Abstract
The presence of 5-HT7 receptor mRNA and protein in 5-HT neurons suggests that this receptor may act as a 5-HT autoreceptor. In this study, the effect of the 5-HT7 receptor antagonist, SB-269970 ((R)-1-[3-hydroxy phenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine), was investigated on 5-HT release in the guinea-pig and rat cortex and the rat dorsal raphe nucleus (DRN), using the techniques of in vitro [3H]-5-HT release or fast cyclic voltammetry, respectively.
Cortical slices were loaded with [3H]-5-HT and release was evoked by electrical stimulation. 5-CT inhibited the evoked release of [3H]-5-HT in a concentration-dependent manner. SB-269970 had no significant effect on [3H]-5-HT release while the 5-HT1B receptor antagonist, SB-224289 significantly potentiated [3H]-5-HT release. In addition, SB-269970 was unable to attenuate the 5-CT-induced inhibition of release while SB-224289 produced a rightward shift of the 5-CT response, generating estimated pKB values of 7.8 and 7.6 at the guinea-pig and rat terminal 5-HT autoreceptors respectively.
Rat DRN slices were electrically stimulated and the evoked 5-HT efflux detected by voltammetric analysis. 8-OH-DPAT inhibited evoked 5-HT efflux and was fully reversed by WAY 100635. SB-269970 had no effect on either 5-HT efflux per se or 8-OH-DPAT-induced inhibition of 5-HT efflux. In addition, 5-CT inhibited 5-HT efflux in a concentration-dependent manner. SB-269970 was unable to attenuate the 5-CT-induced inhibition of 5-HT efflux.
In conclusion, we were unable to provide evidence to suggest a 5-HT autoreceptor role for 5-HT7 receptors. However, investigations with more selective 5-HT7 receptor agonists are needed to confirm the data reported here.
Keywords: 5-HT7, voltammetry, [3H]-5-HT release
Introduction
5-Hydroxytryptamine (5-HT) receptors have been classified into seven families (5-HT1 – 7) based on their structural, functional and pharmacological characteristics (Hoyer et al., 1994; Martin & Humphrey; 1994). The 5-HT7 receptor was recently cloned from several species including rat (Lovenberg et al., 1993; Meyerhof et al., 1993; Ruat et al., 1993; Shen et al., 1993), mouse (Plassat et al., 1993), guinea-pig (Tsou et al., 1994) and human (Bard et al., 1993). 5-HT7 receptor binding has also been demonstrated in native guinea-pig, rat, mouse, pig and human brain (To et al., 1995; Boyland et al., 1996; Atkinson et al., 2000). The receptor has been shown to be positively coupled to adenylyl cyclase in both human clones (Hoyer et al., 1994) and guinea-pig hippocampal tissue (Tsou et al., 1994; Thomas et al., 1999).
The 5-HT7 receptor displays a high degree of interspecies homology (>95% between all species; To et al., 1995) but alternative splicing in both human and rat tissues has been identified. The cDNA encoding the receptor contains two introns: the first located in the gene region corresponding to the second intracellular loop (Bard et al., 1993; Shen et al., 1993; Ruat et al., 1993) and the second in the gene region corresponding to the predicted intracellular carboxyl terminal (Ruat et al., 1993). Alternative splicing of this latter intron has been reported to generate four 5-HT7 receptor isoforms (5-HT7(a); 5-HT7(b); 5-HT7(c); 5-HT7(d)) which differ in their C-terminal intracellular tails (Heidmann et al., 1997). However, there are no reports on these splice variants differing in pharmacology, signal transduction or tissue distribution (Jasper et al., 1997; Heidmann et al., 1998).
In situ hybridization, immunohistochemistry and autoradiography have all concluded that 5-HT7 receptors are present on serotonergic neurones throughout the central nervous system (CNS), in both terminal regions such as the cortex and cell body regions such as the raphe. As the biological role of this receptor still remains to be elucidated the distribution of 5-HT7 receptors has been important in suggesting function.
