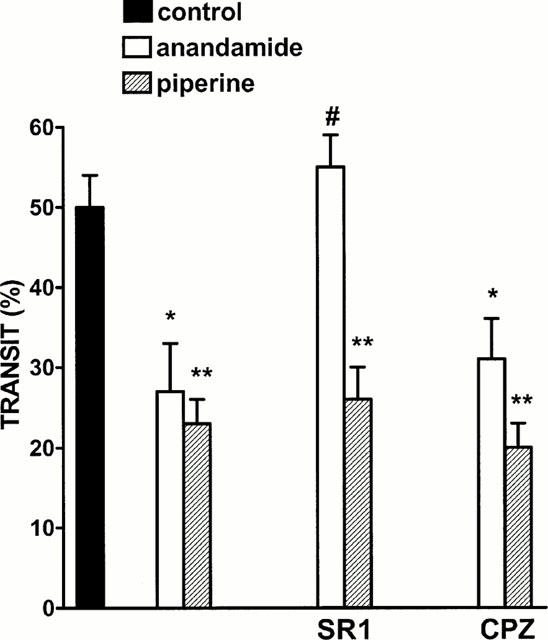
Effect of piperine (10 mg kg−1, i.p.) or anandamide (10 mg kg−1) on upper gastrointestinal transit alone or in mice treated with the cannabinoid CB1 receptor antagonist SR141716A (SR1, 0.3 mg kg−1 i.p.) or the vanilloid receptor antagonist capsazepine (CPZ, 15 mg kg−1, i.p.). Results are mean±s.e.mean of 7 – 8 animals for each experimental group. *P<0.05 and **P<0.01 vs control and #P<0.01 vs anandamide (alone).
As shown in Figure 3, pretreatment of mice with capsaicin (75 mg kg−1 in total, 13 and 14 days before) did not modify significantly gastrointestinal transit. However, piperine (10 mg kg−1), but not anandamide (10 mg kg−1) was without significant effect in mice pretreatment with capsaicin (Figure 3). Vehicle (DMSO 5 μl) for capsaicin did not modify significantly anandamide (10 mg kg−1 i.p.)- or piperine (10 mg kg−1 i.p.)-induced changes in motility (per cent transit: control 49±5, anandamide 28±5, anandamide+DMSO 24±5, piperine 24±4, piperine+DMSO 22±6, n=7 – 8 for each experimental group)
Figure 3.
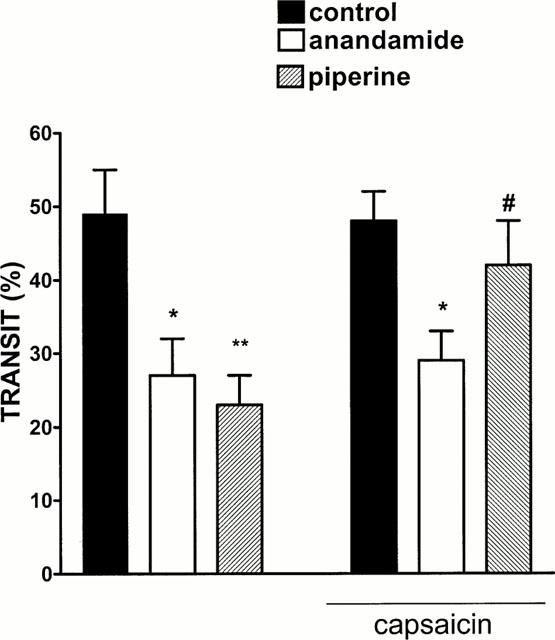
Effect of piperine (10 mg kg−1 i.p.) or anandamide (10 mg kg−1 i.p.) on upper gastrointestinal transit in mice not receiving capsaicin or in capsaicin (75 mg kg−1 s.c., 13 and 14 days before)-treated mice. Each point represents the mean±s.e.mean of 7 – 8 animals for each experimental group. *P<0.05 and **P<0.01 vs corresponding control (animals not treated with anandamide or piperine) and #P<0.05 vs piperine.
