Abstract
These experiments determined whether the housing conditions of rats influenced the effects of nicotine in two animal tests of anxiety, social interaction and elevated plus-maze tests.
In animals housed singly for 7 days, (−)nicotine (0.025 mg kg−1 s.c.) was ineffective, but 0.05, 0.1 and 0.25 mg kg−1 (s.c.) significantly increased the time spent in social interaction, without changing locomotor activity, thus indicating anxiolytic actions. (−)Nicotine (0.45 mg kg−1 s.c.) significantly reduced social interaction, indicating an anxiogenic effect.
However, in group-housed animals, (−)nicotine (0.025 mg kg−1 s.c.) had a significant anxiolytic effect in the social interaction test, but 0.01, 0.05, 0.1, 0.25 and 0.45 mg kg−1 were ineffective. (−)Nicotine (1 mg kg−1) reduced motor activity and social interaction in the group-housed animals.
In the elevated plus-maze, the time-course and the dose-response curve to nicotine were investigated. In both singly- and group-housed rats, (−) nicotine (0.1 – 0.45 mg kg−1 s.c.) decreased the per cent entries into, and per cent time spent on, the open arms, indicating anxiogenic effects.
The housing condition influenced the time course, with significant effects at 5 and 30 min after injection in group-housed rats, and significant effects at 30 and 60 min in singly-housed rats.
In the social interaction test there was no difference in the scores of the first and last rats removed from group cages, whereas the order of removal from the cages did affect the scores in the elevated plus-maze.
These results provide further evidence that the two animal tests model distinct states of anxiety, and show how social isolation powerfully modifies both anxiolytic and anxiogenic effects of nicotine.
Keywords: Nicotine, anxiety, locomotor activity, housing, social isolation, social interaction test, elevated plus-maze
Introduction
Calming and anxiety-reducing effects are frequently cited by smokers as reasons for their smoking, and this is particularly prevalent amongst teenage girls (Royal College of Physicians, 2000). However, some have queried whether nicotine really does have anxiolytic effects, and there is evidence that under some circumstances nicotine can actually increase stress and anxiety (Netter et al., 1998; Newhouse et al., 1990; Parrott et al., 1996). The data from animal studies suggest that even following acute administration, nicotine has complex effects on anxiety.
Anxiolytic effects of acute administration of nicotine have been reported in several experimental models of anxiety in both mice (Cao et al., 1993; Brioni et al., 1993), and rats (Brioni et al., 1994; Vale & Green, 1986), but these have been restricted to a narrow dose range. In the social interaction test of anxiety, it has been shown that the effects of nicotine in rats are dose-dependent, with low doses having anxiolytic and high doses anxiogenic effects (File et al., 1998). The effects are also dependent on the time between injection and testing and Irvine et al. (1999) found that nicotine (0.1 mg kg−1 s.c.) had an anxiogenic effect 5 and 60 min after administration, but an anxiolytic effect at 30 min. Both anxiolytic and anxiogenic effects have also been found in rats tested in the elevated plus-maze. However, it is less clear in this test that the effects are dependent on dose. Ouagazzal et al. (1999a) found low doses (0.001 – 0.1 mg kg−1 i.p) were ineffective, but high doses had an anxiogenic effect (0.5 – 1 mg kg−1 i.p.). In contrast, Brioni et al. (1994) reported an anxiolytic action with 0.3 mg kg−1 nicotine. It is possible that this discrepancy can be explained by intrinsic differences between the rat strains that were used in these two studies. It is also possible that differences in the housing conditions can explain some of the discrepancies in the literature, especially between the findings in rats and mice. Anxiolytic effects have been universally reported in mice, where group housing has always been used (Cao et al., 1993; Costall et al., 1989; Brioni et al., 1993), whereas anxiogenic effects have tended to dominate in rat studies which have used single housing.
Social isolation has been shown to modify the effects of acutely administered drugs. In the elevated plus-maze, an anxiogenic profile and a greater sensitivity to the anxiolytic effects of diazepam was seen in rats that were reared in isolation from weaning (Fone et al., 1996; Morinan & Parker, 1985; Wright et al., 1991; Lopes da Silva et al., 1996). Isolation-reared rats have also been found to be more sensitive to the effects of amphetamine, cocaine and ethanol (Jones et al., 1990; Smith et al., 1997; Fowler et al., 1993; Hall et al., 1998). The effects of a short period of isolation housing in adult rats have not been extensively investigated. Singly-housed rats showed greater locomotor stimulation in response to amphetamine (Ahmed et al., 1995) and enhanced chloride uptake in response to GABA and flunitrazepam (Thielen et al., 1993). In contrast, pair-housed adult rats were more sensitive than singly-housed rats to the anxiolytic effects of diazepam in the social interaction test of anxiety (Gardner & Guy, 1984). The purpose of the present experiments was therefore to determine whether the housing conditions of adult rats would modify the effects of nicotine in two animal tests of anxiety, the social interaction test and the elevated plus-maze. The time-course of nicotine's effects on the plus-maze was also examined in the isolated and socially grouped animals, because this has not been previously studied. The times selected were based on those previously investigated in the social interaction test (Irvine et al., 1999). The dose-range of nicotine was selected on the basis of the doses previously found effective in these two tests (File et al., 1998; Ouagazzal et al., 1999a).