In the rat, high to moderate levels of mRNA were evident in the cortex (Lovenberg et al., 1993; Ruat et al., 1993; Shen et al., 1993; Gustafson et al., 1996; Heidmann et al., 1998) with lower levels in the raphe (Ruat et al., 1993), while in the guinea-pig, moderate levels of mRNA were observed in cortex and low levels in raphe (To et al., 1995). Similarly, moderate levels of 5-HT7 protein was identified in rat cortex (Waeber et al., 1995; Gustafson et al., 1996; Oliver et al., 1999; Atkinson et al., 2000) with varying levels in the raphe (Gustafson et al., 1996; Oliver et al., 1999), while in the guinea-pig high levels of 5-HT7 receptor protein were found in the cortex (To et al., 1995; Thomas et al., 2000) and moderate levels in the raphe (To et al., 1995). A review of this data can be found in Lucas & Hen (1995).
The identification of 5-HT7 receptor mRNA and protein in serotonin cell body and terminal areas raised the possibility that this receptor was another 5-HT autoreceptor which would serve to regulate 5-HT release. Ruat et al. (1993) eluded to a possible autoreceptor role for this class of receptor, stating, ‘It will be of interest to establish whether 5-HT7 receptor mRNAs detected in raphe nuclei reflect the local synthesis of another class of autoreceptors.'
With the emergence of selective 5-HT7 receptor antagonists (Thomas et al., 1998; Hagan et al., 2000) it has now become possible to directly investigate the role of this receptor in the CNS. Therefore, in this study the effect of the 5-HT7 receptor antagonist, SB-269970 (Hagan et al., 2000), was investigated in guinea-pig and rat cerebral cortex and in rat dorsal raphe nucleus (DRN), using the techniques of in vitro [3H]-5-HT release or fast cyclic voltammetry, respectively.
To date, there are no selective 5-HT7 receptor agonists available. Therefore, in the current studies we have used non-selective 5-HT receptor agonists: 5-CT, which has high affinity for 5-HT7 receptors but also 5-HT1A, 5-HT1B, 5-HT1D and 5-HT1F receptors and 8-OH-DPAT, which has moderate affinity at 5-HT7 receptors but high affinity at 5-HT1A receptors (Thomas et al., 1998; 1999). Both 5-CT and 8-OH-DPAT have been demonstrated to act as functional agonists at 5-HT7 receptors, stimulating adenylyl cyclase activity both in a 5-HT7 cloned cell line and in guinea-pig hippocampal tissue. However, while 5-CT was a full agonist in both tissues, 8-OH-DPAT was a partial agonist in guinea-pig tissue, with an intrinsic activity of 0.4 (Thomas et al., 1998; 1999).
Methods
In vitro [3H]-5-HT release
Male guinea-pigs (Harlan Porcellus) or rats (Sprague Dawley) were sacrificed, decapitated and the brains removed. The cortex was rapidly dissected and cross-chopped into 300×300 μm slices on a McIlwain chopper. The slices were incubated with 100 nM [3H]-5-HT in the presence of pargyline (10 μM) at 37°C for 15 min. Slices were washed, suspended in 2 ml of Krebs buffer (mM: NaCl 118; KCl 4.8; CaCl2 1.3; MgSO4 1.2; NaHCO3 25; NaH2PO4 1.2; glucose 10; L-ascorbate 0.06; Na2EDTA 0.03) and 100 μl aliquots transferred to a Brandel superfusion 2000 apparatus. The slices were then superfused at 0.5 ml min−1 with oxygenated Krebs in the presence of paroxetine (1 μM).
After 30 min of superfusion (t=0) samples were collected every 4 min for a duration of 80 min. [3H]-5-HT release was electrically evoked, using stimulation parameters of 1 Hz, 2 min, 20 mA to investigate effects of antagonists or 3 Hz, 1 min, 20 mA to investigate effects of agonists. Slices were stimulated at t=12 (S1) and 56 (S2) min. Compounds were perfused at t=24 min and were present until the end of the experiment.