The inhibitory effect of both piperine (10 mg kg−1) and anandamide (10 mg kg−1) was unchanged in mice pretreated with naloxone (2 mg kg−1), yohimbine (1 mg kg−1), L-NAME (25 mg kg−1) or hexamethonium (1 mg kg−1) (Figure 4).
Figure 4.
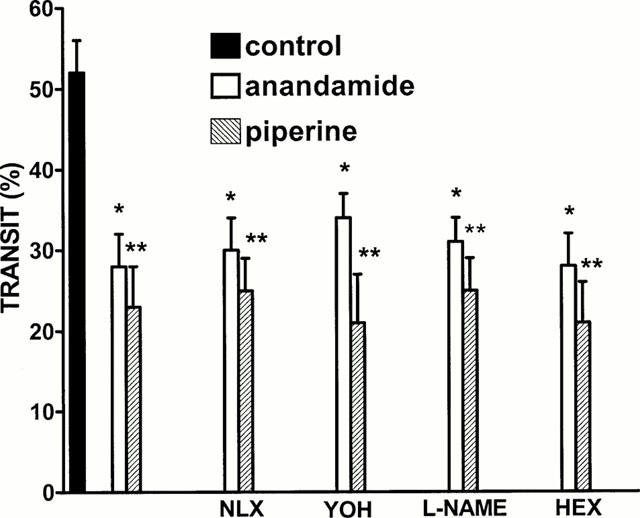
Effect of piperine (10 mg kg−1, i.p.) or anandamide (10 mg kg−1 i.p.) on upper gastrointestinal transit alone or in mice treated with naloxone (NLX, 2 mg kg−1, i.p.), yohimbine (YOH, 1 mg kg−1 i.p.), NG-nitro-L-arginine methyl ester (L-NAME, 25 mg kg−1) and hexamethonium (HEX, 1 mg kg−1 i.p.). Results are mean±s.e.mean of 7 – 8 animals for each experimental group. *P<0.05 and **P<0.01 vs control.
At the dosage used, none of the antagonists tested, i.e. SR141716A, yohimbine, naloxone, hexamethonium, L-NAME, had any significant effect per se on upper gastrointestinal transit (variation of upper gastrointestinal transit: SR141716A +16±6%, yohimbine +14±8, naloxone +2±5%, hexamethonium +15±7%, L-NAME −8±6%, n=7 – 8, P>0.2).
DMSO (10 μl mouse−1) per se did not significantly modify gastrointestinal transit (per cent transit control 51±5, DMSO 48±4, n=6, P>0.2).
Discussion
Primary afferent neurones carry sensory information to the central nervous system and may simultaneously release neurotransmitters able to affect enteric neurotransmission (Torsoli et al., 1993; Goyal & Hirano, 1996). In recent years it has been shown that capsaicin and other active ingredient present in ‘hot spices' bind to specific receptors, named vanilloid receptors, mostly located on the cell membrane of primary afferent neurones (Szallasi & Blumberg, 1999). Previous investigators have shown that piperine and capsaicin, which act via vanilloid receptors (Sterner & Szallasi, 1999), affect gastric and intestinal motility in vitro (Maggi et al., 1986a,1986b; Jin et al., 1990; Takaki et al., 1990; Lefebvre et al., 1991; Allesher et al., 1992; Holzer-Petsche et al. 1989; Barthò et al., 2000). In addition, the endocannabinoid anandamide, which is know to inhibit enteric excitatory transmission in vitro (Izzo et al., 1998) and intestinal motility in vivo (Fride, 1995; Calignano et al., 1997), has been recently identified as an endogenous vanilloid ligand (Zygmunt et al., 1999; Smart et al., 2000). These results could suggest an involvement of vanilloid receptors in the control of intestinal motility.