Methods
Animals
A total of 447 male Lister hooded rats (Charles River, Margate, Kent, U.K.) weighing between 250 – 300 g were used. Rats were housed either in isolation or in social groups of five for 7 days prior to the start of behavioural testing. The duration of isolation housing was chosen to be the same as that used in previous studies (File et al., 1998; Ouagazzal et al., 1999a). Isolation housed rats were housed singly in a cage, 45 cm×28 cm×20 cm high. The group housed cages were 56 cm×38 cm×20 cm high. All the cages were in racks that allowed the rats to see, hear and smell other rats. Food and water were freely available to all the animals. The room in which animals were housed was lit with dim light and maintained at 22°C. Lights were on from 0700 – 1900. The experimental procedures carried out in this study were in compliance with the U.K. Animals (Scientific Procedures) Act 1986 (Home Office Project License Number 70/4041).
Apparatus
The social interaction test arena was a wooden box 60×60 cm, with 35 cm walls, and was lit by high light (300 lux). A camera was mounted vertically above the arena and the rats were observed on a monitor in an adjacent room. The time spent in social interaction (sniffing, following, grooming the partner, boxing and wrestling) provided the measure of anxiety and was scored by an observer who was blind to the drug treatment. The interruption of infrared beams from photocells mounted in the walls 3.5 cm from the floor provided an automated measure of locomotor activity (for details see File & Hyde, 1978; File, 1980, 1997).
The elevated plus-maze was made of wood and consisted of two opposite open arms 50×10 cm, and two opposite equal sized arms enclosed by 40 cm high walls. The arms were connected by a central 10×10 cm square, and thus the maze formed a ‘plus' shape. The maze was elevated 50 cm from the floor and lit by dim light. A closed-circuit TV camera was mounted vertically over the maze, and the behaviour was scored from a monitor in an adjacent room. All scores were entered directly into an IBM computer. The percentage of time spent, and the percentage of entries onto the open arms of the maze provide the measure of anxiety, and the number of closed arm entries provides the best measure of locomotor activity in this test (Pellow et al., 1985; File, 1992; Fernandes & File, 1996).
Drug
(−)-Nicotine hydrogen tartrate (Sigma, Poole, U.K.) was dissolved in distilled water, and doses of nicotine were calculated as the base. All drug injections were given subcutaneously (s.c.), in a volume of 1 ml kg−1 body weight. Control animals received equal volume injections of distilled water.
Procedure
Social interaction
In the social interaction test, the light level and familiarity of the test arena can be varied in order to modify the level of anxiety generated by the test. A moderate level of anxiety is generated by testing in a brightly lit arena with which rats have been familiarized. This is the test condition selected for this experiment, since it had proved sensitive to both the anxiolytic and anxiogenic effects of nicotine (File et al., 1998; Irvine et al., 1999). Therefore, each rat was placed individually in the test arena for a 5 min familiarization trial on the day prior to the social interaction test. Rats were allocated to test partners on the basis of weight, so they did not differ by more than 10 g. For the group-housed rats, the test partner was always taken from a different cage. After testing, the rats were returned to their home cages, so that group-housed animals always remained in a group of at least four. In the main study, 48 pairs of singly-housed rats and 42 pairs of group-housed rats were randomly allocated among the six drug groups (vehicle or (−) nicotine 0.025, 0.05, 0.1, 0.25 or 0.45 mg kg−1). On the basis of the results, a further 21 pairs of animals were housed in social groups for 7 days and then tested with vehicle or (−) nicotine (0.01 or 1 mg kg−1 s.c.). In all experiments, both members of a test pair received the same dose of nicotine on the test day. On the test days, 30 min after injection, pairs of rats were placed in the centre of the arena and their behaviour scored for 4.5 min from a monitor in the adjacent room, by an observer with no knowledge of the drug treatment. The scores that were analysed were the total times spent interacting by each pair of rats (i.e. a single score for each pair of rats). This is because the behaviour of one rat cannot be considered to be independent of its partner's behaviour. At the end of the trial any faeces were removed and the arena wiped with a damp cloth. Rats were tested between 0900 – 1300 h.
Elevated plus-maze
For the plus-maze experiments, 119 singly-housed rats and 106 group-housed rats were randomly allocated among the five drug groups (vehicle or nicotine 0.05, 0.1, 0.25 or 0.45 mg kg−1 s.c.). On test days, the rats receiving each drug treatment were further sub-divided into three groups, and were tested either 5, 30 or 60 min after their injection. Each rat was placed individually in the central square of the plus maze facing the closed arm, and its behaviour was scored for 5 min by an observer blind to the drug treatment. The number of entries onto the open and closed arms, and the time spent on the open arms, closed arms and in the central square was scored. Testing took place between 0900 and 1300 h in an order randomized for drug treatment. The arena was wiped with a damp tissue after each trial. After testing each rat was returned to its home cage.