At the end of the experiment the radioactivity in the slices and superfusate samples were determined by scintillation spectrometry. Fractional release (FR) for each sample was calculated and data expressed as either an S2/S1 ratio or as a per cent of control (Roberts et al., 1996).
In vitro fast cyclic voltammetry
Male rats (Sprague Dawley) were killed by terminal anaesthesia (halothane) and decapitated. The brain was rapidly removed and a 350 μm brain slice containing the DRN was taken under ice-cold ‘slicing' buffer (mM: KCl 2.5; NaHCO3 26; MgCl2 5; NaH2PO4 1.2; CaCl2 0.1; sucrose 189; glucose 10). The slice was allowed to recover in oxygenated artificial CSF (mM: a.CSF: NaCl 120; KCl 2.5; NaHCO3 26; NaH2PO4 1.2; MgCl2 1.3; CaCl2 2.4; glucose 10) at room temperature for 60 min. The slice was then transferred to a brain slice chamber where it was perfused at 2.5 ml min−1 with oxygenated a.CSF at 32°C. A stimulating (bipolar tungsten, 100 μm tip diameter, 150 μm tip separation) and voltammetric (carbon fibre, 8 μm tip width, 100 μm tip length) electrode was placed in the ventral DRN at a depth of 100 μm. The voltammetric electrode tip was positioned between those of the stimulating electrode, just off linearity.
A triangular voltage waveform (−1.0 to +1.4 V, 1.5 cycles at a rate of 480 V s−1) was applied to the carbon fibre microelectrode (CFe). The voltammetric scan was applied at a frequency of 2 Hz and the current sampled at 525 mV. This signal was fed into a chart recorder and a CED 1401. Data captured with the CED 1401 was analysed using CED ‘Signal' software.
5-HT efflux was evoked by electrical stimulation (100 Hz, 10 mA, 0.1 ms, 20 pulses every 5 min, 190 ms duration). 5-HT efflux was taken as the peak signal attained following stimulation. Electrodes were calibrated with standard 5-HT solutions at the end of the experiment.
The slice was stimulated every 5 min and compounds were added when four consecutive stable 5-HT efflux events were obtained. Subsequent 5-HT efflux was then expressed as a percentage of this pre-compound level. Agonists and antagonists were perfused for at least 20 mins.
Materials
8-OH-DPAT, 5-CT (5-carboxamidotryptamine), 5-HT and WAY 100635 were supplied by RBI. SB-269970 ((R)-1-[(3-Hydroxyphenyl) sulfonyl]-2- [2- (4-methyl-1-piperidinyl) ethyl] pyrrolidine) and SB-224289 (2,3,6,7-tetrahydro-1′-methyl-5-{2′- methyl-4′- [(5- methyl-1,2,4- oxadiazole-3-yl) biphenyl-4 -yl] carbonyl} furo[2,3-f]indole-3-spiro-4′-piperidine oxalate) were synthesized at SmithKline Beecham Pharmaceuticals. All other chemicals were supplied by Sigma.
Results
[3H]-5-HT release from guinea-pig and rat cortex
The 5-HT7 receptor antagonist, SB-269970 (10 nM – 1 μM), had no effect on [3H]-5-HT release in tissue from either species while the 5-HT1B receptor antagonist, SB-224289, significantly potentiated the evoked release of [3H]-5-HT to a maximum of 138 and 168% at 1 μM in rat and guinea-pig respectively (P<0.05; Figure 1). The non-selective 5-HT receptor agonist, 5-CT, inhibited the evoked release of [3H]-5-HT in a concentration-dependent manner, with a maximum inhibition of 60±4% (n=8) and 61±4% at 100 nM in rat and guinea-pig respectively (Figures 1 and 2). At a lower stimulation frequency of 1 Hz, to maximize agonist response and minimize antagonist potentiation, SB-269970 was unable to attenuate the 5-CT inhibition of release whereas SB-224289 produced a rightward shift of the 5-CT concentration curve, generating an estimated pKB of 7.83±0.06 (n=3, Figure 2A) and 7.60±0.06 (n=3, Figure 2B) for guinea-pig and rat tissue respectively, consistent with 5-HT1B receptor blockade.