In the present study, we have shown that piperine and anandamide, which activate vanilloid receptors (Liu & Simon, 1997; Zygmunt et al., 1999; Smart et al., 2000), are able to produce a dose-dependent reduction of upper gastrointestinal transit. However, it is unlikely that this effect is due to activation of vanilloid receptors as the inhibitory effect of both piperine and anandamine was not modified by capsazepine, a specific vanilloid receptor antagonist. In addition, capsaicin (up to 3 mg kg−1), another vanilloid receptor agonist (Sterner & Szallasi, 1999) did not affect upper gastrointestinal transit thus confirming the lack of involvement of vanilloid receptors in the control of upper gastrointestinal transit. Higher doses of capsaicin (i.p.) were not studied as they were toxic. Others have shown that lower doses of capsaicin (<3 mg kg−1) affected gastric motility (Kang et al., 1993) and gastric blow flow (Abdel Salam et al., 1996) in rats.
Previous investigators have shown that chronic treatment with capsaicin (to ablate capsaicin-sensitive afferent neurons) does not affect gastrointestinal propulsion in physiological states, while it reduced the inhibition of gastrointestinal transit due to surgical trauma or peritoneal administration (Holzer, 1986; Holzer et al., 1987). When given acutely (as in our study) capsaicin reduced intestinal transit in the rat (Miller et al., 1981; Chang et al., 1999). In the present study performed in the mouse, capsaicin did modify upper gastrointestinal transit. The use of a different animal specie (rat vs mouse) or different regions of the gut studied (intestinal transit vs gastrointestinal transit) could explain the discrepancy between our results (no effect of capsaicin on motility) and those reported in the rat (delaying effect of capsaicin on motility). However, others have shown that capsaicin did not modify upper gastrointestinal transit in the rat (Kang et al. 1993).
Capsaicin has been used systematically to ablate all capsaicin-sensitive C fibres to produce sensory pathway-specific ablation in various animal species, including the mouse (Barrachina et al., 1997). In the digestive tract, capsaicin-sensitive afferent innvervation participates in nocioception, gastroprotection and intestino-intestinal activation of inhibitory reflexes (Holzer, 1991; Holzer et al., 1991). Gastroprotection by oral capsaicin could result from increase of mucosal blood flow and inhibition of gastric motility (Takeuchi et al., 1991). In order to verify whether the vanilloid drugs-induced changes in motility were due to an effect on capsaicin-sensitive nerve terminals, the effect of anandamide and piperine was evaluated in mice desensitized by systemic capsaicin doses. Thus, we have shown that the inhibitory effect of piperine, but not anandamide, was markedly attenuated by the pretreatment with capsaicin (13 and 14 days before). These results suggests that capsaicin-sensitive sensory nerves are, at least in part, involved in the inhibition of intestinal transit by piperine. Consistent with these in vivo results, Takaki et al. (1990) have shown that pretreatment of the isolated ileum with capsaicin prevented piperine-induced motility changes. If we assume that piperine modify gastrointestinal motility by acting on capsaicin-sensitive neurones, at present it is not clear why capsaicin, which, like piperine, acts on capsaicin-sensitive neurones does not affect intestinal motility; thus, we can hypothesize that there are sites on sensory neurones which are selectively recognized by piperine, but not by capsaicin. Activation of these sites can delay gastrointestinal motility and their identification could represent a novel target for therapeutic drugs. It is unlikely that the vanillyl moiety is essential for this activity as both capsaicin and piperine share this chemical group. Therefore, the different pharmacological response evoked by capsaicin and piperine in this study could be explained, at least in part, by the existence of different subtypes of vanilloid receptors (Acs et al., 1997; Szolcsanyi, 2000) or perhaps by the fact that both capsaicin and piperine possess non-specific actions (i.e. not restricted to primary afferent neurones) on nerves and smooth muscle (Holzer, 1991; Takaki et al., 1990). It is unlikely that the difference between capsaicin and piperine is due to a different activation and desensitisation kinetics (i.e. effect of capsaicin, due to a more rapid desensitization, is much shorter-lasting than that of piperine) as capsaicin was inactive even when given immediately (t=0) or 10 min (t=10) before charcoal administration.