Statistics
Data for the dose-response to nicotine in the social interaction test were analysed by a two-factor parametric analysis of variance (Factor 1, housing; Factor 2, drug treatment). A significant housing×drug interaction would show that the housing condition significantly influenced the drug effect. The data for the additional doses investigated only in the group-housed rats were analysed in a one-way analysis of variance. The plus-maze data were analysed by a three-factor analysis of variance (Factor 1, housing; Factor 2, drug treatment; Factor 3, pre-treatment time.) After the analyses of variance, comparisons between individual groups were made with Fisher's post-hoc tests and it is the significances of these that are shown in the figures and tables. A subsidiary analysis was conducted in order to determine whether the order of removal from the group influenced the scores in the two tests. The scores of the first and last rats to be removed from each cage were compared in all the groups in which nicotine had no effect. Thus, in the social interaction test the comparison included all groups, except for 0.025 mg kg−1 nicotine, and in the plus-maze the comparison included all the groups tested at the 60 min time-point.
Results
Social interaction
Overall, the group-housed rats spent significantly less time in social interaction than did those that were singly housed [F(1,78)=188.6, P<0.00001]. There were dose-dependent effects of nicotine, but these varied significantly in the two housing conditions [housing×drug interaction, F(5,78)=18.7, P<0.00001]. In the singly-housed rats, nicotine (0.05, 0.1, 0.25 mg kg−1) significantly increased social interaction, whereas 0.45 mg kg−1 significantly decreased it, see Figure 1. In comparison, in the group-housed rats, only 0.025 mg kg−1 nicotine significantly increased social interaction, see Figure 1. In the group-housed rats, the lowest dose of nicotine (0.01 mg kg−1) non-significantly increased, and the highest dose (1 mg kg−1) significantly decreased, social interaction (see Table 1).
Figure 1.
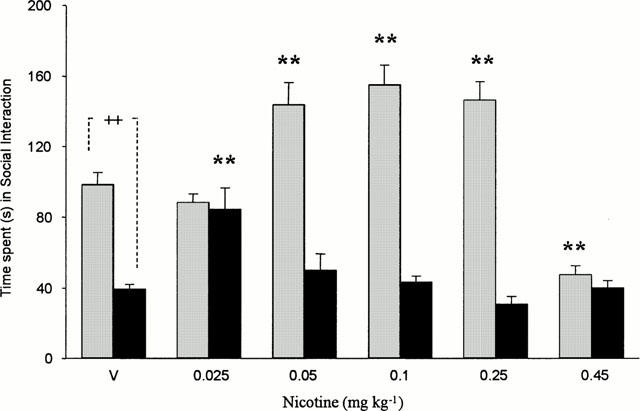
Mean (±s.e.mean) time(s) spent in social interaction by rats housed singly (hatched bars) or in social groups of five (black bars) for 7 days and then tested 30 min after injection with vehicle or (−) nicotine (0.025 – 0.45 mg kg−1 s.c.). **P<0.01 compared with appropriate vehicle control group. ++P<0.01 comparing vehicle-injected singly and group housed animals.
Table 1.
Social interaction and locomotor activity of group-housed rats
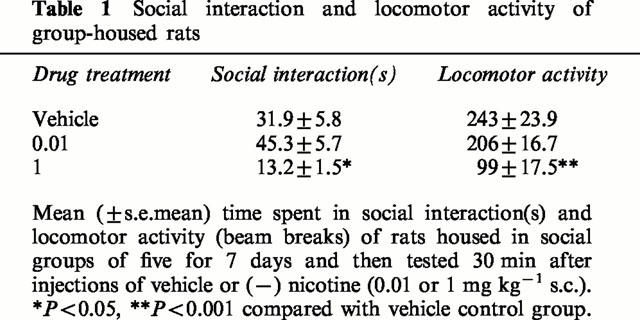
Overall, the group-housed rats had lower locomotor activity than the singly-housed rats (Table 2: F(1,78)=7.5, P<0.01). Nicotine produced a dose-related decrease in locomotor activity (F(5,78)=2.8, P<0.05), that reached significance in both housing conditions at the 0.45 mg kg−1 dose and in the singly housed rats also at the 0.25 mg kg−1 dose (Table 2). There was no significant housing×nicotine interaction on locomotor activity (F<1.0).
Table 2.