Figure 1.
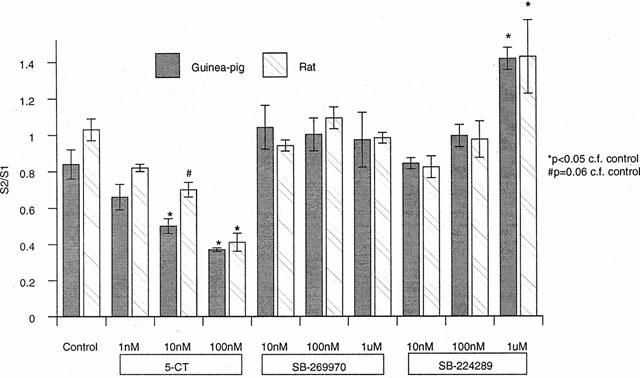
Effect of 5-CT, SB-269970 and SB-224289 on electrically evoked [3H]-5-HT release from guinea-pig and rat cortical slices. Data are expressed as an S2/S1 ratio±s.e.mean and are the mean of at least three determinations. Statistical analysis was by one-way ANOVA, followed by a post-hoc t-test (least squares difference).
Figure 2.
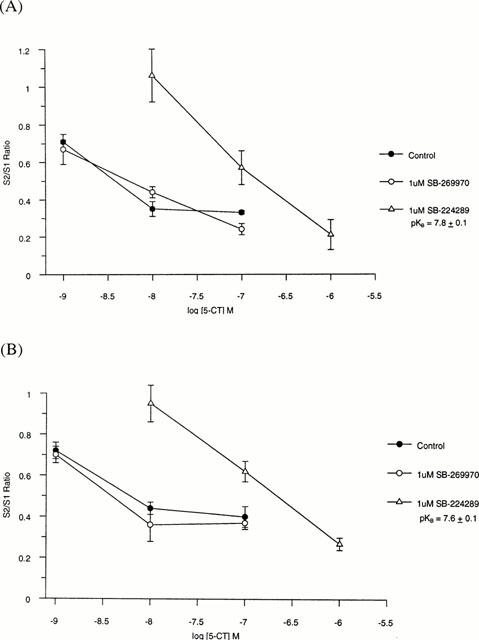
Inhibition of electrically evoked [3H]-5-HT release from (A) guinea-pig or (B) rat cortex with 5-CT, or 5-CT in the presence of 1 μM SB-269970 or 1 μM SB-224289. Data are expressed as an S2/S1 ratio±s.e.mean and are the mean of at least three determinations.
5-HT efflux from rat DRN
Control stimulations of 20 pulses every 5 min (100 Hz, 10 mA, 0.1 ms width) generated a peak 5-HT efflux of 22±2 nM (n=16). The mixed 5-HT receptor agonist, 8-OH-DPAT (100 nM), inhibited 5-HT efflux to a minimum of 40% of control (Figure 3). Neither the 5-HT1A receptor antagonist, WAY 100635 (100 nM), nor the 5-HT7 receptor antagonist, SB-269970 (1 μM), had any effect on 5-HT efflux when superfused alone (Figure 3). SB-269970 (1 μM) was unable to attenuate the 8-OH-DPAT effect whereas WAY 100635 (100 nM) fully reversed the 8-OH-DPAT-induced inhibition of stimulated 5-HT efflux (Figure 4).
Figure 3.