Activation of prejunctional cannabinoid CB1 is known to inhibit enteric excitatory transmission (Pertwee et al., 1996; Izzo et al., 1998) and peristalsis (Heinemann et al., 1999; Izzo et al., 2000a) in the isolated guinea-pig ileum. Cannabinoid receptor agonists reduce while the cannabinoid receptor antagonist SR141716A increase intestinal motility in vivo by activating peripheral (enteric) cannabinoid CB1 receptors (Izzo et al., 2000b). In the present study we have shown that the effect of anandamide, but not of piperine, was counteracted by a per se non effective dose of the selective cannabinoid CB1 antagonist SR141716A (Rinaldi-Carmona et al., 1995), indicating an involvement of cannabinoid CB1 receptors in the inhibitory effect of anandamide. However, other prejunctional or presynaptic systems, such as opioid or α2-adrenergic receptors, which are known to be involved in the regulation of intestinal motility (Dockray, 1994; Burks, 1994) are not involved in the inhibitory effect of the two vanilloid ligands as the inhibitory effect of both anandamide and piperine was not modified by naloxone, an opioid receptor antagonist or yohimbine, an α2-adrenergic receptor antagonist.
Previous investigators have shown that capsaicin can depress intestinal peristalsis with a mechanism involving nitric oxide (Bartho & Holzer, 1995); in addition, anandamide stimulates nitric oxide production in neural tissues (Stefano et al., 1997). Nitric oxide is now recognized as perhaps the major mediator of relaxation induced by enteric inhibitory neurones (Burks, 1994). Reduction of gastrointestinal motility in vivo can either results from inhibition of nitric oxide synthesis or from formation of excess nitric oxide (Orihata & Sarna, 1994; De Winter et al., 1997). In the present study we have shown that L-NAME, a nitric oxide synthase inhibitor, at dose previously shown to be effective (Izzo et al., 1994), was not able to modify the inhibitory effect of both anandamide and piperine. Thus, an involvement of nitric oxide in the motility changes associated with these vanilloid drugs seems unlikely.
In conclusion, the present study indicates that vanilloid ligands have a different effect on intestinal motility. Piperine may decrease gastrointestinal transit through actions on capsaicin-sensitive neurones; anandamide decreases intestinal motility through an action on cannabinoid CB1 receptors (but not on vanilloid receptors or on capsaicin-sensitive neurones) while capsaicin, another activator of vanilloid receptors, was without effect. The effect of both anandamide and piperine probably involves peripheral mechanisms (as suggested by the lack of effect of hexamethonium) but does not involve nitric oxide generation or activation of α2- adrenergic or opioid receptors. The lack of effect of the vanilloid receptor antagonist capsazepine to affect per se intestinal motility or to counteract the inhibitory effect of the vanilloid ligands (together with the ineffectiveness of the vanilloid receptor agonist capsaicin) suggests that endogenous or exogenous activation of vanilloid receptors does not play a role in the control of upper gastrointestinal transit under physiological states.