Locomotor activity of singly and group-housed rats
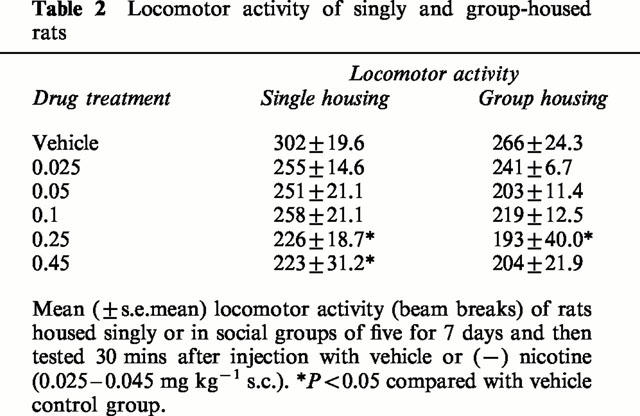
Elevated plus-maze
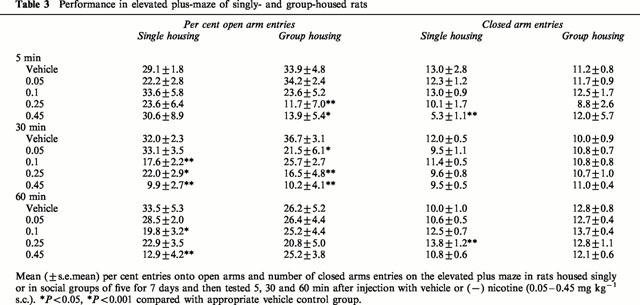
In the elevated plus-maze there was no overall effect of the housing conditions on any of the measures (F<1.4 in all cases). However, the effects of nicotine on the measures of anxiety depended on both the housing conditions and the pre-treatment time (drug×housing×pre-treatment time, F(8,195)=2.4, P<0.05 for per cent time on open arms and F(8,195)=2.6, P<0.01 for per cent open arm entries). It can be seen from Figure 2 and Table 3 that in both singly- and group-housed rats the doses 0.1 – 0.45 mg kg−1 nicotine significantly decreased the percentage of time spent on the open arms and the percentage of open arm entries. However, these anxiogenic effects of nicotine were time-related and in the group housed animals were more evident at the 5 and 30 min times of testing, whereas in the singly-housed animals they were significant at 30 and 60 min. Neither nicotine nor the housing conditions significantly affected the number of closed arm entries (see Table 3) and therefore nicotine had a specific anxiogenic effect in this test.
Figure 2.
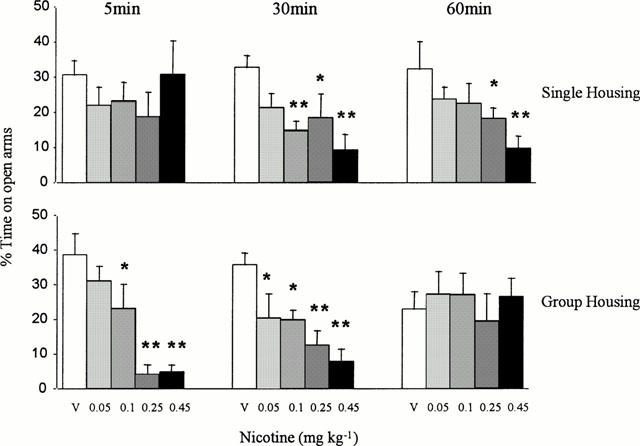
Mean (±s.e.m.) per cent time spent on the open arms of the elevated plus maze by rats housed singly or in social groups of five for 7 days and then tested 5, 30 or 60 min after injection with vehicle or (−) nicotine (0.05 – 0.45 mg kg−1). *P<0.05, **P<0.001 compared with appropriate vehicle control group.
Table 3.
Performance in elevated plus-maze of singly- and group-housed rats
Effect of order of removal from the group
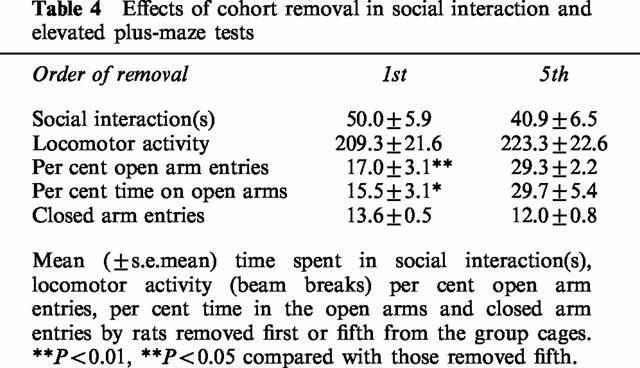
It can be seen from Table 4 that there was no difference in the time spent in social interaction or in the locomotor scores between the rats removed first from each cage and those removed last. However, in the elevated plus-maze the rats removed first showed an anxiogenic effect, indicated by decreased per cent of open arm entries (F(1,13)=9.8, P<0.01) and per cent time in the open arms (F(1,13)=5.5, P<0.05), see Table 4. There was no effect on the number of closed arm entries.
Table 4.