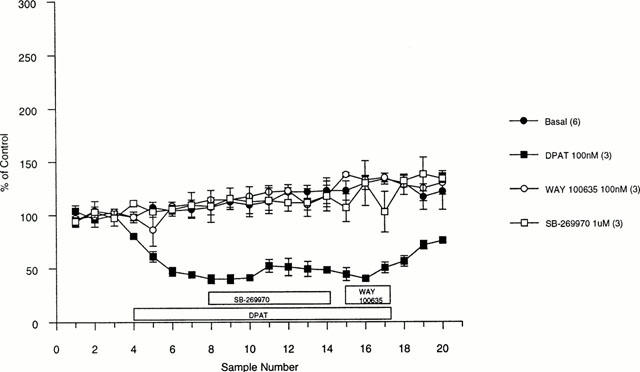
Effect of 100 nM DPAT, 100 nM WAY 100635 and 1 μM SB-269970 on electrically evoked 5-HT efflux from rat DRN as measured by fast cyclic voltammetry. Data are expressed as a per cent of control±s.e.mean and are the mean of at least three determinations.
Figure 4.
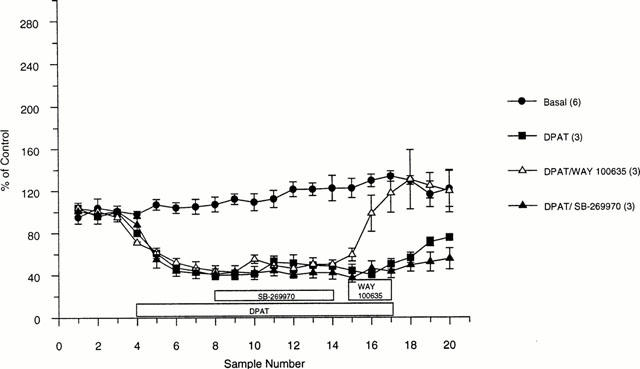
Effect of 1 μM SB-269970 and 100 nM WAY 100635 on the 8-OH-DPAT-induced inhibition of 5-HT efflux from rat DRN as measured by fast cyclic voltammetry. Data are expressed as a per cent of control±s.e.mean and are the mean of at least three determinations.
5-CT (1 nM – 100 nM) also inhibited 5-HT efflux in a concentration dependent manner (Figure 5). SB-269970 (1 μM) had no significant effects on the 5-CT inhibition of 5-HT efflux (Figure 5).
Figure 5.
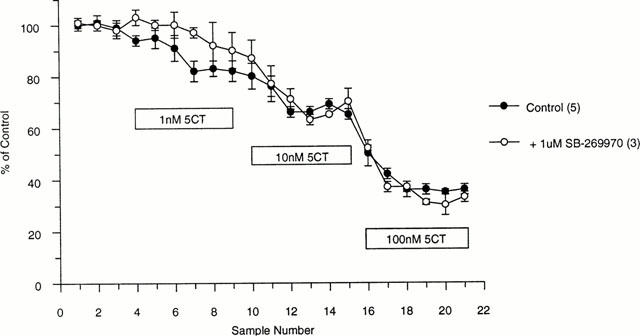
Effect of 5-CT on 5-HT efflux from the rat DRN in the presence or absence of SB-269970 (1 μM), as measured by fast cyclic voltammetry. Data are expressed as a per cent of control±s.e.mean and are the mean of at least three determinations.
Discussion
The 5-HT7 receptor is the most recently identified member of the family of G-protein-coupled 5-HT receptors. Until recently investigations into the possible role of this receptor has relied on the use of non-selective ligands such as 5-CT, 8-OH-DPAT, risperidone, methiothepin and clozapine but the discovery of the first selective 5-HT7 receptor antagonist, SB-266970 (Thomas et al., 1998; 1999; Hagan et al., 2000), has been an important first step in determining the biological function of the 5-HT7 receptor subtype. Hypotheses for a possible role for the 5-HT7 receptor have been based on the distribution of the receptor mRNA and protein. In situ hybridization, immunohistochemistry and autoradiography have all concluded that 5-HT7 receptors are present on serotonergic neurones in the CNS. Therefore, this raises the possibility for a role of 5-HT7 receptors as autoreceptors, controlling 5-HT release either through a direct action on serotonergic terminals and somatodendritic regions or through inhibition of serotonergic cell firing. In this present study we investigated these possibilities at both the serotonergic terminal and cell body regions, using the techniques of in vitro [3H]-5-HT release and fast cyclic voltammetry respectively.