Acknowledgments
This work was supported by Indena (Milano), Cofinanziamento Murst and Enrico and Enrica Sovena Foundation (Roma). SR141716A was a kind gift from SANOFI (Montpellier, France)
Abbreviations
- DMSO
dimethyl sulphoxide
- L-NAME
NG-nitro-L-arginine methyl ester
- VR1
vanilloid receptor (subtype 1)
References
- ABDEL SALAM O.M., SZOLCSANYI J., PORSZASZ R., MOZSIK G. Effect of capsaicin and resiniferatoxin on gastrointestinal blood flow in rats. Eur. J. Pharmacol. 1996;305:127–136. doi: 10.1016/0014-2999(96)00147-1. [DOI] [PubMed] [Google Scholar]
- ACS G., BIRO T., ACS P., MODARRES S., BLUMBERG P.M. Differential activation and desensitization of sensory neurons by resiniferatoxin. J. Neurosci. 1997;17:5622–5628. doi: 10.1523/JNEUROSCI.17-14-05622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLESCER H.-D., SATTLER D., PILLER C., SCHUSDZIARRA V., CLASSEN M. Ascending neural pathways in the rat ileum in vitro: effect of capsaicin and involvement of nitric oxide. Eur. J. Pharmacol. 1992;217:153–162. doi: 10.1016/0014-2999(92)90839-v. [DOI] [PubMed] [Google Scholar]
- BARRACHINA M.D., MARTINEZ V., WANG L., WEI J.Y., TACHE' Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTHO' L., HOLZER P. The inhibitory modulation of guinea-pig intestinal peristalsis caused by capsaicin involves calcitonin gene-related peptide and nitric oxide. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;353:102–109. doi: 10.1007/BF00168922. [DOI] [PubMed] [Google Scholar]
- BARTHO' L., LAZAR Z., LENARD L., JR, BENKÒ R., TOTH G., PENKE B., SZOLCSANYI J., MAGGI C.A. Evidence for the involvement of ATP, but not of VIP/PACAP or nitric oxide, in the excitatory effect of capsaicin in the small intestine. Eur. J. Pharmacol. 2000;392:183–188. doi: 10.1016/s0014-2999(00)00137-0. [DOI] [PubMed] [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKS T.S. Physiology of the Gastrointestinal Tract. ed. Johnson, L.R. pp. New York, Raven Press; 1994. Neurotransmission and neurotransmitters; pp. 211–242. [Google Scholar]
- CALIGNANO A., LA RANA G., MAKRIAYANNIS A., LIN S.Y., BELTRAMO M., PIOMELLI D. Inhibition of intestinal motility by anandamide, an endogenous cannabinoid. Eur. J. Pharmacol. 1997;340:R7–R8. [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVEIN J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHANG F.Y., CHEN C.Y., CHEN T.S., LEE S.D., DOONG M.L., WANG P.S. Variation of capsaicin-sensitive motor activities along the rat gastrointestinal tract. Chin. J. Physiol. 1999;31:41–45. [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;277:119–131. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DE WINTER B.Y., BOECKXSTAENS G.E., DE MAN J.G., MOREELS T.G., HERMAN A.G., PELCKMANS P.A. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br. J. Pharmacol. 1997;120:464–468. doi: 10.1038/sj.bjp.0700913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCKRAY G.J.Physiology of enteric neuropeptides Physiology of the Gastrointestinal Tract 1994New York, Raven Press; 169–210.ed. Johnson, L.R. pp [Google Scholar]
- FRIDE E. Anandamides: tolerance and cross-tolerance to delta-9-tetrahydrocannabinol. Brain Res. 1995;697:83–90. doi: 10.1016/0006-8993(95)00790-w. [DOI] [PubMed] [Google Scholar]
- GOYAL R.K., HIRANO I. The enteric nervous system. N. Eng. J. Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- HEINEMANN A., SHAHBAZIAN A., HOLZER P. Cannabinoid inhibition of guinea-pig intestinal peristalsis via inhibition of excitatory and activation of inhibitory neural pathways. Neuropharmacology. 1999;38:1289–1297. doi: 10.1016/s0028-3908(99)00056-8. [DOI] [PubMed] [Google Scholar]