Effects of cohort removal in social interaction and elevated plus-maze tests
Discussion
The increased social interaction in the singly-housed rats confirmed earlier findings on the effects of social isolation in adulthood (File & Pope, 1974; Niesink & Van Ree, 1982; Varlinskaya et al., 1999), and since weaning (Wongwitdecha & Marsden, 1996). The baseline scores of the singly-housed rats were very similar to those found in previous studies (File et al., 1998; Irvine et al., 1999). This increased interaction is the main reason for routinely using singly-housed rats in this test. However, this test has been used in group-housed rats (Jones et al., 1988; Dunn et al., 1991; Costall et al., 1993), and it is possible to detect anxiolytic effects of benzodiazepines in both housing conditions (File & Hyde, 1978; File, 1980; Dunn et al., 1991; Gardner & Guy, 1984). The present experiment showed that an anxiolytic effect of nicotine can be detected in both group- and singly-housed rats, but it was manifested at a much wider dose-range in the singly-housed rats. This is perhaps surprising, since in general it is easier to detect increases in behaviour when baseline scores are low (e.g. Crawley & Davis, 1982). Thus, it would seem to be the stress, or some other effect, of social isolation, rather than the baseline scores per se, that was enhancing the anxiolytic effects of nicotine in this test. Interestingly, the anxiogenic effects of nicotine were also enhanced in the isolated rats, which perhaps argues against an interpretation simply in terms of social isolation acting as a stressor.
Our results also showed that short-term social isolation in adult rats enhanced locomotor activity, as has been shown previously for rats reared in social isolation (Smith et al., 1997; Hall et al., 1998). This raises the possibility that the increased activity is a response to the current housing conditions and not necessarily a consequence of the long-term effects of isolation rearing. However, the current housing conditions did not modify nicotine's effects on locomotor activity, whereas isolation since weaning did enhance the locomotor stimulant effects of amphetamine and ethanol (Smith et al., 1997; Hall et al., 1998).
In contrast to the social interaction test, the housing conditions did not modify behaviour in the elevated plus-maze, as has been previously found in mice (Rodgers & Cole, 1993). The baseline scores of the singly housed rats were very similar to those found in previous experiments (Ouagazzal et al., 1999a). These results are also in general agreement with previous studies in which other test condition manipulations, such as extra-maze cues and lighting, were found not to influence baseline scores (Pellow et al., 1985; Baldwin & File, 1986; Falter et al., 1992; Becker & Grecksh, 1996; Rodgers et al., 1997). It is therefore even more striking that the order of removal from the group did seem to affect behaviour, with the rats that were removed first having scores in the plus-maze that indicated increased anxiety. It is possible that the effects we observed were caused, not by cohort removal, but by the initial disturbance to the cage, to which the rats habituated after several disturbances. Further studies are clearly needed in this area. Another contrast between the two tests of anxiety was that the housing conditions did not influence the dose-response to nicotine in the plus-maze and only anxiogenic effects were detected in this test. The housing conditions did, however, alter the time at which nicotine's effects were detected, with the effects being delayed in the singly-housed rats. Previously, rats reared in isolation have shown an altered time course of amphetamine-induced locomotor activity (Jones et al., 1992). The shift in the time course in the present study does not appear to be dependent on baseline differences between singly- and group-housed rats. There is convincing evidence that the anxiogenic effects of nicotine in the elevated plus-maze are primarily mediated by 5-HT1A serotonergic receptors in the lateral septum (Cheeta et al., 2000). The present results therefore indicate possible alteration of serotonergic dependent function of the lateral septum following isolation housing of rats. However, it is also possible that isolation housing altered the pharmacokinetic effects of nicotine, and that the later onset of nicotine's anxiogenic effect was due to changes in nicotine absorption and metabolism, although as yet this still has to be determined.
Several factor analysis studies have provided evidence that different animal tests of anxiety are reflecting different underlying factors, and hence may be modelling different anxiety disorders (File, 1992; Belzung & Le Pape, 1994; Ramos et al., 1997; Flaherty et al., 1998). Inbred rat lines have showed different effects in different tests of anxiety (Chaouloff et al., 1994) and further differences between these tests have been shown in the effects of brain lesions and central drug administration (Menard & Treit, 1999). Differential effects have also been found following systemically administered drugs (Treit et al., 1993; Fernanez-Guasti et al., 1999) and after the stress of inescapable shock (Steenbergen et al., 1990).
We have found differences between the social interaction test and the elevated plus-maze in the effects of nicotine. Thus, in our strain of rat and test conditions, although low doses of nicotine were anxiolytic in the social interaction test, we have only ever found anxiogenic effects in the plus-maze (this experiment and File et al., 1998; Ouagazzal et al., 1999a). It is perhaps most striking that, in singly-housed rats, nicotine (0.1 mg kg−1 30 min after injection) had an anxiolytic action in the social interaction test, yet an anxiogenic effect in the plus-maze. (Kenny et al., 2000; Ouagazzal et al., 1999a). We failed to find any dose of nicotine that had an anxiolytic effect in the plus-maze after acute administration. The lowest dose investigated in the present study was 0.025 mg kg−1, but Ouagazzal et al. (1999a) also found no effects with doses as low as 0.01 mg kg−1. A further difference seems to lie in the brain regions mediating nicotine's anxiogenic effects in the two tests, which implies that different brain regions may be activated by the two tests. Thus, while both the dorsal hippocampus and the lateral septum mediate the anxiogenic effects of nicotine in the social interaction test, only the latter structure mediates effects in the plus-maze (Kenny et al., 2000; Ouagazzal et al., 1999a,1999b; Cheeta et al., 2000). In all cases the anxiogenic effects were antagonized by co-administration of the 5HT1A antagonist, WAY 100635 (Kenny et al., 2000; Cheeta et al., 2000). Since the group-housed rats did not show any anxiogenic effects to nicotine in the social interaction test, this implies that the housing conditions might have modified the nicotinic cholinergic and/or serotonergic systems in these brain regions.