The technique of in vitro [3H]-5-HT release studies the effects at serotonergic terminal autoreceptors. Using this method, the 5-HT agonist, 5-CT, decreased the evoked release of [3H]-5-HT from cortical slices through activation of a 5-HT autoreceptor (Roberts et al., 1996). The magnitude of this effect was stimulation frequency dependent: 5-CT inhibition of release was greater at 1 Hz than 3 Hz. This was probably a reflection of the biophase concentration of endogenous 5-HT; higher frequencies of stimulation results in a greater endogenous 5-HT tone on the system at hence a smaller inhibition from exogenously applied 5-HT receptor agonists.
In the present study, the selective 5-HT1B receptor antagonist, SB-224289, increased the evoked release of 5-HT through antagonism of endogenous 5-HT at the autoreceptor (see also Selkirk et al., 1998) whereas the 5-HT7 receptor antagonist, SB-269970, had no effect. SB-224289 compound also attenuated the 5-CT response, shifting the concentration response curve to the right. The calculated pKB values of 7.8 and 7.6, for guinea-pig and rat respectively, were consistent with competitive antagonism of 5-HT1B receptors (Selkirk et al., 1998). SB-269970 had no effect on the 5-CT-induced inhibition of release. Therefore, although the 5-HT7 receptor is present in the cortex, these data indicate that the serotonergic terminal autoreceptor is of the 5-HT1B receptor subtype and there is no evidence for an autoreceptor function for the 5-HT7 receptor. Thus cortical 5-HT7 receptors are unlikely to be present on the terminals of serotonergic projections and are presumably located postsynaptically on cortical neurones.
This study was then extended to investigate effects of 5-HT receptor agonists and antagonists at serotonergic autoreceptors located in the cell body region, the DRN, the brain region responsible for the serotonergic innervation of the frontal cortex (Kosofsky & Molliver, 1987), using the technique of fast cyclic voltammetry. The 5-HT agonists, 5-CT and 8-OH-DPAT, both inhibited 5-HT efflux through activation of the cell body autoreceptors.
The 8-OH-DPAT-induced inhibition of 5-HT efflux was fully reversed by WAY 100635, a selective 5-HT1A receptor antagonist, confirming that the 5-HT1A receptor regulates 5-HT release at the cell body (Davidson & Stamford, 1995; Starkey & Skingle, 1995). WAY 100635 had no effect per se, which may indicate either a low level of endogenous 5-HT or that the effect of 5-HT1A receptor antagonism may be limited by the presence of additional autoreceptors e.g. 5-HT1B or 5-HT1D (Roberts et al., 1998). That is, endogenous 5-HT may act as an agonist at 5-HT1B or 5-HT1D receptors, to negatively feedback and reduce the 5-HT level.
SB-269970 had no effect on basal 5-HT efflux and, in contrast to WAY 100635, was unable to significantly reverse either the 5-CT or 8-OH-DPAT inhibition of 5-HT efflux. These data imply that the 5-HT7 receptor does not function to regulate 5-HT release in this cell body region.
To conclude, we were unable to provide evidence for the existence of 5-HT7 serotonergic autoreceptors in either the terminal or cell body regions. However, the location of 5-HT7 receptors in regions known to contain serotonergic autoreceptors raises the question of whether they serve to modulate the action of 5-HT1A, 5-HT1B or 5-HT1D autoreceptors. Recently Thomas et al. (1999) demonstrated an interaction of 5-HT1A and 5-HT7 receptors in the hippocampus. Both receptors stimulate adenylyl cyclase in the hippocampus but the data suggested that the 5-HT1A effect was indirect, that is, 5-HT1A receptors do not directly stimulate adenylyl cyclase but serve to augment the 5-HT7-mediated stimulation. In serotonergic neurones the converse may be true, 5-HT7 receptors may play an indirect role through other 5-HT autoreceptors. However, such studies are not possible with the currently available, non-selective 5-HT receptor agonists.