- HELLIWELL R.J.A., MCLATCHIE L.M., CLARKE M., WINTER J., BEVAN S., MCINTYRE P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin-sensitive afferent neurones and gastrointestinal propulsion in the rat. Naunyn-Schmiedebergs Arch. Pharmacol. 1986;332:62–65. doi: 10.1007/BF00633198. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanism of action, and selectivity of thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HOLZER P., LIVINGSTON E.H., SARIA A., GUTH P.H. Sensory neurons mediate protective vasodilatation in rat gastric mucosa. Am. J. Physiol. 1991;260:G363–G370. doi: 10.1152/ajpgi.1991.260.3.G363. [DOI] [PubMed] [Google Scholar]
- HOLZER P., PABST M.A., LIPPIE I.T., PESKAR B.M., PESKAR B.A., LIVINGSTON E.H., GUTH P.H. Afferent nerve-mediated protection against deep mucosal damage in the rat stomach. Gastroenterology. 1990;98:839–849. doi: 10.1016/0016-5085(90)90005-l. [DOI] [PubMed] [Google Scholar]
- HOLZER P., SCHLUET W., LIPPE I.T., SAMETZ W. Involvement of capsaicin-sensitive sensory neurons in gastrointestinal function. Acta Physiol. Hung. 1987;69:403–411. [PubMed] [Google Scholar]
- HOLZER-PETSCHE U., SEITZ H., LEMBECK F. Effect of capsaicin on gastric corpus smooth muscle of the rat in vitro. Eur. J. Pharmacol. 1989;162:29–36. doi: 10.1016/0014-2999(89)90600-6. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., GAGINELLA T.S., MASCOLO N., CAPASSO F. Nitric oxide as a mediator of the laxative action of magnesium sulphate. Br. J. Pharmacol. 1994;113:228–232. doi: 10.1111/j.1476-5381.1994.tb16198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., BORRELLI F., CAPASSO F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid CB1 receptor. Br. J. Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., BORRELLI F., CAPASSO F. Defaecation, intestinal fluid accumulation and motility in rodents: implications of cannabinoid CB1 receptors. Naunyn-Schmiedeberg's Arch., Pharmacol. 1999;359:65–70. doi: 10.1007/pl00005325. [DOI] [PubMed] [Google Scholar]
- IZZO A.A., MASCOLO N., TONINI M., CAPASSO F. Modulation of peristalsis by cannabinoid CB1 ligands in the isolated guinea-pig ileum. Br. J. Pharmacol. 2000a;129:984–990. doi: 10.1038/sj.bjp.0703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO A.A., PINTO L., BORRELLI F., CAPASSO R., MASCOLO N., CAPASSO F. Central and peripheral cannabinoid modulation of gastrointestinal transit in physiological states or during the diarrhoea induced by croton oil. Br. J. Pharmacol. 2000b;129:1627–1632. doi: 10.1038/sj.bjp.0703265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG J.Y., ALEXANDER B., MATH M.V., WILLIAMSON R.C. The effect of chilli and its pungent ingredient capsaicin on gastrointestinal transit in the rat. J. Gatroenterol. Hepatol. 1993;8:513–516. doi: 10.1111/j.1440-1746.1993.tb01644.x. [DOI] [PubMed] [Google Scholar]
- JIN J.-G., TAKAKI M., NAKAYAMA S. Inhibitory effect of capsaicin on the ascending pathway of the guinea-pig ileum and antagonism of this effect by ruthenium red. Eur. J. Pharmacol. 1990;180:13–19. doi: 10.1016/0014-2999(90)90587-v. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., DE BEURME F.A., SAS S. Relaxant effect of capsaicin in the rat gastric fundus. Eur. J. Pharmacol. 1991;195:131–137. doi: 10.1016/0014-2999(91)90390-c. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Similarities and differences in the currents activated by capsaicin, piperine and zingerone in rat trigeminal ganglion cells. J. Neurophysiol. 1997;76:1858–1869. doi: 10.1152/jn.1996.76.3.1858. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MELI A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., MANZINI S., GIULIANI S., SANTICIOLI P., MELI A. Extrinsic origin of the capsaicin-sensitive innervation of rat duodenum: possible involvement of calcitonin gene-related peptide (CGRP) in the capsaicin-induced activation of intramural non-adrenergic non-cholinergic neurons. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986a;334:172–180. doi: 10.1007/BF00505818. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., MANZINI S., MELI A. Capsaicin activates neurogenic non-adrenergic non-cholinergic relaxation of the isolated rat duodenum. Eur. J. Pharmacol. 1986b;120:367–370. doi: 10.1016/0014-2999(86)90480-2. [DOI] [PubMed] [Google Scholar]