In conclusion, our results showed that even a few days of social isolation in adulthood can profoundly affect baseline responses in tests of anxiety and responses to nicotine. Singly-housed rats have also shown greater physiological reactions to stress than did group-housed rats (Baldwin et al., 1995; Ruis et al., 1999). However, an increased stress response to the behavioural tests in an insufficient explanation for the pattern of results that we have found with nicotine.
Abbreviations
- GABA
γ-Aminobutyric acid
- 5-HT
5-Hydroxytryptamine
- i.p.
intraperitoneal
- s.c.
sub-cutaneous
References
- AHMED S.H., STINUS L., LE MOAL M., CADOR M. Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology. 1995;117:116–124. doi: 10.1007/BF02245106. [DOI] [PubMed] [Google Scholar]
- BALDWIN H.A., FILE S.E. The elevated plus-maze test of anxiety: Further behavioural validation. Psychopharmacology. 1986;89:S9. [Google Scholar]
- BALDWIN D.R., WILCOX Z.C., BAYLOSIS R.C. Impact of differential housing on humoral immunity following exposure to an acute stressor in rats. Physiol. Behav. 1995;57:649–653. doi: 10.1016/0031-9384(94)00313-0. [DOI] [PubMed] [Google Scholar]
- BECKER A., GRECKSCH G. Illumination has no effect on rats' behavior in the elevated plus-maze. Physiol. Behav. 1996;59:1175–1177. doi: 10.1016/0031-9384(95)02224-4. [DOI] [PubMed] [Google Scholar]
- BELZUNG C., LE PAPE G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol. Behav. 1994;56:623–628. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- BRIONI J.D., O'NEILL A.B., KIM D.J.B., BUCKLEY M.J., DECKER M.W., ARNERIC S.B. Anxiolytic-like effects of the novel cholinergic channel activator ABT-418. J. Pharmacol. Exp. Ther. 1994;271:353–361. [PubMed] [Google Scholar]
- BRIONI J.D., O'NEILL A.B., KIM D.J.B., DECKER M.W. Nicotinic receptor agonists exhibit anxiolytic-like effects on the elevated plus-maze test. Eur. J. Pharmacol. 1993;238:1–8. doi: 10.1016/0014-2999(93)90498-7. [DOI] [PubMed] [Google Scholar]
- CAO W., BURKHOLDER T., WILKINS L., COLLINS A.C. A genetic comparison of the behavioral actions of ethanol and nicotine in the mirrored chamber. Pharmacol. Biochem. Behav. 1993;45:803–809. doi: 10.1016/0091-3057(93)90124-c. [DOI] [PubMed] [Google Scholar]
- CHAOULOFF F., CASTANON N., MORMEDE P. Paradoxical differences in animal models of anxiety among the Roman rat lines. Neurosci. Lett. 1994;182:217–221. doi: 10.1016/0304-3940(94)90801-x. [DOI] [PubMed] [Google Scholar]
- CHEETA S., KENNY P.J., FILE S.E. The role of 5−HT1A receptors in mediating the anxiogenic effects of nicotine following lateral septal administration. Eur. J. Neurosci. 2000;12:3797–3802. doi: 10.1046/j.1460-9568.2000.00246.x. [DOI] [PubMed] [Google Scholar]
- COSTALL B., DOMENEY A.M., KELLY M.E., TOMKINS D.M., NAYLOR R.J., WONG E.H.F., SMITH W.L., WHITING R.L., EGLEN R.M. The effect of the 5-HT3 receptor antagonist, RS-42358-197, in animal models of anxiety. Eur. J. Pharmacol. 1993;234:91–99. doi: 10.1016/0014-2999(93)90710-y. [DOI] [PubMed] [Google Scholar]
- COSTALL B., KELLY M.E., NAYLOR R.J., ONAIVI E.S., WONG E.H., SMITH W.L., WHITING R.L., EGLIN R.M. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol. Biochem. Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- CRAWLEY J.N., DAVIS L.G. Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res. Bull. 1982;8:609–612. doi: 10.1016/0361-9230(82)90087-9. [DOI] [PubMed] [Google Scholar]
- DUNN R.W., CARLEAON W.A., CORBETT R. Preclinical anxiolytic versus antipsychotic profiles of the 5−HT3 antagonists ondansetron, zacopride, 3α-tropanyl-1H-indole-3-carboxylic acid ester, and 1αH, 3α, 5αH-tropan-3-yl-3,5-dichlorobenzoate. Drug Develop. Res. 1991;23:289–300. [Google Scholar]
- FALTER U., GOWER A.J., GOBERT J. Resistance of baseline activity in the elevated plus-maze to exogenous influences. Behav. Pharmacol. 1992;3:123–128. [PubMed] [Google Scholar]
- FERNANDES C., FILE S.E. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol. Biochem. Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-GUASTI A., MARTINEZ-MOTA L., ESTRADA-CAMARENAS E., CONTRERAS C.M., LOPEZ-RUBALCAVA C. Chronic treatment with desipramine induces an estrous cycle-dependent anxiolytic-like activity in the burying behavior, but not in the elevated plus-maze test. Pharmacol. Biochem. Behav. 1999;63:13–20. doi: 10.1016/s0091-3057(98)00231-7. [DOI] [PubMed] [Google Scholar]