Abbreviations
- a. CSF
artificial cerebrospinal fluid
- CFe
carbon fibre electrode
- DRN
dorsal raphe nucleus
- FR
fractional release
References
- ATKINSON P.J., THOMAS D.R., HAGAN J.J., MIDDLEMISS D.N., PRICE G.W. [3H]-SB-269970 selectively radiolabels 5-HT7 receptors in mouse, rat and pig brain membranes. Br. J. Pharmacol. 2000;129:132P. [Google Scholar]
- BARD J.A., ZGOMBICK J., ADHAM N., VAYSSE P., BRANCHEK T.A., WEINSHANK R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;269:23422–23426. [PubMed] [Google Scholar]
- BOYLAND P.S., EASTWOOD S., ELLIS C., BERGSMA D., JONES B.J., GLOGER I.S., UPTON N., MIDDLEMISS D.N. High specific activity of [3H]-5-CT binding: correlation of guinea-pig cortex with human cloned 5-HT7 receptors. Br. J. Pharmacol. 1996;117:132P. [Google Scholar]
- DAVIDSON C., STAMFORD J.A. Evidence that 5-HT release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br. J. Pharmacol. 1995;114:1107–1109. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSON E.L., DURKIN M.M., BARD J.A., ZGOMBICK J., BRANCHEK T.A. A receptor autoradiographic and in situ hybridisation analysis of the distribution of the 5-HT7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN J.J., PRICE G.W., JEFFREY P., DEEKS N.J., STEAN T., PIPER D., SMITH M.I., UPTON N., MEDHURST A.D., MIDDLEMISS D.N., RILEY G.J., LOVELL P.J., BROMIDGE S.M., THOMAS D.R. Characterisation of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIDMANN D.E.A., METCALF M.A., KOHEN R., HAMBLIN M.W. Four 5-HT7 receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organisation. J. Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- HEIDMANN D.E.A., SZOT P., KOHLEN R., HAMBLIN M.W. Function and distribution of three rat 5-HT7 receptor isoforms produced by alternative splicing. Neuropharmacol. 1998;37:1621–1632. doi: 10.1016/s0028-3908(98)00070-7. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA F.R., HUMPHREY P.P.A. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–243. [PubMed] [Google Scholar]
- JASPER J.R., KOSAKA A., TO Z.P., CHANG D.J., EGLEN R.M. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7(b)) Br. J. Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSOFSKY B.E., MOLLIVER M.E. The serotonergic innervation of cerebral cortex: different classes of axon terminal arise from dorsal and median raphe nuclei. Synapse. 1987;1:153–168. doi: 10.1002/syn.890010204. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., BARON B.M., DE LECEA L., MILLER J.D., PROSSER R.A., REA M.A., FOYE P.E., RACKE M., SLONE A.L., SIEGEL B.W., DANIELSON P.E., SUTCLIFFE J.G., ERLANDER M.G. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- LUCAS J., HEN R. New players in the 5-HT receptor field: genes and knockouts. T.I.P.S. 1995;16:246–252. doi: 10.1016/s0165-6147(00)89034-3. [DOI] [PubMed] [Google Scholar]
- MARTIN G.G., HUMPHREY P.P.A. Classification review: receptors for 5-hydroxytryptamine: current perspectives on classification and nomenclature. Neuropharmacol. 1994;33:261–273. doi: 10.1016/0028-3908(94)90058-2. [DOI] [PubMed] [Google Scholar]
- MEYERHOF W., OBERMULLER F., FEHR S., RICHTER D. A novel rat serotonin receptor: primary structure, pharmacology and expression pattern in distinct brain regions. DNA Cell Biol. 1993;12:401–416. doi: 10.1089/dna.1993.12.401. [DOI] [PubMed] [Google Scholar]