- MILLER M.S., GALLIGAN J.J., BURKS T.F. Accurate measurement of intestinal transit in the rat. J. Pharmacol. Methods. 1981;6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- MATSUDA H., LY Y., YAMAHARA J., YOSHIKAWA M. Inhibition of gastric emptying by triterpene saponin, momordin Ic, in mice: roles of blood glucose, capsaicin-sensitive sensory nerves, and central nervous system. J. Pharmacol. Exp. Ther. 1999;289:729–734. [PubMed] [Google Scholar]
- ORIHATA M., SARNA S.K. Inhibition of nitric oxide synthase delays gastric emptying of solid meals. J. Pharmacol. Exp. Ther. 1994;271:660–667. [PubMed] [Google Scholar]
- PERKINS M.N., CAMPBELL E.A. Capsazepine reversal of the antinociceptive action of capsaicin in vivo. Br. J. Pharmacol. 1992;107:329–333. doi: 10.1111/j.1476-5381.1992.tb12746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R., NASH J.E., COUTTS A.A. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br. J. Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., ALFONSO R., SHIRE D., CONGY C., SOBRIE' P., BRELIERE J.-C., LE FUR G. Biochemical and pharmacological characterization of SR141716A, the first potent and selective brain cannabinoid receptor antagonist. Life Sci. 1995;56:1941–1947. doi: 10.1016/0024-3205(95)00174-5. [DOI] [PubMed] [Google Scholar]
- SANTOS F.A., RAO V.S.N. Quinine-induced inhibition of gastrointestinal transit in mice: possible involvement of endogenous opioids. Eur. J. Pharmacol. 1999;364:193–197. doi: 10.1016/s0014-2999(98)00842-5. [DOI] [PubMed] [Google Scholar]
- SASAMURA R., SASAKI M., TOHDA C., KURAISHI Y. Existence of capsaicin-sensitive glutamatergic terminals in rat hypothalamus. Neuroreport. 1998;9:2045–2048. doi: 10.1097/00001756-199806220-00025. [DOI] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIDS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEFANO G.B., SALZET B., RIALAS C.M., POPE M., KUSTKA A., NEENAN K., PRYOR S., SALZET M. Morphine- and anandamide-stimulated nitric oxide production inhibits presynaptic dopamine release. Brain Res. 1997;763:63–68. doi: 10.1016/s0006-8993(97)00403-4. [DOI] [PubMed] [Google Scholar]
- STERNER O., SZALLASI A. Novel natural vanilloid receptor agonists: new therapeutic targets for drug development. Trends Pharmacol. Sci. 1999;20:459–464. doi: 10.1016/s0165-6147(99)01393-0. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- SZOLCSANYI J. Are cannabinoids endogenous ligands for the VR1 capsaicin receptor. Trends Pharmacol. Sci. 2000;21:41–42. doi: 10.1016/s0165-6147(99)01436-4. [DOI] [PubMed] [Google Scholar]
- TAKAKI M., JIN J., LU Y.F., NAKAYAMA S. Effects of piperine on the motility of the isolated guinea-pig comparison with capsaicin. Eur. J. Pharmacol. 1990;186:71–77. doi: 10.1016/0014-2999(90)94061-2. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI K., NIIDA H., MATSUMOTO J., UESHIMA K., OKABE S. Gastric motility changes in capsaicin-induced cytoprotection in the rat stomach. Jpn. J. Pharmacol. 1991;55:147–155. doi: 10.1254/jjp.55.147. [DOI] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- TORSOLI A., ANNESE V., CORAZZIARI E., CUCCHIARA S., RENZI D., SEVERI C., STANCHELLINI V., STERNINI C., SURRENTI C. Neuroendocrine regulation of gastrointestinal motility. Normal and abnormal physiology. Ital. J. Gastroenterol. 1993;25:123–134. [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilatator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