- FILE S.E. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J. Neurosci. Meth. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- FILE S.E. Usefulness of animal models with newer anxiolytics. Clin. Neuropharmacol. 1992;15:525A–526A. doi: 10.1097/00002826-199201001-00273. [DOI] [PubMed] [Google Scholar]
- FILE S.E.Animal measures of anxiety Current Protocols in Neuroscience 1997New York: John Wiley & Sons Inc; 8.3.1–8.3.15.Ed. Crawley, J., Gerfen, C., McKay, R., Rogawski, M.A., Sibley, D. & Skolnick, P. pp [Google Scholar]
- FILE S.E., HYDE J.R. Can social interaction be used to measure anxiety. Br. J. Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILE S.E., KENNY P.J., OUAGAZZAL A-M. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav. Neurosci. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- FILE S.E., POPE J.H. Social interaction between drugged and undrugged rats. Ani. Learn. Behav. 1974;2:161–164. [Google Scholar]
- FLAHERTY C.F., GREENWOOD A., MARTIN J., LESZCZUK M. Relationship of negative contrast to animal models of fear and anxiety. Anim. Learn. Behav. 1998;26:397–407. [Google Scholar]
- FONE K.C., SHALDERS K., FOX Z.D., ARTHUR R., MARSDEN C.A. Increased 5-HT2C receptor responsiveness occurs on rearing rats in social isolation. Psychopharmacology. 1996;123:346–352. doi: 10.1007/BF02246645. [DOI] [PubMed] [Google Scholar]
- FOWLER S.C., JOHNSON J.S., KALLMAN M.J., LION J.-R., WILSON M.C., HIKAL A.H. In a drug-discrimination procedure isolation reared rats generalize to lower doses of cocaine and amphetamine that rats reared in an enriched environment. Psychopharmacology. 1993;110:115–118. doi: 10.1007/BF02246959. [DOI] [PubMed] [Google Scholar]
- GARDNER C.R., GUY A.P. A social interaction model of anxiety sensitive to acutely administered benzodiazepines. Drug Develop. Res. 1984;4:207–216. [Google Scholar]
- HALL F.S., HUANG S., FONG G.W., PERT A., LINNOILA M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology. 1998;139:203–209. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- IRVINE E.E., CHEETA S., FILE S.E. Time-course of changes in the social interaction test of anxiety following acute and chronic administration of nicotine. Behav. Pharmacol. 1999;10:691–697. doi: 10.1097/00008877-199911000-00016. [DOI] [PubMed] [Google Scholar]
- JONES B.J., COSTALL B., DOMENEY A.M., KELLY M.E., NAYLOR R.J., OAKLEY N.R., TYERS M.B. The potential anxiolytic activity of GR38032F, a 5-HT3 receptor antagonist. Br. J. Pharmacol. 1988;93:985–993. doi: 10.1111/j.1476-5381.1988.tb11489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES G.H., HERNANDEZ T.D., KENDALL D.A., MARSDEN C.A., ROBBINS T.W. Dopaminergic and serotonergic function following isolation rearing in rats: Study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol. Biochem. Behav. 1992;43:17–35. doi: 10.1016/0091-3057(92)90635-s. [DOI] [PubMed] [Google Scholar]
- JONES G.H., MARSDEN C.A., ROBBINS T.W. Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms in the nucleus accumbens. Psychopharmacology. 1990;102:364–372. doi: 10.1007/BF02244105. [DOI] [PubMed] [Google Scholar]
- KENNY P.J., CHEETA S., FILE S.E. Anxiogenic effects of nicotine in the dorsal hippocampus are mediated by 5-HT1A and not by muscarinic M1 receptors. Neuropharmacology. 2000;39:300–307. doi: 10.1016/s0028-3908(99)00114-8. [DOI] [PubMed] [Google Scholar]
- LOPES DA SILVA N., FERREIRA V.M.M., CAROBREZ A.P., MORATO G.S. Individual housing from rearing modifies the performance of young rats on the elevated plus-maze apparatus. Physiol. Behav. 1996;60:1391–1396. doi: 10.1016/s0031-9384(96)00254-5. [DOI] [PubMed] [Google Scholar]
- MENARD J., TREIT D. Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci. Biobehav. Rev. 1999;23:591–613. doi: 10.1016/s0149-7634(98)00056-6. [DOI] [PubMed] [Google Scholar]
- MORINAN A., PARKER V. Are socially isolated rats anxious. Br. J. Pharmacol. 1985;86:460. [Google Scholar]