- OLIVER K.R., KINSEY A.M., WAINWRIGHT G., MCALLISTER G., SIRINATHSINGHJI D. Localisation of 5-HT7 and 5-HT5A receptor immunoreactivity in the rat brain. British Neurosci. Assoc. Abstr. 1999;15:79. [Google Scholar]
- PLASSAT J.-L., AMLAIKY N., HEN R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- ROBERTS C., WATSON J., BURTON M., PRICE G.W., JONES B.J. Functional characterisation of the 5-HT terminal autoreceptor in the guinea-pig cortex. Br. J. Pharmacol. 1996;117:384–388. doi: 10.1111/j.1476-5381.1996.tb15203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS C., BELENGUER A., MIDDLEMISS D.N., ROUTLEDGE C. Differential effects of 5-HT1B/1D receptor antagonists in dorsal and median raphe innervated brain regions. Eur. J. Pharmacol. 1998;346:175–180. doi: 10.1016/s0014-2999(98)00061-2. [DOI] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACONBE J., DIAZ J., ARRANG J-M., SCHWARTZ J-C. Molecular cloning, characterisation and localisation of a high affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELKIRK J.V., SCOTT C., HO M., BURTON M.J., WATSON J., GASTER L.M., COLLIN L., JONES B.J., MIDDLEMISS D.N., PRICE G.W. SB-224289 - a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br. J. Pharmacol. 1998;125:202–208. doi: 10.1038/sj.bjp.0702059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J., METCALF M.A., JOSE P.A., HAMBLIN M.W., SIBLEY D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- STARKEY S.J., SKINGLE M. 5-HT1D as well as 5-HT1A autoreceptors modulate 5-HT release in the guinea-pig dorsal raphe nucleus. Neuropharmacol. 1995;33:393–402. doi: 10.1016/0028-3908(94)90069-8. [DOI] [PubMed] [Google Scholar]
- THOMAS D.R., GITTINS S.A., COLLIN L.L., MIDDLEMISS D.N., RILEY G., HAGAN J., GLOGER I., ELLIS C.E., FORBES I.T., BROWN A.M. Functional characterisation of the human cloned 5-HT7 receptor (long form) - antagonist profile of SB-258719. Br. J. Pharmacol. 1998;124:1300–1306. doi: 10.1038/sj.bjp.0701946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS D.R., MIDDLEMISS D.N., TAYLOR S.G., NELSON P., BROWN A.M. 5-CT stimulation of adenylyl cyclase activity in guinea-pig hippocampus: evidence for involvement of 5-HT7 and 5-HT1A receptors. Br. J. Pharmacol. 1999;128:158–164. doi: 10.1038/sj.bjp.0702759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS D.R., ATKINSON P.J., HO M., BROMIDGE S.M., LOVELL P.J., VILLANI A.J., HAGAN J.J., MIDDLEMISS D.N., PRICE G.W. [3H]-SB-269970- a selective antagonist radioligand for 5-HT7 receeptors. Br. J. Pharmacol. 2000;130:409–417. doi: 10.1038/sj.bjp.0703318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TO Z.P., BONHAUS D.W., EGLEN R.M., JAKEMAN L.B. Characterisation and distribution of putative 5-HT7 receptors in guinea-pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSOU A.P., KOSADA A., BACH C., ZUPPAN P., YEE C., TOM L., ALVAREZ R., RAMSEY S., BONHAUS D.W., STEINFANICH E., JAKEMAN L., EGLEN R.M., CHAN H.W. Cloning and expression of a 5-hydroxytryptamine7 receptor positively linked to adenylyl cyclase. J. Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- WAEBER C., MOSKOWITZ M.A. Autoradiographic visualisation of [3H]5-carboxamidotryptamine binding sites in the guinea-pig and rat brain. Eur. J. Pharmacol. 1995;283:31–46. doi: 10.1016/0014-2999(95)00275-p. [DOI] [PubMed] [Google Scholar]