- NETTER P., HENNIG J., HUWE S., OLBRICH R. Personality related effects of nicotine, mode of application, and expectancies on performance, emotional states, and desire for smoking. Psychopharmacology. 1998;135:52–62. doi: 10.1007/s002130050485. [DOI] [PubMed] [Google Scholar]
- NEWHOUSE P.A., SUNDERLAND T., NARANG P.K., MELLOW A.M., FERTIG J.B., LAWLOR B.A., MURPHY D.L. Neuroendocrine, physiologic, and behavioral responses following intravenous nicotine in nonsmoking healthy volunteers and in patients with Alzheimer's disease. Psychoneuroendocrinology. 1990;15:471–484. doi: 10.1016/0306-4530(90)90070-p. [DOI] [PubMed] [Google Scholar]
- NIESINK R.J., VAN REE J.M. Short-term isolation increases social interactions of male rats: a parametric analysis. Physiol. Behav. 1982;29:819–825. doi: 10.1016/0031-9384(82)90331-6. [DOI] [PubMed] [Google Scholar]
- OUAGAZZAL A.-M., KENNY P.J., FILE S.E. Modulation of anxiety on trials 1 and 2 in the elevated plus-maze after systemic and hippocampal administration of nicotine. Psychopharmacology. 1999a;144:54–60. doi: 10.1007/s002130050976. [DOI] [PubMed] [Google Scholar]
- OUAGAZZAL A.-M., KENNY P.J., FILE S.E. Stimulation of nicotinic receptors in the lateral septal nucleus increases anxiety. Eur. J. Neurosci. 1999b;11:3957–3962. doi: 10.1046/j.1460-9568.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- PARROTT A.C., GARNHAM N.J., WESNES K., PINCOCK C. Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum. Psychopharmacol. 1996;11:391–400. [Google Scholar]
- PELLOW S., CHOPIN P.H., FILE S.E., BRILEY M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Meth. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- RAMOS A., BERTON O., MORMEDE P., CHAOULOFF F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav. Brain Res. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- RODGERS R.J., COLE J.C. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol. Behav. 1993;54:729–736. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- RODGERS R.J., JOHNSON N.J.T., CARR J., HODGSON T.P. Resistance of experientially-induced changes in murine plus-maze behaviour to altered retest conditions. Beh. Brain Res. 1997;86:71–77. doi: 10.1016/s0166-4328(96)02248-6. [DOI] [PubMed] [Google Scholar]
- ROYAL COLLEGE OF PHYSICIANS . Nicotine addiction in Britain: A report of the tobacco advisory group. Suffolk: The Lavenham Press Ltd; 2000. [Google Scholar]
- RUIS M.A., TE BRAKE J.H., BULWADA B., DE BOER S.F., MEERLO P., KORTE S.M., BLOKHUIS H.J., KOOLHAAS J.M. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- SMITH J.K., NEILL J.C., COSTALL B. Post-weaning housing conditions influence the behavioural effects of cocaine and d-amphetamine. Psychopharmacology. 1997;131:23–33. doi: 10.1007/s002130050261. [DOI] [PubMed] [Google Scholar]
- STEENBERGEN H.L., HEINSBROEK R.P., VAN HEST A., VAN DE POLL N.E. Sex-dependent effects of inescapable shock administration on shuttlebox-escape performance and elevated plus-maze behavior. Physiol. Behav. 1990;48:571–576. doi: 10.1016/0031-9384(90)90302-k. [DOI] [PubMed] [Google Scholar]
- THIELEN R.J., McBRIDE W.J., LUMENG L., LI T.K. Housing conditions alter GABAA receptor of alcohol-preferring and -nonpreferring rats. Pharmacol. Biochem. Behav. 1993;46:723–727. doi: 10.1016/0091-3057(93)90568-e. [DOI] [PubMed] [Google Scholar]
- TREIT D., ROBINSON A., ROTZINGEER S., PESOLD C. Anxiolytic effects of serotonergic interventions in the shock-probe burying test and the elevated plus-maze test. Behav. Brain Res. 1993;54:23–34. doi: 10.1016/0166-4328(93)90045-r. [DOI] [PubMed] [Google Scholar]
- VALE A.L., GREEN S. Effects of chlordiazepoxide, nicotine and d-amphetamine in the rat potentiated startle model of anxiety. Behav. Pharmacol. 1986;7:138–143. [PubMed] [Google Scholar]
- VARLINSKAYA E.I., SPEAR L.P., SPEAR N.E. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol. Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- WONGWITECHA N., MARSDEN C.A. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav. Brain. Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- WRIGHT I.K., UPTON N., MARSDEN C.A. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol. Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]


